Abstract
Currently, 9 out of 10 experimental drugs fail in clinical studies. This has caused a 40% plunge in the number of drugs approved by the US Food and Drug Administration (FDA) since 2005. It has been suggested that the mechanistic differences between human diseases modeled in animals (mostly rodents) and the pathophysiology of human diseases might be one of the critical factors that contribute to drug failure in clinical trials. Rapid progress in the field of human stem cell technology has allowed the in-vitro recreation of human tissue that should complement and expand upon the limitations of cell and animal models currently used to study human diseases and drug toxicity. Recent success in the identification and isolation of human intestinal epithelial stem cells (Lgr5+) from the small intestine and colon has led to culture of functional intestinal epithelial units termed organoids or enteroids. Intestinal enteroids are comprised of all four types of normal epithelial cells and develop a crypt–villus differentiation axis. They demonstrate major intestinal physiologic functions, including Na+ absorption and Cl− secretion. This review discusses the recent progress in establishing human enteroids as a model of infectious diarrheal diseases such as cholera, rotavirus, and enterohemorrhagic Escherichia coli, and use of the enteroids to determine ways to correct the diarrhea-induced ion transport abnormalities via drug therapy.
Keywords: Enteroids, intestinal pathophysiology, intestinal physiology, host–pathogen interaction
Introduction
Recent development of a procedure to propagate three-dimensional (3-D) “mini-intestines” from isolated human adult intestinal stem cells has provided an exciting opportunity to advance gastrointestinal (GI) research and therapy by increasing our understanding of human intestinal physiology, pathophysiology, molecular and cell biology, pharmacology, developmental biology, microbial replication, epithelial cell responses, and regenerative medicine. A group led by Hans Clevers at the Hubrecht Institute in Utrecht, the Netherlands, pioneered the primary culturing of indefinitely propagating mouse intestine,1 and subsequently human intestine2,3 to produce self-organizing mini-intestines, termed enteroids, that add a unique functional companion to models using animal intestine and immortalized polarized intestinal cancer-derived cell lines. One area that has the potential to benefit from use of this approach relates to drug development. Currently, approximately 90% of pharmaceuticals based on studies in animal intestine and cell lines ultimately fail when tested in humans, largely due to differences in toxicity or efficacy.4 Infrequent successes have led to huge costs for new drug development.
Enteroids are a promising pre-clinical model with the potential to overcome the deficiencies of current research models by facilitating faster and more cost-effective drug development. Because these are an ex-vivo human intestinal preparation, they may predict drug toxicity and human efficacy more accurately than other models. We describe recent advances using human small intestinal enteroids applied to understanding digestive physiology, diarrheal pathophysiology, and drug therapy, as well as highlight features for consideration when applying the model for drug target discovery and drug validation.
Comparing this new 3-D culture methodology to the traditional human tissue culture models of cancer-derived intestinal epithelial cells is revealing. The most widely used cell lines for GI research originated from human colon cancers. T84 cells have extensive (over 30 years) validation in anion secretion studies, although new aspects of anion secretion and its regulation continue to be defined. Caco-2 and HT29 cells have been used to study intestinal absorption, drug transport, and other aspects of transport physiology. Caco-2 cells have been used to represent small intestinal absorptive epithelial cells since 1989, when they were shown to reproduce characteristics of human intestinal permeability5 and mucosal transport6 in vitro.
However, over the next 20 years, many derivatives of Caco-2 cells were subcloned that evolved and exhibited great diversity in protein expression and localization. Recent analysis of drug transporter gene expression and functionality of 14 ATP-binding cassette (ABC) transporters and 58 solute carrier (SLC) transporters was reported in Caco-2 cells from 10 laboratories.7 Although the specific proteins expressed were not altered, there was up to 100-fold variation in levels of mRNA and protein expression, and expression of all tested transporters was increased in the presence of collagen-coated cell culture surfaces. In addition, even small differences in culture conditions exerted a major impact on gene expression.8 Membrane support and scaffold material significantly influence the transepithelial resistance (TER) and paracellular permeability of Caco-2 monolayers. Growth on polyethylene terephthalate (PET) or polyester (PE) membranes shows the highest TER and lowest permeability compared to monolayers grown on polycarbonate. This range of results complicates the comparison of results using Caco-2 monolayers from laboratory to laboratory, although many important and validated findings have emerged by a using single cell line as a control for studies in the same laboratory. While it remains unclear if these differences are related to the fact that Caco-2 is a cancer cell line, there is a need to establish highly reproducible and stable epithelial cell models of non-transformed human intestinal cultured cells that can be studied and yield uniform results in different laboratories.
