Abstract
To clarify the specific molecular events of progressive tubular damage in chronic renal failure (CRF), we conducted microarray analyses using isolated proximal tubules from subtotally nephrectomized (Nx) rats as a model of CRF. Our results clearly demonstrated time-dependent changes in gene expression profiles localized to proximal tubules. The expression of mitosis-specific genes Cyclin B2 and Cell division cycle 2 (Cdc2) was significantly and selectively increased in the proximal tubules during the compensated period but decreased to basal level in the end-stage period. Administration of everolimus, a potent inhibitor of mammalian target of rapamycin, markedly reduced compensatory hypertrophy and hyperplasia of epithelial cells, which was accompanied by complete abolishment of the expression of Cyclin B2 and Cdc2 enhancement; renal function was then severely decreased. Treatment with the Cdc2 inhibitor 2-cyanoethyl alsterpaullone clearly decreased epithelial cell hyperplasia, based on staining of phosphorylated histone H3 and Ki-67, while hypertrophy was not inhibited. In conclusion, we have demonstrated roles of Cyclin B2 and Cdc2 in the epithelial hyperplasia in response to Nx. These results advance the knowledge of the contribution of cell cycle regulators, especially M phase, in pathophysiology of tubular restoration and/or degeneration, and these two molecules are suggested to be a marker for the proliferation of proximal tubular cells in CRF.
Keywords: proximal tubule, microarray, cell cycle, G2-M, hypertrophy
in addition to glomerular filtration, renal tubular cells are important for the reabsorption and secretion of various compounds to maintain the homeostasis of body fluid. In chronic renal failure (CRF), loss of nephron mass results in compensating responses with respect to the function and structure of the kidney for minimizing the reduction in the total glomerular filtration rate (GFR); the hyperreactivity of the residual nephron results in irreversible glomerular sclerosis, tubular atrophy, and subsequent tubulointerstitial fibrosis (11, 15, 35). Previous studies have demonstrated that the decline in GFR is not associated with a decline in tubular function (13) and that the renal epithelium is intrinsically susceptible to injury because it concentrates many toxins (1). These findings suggest the importance of understanding the pathogenesis of dysfunction of tubular cells in CRF.
The subtotally nephrectomized (Nx) rat is a well-established model for understanding the pathological changes in CRF (15). With the use of this model, the effects on proximal tubular functions were examined. Several studies, including ours, have previously demonstrated the expressional and functional changes of membrane transporters in the compensated remnant kidneys and the effect of transporter levels on renal handling of ionic drugs in progressive CRF (14, 17, 20, 29, 30, 38). However, these changes were studied from a phenomenological perspective, and therefore there is only limited information on the basic molecular mechanisms and/or networks involved in the progression of tubular damage in CRF. Some candidate genes responsible for progressive renal injury were identified by microarray analysis using whole kidney samples; however, reproducibility of this strategy has been poor (40, 44).
To overcome these problems and find the responsible genes affecting tubular pathological changes in compensative CRF, we conducted a microarray analysis with isolated proximal tubules at several time points after Nx and found the expression of the mitosis-specific genes Cyclin B2 and Cell division cycle 2 (Cdc2), which are M-phase regulators, to be transiently upregulated in the proximal tubules of the compensatory kidney. Although cell cycle regulation has been demonstrated to play important roles in renal pathophysiology, these reports mainly focused on the molecules that played roles in G1-S phase among the four stages of the cell cycle, G1, S, G2, and M (23, 31, 33, 42). Therefore, it remains unclear which molecules and/or regulators are critical in the progress and restoration of tubular damage in CRF. On the basis of these findings, we focused on the M-phase regulators and then examined in vivo effects of a specific inhibitor for Cdc2 on their expression and renal function. The time-dependent expression profile of Cyclin B2-Cdc2 has been suggested to explain, in part, the balance of epithelial hypertrophy and hyperplasia in the remnant proximal tubules in progressive CRF.
MATERIALS AND METHODS
Animals.
Male Wistar/ST rats (180–200 g) were subtotally nephrectomzed as described previously (17, 38). In brief, the right kidney was removed, and the posterior and anterior apical segmental branches of the left renal artery were individually ligated. Sham-operated rats at 2 wk after surgery were used as controls. Everolimus (LC Laboratories, Woburn, MA; 2 mg/kg body weight, E+), 2-cyanoethyl alsterpaullone [CE-ALP; Calbiochem, Darmstadt, Germany; 0.5 mg/kg body wt, dissolved in Cremophor-EtOH-DMSO-saline (12.5:12.5:5:70 vol/vol/vol/vol), A+], or vehicle (E− or A−) was subcutaneously administered to Nx rats every day for 2 wk after surgery. The animals were allowed free access to water and standard chow. To examine renal function, the levels of blood urea nitrogen (BUN) and plasma creatinine (PCr) and urine creatinine were determined. For measurements, we used an assay kit from Wako Pure Chemical Industries (Osaka, Japan). The urinary concentration of albumin was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Nephrat II, Exocell, Philadelphia, PA). For histological examinations, the kidney was stained with periodic acid Schiff (PAS) reagent by Sapporo General Pathology Laboratory (Hokkaido, Japan). The glomerular diameter and the height of epithelial cells were determined as mean values from 30 glomeruli and 20 proximal tubules in 3 rats, respectively. All protocols had been approved previously by the Animal Research Committee, Graduate School of Medicine, Kyoto University.
Isolation of proximal tubules by microdissection.
To obtain renal proximal tubules, the rat nephron was microdissected as described previously (26). In brief, the left kidney was perfused and removed, and slices of the kidney were cut along the medullary axis and incubated with collagenase; the renal proximal tubules were then microdissected and isolated with sharp needles under a light microscope. After microdissection, the tubules (20 mm) were transferred into a microtube to isolate total RNA with the RNeasy mini kit (Qiagen, Hilden, Germany). The quality of total RNA was validated with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). To examine the purity of isolated proximal tubules, RT-PCR and microarray analysis were performed with isolated proximal tubules and whole kidney specimens from normal rats (Supplemental Fig. S1).1
Microarray analysis using isolated proximal tubules.