Another important limitation of current cell lines is that they consist of a single epithelial cell population and thus fail to recapitulate the diversity of cell types that are present in the normal intestinal epithelium. Each single type of intestinal epithelial cell frequently exerts influence on other cell types (cross-talk) in health and disease by multiple mechanisms including paracrine and autocrine signaling. An example of the importance of having the normal intestinal cell population comes from studies of a model of fatal diarrhea caused by Citrobacter rodentium in certain susceptible mouse strains.9 This colonic infection is associated with induced production of R-spondin2, a ligand of the Lgr5 (leucine-rich repeat-containing G-protein-coupled receptor 5) receptor, in mice harboring a modified Rspo2 promoter via an unknown mechanism. Increased R-spon-din2 triggers potent Wnt-mediated hyperproliferation of immature colonic crypt cells that apparently lack absorptive function and contribute to severe diarrhea. Thus, a model with the potential to represent the multiple cell types within normal adult human intestinal epithelium would be of great value.
Intestinal organoid/enteroid cultures
Enteroids are new ex-vivo 3-D culture systems that are expected to recapitulate the multiple cell types of normal intestinal epithelium and should overcome recognized limitations of cancer cell-based systems. There are two stem cell-based methods to culture human “mini-intestines”; one approach uses human-induced pluripotent stem cells (iPSCs),10 while the other procedure employs adult stem cells from intestinal crypts isolated from human surgical specimens or endoscopic biopsies. Another stem cell-oriented approach, demonstrated primarily for application with neonatal mouse tissue and not yet described for human specimens, cultures minced intestine in a collagen gel at the air–liquid interface to produce organoids in co-culture with myofibroblasts.11 Table 1 compares properties of mini-intestines obtained by each approach, each of which yields all the major epithelial cell types (enterocytes of absorptive and secretory subtypes, Paneth cells, goblet cells, enteroendocrine cells, M cells, and tuft cells). The technique for isolation of Lgr5/Paneth cell-containing crypts was initially described for mouse tissues by Sato et al.,1 and was subsequently optimized for use with human tissues under modified conditions (including different growth factors and small molecule inhibitors) to enhance the efficiency of enteroid formation and long-term viability of each intestinal segment.2,3
Table 1.
Comparison of intestinal stem cell culture approaches
| Characteristics | Crypt-derived enteroids1–3 | Tissue-derived organoids11 | iPSC/ESC-derived organoids10 |
|---|---|---|---|
| Starting material | Biopsy or tissue | Murine neonatal or adult tissue | ES and iPS cells |
| Indefinitely expandable | Yes | No; proliferation potential decreases over time | No; limited to finite number of passages |
| Phenotype | Still under characterization | Neonatal/adult | More embryonic |
| Mesenchymal cells present | No | Yes | Yes |
| Production capabilities | Expand quickly | Dependent on tissue availability | Not dependent on tissue availability |
| Length of time to generate cultures | Less than 1 week | Less than 1 week | >1 month |
| Organization | Mimic crypt/villus unit | Mimic crypt/villus unit | Crypt/villus units with some random cell distribution |
This breakthrough in the ability to culture normal human intestinal epithelia emerged from fundamental molecular characterization studies of intestinal stem cells (ISCs) in mice. At least two different populations of ISCs are currently recognized in the crypt. Lgr5 is a marker for the crypt base columnar (CBC) cells that are an anatomically distinct, actively cycling population of ISCs capable of giving rise to all the differentiated cell types of the intestinal epithelium.12 Different populations of quiescent or infrequently dividing ISCs at the +4 position from the crypt base in the mouse intestine were identified by studying mice lacking Lgr5-containing cells in conditions in which the intestine was damaged, primarily by whole body radiation.13 The +4 cell population is identified based on the expression of several markers including Bmi1, mTert, Hopx, and Lrig1. Although the +4 cells were initially thought of as a reserve population,14 these two ISC populations exhibit remarkable plasticity and a bi-directional relationship in which one population can interconvert into the other under some circumstances.13,15,16 Thus, the intestine is highly adaptable with built-in redundancies to assure intestinal regeneration after damage. Several methods for separating these stem cell populations have since been described in mice17,18 and more recently in human enteroids,19 which include using a variety of stem cell marker fluorescence-activated cell sorting (FACS)-based separations. The interactions of the rapidly cycling Lgr5+ population and the quiescent stem cell population at the +4 position continue to be studied and are providing important insights about ISC function following intestinal stress (e.g. radiation,15,20–24 and likely also as a result of hypoxia, inflammation, surgical injury, drug toxicity, fasting, or undernutrition). However, ISC function in physiologic and host–pathogen interactions remain largely unstudied.
Current status of human enteroid-based studies
Enteroids/organoids are currently being assessed as 3-D structures to determine whether they reproduce characteristics of normal human intestine. Our studies employ the isolation methods and culture conditions outlined by Sato et al.3 to generate enteroids from whole intestinal crypts isolated from biopsies or surgically resected tissues. Several aspects related to the use of enteroids for functional physiological studies are examined as follows.
(a) Development of polarity
We and others have demonstrated that from very early passage and beyond 40 passages, small intestinal human enteroids polarize with brush border resident proteins villin and ezrin present apically, basolateral membrane (BLM) resident proteins Na+/K+-ATPase, NKCC1, β-catenin, and E-cadherin present basolaterally, and separation of apical and BLM documented by the presence of the tight junctional protein ZO-1 (unpublished results).
(b) Differentiation
Enteroid differentiation induced by Wnt3a withdrawal expands the cellular repertoire to include enterocyte, goblet, and enteroendocrine cells in addition to Lgr5+ cells and Paneth cells.3 In proximal small intestinal enteroids, differentiation changes expression of the ion transport proteins NHE3, CFTR, DRA (all present apically), and NKCC1 (present basolaterally) as observed by immunofluorescence microscopy, immunoblot, and qRT-PCR. Under differentiation conditions, NKCC1 and CFTR message increases minimally, whereas NHE3 mRNA increases 3-fold and DRA message jumps by 20-fold (unpublished results). The presence of multiple cells types, the anatomic separation of crypt-like domains and villus-like protrusions that express proteins involved in Na+ absorption, and the polar plasma membrane distribution of transport proteins involved in Na+ absorption and Cl− secretion document several aspects of differentiation in enteroids that mimic differentiation in mature intact human intestine. However, these 3-D enteroid cultures lack true villus structures to represent in-vivo crypt–villus architecture. Villus-like structures have been reported after subcutaneous transplantation of enteroids and myofibroblasts in mice,25 or implanting scaffolded multicellular organoid units in the abdomen of mice,26 rats,27 or pigs,28 indicating that further differentiation of enteroids can be achieved under certain circumstances. Additional studies are needed to measure multiple aspects of differentiation and to fully characterize the differentiation model achieved by Wnt3a removal as well as to identify conditions to prompt further differentiation of these “mini-intestines”.
(c) Physiologic Na+/Cl− absorption and Cl− secretion
Our studies use human enteroids as models to understand normal transport physiology and host–pathogen interactions. In this section we summarize the current status of human small intestinal enteroids as a model to understand normal intestinal salt and water transport physiology. In the next section, we describe the use of enteroids to understand diarrhea-related pathophysiology including several examples of host–pathogen interactions.
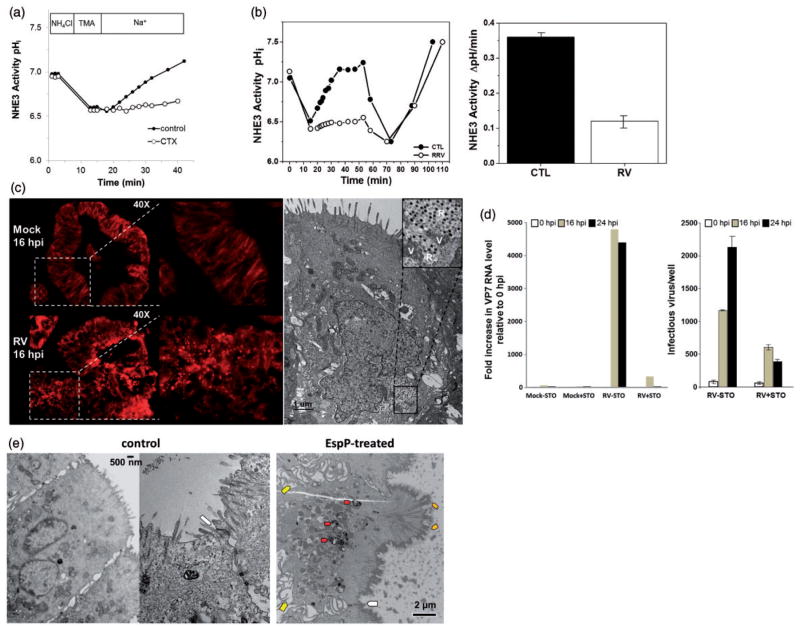
The transporters required for apical Na+ and Cl− absorption (NHE3 and DRA, respectively), stimulated Cl− secretion (apical membrane CFTR and BLM NKCC1), as well as basolateral Na+/K+-ATPase and apical and BLM K+ channels are present in enteroids shown by semi-quantitative RT-PCR and immmunodetection (unpublished results). Differentiated duodenal and jejunal enteroids contain active NHE3, defined as HOE-694-insensitive and S3226-inhibitable Na+-dependent intracellular alkalinization (unpublished results) (Figure 1). NHE3 activity is stable over at least 13 passages (passages 7–20). CFTR-mediated Cl− secretion, which can be assayed as either forskolin-induced, CFTRinh-172-sensitive luminal dilatation or forskolin-induced membrane depolarization, has also been studied in enteroids. Liu et al. found that activation of cAMP signaling increased the membrane potential in wild type but not CFTR knockout or CFTRinh-172-treated murine enteroids.29 Additionally, forskolin treatment causes apical fluid secretion which leads to luminal swelling and enterocyte shrinkage, introducing a useful quantitative tool to monitor CFTR-related fluid secretion in enteroids. Forskolin-induced swelling (FIS) was employed by Dekkers et al. to examine the efficacy of CFTR correctors in human rectal enteroids derived from CF patients.30 Surprisingly, enteroids from patients with the same CFTR-F508del mutation responded variably to the same correctors, suggesting the need to develop personalized therapeutic approaches for each patient. The quantitative FIS assay has an advantage compared to short-circuit current measurements because it can be used in a high-throughput fashion to generate drug response data sets for both drug discovery and personalized medicine related to anti-diarrheal therapy. This model should also allow in-depth understanding of the transport processes that regulate anion secretion in human intestine as well as aid determination of whether CFTR is rate limiting for Cl− secretion, information needed to develop a strategy for diarrheal drug therapy.