Digoxigenin (DIG)-UTP-labeled complementary (c)RNA was generated from the proximal tubular total RNA of four control rats (sham-operated rats at 2 wk after surgery) and Nx rats (1, 2, 4, and 8 wk after surgery) with Applied Biosystems Chemiluminescent RT-IVT Labeling Kit v2.0 (Applied Biosystems, Foster City, CA). Array hybridization, chemiluminescence detection, and image acquisition were performed according to the manufacturer's directions. In brief, 10 μg of cRNA was hybridized to Rat Genome Survey Microarrays (Applied Biosystems) at 55°C for 16 h. After the arrays were washed, anti-DIG-alkaline phosphatase (DIG-AP; Roche Diagnostic, Mannheim, Germany) was hybridized at room temperature for 20 min. Enhanced chemiluminescence signals were generated by adding the substrate solution and scanned with the ABI 1700 Chemiluminescent Microarray Analyzer (Applied Biosystems). The results of the microarray assay were analyzed with Spotfire software (TIBCO Software, Palo Alto, CA). After the bad spots were flagged, the assay signals were normalized across the experiments with each median. Furthermore, detectable signals were selected with a signal-to-noise (S/N) threshold (a gene with an S/N threshold >3 in 3 or 4 assays was considered to be detectable). The ratio of the signal from the Nx rat assay to the control signal was calculated, and a profile ANOVA was performed. Genes with a P value <0.01 and a ratio >2 or <0.5 were considered to be significantly upregulated or downregulated, respectively. Finally, these genes were classified with MetaCore Software (GeneGo, St. Joseph, MI) according to their function. A filled column in Fig. 1 indicates that the false discovery rate was <0.01.
Fig. 1.
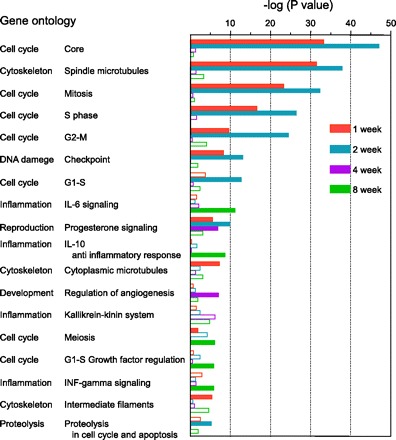
Biological function of the genes significantly changed in the microarray analysis. To assess the results of the microarray analysis in terms of biological function, the genes that significantly changed each week after subtotal nephrectomy (Nx) were classified according to their Gene Ontology and P values were calculated with MetaCore software. A filled column implies that the false discovery rate was <0.01.
Real-time PCR.
Whole kidney total RNA was extracted with the MagNA Pure LC RNA Isolation Kit II (Roche Diagnostic). Total RNA was reverse transcribed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and subjected to digestion with RNase H (Invitrogen, Carlsbad, CA). Real-time PCR was performed with the ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The primer-probe set used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and other genes were Predeveloped TaqMan Assay Reagents (Applied Biosystems) and Premade TaqMan Assay Reagents, respectively. GAPDH mRNA expression was measured as an internal control.
Measurement of Cdc2 activity in kidney.
The activities of Cdc2 in the kidney were examined with the MESACUP cdc2 Kinase Assay Kit (MBL, Nagoya, Japan) according to the manufacturer's instructions with slight modification. The kidney was homogenized in lysis buffer [in mM: 50 Tris · HCl pH 7.5, 150 NaCl, 10 NaF, 1 Na4P2O7, and 100 Na3VO4, with 1% NP-40, 1% protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan), and phosphatase inhibitor (PhosSTOP; Roche Diagnostic)]. The tissue lysate was clarified by centrifugation, and protein concentrations were determined with the Bradford protein assay. The phosphorylation reaction was performed with the lysate (50 μg) in the presence of 0.1 mM ATP at 30°C for 5 min. The phosphorylated substrates were detected by ELISA.
In situ hybridization.
Fixed paraffin-embedded blocks and sections of rat kidney for in situ hybridization were obtained from Genostaff (Tokyo, Japan). After dewaxing and rehydration, the sections (6 μm) were fixed with 4% paraformaldehyde. The sections were treated with proteinase K, washed with PBS, and placed in 0.2 N HCl for 10 min. After washing, the sections were acetylated by incubation in 0.1 M triethanolamine-HCl and 0.25% acetic anhydride for 10 min. Hybridization was performed with probes specific for Cyclin B2 and Cdc2 (300 ng/ml) at 60°C for 16 h. The sections were then washed in HybriWash (Genostaff) before being subjected to RNase treatment. After treatment with 0.5% blocking reagent (Roche Diagnostic), the sections were incubated with anti-DIG-AP conjugate (Roche Diagnostic) for 2 h. Coloring reactions were performed with nitro blue tetrazolium chloride-5-bromo-4-chloro-3-indolyl phosphatase (NBT-BCIP; Sigma-Aldrich, St. Louis, MO) overnight. They were then counterstained with Kernechtrot stain solution.
Immunofluorescent analysis.
The fixed tissue sections were prepared and immunofluorescent analysis was performed as described previously with slight modification (29, 30). The animals were anesthetized, and the kidneys were perfused via the abdominal aorta, first with saline containing 50 U/ml of heparin and then with 4% paraformaldehyde in PBS. Fixed tissues were embedded in Tissue-Tek OCT compound (Sakura Finetek, Tokyo, Japan) and frozen rapidly in liquid nitrogen. Sections (5 μm thick) were cut and covered with 5% BSA and 0.3% Triton X-100 in PBS for Ki-67, rabbit serum (Invitrogen) for Cyclin B2, and SuperBlock Blocking Buffer (Thermo Fisher Scientific, Waltham, MA) for Cdc2 at 37°C for 60 min. The covered sections were incubated with antiserum specific for Ki-67 (1:500; Abcam, Cambridge, UK), Cyclin B2 (1:200, Abcam), and Cdc2 (1:200, Cell Signaling Technology, Danvers, MA) at 4°C overnight. After further washing, the sections were incubated with Alexa Fluor 546-labeled goat anti-rabbit IgG or Alexa Fluor 488-labeled anti-mouse IgG, Alexa Fluor 488-labeled phalloidin or Alexa Fluor 594-labeled phalloidin (Invitrogen), and 4′,6-diamidino-2-phenylindole (DAPI; Wako) at 37°C for 60 min.
Immunohistochemistry of phospho-histone H3 at serine 10.
The sections of immunohistochemistry were obtained from Genostaff. After deparaffinization and rehydration, the sections (5 μm) were treated with proteinase K and 0.3% hydrogen peroxide. The sections were blocked with Protein Block (Dako) and the Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA) followed by incubation with anti-phospho-histone H3 (serine 10) (p-histone H3) antibody (Abcam) at 4°C overnight. After washing, the sections were incubated with biotinylated goat anti-rabbit IgG (Dako). After further washes, the sections were incubated with an avidin-biotinylated horseradish peroxidase complex (Nichirei Biosciences, Tokyo, Japan). Coloring reactions were performed with hydrogen peroxide containing 3,3′-diaminobenzidine (DAB-H2O2). The sections were then counterstained with hematoxylin stain solution.
Image analysis.
The images of in situ hybridization and immunofluorescent and immunohistochemical analysis were captured and examined with BZ-9000 (Keyence, Osaka, Japan). To evaluate the levels of Ki-67, Cyclin B2, Cdc2, and p-histone H3, three or six independent photographs in the renal cortex were taken at 100-fold magnification (each total area 1.58 μm2), and the stained nuclei in the proximal tubules were counted.
Statistical analysis.
All data are expressed as means ± SE. Comparisons were performed with the unpaired t-test. Multiple comparisons were performed with a one-way ANOVA. Probability values of <0.05 were considered statistically significant.
RESULTS
Gene expression profile of renal proximal tubules in CRF rats.
Before microarray analyses were performed with the isolated proximal tubules, the purity of the proximal tubules was examined by RT-RCR (Supplemental Fig. S1A). The band for Na+-glucose cotransporter (SGLT)2, a marker for proximal tubules (46), was clearly found in the proximal tubules, while those for podocin, Na+-K+-Cl− cotransporter (NKCC)2, and aquaporin (AQP)-2, markers for glomeruli (18), thick ascending limbs (8), and collecting ducts (7), respectively, were not amplified in the proximal tubules, whereas all bands were found in the whole kidney. Simultaneously, the expressional profiles in the isolated proximal tubules and whole kidney were compared by microarray analysis (Supplemental Fig. S1B). Consistent with the results of RT-PCR, the intensity of SGLT2 in the proximal tubules was much higher than in whole kidney. On the other hand, the genes that were not contained in the proximal tubules such as hemoglobin showed low intensity in the proximal tubules. Consequently, the purity of the isolated proximal tubules was well confirmed.
After Nx, urine volume and PCr significantly increased, BUN and urinary albumin excretion time-dependently increased, and creatinine clearance (CCr) markedly decreased compared with sham-operated rats (Table 1). Therefore, marked renal insufficiency and its progression were confirmed in Nx rats. Next, microarray analyses were performed with isolated renal proximal tubules of these sham-operated and Nx rats. To establish distinctive expressional profiles of proximal tubules in progressive CRF, genes were selected according to the criteria described in materials and methods. The numbers of selected genes after statistical analysis are summarized in Table 2. Among these, the total numbers of genes whose expression was significantly changed more than twofold compared with control at 1, 2, 4, and 8 wk after surgery were 239, 161, 278, and 389, respectively. On the basis of the total number of genes, we classified them according to their Gene Ontology, using MetaCore software to identify the biological function involved in the progression of CRF in the proximal tubules. The results revealed that the cell cycle- or cytoskeleton-related genes were significantly altered at 1 and 2 wk after Nx (Fig. 1). On the other hand, inflammation-related genes were frequently detected at 8 wk after Nx.
Table 1.
Biochemical parameters in sham-operated and subtotally nephrectomized rats
| Nx |
|||||
|---|---|---|---|---|---|
| Sham (n = 10) | 1 wk (n = 7) | 2 wk (n = 13) | 4 wk (n = 11) | 8 wk (n = 9) | |
| Body weight, g | 264.1 ± 6.2 | 210.7 ± 6.0† | 231.5 ± 5.9* | 288.4 ± 7.7 | 323.9 ± 12.2† |
| Urine volume, ml/day | 6.9 ± 1.0 | 24.8 ± 1.9† | 23.6 ± 1.7† | 26.4 ± 1.8† | 25.3 ± 3.4† |
| BUN, mg/dl | 13.5 ± 0.5 | 44.1 ± 6.5 | 57.4 ± 6.5† | 71.0 ± 12.0† | 85.0 ± 15.3† |
| PCr, mg/dl | 0.52 ± 0.02 | 1.22 ± 0.18* | 1.23 ± 0.10* | 1.26 ± 0.15* | 1.96 ± 0.32† |
| CCr, ml·min−1·kg−1 | 4.42 ± 0.23 | 2.70 ± 0.36† | 2.31 ± 0.22† | 2.46 ± 0.28† | 1.69 ± 0.30† |
| Albumin excretion, mg/day | 0.5 ± 0.1 | 3.5 ± 0.3 | 21.5 ± 4.7 | 67.4 ± 4.5* | 128.6 ± 45.2† |
Data represent means ± SE for n rats. Sham, sham-operated rats at 2 wk after surgery; Nx, 5/6 nephrectomized rats; BUN, blood urea nitrogen; PCr, plasma creatinine; CCr, creatinine clearance. Multiple comparison was performed with Dunnett's 2-tailed test after a 1-way ANOVA.
P < 0.05,
P < 0.01, significantly different from Sham.
Table 2.
Number of genes in microarray analysis
| 1 wk | 2 wk | 4 wk | 8 wk | |
|---|---|---|---|---|
| Total number | 239 | 161 | 278 | 389 |
| Up/down | 191/48 | 79/82 | 176/102 | 268/117 |
| Unique number | 103 | 37 | 121 | 246 |
| Up/down | 98/5 | 24/13 | 97/24 | 206/40 |
| Common number | 36 | |||
| Up/down | 3/33 | |||
Sham-operated rats at 2 wk after surgery were used as controls. Genes were selected when the ratio to control was >2 or <0.5 and the P value was <0.01. Unique and common imply that the genes specifically changed in each week and throughout the experimental period, respectively.
Validation of microarray analysis revealed activation of mitosis-specific genes in remnant kidney immediately after Nx.
To examine whether renal epithelial cells entered into the cell cycle immediately after Nx, the expression of Ki-67, a proliferation marker (9), was examined. Comparable to the results of microarray analysis, the proximal tubules of Nx rats at 1, 2, and 4 wk after surgery showed abundant staining for Ki-67 compared with those in the sham-operated rats; however, the number of stained nuclei in the proximal tubules was markedly decreased to approximately control level at 8 wk after surgery (Fig. 2). Furthermore, the mRNA levels of some cell cycle regulators were examined by real-time PCR using cDNA of the remnant kidney to confirm whether the expression of these genes was also detected in whole kidney samples. Among eight cell cycle-related genes such as Cyclins (Cyclin A2, B2, D1, and E2) and Cyclin-dependent kinases (Cdk; Cdk2, 4, 6 and Cdc2), the changes in the levels of Cyclin B2 and Cdc2 at 1 and 2 wk after Nx corresponded well with the results of the microarray analysis (Fig. 3A). The activities of Cdc2 were significantly increased in Nx rats at 1 wk after surgery compared with sham-operated rats, and the increased activity was retained at 2 wk after Nx (Fig. 3B). Furthermore, Cdc2 activity was decreased almost to the sham-operated level with the progression of CRF.
Fig. 2.