Figure 1.
Na+/H+ transport assays were performed using the cell-permeable pH-sensitive fluorophore SNARF-4 F acetoxymethyl ester and multi-photon laser scanning microscopy. Differentiated human duodenal enteroids were first incubated in the presence of NH4Cl buffer, then pre-pulsed with Na+-free buffer (tetramethylammonium chloride, TMA) to induce intracellular pH acidification, and finally transferred to Na+ buffer to follow the rate of alkalinization. Specific inhibition of NHE3 with S3226 (20 μmol/L) dramatically decreased the rate of Na+ driven alkalinization (NHE activity) in 3-D differentiated human duodenal enteroids relative to specimens treated with HOE-694 (50 μmol/L) to block activity of other NHE isoforms. Please note the similarity of Na+-dependent alkalinization in the presence of HOE-694 plus S3226 and S3226 alone, supporting that nearly all NHE activities are related to NHE3. Graph depicts a representative experiment for each condition; each condition was evaluated in triplicate. (A color version of this figure is available in the online journal.)
(d) Host–pathogen interactions
We are developing human enteroids as models of host–pathogen interactions in diarrheal diseases. Our initial experiments have been performed in human small intestinal enteroids to study three of the major causes of death in the world from diarrhea, cholera, rotavirus, and entero-hemorrhagic Escherichia coli (EHEC).31
Most of the large volume diarrhea of cholera is thought to be due to luminal release of cholera toxin, which inhibits NHE3 and stimulates CFTR in many cell and animal models. Cholera toxin exposure to proximal small intestinal human enteroids inhibits NHE3 activity (unpublished results) (Figure 2a) and induces luminal dilatation that can be blocked by CFTR inhibitors, which is consistent with the anion secretion model reported in human rectal enteroids.30
Figure 2.
(a) Differentiated human duodenal enteroids treated with cholera toxin (0.1 μg/mL, 2 h) have markedly inhibited NHE3 activity, which is similar to observations in human disease. The graph shows a representative experiment, n =3. (b) Exposure of differentiated human duodenal enteroids to rotavirus (1 h) inhibited NHE3 activity by >65%. NHE3 activity was measured in the presence of HOE694 (50 μmol/L) to inhibit all other NHE isoforms (bar graphs are mean ± SEM of three experiments). (c) Rotavirus infects human enteroids. (Left panel) Human jejunal enteroids were metabolically labeled with fluorescent-tagged BODIPY-fatty acid for 1 h prior to mock or rotavirus infection. Enteroids imaged by epifluorescence at 16 h post infection show BODIPY-fatty acid is retained in the endoplasmic reticulum lipid bilayer of mock-infected enteroids. In contrast, rotavirus infection induces lipid droplet formation indicated by the round, red lipid droplets. The inset on the right is a magnification of the boxed area. (Right panel) Electron micrograph of a rotavirus-infected human jejunal enteroid shows that rotavirus infects and replicates in the enteroids. (Inset) Magnification of the boxed area show viroplasms (V), sites of immature particle assembly and genome replication, and rotavirus particles (R). (d) The calcium/calmodulin kinase kinase 2 (CAMKK2) inhibitor STO-609 reduces the yield of rotavirus. Mock- and rotavirus-infected human jejunal enteroids were cultured in the absence or presence of STO-609 (STO). The enteroids were harvested at the indicated times. (Left panel) qRT-PCR was used to quantify the level of rotavirus VP7 gene, normalized to GAPDH, and expressed relative to 0 h post infection. (Right panel) Infectious virus was quantified in the enteroids by fluorescent focus assay. No infectious virus was observed in mock-infected enteroids and no drug toxicity was observed (data not shown). (e) Normal enterocytes in enteroids adopt a columnar shape with a clear brush border and tight junctions (white arrow, control). Enteroids incubated with serine protease EspP, an important virulence factor from EHEC bacteria that may facilitate systemic transepithelial Shiga toxin delivery in human disease, caused actin remodeling of the brush border and basolateral membranes. Yellow arrows indicate actin rearrangement in basolateral membranes; orange arrows denote clumps of microvilli with prominent rootlets and terminal webs; red arrows mark macropinosomes containing internalized horseradish peroxidase (darkened areas) and there are also wide-opened lateral intercellular spaces. (A color version of this figure is available in the online journal.)