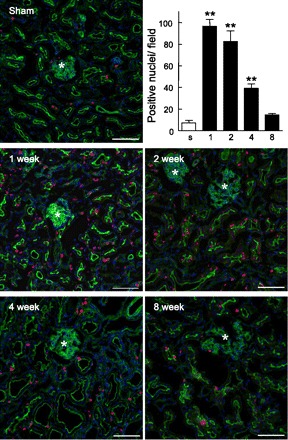
Expression of Ki-67 in the kidney. Immunofluorescent labeling of Ki-67 in sham-operated and Nx rats at 1, 2, 4, and 8 wk after surgery is shown. Red signals for Ki-67 merged with green signals for phalloidin and with blue signals for 4′,6-diamidino-2-phenylindole (DAPI). *, Glomeruli. Scale bars 100 μm. Stained nuclei in the proximal tubules were counted in 3 independent regions at 100-fold magnification. s, Sham-operated rats; 1, 2, 4, and 8, Nx rats at 1, 2, 4, and 8 wk after surgery. Multiple comparisons were performed with Dunnett's 2-tailed test after a 1-way ANOVA. **P < 0.01, significantly different from sham-operated rats.
Fig. 3.
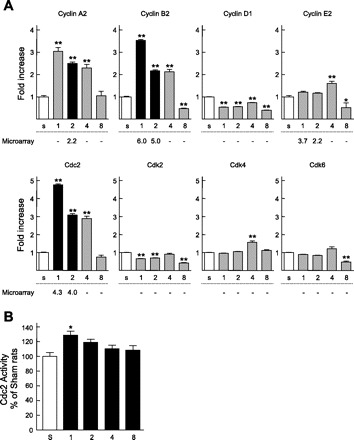
Expression levels of cell cycle-related genes and the activities of Cell division cycle 2 (Cdc2) in the remnant kidney. A: mRNA levels of Cyclin A2, Cyclin B2, Cyclin D1, Cyclin E2, Cdc2, Cyclin-dependent kinase (Cdk)2, Cdk4, and Cdk6. An equal amount of cDNA was pooled from the remnant kidney of each rat, and the expressional changes of mRNA were measured by real-time PCR and analyzed by the ΔΔCt method (where Ct is threshold cycle). Numbers below each column show the fold change in the microarray analysis; − indicates that expressional change was not significant in the microarray analysis. Open columns represent levels in sham-operated rats; black and gray columns represent results of the microarray analysis validated by real-time PCR analysis or not, respectively. B: kinase activities of Cdc2. Multiple comparisons were performed with Dunnett's 2-tailed test after a 1-way ANOVA. *P < 0.05, **P < 0.01, significantly different from sham-operated rats.
Cyclin B2 and Cdc2 were specifically expressed and upregulated in renal proximal tubules of CRF rats.
On the basis of the validation of microarray analysis and the enhancement of Cdc2 activity in the remnant kidney, we focused on Cyclin B2 and Cdc2 and further confirmed the expression of these genes in individual sham-operated and early-stage Nx rats. The mRNA levels of Cyclin B2 and Cdc2 were significantly increased in the remnant kidney at 1 and 2 wk after Nx compared with the sham-operated rats (Fig. 4, A and B). On the basis of the in situ hybridization analysis, the mRNAs of Cyclin B2 and Cdc2 were specifically visualized in a portion of the epithelial cells in the proximal tubules in sham-operated rats, and their expression levels were markedly increased in the proximal tubules of Nx rats at 2 wk after surgery (Fig. 4C, arrows). The number of stained nuclei in the proximal tubules of renal cortex significantly increased to approximately threefold in the Nx rats, which was consistent with the results of real-time PCR (Fig. 4, D and E). We examined the relationship between the levels of Cyclin B2 and Cdc2 because both proteins function as heterodimers in the mitotic phase (16). The mRNA level of Cyclin B2 correlated well with that of Cdc2 (coefficient of correlation r2 = 0.80, P < 0.0001) (Fig. 4F). Furthermore, most of the signals for Cyclin B2 and Cdc2 were simultaneously observed in the proximal tubular cells (Fig. 4G, arrows), and a portion of them were independently detected (Fig. 4G, open and filled arrowheads).
Fig. 4.
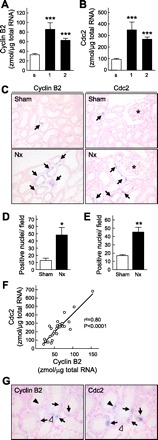
Detection of Cyclin B2 and Cdc2 in the kidney by real-time PCR and in situ hybridization analysis. A and B: mRNA levels of Cyclin B2 (A) and Cdc2 (B) were measured by real-time PCR. s, Sham-operated rats; 1 and 2, Nx rats at 1 and 2 wk after surgery. Multiple comparisons were performed with Dunnett's 2-tailed test after a 1-way ANOVA. ***P < 0.001, significantly different from sham-operated rats. C: in situ hybridization of Cyclin B2 (left) and Cdc2 (right). *, Glomeruli. Magnification ×200. D and E: Cyclin B2 (D)- and Cdc2 (E)-positive nuclei in the renal cortex were counted. *P < 0.05, **P < 0.01, significantly different from sham-operated rats. F: correlation between mRNA levels of Cyclin B2 and Cdc2. Linear regression analysis was performed, and the correlation coefficient (r) was calculated. G: in situ hybridization analysis of Cyclin B2 and Cdc2 with serial sections in Nx rats was carried out. Arrows, positive staining for both Cyclin B2 and Cdc2; open arrowhead, positive staining for Cyclin B2 without Cdc2; filled arrowhead, positive staining for Cdc2 without Cyclin B2.
On immunofluorescent analysis with serial sections, Cyclin B2 and Cdc2 signals were slightly detected in the proximal tubules, but no signals were observed at the glomeruli or other tubular segments in the sham-operated rats (Fig. 5, A and B). In Nx rats, Cyclin B2 and Cdc2 signals were markedly increased and aggregated in the proximal tubules (Fig. 5, C and D). As indicated by in situ hybridization, the localization of Cyclin B2 and Cdc2 was identified in the same epithelial cells (Fig. 5, C and D, arrows).
Fig. 5.
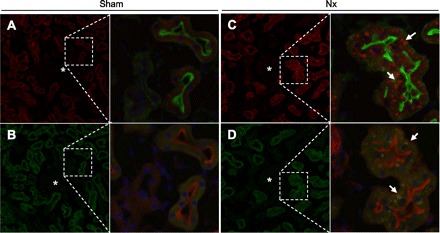
Immunofluorescent analysis of Cyclin B2 and Cdc2 in the kidney. The kidney was perfused, fixed, and then embedded. Sections (5 μm) were stained with a specific antibody for Cyclin B2 (A and C, red) or Cdc2 (B and D, green), phalloidin (A and C, green; B and D, red), and DAPI (blue). A and B: series of sections from sham-operated rat kidney. C and D: series of sections from Nx rat kidney at 2 wk after surgery. Arrows, aggregation of signals for Cyclin B2 or Cdc2; *, glomeruli. Magnification ×200 and ×400.
Effects of mammalian target of rapamycin inhibitor in CRF rats.
Next, we examined the effects of the mammalian target of rapamycin (mTOR) inhibitor everolimus on the expression levels of Cyclin B2 and Cdc2 and renal function of Nx rats at 2 wk after surgery. Everolimus markedly reduced the mRNA expression of Cyclin B2 and Cdc2 in Nx rats to levels similar to those in sham-operated rats (Fig. 6, A and B, vs. Fig. 4, A and B). Immunofluorescent analysis revealed that Ki-67-positive proximal tubular epithelial cells were decreased to 20% in everolimus-treated (E+) rats compared with those in vehicle-treated (E−) rats (Fig. 6, C and D). As shown in Fig. 6E, compensatory renal hypertrophy was significantly inhibited by the administration of everolimus. Correspondingly, the kidney-to-body weight ratio was significantly decreased by everolimus treatment, although the body weight of these rats was markedly decreased compared with that of the vehicle-treated rats (Table 3, E− vs. E+). Histological examination of the remnant kidneys also showed inhibition of glomerular and tubular hypertrophy in E+ rats (Fig. 6F). Moreover, the glomerular diameter and height of epithelial cells were significantly decreased in E+ rats (Fig. 6, G and H). At this time, administration of everolimus markedly increased the level of BUN and PCr and decreased the level of CCr (Table 3). However, urinary excretion of albumin was lowered in the E+ rats to ∼30% of that in the E− rats.
Fig. 6.
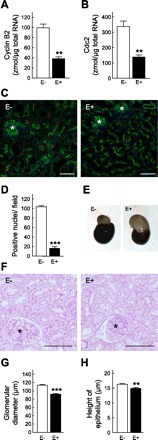
Effects of administration of everolimus in Nx rats. Nx rats were subcutaneously treated with vehicle (E−) or everolimus (E+, 2 mg/kg) for 14 days immediately after Nx. A and B: detection of mRNA of cyclin B2 (A) and Cdc2 (B) by real-time PCR. C: immunofluorescent analysis of Ki-67. D: numbers of stained nuclei for Ki-67 in the proximal tubules were counted in 3 independent regions at 100-fold magnification. E: appearance of remnant kidney. F: representative photographs of periodic acid Schiff (PAS) staining of the remnant kidney. G and H: measurement of glomerular diameter (G) and the height of epithelial cells (H). *, Glomeruli. Scale bars 100 μm. **P < 0.01, ***P < 0.001, significantly different from vehicle-treated (E−) rats.
Table 3.
Effects of administration of everolimus and Cdc2 inhibitor on renal function in Nx rats
| Everolimus |
2-Cyanoethyl Alsterpaullone |
|||
|---|---|---|---|---|
| E− (n = 8) | E+ (n = 6) | A− (n = 11) | A+ (n = 9) | |
| Body weight, g | 226.1 ± 5.3 | 171.9 ± 4.5‡ | 242.5 ± 3.6 | 222.7 ± 8.8* |
| Kidney weight/body weight, % | 0.26 ± 0.01 | 0.22 ± 0.01† | 0.24 ± 0.01 | 0.24 ± 0.01 |
| Urine volume, ml/day | 15.4 ± 1.8 | 17.7 ± 3.9 | 25.3 ± 2.0 | 29.1 ± 2.3 |
| BUN, mg/dl | 41.9 ± 4.8 | 68.1 ± 5.0† | 39.2 ± 2.0 | 54.2 ± 7.2* |
| PCr, mg/dl | 0.90 ± 0.07 | 1.17 ± 0.08† | 1.07 ± 0.05 | 1.22 ± 0.01 |
| CCr, ml·min−1·kg−1 | 2.83 ± 0.17 | 1.41 ± 0.16‡ | 2.69 ± 0.19 | 2.44 ± 0.27 |
| Albumin excretion, mg/day | 8.4 ± 4.5 | 2.4 ± 1.0 | 5.7 ± 1.5 | 11.8 ± 3.9 |
Data represent means ± SE for n rats. E− and A−, Nx rats administered vehicle; E+ and A+, Nx rats daily administered everolimus (2 mg/kg) and 2-cyanoethyl alsterpaullone (0.5 mg/kg) for 14 days, respectively.
P < 0.05,
P < 0.01,
P < 0.001, significantly different from vehicle-treated rats.
Effect of Cdc2 inhibitor in CRF rats.
A Cdc2-specific inhibitor, CE-ALP, was administered to Nx rats to obtain more direct information about the significance of cell cycle-related molecules during the compensative period. The mRNA levels of Cyclin B2 and Cdc2 showed no difference with or without administration of the inhibitor (Fig. 7, A and B). However, administration of CE-ALP significantly decreased the number of nuclei with positive signals for Ki-67 to ∼60% in the proximal tubules (Fig. 7, C and D). CE-ALP also deteriorated renal function of Nx rats, although the effects were less than those of everolimus. The level of BUN was significantly increased, the urine volume and PCr were also increased, and CCr was decreased in the CE-ALP-treated rats (Table 3, A− vs. A+). The excretion of albumin tended to be high, suggesting that the kidney was further damaged by Cdc2 inhibition. Histological examination of the remnant kidneys revealed that the glomerular diameter was not affected, but the height of epithelial cells was significantly increased by the treatment with CE-ALP (Fig. 7, E–G).
Fig. 7.
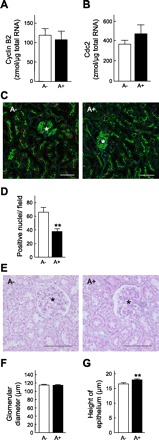
Effects of administration of 2-cyanoethyl alsterpaullone (CE-ALP) in Nx rats. Nx rats were subcutaneously treated with vehicle (A−) or CE-ALP (A+, 0.5 mg/kg) for 14 days immediately after Nx. A and B: measurement of mRNA levels of Cyclin B2 (A) and Cdc2 (B) by real-time PCR. C: immunofluorescent analysis of Ki-67. D: numbers of stained nuclei in the proximal tubules were counted in 6 independent regions at 100-fold magnification. E: representative photographs of PAS staining of the remnant kidney. F and G: measurement of glomerular diameter (F) and height of epithelial cells (G). *, Glomeruli. Scale bars 100 μm. **P < 0.01, significantly different from vehicle-treated (A−) rats.
Effects of everolimus and CE-ALP on expression of p-histone H3 and activity of Cdc2.