We have begun exploring the use of enteroids to understand the pathophysiology of rotavirus diarrhea. Previous work demonstrated that iPSC-derived organoids are subject to rotaviral infection.32 We now show that adult intestinal crypt-derived enteroids from each segment of human small intestine can be infected by rotavirus. Similar to the effects of cholera toxin, rotavirus exposure inhibits NHE3 in proximal intestinal enteroids and this occurs within 1 h of exposure (Figure 2b). Studies on the mechanism of rotaviral alteration in intestinal transport have been performed in parallel with studies on viral replication. In human enteroids, viral replication occurs over at least 96 h and is detected based on quantitation of increasing viral RNA by qRT-PCR, virus-specific protein expression by Western blot analysis, and production of infectious virus by fluorescent focus assays.33 Laboratory strains of rotavirus as well as human clinical isolates in fecal samples can undergo replication in enteroids, which subsequently produce infectious rotavirus. Intracellular changes typical of rotavirus replication in cultured cells are seen in infected enteroids and include the induction of lipid droplets (Figure 2c, left panel), formation of replication compartments (viroplasms) (Figure 2c, right panel), and visualization of nascent virus particles within intracellular vesicles by electron microscopy (Figure 2c, right panel inset). Treatment of enteroids with the calcium/calmodulin kinase kinase 2 (CAMKK2) inhibitor STO-609 significantly inhibits rotavirus infectivity based on quantitating viral genome (Figure 2d, left panel) or infectious virus particle production in cells (Figure 2d, right panel) and the drug is not toxic to enteroids as seen previously in monkey kidney cells.34 These rapid advances in understanding how rotavirus affects intestinal cells in human enteroids, which support studies in human intestine, suggest that this model will lead to a deeper understanding of this disease than we currently have with potential applicability to other viral GI disorders.
In addition, we have begun studies on the pathophysiology of EHEC-related diarrhea using human enteroids. EHEC forms characteristic attachment and effacement lesions in the distal small intestine and colon, and it separately induces major rearrangement in the actin cytoskeleton to induce macropinocytosis. Human enteroids exposed to a product of EHEC, the serine protease EspP, undergo actin cytoskeletal changes resembling macropinocytosis (unpublished results) (Figure 2e). The actin changes involve both the apical and basolateral surfaces and appear to allow both uptake and transcytosis of Shiga toxins 1 and 2, which leads to Shiga toxin entry into the systemic circulation and almost certainly contributes to the pathobiology of EHEC-related hemolytic uremic syndrome.
(e) Growth as monolayers
Current protocols support organoid and enteroid growth in Matrigel as 3-D spheroids with either cystic morphology or crypt-like projections. Currently, important functional studies must be performed on enteroids in 3-D cultures that extend beyond the Matrigel surface since intracellular pH- and Ca2+-sensitive dyes diffuse very slowly through Matrigel. In addition, the luminal surface of spheroids faces inwards and the BLM is in contact with the Matrigel, thus limiting apical exposure to ligands and retarding responses to changes in environment, such as those related to quantitation of Na+/H+ exchange rates and drug exposure. Thus, there would be multiple advantages to growing enteroids as polarized monolayers instead of spheroids, as this would permit direct apical exposure to nutrients, pathogens, and oral drugs, as well as enable the prospect of growing the cells inside or outside of tubes for use in intestinal repair or regenerative medicine to treat intestinal insufficiency.
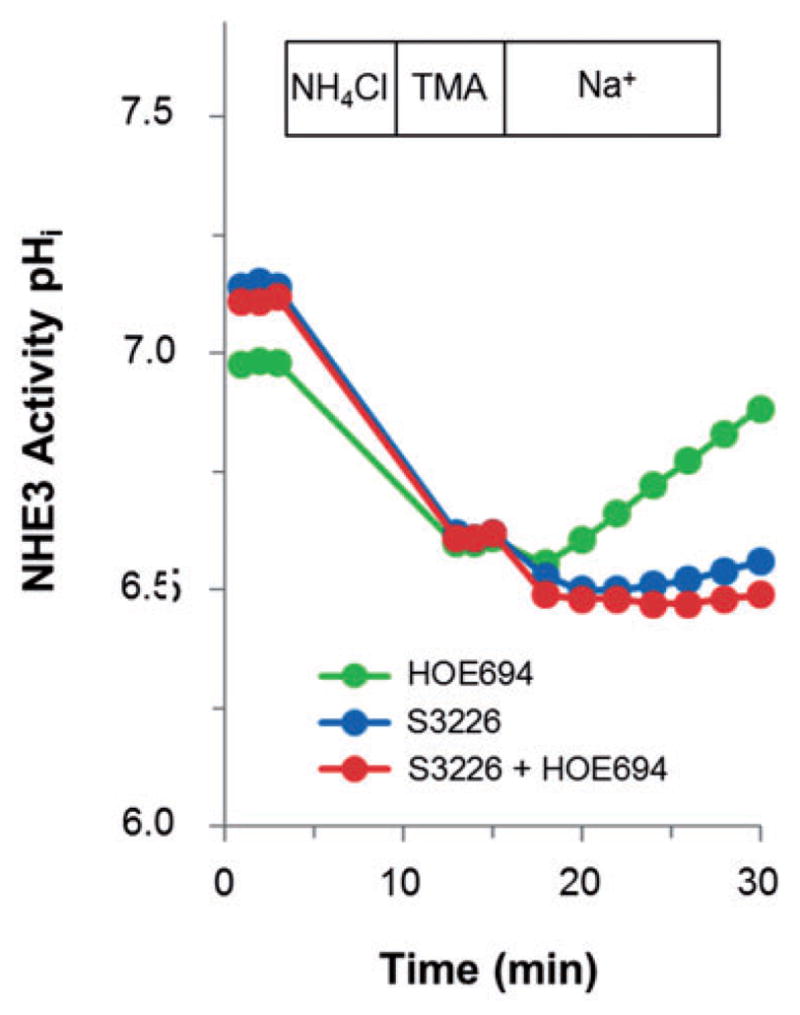
It was recently reported that replacement of Matrigel with type I collagen causes murine enteroids to spread spontaneously into epithelial sheets and form a mixed culture of cyst-like enteroids surrounded by a monolayer.35 The transepithelial electrical resistance (TER) of this preparation was not reported, so it is unknown whether these collagen-grown murine monolayers were confluent. We have grown human enteroids on porous Transwell membranes coated with a fibronectin-based peptide leading to formation of confluent monolayers that have the apical surface facing outward and the BLM surface attached to the substrate, exhibit a high TER (600–1000 Ωcm−2), and polarize as demonstrated by the presence of apical villin and basolateral Na+/K+-ATPase (Figure 3a) (unpublished results). In addition, these 2-D cultures exhibit a similar polar distribution of transporters as occurs in 3-D enteroids (Figure 3b).
Figure 3.
(a) Human jejunal enteroids plated on a fibronectin-based peptide-coated Transwell insert formed flat sheets of cells that merged to yield a monolayer with TER ~1000 Ωcm−2. Immunostaining with apical (villin), basolateral (Na+/K+-ATPase), and nuclear markers (Hoescht 33342) verified the polarized character of the single cell layer. (b) A similar differentiated monolayer of human duodenal enteroids expresses apical NHE3 and CFTR. (A color version of this figure is available in the online journal.)
Monolayers representing enteroids from multiple normal subjects or a broad spectrum of human genetic variants could be used in microfluidic chambers of intestinal epithelial tissue arrays to generate a tool appropriate for high-throughput drug screening. The choice of both support scaffold and coating for cell attachment is important, as they may influence protein expression, cell proliferation, cell differentiation, or tight junction development and therefore may affect results of drug testing. Polymer rigidity, membrane thickness, drug adsorption to the surface, and material resistance to small molecule adhesion/interaction36 are a few hurdles this technology faces.
(f) Genetic manipulations
An important utility for molecular physiologic studies in enteroids is transcriptional knock-down, knock-out, knock-in, or expression of exogenous proteins. The first peer reviewed report of viral transgene delivery in mouse enteroids used a modified murine stem cell retrovirus to achieve knock-down or overexpression via 4-hydroxyta-moxifen-inducible Cre recombinase.37 We have achieved GIPZ lentiviral shRNA transduction via the spinoculation procedure described by Koo et al.37 in human small intestinal enteroids to establish stable expression of enhanced green fluorescent protein (eGFP) over at least eight passages (Figure 4) (unpublished results), supporting reports that human enteroids are amenable to viral-based genetic manipulation. Additional techniques have been illustrated by the recent successful transfection of human enteroids using lipofectamine or low voltage electroporation.38 The lipofection protocol facilitated use of the CRISPR/Cas9 system to edit the genome of intestinal organoids derived from CF patients and restored CFTR response to elevated cAMP in an in-vitro swelling assay.39 Similar applications using ZFNs or TALENs for genetic engineering are anticipated to be equally plausible in enteroids.