To examine whether renal proximal tubular cells underwent mitosis in compensative CRF, the levels of p-histone H3, a mitosis marker (12), were examined and the positive nuclei were counted (Fig. 8, A and B). p-Histone H3 staining in the proximal tubular nuclei was abundantly observed in Nx rats compared with sham-operated rats. Administration of everolimus abolished the level of p-histone H3 almost to the level of the sham-operated rats. In addition, treatment with CE-ALP clearly decreased the level of p-histone H3 to ∼50% in Nx rats. Consistent with the immunohistochemical analysis of p-histone H3, the activities of Cdc2 after the administration of everolimus and CE-ALP were significantly decreased compared with those of the vehicle-treated Nx rats (Fig. 8C).
Fig. 8.
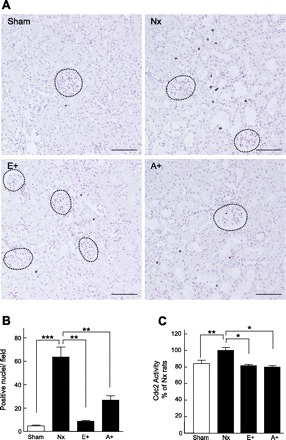
Effects of everolimus and CE-ALP on expression of phospho (p)-histone H3 and activities of Cdc2 in the kidney. A: representative photograph of kidney stained with p-histone H3 in sham-operated rats, Nx rats, and Nx rats treated with everolimus (E+) or CE-ALP (A+) at 2 wk after surgery. Dotted circles, glomeruli. Scale bars 100 μm. B: numbers of nuclei stained for p-histone H3 in the proximal tubules were counted at 100-fold magnification. C: activities of Cdc2 after administration of everolimus and CE-ALP. Multiple comparisons were performed with Bonferroni's test after a 1-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different.
DISCUSSION
The kidney is a heterogeneous organ with many types of cells; therefore, it is difficult to clarify the cell-specific changes in molecular events in CRF. Almost no microarray analyses have been undertaken with nephron segment-selective samples. In statistical analyses of microarray experiments, strong expression signals derived from other segments often disturb the detection of responses in intact proximal tubular epithelial cells. Previous gene expression profiles focusing on tubular cells were obtained with rat primary proximal tubular cells (36, 41) or human proximal tubule-derived HK-2 cells (22) to study the responses to nephrotoxic drugs or hypoxia. Therefore, these previous reports could not explain the pathophysiology of progressive CRF in vivo. The present study was designed to investigate these issues by continuously isolating viable proximal tubules during CRF progression. The histology of the remnant kidneys showed severe glomerular sclerosis, tubular dilation, and interstitial fibrosis at 4–8 wk after Nx (Supplemental Fig. S2A). The accumulation of ED1 (a marker of infiltrated macrophage) and α-smooth muscle actin (a fibrotic marker) was also observed in the later phase of Nx rats (Supplemental Fig. S2, D and E). However, the molecules related to the fibrotic response, such as transforming growth factor-β or connective tissue growth factor (2, 5), were not increased in the microarray analysis of proximal tubules even 8 wk after Nx. Furthermore, interleukin-1β and tumor necrosis factor-α were also undetectable, although they were reported to be increased in Nx rats in a study using whole kidney samples (37). Therefore the present microarray data may be considered to accurately reflect the responses to CRF in the proximal tubules, avoiding contamination from other nephron segments and infiltrating immune cells.
Immediately after Nx, significant changes were found in the expression of cell cycle-related genes (Fig. 1). The mammalian cell cycle is positively regulated by complexes of cell cycle proteins consisting of a catalytic subunit (called Cdk) and a regulatory subunit (called cyclin). In addition, the cyclin-Cdk complexes are negatively regulated by proteins called Cdk inhibitors (10). Several studies showed that cell cycle regulators are involved in renal pathophysiology. In acute renal injury, it is considered that cell cycle activation focusing on Cdk2 and Cdk inhibitor p21WAF1/Cip1 contributes to both cellular life and death in the renal tubules (31). Megyesi et al. (27) reported that p21WAF1/Cip1-knockout mice did not develop CRF such as glomerular sclerosis and systemic hypertension. However, there is no clear difference in renal function between the presence and absence of p21WAF1/Cip1 immediately after renal ablation. In the present study, the increase in expression of p21WAF1/Cip1 was found in both microarray analysis and real-time PCR and the enhancements were retained throughout the experimental period (Supplemental Fig. S3). Furthermore, p21WAF1/Cip1 affects several phases of the cell cycle through the inhibition and interaction of Cdks (43). On the basis of these results and reports, further study might be needed to clarify the specific contribution of p21WAF1/Cip1 to compensative CRF.
It is reported that the renal hypertrophy is modulated by a cell cycle-dependent mechanism whereby Cyclin D-Cdk4/6 is activated without subsequent Cyclin E-Cdk2 involvement; thus the cell cycle is arrested in late G1, resulting in cellular hypertrophy (23, 33). Other studies have demonstrated that the induction and accumulation of Cdk inhibitor p27Kip1 play pivotal roles in the arrest in G1 by using an in vitro model of hypertrophy (42). Considering the expression profiles, the levels of D-type cyclins, E-type cyclins, Cdk2, Cdk4, Cdk6, as well as p27Kip1 were not definitively changed in the compensatory period in Nx rats because of the lowered fold changes or S/N threshold. In contrast, the expression of Cyclin B2 and Cdc2, which mediated the M-phase transition, was significantly upregulated and the activities of Cdc2 were increased in the remnant kidney, especially at early stages (Fig. 3). Furthermore, the two were colocalized to the same epithelial cells in Nx rats (Figs. 4G and 5, C and D). It is known that two B-type cyclins (B1 and B2) are able to bind and activate Cdc2. We also examined the level of Cyclin B1, although statistically significant changes were not observed in the microarray analysis (Supplemental Fig. S4). Interestingly, the expression pattern after Nx and response to everolimus in Cyclin B1 was quite similar to those in Cyclin B2 and Cdc2. These results clearly indicated the coordination of their functions and the activation of a state of M phase in the epithelial cells in the Nx rat kidneys.