Figure 4.
Human jejunal enteroids exposed to lentivirus stably express vector-encoded eGFP over a period of at least four weeks, suggesting transduction of Lgr5+ stem cells. Lentivirus transduction was carried out by spinoculation with mechanically dissociated enteroids. The panels show a single enteroid viewed by fluorescence (left panel) and bright field microscopy (right panel) obtained after four weekly passages. (A color version of this figure is available in the online journal.)
(g) Assessment of cell viability
Cells in the intestinal epithelium exhibit varying degrees of sensitivity to exogenous agents, pathogens, and internal signals. The interactions of the various differentiated and undifferentiated cell types and how they contribute to the maintenance of cell viability is fundamental to intestinal homeostasis and is important to evaluate as enteroids are assessed as pre-clinical models for drug testing. Cell viability is difficult to assess and follow in vivo. Although many studies have utilized the traditional 2-D cell culture models to study cell viability of intestinal cells, the conclusions have limited applicability because many of these traditional models have been derived from cancer cells and they lack the different cell types that comprise the intestinal epithelium in vivo. One approach, enzymatic digestion of the enteroids resulting in single cell suspensions, allows live cells with intact membranes to be distinguished from dead or damaged cells based on their ability to exclude fluorescent dyes. We have recently utilized flow cytometric assessment of dye exclusion to determine changes in membrane permeability that accompany changes in cell viability within the context of the human enteroid model (unpublished results). Cell death from virus infection can be quantitated (Figure 5) and followed over time. These and similar studies using the human enteroid models with real-time and high-throughput measurements of cell death and recovery will be important in determining toxicity. Such studies can compare responses to various insults, identify relationships between cell behavior and cell number, and provide insight into many biological interactions that occur between the different cell types that comprise the intestinal epithelium.
Figure 5.
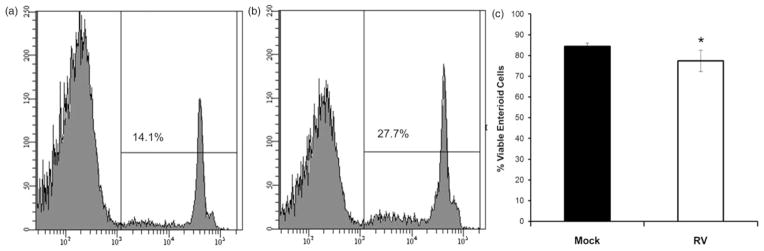
Assessment of cell viability in enteroids following exposure to rotavirus. Human jejunal enteroids were mock treated (a) or treated with rotavirus (b) for 24 h. Single cell suspensions were incubated with propidium iodide (PI) and 50,000 events examined for the presence of PI fluorescence using an LSRII flow cytometer. (a) and (b) Representative histograms showing percentage of cells that take up PI (non-viable cells) after each treatment. (c) Quantitation of viable cells after each treatment (n =3 ± SD). *P <0.05 using Mann–Whitney test
Unresolved issues related to use of enteroids/organoids as models of human intestine
While a vast array of studies utilizing human enteroid models will be emerging in the near future, most aspects of the technology lack agreement regarding standardization, reproducibility, and consideration of human biological diversity. The following includes details that should be addressed to prevent the deficiencies of current cell models from being reproduced in this emerging field.
(a) Basic culture conditions
Multiple protocols based on the original Sato and Clevers methods have been used to establish and culture enteroids. The myriad growth factors required for maintaining the enteroids have multiple sources (conditioned media from cell lines, purified recombinant proteins) with varying quality and quantitation. Standards should be developed regarding growth media formulation so that studies are cross-comparable. The emergence of alternative protocols may offer physiological advantages, but they may also introduce cell culture-induced variations like those seen in multiple Caco-2 cell clones.7
(b) Methods for differentiation of enteroids
These include removing Wnt3a and other growth factors3 for a period of time or transplanting an enteroid and myofibroblast co-culture subcutaneously in animals.25,40 Specific markers or functional aspects must be agreed upon to define differentiation, whether that criteria is expression profiling in enterocytes, validating the presence, and relative percentage of each of the different intestinal cell types, monitoring changes in polarized expression of plasma membrane transport proteins involved in Na+, K+, and Cl− transport, or demonstrating crypt–villus axis formation, which mimics the organized structure of normal intestine.