Among five Cdks, only disruption of Cdc2 caused embryonic lethality in the studies with gene knockout mice, indicating that the G2-M regulator is a crucial factor in embryonic development (32). We confirmed that the mRNA levels of Cdc2 as well as Cyclin B1 and B2 were high during renal development and decreased with growth (Supplemental Fig. S5, A–C). Furthermore, the activities of Cdc2 were well correlated with the mRNA levels of Cdc2 (r2 = 0.86, P < 0.0001), suggesting that the mRNA level might reflect the activity of Cdc2 (Supplemental Fig. S5D). Thus the M-phase regulator Cdc2, in association with Cyclin B, is essential for the promotion of the cell cycle, and we suggest that these regulators are responsible for compensative proliferation of the proximal tubular epithelium itself. On the contrary, the expression levels of Cyclin B2 and Cdc2 and the activity of Cdc2 in the remnant kidney were reduced almost to the sham-operated level, and the Ki-67-positive tubules were markedly decreased at 8 wk after surgery. These phenomena might be associated with the expression profile of Cyclin B-Cdc2 in that the epithelial cells in the fibrotic period could not proliferate because of the lack of M-phase cyclins. Several reports demonstrated that the mitotic cyclins were mainly regulated at the level of transcription by some transcriptional factors such as B-MYB, E2F, FOXM1, and NF-Y (6). In the present study, the expression of these transcriptional factors was not significantly changed by Nx treatment in the microarray analysis, and therefore further examinations would be needed to clarify the molecular mechanisms of the increase in Cyclin B2-Cdc2 after Nx.
The mTOR pathway has been demonstrated to contribute to the tissue hypertrophy (21) and pathophysiology of several kidney diseases such as diabetic nephropathy (24, 45) and polycystic kidney disease (34, 39). Treatment with the mTOR inhibitor rapamycin (sirolimus) was reported to attenuate renal hypertrophy in unilaterally nephrectomized mice by modulating RNA and protein synthesis (3). Most recently, it was demonstrated that S6 kinase 1 (S6K1), a downstream effector of mTOR, plays a major role in the development of compensatory renal hypertrophy in the same model using S6K1-knockout mice (4). Thus the molecular mechanisms of renal hypertrophy on the mTOR signaling pathway have been elucidated in detail. In this study, the increased expression in Cyclin B2 and Cdc2 was completely abolished, and the signals for Ki-67 and p-histone H3 in the epithelial cells were clearly decreased by treatment with everolimus in the Nx rats (Figs. 6 and 8). Therefore it can be inferred that everolimus inhibited both cellular hypertrophy and hyperplasia by modulating RNA and protein synthesis and enhanced cell division arrest by reducing the levels of Cyclin B-Cdc2, resulting in severe renal damage in compensative CRF.
Because the mTOR pathway affects both hypertrophy and hyperplasia, it is difficult to evaluate the specific contribution of the upregulation of Cyclin B-Cdc2 to the epithelial proliferation. Treatment with CE-ALP, a specific inhibitor of Cyclin B-Cdc2 with an IC50 value of 0.23 nM (19), resulted in further deterioration of renal function, as indicated by the significant increase of BUN, the moderate increase in PCr and albuminuria, and the decreased CCr (Table 3). Moreover, the proliferation of epithelial cells in CE-ALP-treated rats assessed by Ki-67-positive nuclei as well as immunohistochemistry of p-histone H3 was decreased to 50–60% of that of vehicle-treated rats (Figs. 7 and 8). These results indicated that the enhanced expression of Cyclin B2-Cdc2 actually acted as an M-phase regulator to retain the renal tubular function in rat kidney. On the other hand, the height of epithelial cells was significantly increased by the treatment with CE-ALP (Fig. 7G). Two recent studies revealed that cell cycle polarity protein kinase, which is an upstream molecule of Cdc2, serves as a sensor for cellular length to regulate mitotic entry in the fission yeast (25, 28). Although further investigation of a precise molecular mechanism is required in mammalian cells, the inhibition of Cdc2 by CE-ALP in the compensative period might associate with the growth of epithelium size in proximal tubules.
In the present study, molecular events specific to the renal proximal tubules have been clearly observed in Nx rats with CRF progression. On the basis of these transcriptome data and subsequent examinations, Cyclin B2-Cdc2 was revealed to be important to the proliferative response of proximal tubular cells in the compensated kidney. Therefore, the present results advance the knowledge of the contribution of cell cycle regulators to pathophysiology of tubular restoration and/or degeneration in progressive CRF. The hypothesized molecular mechanisms of proliferation inhibitors and proximal tubular hyperplasia and hypertrophy in CRF are summarized in Fig. 9. In the normal kidney, a portion of epithelial cells in the proximal tubules was positive for Cyclin B-Cdc2 (Fig. 9B). After renal ablation, hyperplasia via the induction of the M-phase regulator Cyclin B-Cdc2 and hypertrophy occur simultaneously in an attempt to recover the reduction in nephron mass in early-stage CRF (Fig. 9C). However, in end-stage CRF, the enhancement of Cyclin B-Cdc2 was reduced. The remnant viable tubules may lose the ability to regenerate new cells at the fibrotic stage, and thus further tubular atrophy and degeneration occur (Fig. 9D). The severe renal deterioration caused by treatment with the mTOR inhibitor might be mediated by inhibition of both hyperplasia and hypertrophy because the mTOR pathway affects various factors involved in the cell cycle, RNA modulation, and protein synthesis (Fig. 9E). Administration of a specific inhibitor for Cdc2 selectively inhibited hyperplasia of epithelial cells, resulting in a moderate reduction in renal function (Fig. 9F).
Fig. 9.
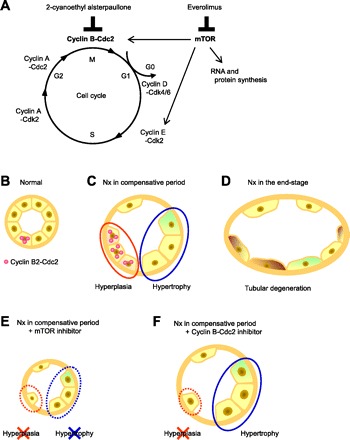
The hypothesized scheme of molecular responses in tubular epithelial hyperplasia and hypertrophy in chronic renal failure (CRF). A: molecular targets of mammalian target of rapamycin (mTOR) inhibitor and Cdc2 inhibitor in the cell cycle and mTOR pathway. B–F: renal proximal tubules of normal rats (B) and Nx rats (C–F). A portion of the renal epithelial cells in the proximal tubules was positive for Cyclin B2-Cdc2 (B). Hyperplasia via induction of Cyclin B2-Cdc2, G2-M cyclins, and hypertrophy simultaneously occurred in the compensative period in CRF rats (C). In end-stage CRF, tubular atrophy and degeneration were observed (D). E: proximal tubules treated with the mTOR inhibitor. Neither hyperplasia nor hypertrophy of tubular epithelial cells occurred, resulting in severe renal damage. F: proximal tubules treated with the Cyclin B-Cdc2 inhibitor. Only hyperplasia was inhibited, and a moderate reduction in renal function was observed.
Considering the time-dependent changes in the gene expression profile of Cyclin B2 and Cdc2 after Nx and the balance between tubular hyperplasia and hypertrophy, the temporal induction of Cyclin B2 and Cdc2 was suggested to be a marker and one of the crucial mechanisms for proliferating proximal tubular cells in early-stage CRF. If we can discover another specific inhibitor of hypertrophy without effects on Cyclin B-Cdc2, it may be a potential regenerative agent that accelerates compensative hyperplasia in the early stage of ablation of nephron mass.
GRANTS
This work was supported in part by a grant-in-aid for Research on Biological Markers for New Drug Development and Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan (08062855 to S. Masuda); by the Japan Health Science Foundation “Research on Health Sciences Focusing on Drug Innovation” (KH23303 to S. Masuda); and by a Grant-in-Aid for Scientific Research (A) (20249036 to K. Inui), a Grant-in-Aid for Young Scientists (A) (21689017 to S. Masuda), and a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows (20-2438 to K. Nishihara) from the Ministry of Education, Science, Culture, Sports and Technology of Japan (MEXT). K. Nishihara is a Research Fellow of the JSPS.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14, Suppl 1: S55–S61, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol 16: 1384–1391, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Chen JK, Chen J, Thomas G, Kozma SC, Harris RC. S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 297: F585–F593, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frazier KS, Paredes A, Dube P, Styer E. Connective tissue growth factor expression in the rat remnant kidney model and association with tubular epithelial cells undergoing transdifferentiation. Vet Pathol 37: 328–335, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Fung TK, Poon RYC. A roller coaster ride with the mitotic cyclins. Semin Cell Dev Biol 16: 335–342, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. [DOI] [PubMed] [Google Scholar]
- 8. Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 9. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715, 1984. [PubMed] [Google Scholar]
- 10. Harper JV, Brooks G. The mammalian cell cycle: an overview. Methods Mol Biol 296: 113–153, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Hayslett JP. Functional adaptation to reduction in renal mass. Physiol Rev 59: 137–164, 1979. [DOI] [PubMed] [Google Scholar]
- 12. Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106: 348–360, 1997. [DOI] [PubMed] [Google Scholar]
- 13. Hori R, Okumura K, Kamiya A, Nihira H, Nakano H. Ampicillin and cephalexin in renal insufficiency. Clin Pharmacol Ther 34: 792–798, 1983. [DOI] [PubMed] [Google Scholar]
- 14. Horiba N, Masuda S, Takeuchi A, Saito H, Okuda M, Inui K. Gene expression variance based on random sequencing in rat remnant kidney. Kidney Int 66: 29–45, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol Renal Fluid Electrolyte Physiol 241: F85–F93, 1981. [DOI] [PubMed] [Google Scholar]
- 16. Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J 14: 1646–1654, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji L, Masuda S, Saito H, Inui K. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int 62: 514–524, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol 13: 46–56, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Kunick C, Zeng Z, Gussio R, Zaharevitz D, Leost M, Totzke F, Schachtele C, Kubbutat MH, Meijer L, Lemcke T. Structure-aided optimization of kinase inhibitors derived from alsterpaullone. Chembiochem 6: 541–549, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Kwon TH, Frokiaer J, Knepper MA, Nielsen S. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol Renal Physiol 275: F724–F741, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47: 443–467, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem 278: 40296–40304, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Liu B, Preisig PA. Compensatory renal hypertrophy is mediated by a cell cycle-dependent mechanism. Kidney Int 62: 1650–1658, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17: 1395–1404, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 459: 852–856, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Masuda S, Saito H, Nonoguchi H, Tomita K, Inui K. mRNA distribution and membrane localization of the OAT-K1 organic anion transporter in rat renal tubules. FEBS Lett 407: 127–131, 1997. [DOI] [PubMed] [Google Scholar]
- 27. Megyesi J, Price PM, Tamayo E, Safirstein RL. The lack of a functional p21WAF1/CIP1 gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci USA 96: 10830–10835, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 459: 857–860, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Nakagawa S, Masuda S, Nishihara K, Inui K. mTOR inhibitor everolimus ameliorates progressive tubular dysfunction in chronic renal failure rats. Biochem Pharmacol 79: 67–76, 2010. [DOI] [PubMed] [Google Scholar]
- 30. Nishihara K, Masuda S, Ji L, Katsura T, Inui K. Pharmacokinetic significance of luminal multidrug and toxin extrusion 1 in chronic renal failure rats. Biochem Pharmacol 73: 1482–1490, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28: 2925–2939, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Shankland SJ, Wolf G. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am J Physiol Renal Physiol 278: F515–F529, 2000. [DOI] [PubMed] [Google Scholar]
- 34. Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimamura T, Morrison AB. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol 79: 95–106, 1975. [PMC free article] [PubMed] [Google Scholar]
- 36. Suzuki H, Inoue T, Matsushita T, Kobayashi K, Horii I, Hirabayashi Y, Inoue T. In vitro gene expression analysis of nephrotoxic drugs in rat primary renal cortical tubular cells. J Appl Toxicol 28: 237–248, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Taal MW, Zandi-Nejad K, Weening B, Shahsafaei A, Kato S, Lee KW, Ziai F, Jiang T, Brenner BM, MacKenzie HS. Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int 58: 1664–1676, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Takeuchi A, Masuda S, Saito H, Doi T, Inui K. Role of kidney-specific organic anion transporters in the urinary excretion of methotrexate. Kidney Int 60: 1058–1068, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Tsuji M, Monkawa T, Yoshino J, Asai M, Fukuda S, Kawachi H, Shimizu F, Hayashi M, Saruta T. Microarray analysis of a reversible model and an irreversible model of anti-Thy-1 nephritis. Kidney Int 69: 996–1004, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Weiland C, Ahr HJ, Vohr HW, Ellinger-Ziegelbauer H. Characterization of primary rat proximal tubular cells by gene expression analysis. Toxicol In Vitro 21: 466–491, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Wolf G, Stahl RA. Angiotensin II-stimulated hypertrophy of LLC-PK1 cells depends on the induction of the cyclin-dependent kinase inhibitor p27Kip1. Kidney Int 50: 2112–2119, 1996. [DOI] [PubMed] [Google Scholar]
- 43. Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 366: 701–704, 1993. [DOI] [PubMed] [Google Scholar]
- 44. Yamada M, Katsuma S, Adachi T, Hirasawa A, Shiojima S, Kadowaki T, Okuno Y, Koshimizu T, Fujii S, Sekiya Y, Miyamoto Y, Tamura M, Yumura W, Nihei H, Kobayashi M, Tsujimoto G. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc Natl Acad Sci USA 102: 7736–7741, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, Chen Y, Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27: 495–502, 2007. [DOI] [PubMed] [Google Scholar]
- 46. You G, Lee WS, Barros EJG, Kanai Y, Huo TL, Khawaja S, Wells RG, Nigam SK, Hediger MA. Molecular characteristics of Na+-coupled glucose transporters in adult and embryonic rat kidney. J Biol Chem 270: 29365–29371, 1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