(c) Impact of stretch and flow on intestinal epithelial cell characteristics
Studies with enteroids have begun considering the mechanical forces which affect the intestinal epithelium under physiologic conditions. These physical forces include those generated by peristalsis and mechanical effects of luminal fluid pressure to create shear stress. Peristalsis is defined as waves of contraction that originate from pacemaker cells in circular smooth muscle and propagate along the GI tract for various distances. Repetitive stretching and contraction result in mixing luminal food and fluid content and drive oral to anal movement. Luminal food and fluid flow generate diverse patterns of shear stress, pressure, and friction during passage along the bowel wall. The importance of interactions between luminal contents and intestinal epithelial cells on gut homeostasis is illustrated by the fact that subjects fed only total parenteral nutrition develop mucosal atrophy.41
From non-intestinal studies, it is well established that cells exposed to mechanical loads respond differently to stretch or deformation. Understanding the role of the physical forces in intestinal physiology has been difficult in living animals. There have been numerous attempts to model these complex mechanical forces in vitro by applying luminal flow and stretch. Deformation–relaxation cycling increases proliferation of both Caco-2 cells grown on collagen and non-transformed human primary intestinal epithelial cells.42 Moreover, the same mechanical forces can generate different responses based on the segment of the GI tract studied. The application of low frequency repetitive mechanical stretch to type-I collagen-grown rat gastric epithelial cells inhibited both proliferation and cell motility.43
The mechanisms behind the transformation of mechanical signaling into molecular responses are just emerging. Protein kinase C, a tyrosine kinase, and focal adhesion kinase (FAK) associated with extracellular matrix protein integrins are activated by stretching.44 The involvement of an integrin in the mechanical response links stretch with matrix effects on intestinal epithelial cells. Indeed, it has been shown that growing cells on fibronectin or a mixture of fibronectin and collagen completely eliminated the mitogenic effect of stretch in Caco-2 cells and in primary non-malignant human intestinal epithelial cells.45
The effects of these physical forces on enteroids have not yet been reported. However, the effects of a first generation microfluidic “gut-on-a-chip” technology that combines peristalsis-like stretch with apical and basolateral liquid flow has been reported recently using Caco-2 cells.46 The presence of both stretch and flow in the microfluidic system allowed Caco-2 cells to differentiate, by measure of specific aminopeptidase activity, after 5 days, compared to 21 days for Caco-2 cells grown on a static Transwell support. Average cell height was also three- to four-fold greater under the stretch and flow system than was obtained after 21 days in static culture. According to these studies, Caco-2 cells grown under stretch and flow conditions demonstrate significant increase in P450 3A4-isoform-based drug metabolism, which might affect drug efficacy and toxicity tests.47 Additionally, the authors asserted that cells underwent morphogenesis into 3-D intestinal villi and basal proliferative crypts. The Caco-2 cells, which under static conditions represent a monoculture of differentiated enterocytes, were reported to turn into three other major types of intestinal epithelial cells, including mucus-producing goblet cells, enteroendocrine cells and lysozyme-producing Paneth cells. These results indicate the profound effect of stretch and flow on intestinal epithelial cell biology and suggest the ability to manipulate cancer stem cells by altering physical forces. However, it is unclear if this model generates actual villi since the images lack nuclear staining to verify whether the Caco-2 cells along the villus-like structure are a monolayer or multilayered cell mounds. Further experimental evidence is necessary to justify the claim that stretch and flow can prompt true crypt–villus axis formation.
Based on these considerations, an ideal microfluidic device supporting ex-vivo human primary cell cultures should consist of enteroid monolayers grown on a coated permeable scaffold in a perfusion chamber with digitally-controlled apical and basolateral flow to introduce shear stress and application of stretch to mimic peristalsis, with the nature of how to use stretch to mimic peristalsis still to be determined.
(d) Human intestine is more than a collection of epithelial cells
The assumption in drug delivery is that differences between cell lines or animal experiments and actual effects in humans are tied to altered drug target location, dose, associating regulators, amino acid composition, drug sensitivity, metabolism, or some combination and these may explain drug development failures. Human enteroids will enable critical testing related to this issue. However, enteroids do not mimic all aspects of native intestine, as they lack other components present in normal intestine, including nerves, immune cells, the microbiome, vasculature, and mesenchyme. These components are likely to be incorporated in the future as human mini-intestine systems continue to be optimized. Organoids made from iPS cells already contain mesenchyme, and cultures from intact tissue have the potential to yield myofibroblasts and nerve cells.
Present methods for enteroid culture do not include nerves, yet these are important for many physiologic and pathophysiologic responses. For example, 50% of fluid secretion in model cholera and rotaviral diarrheas are neurally mediated.48–50 Thus, co-culture approaches are in development. Physiologic and pathophysiologic studies would likely be more meaningful if enterocyte cultures incorporated nerves, inflammatory cells, and immune cells to model host–pathogen interactions in enteric diseases. Establishing methods to study epithelial–nerve interactions and their long-term relationships and effects on Na+ absorption and Cl− secretion is an important area with potentially great relevance to drug development.
Interactions of intestinal epithelial cells with the luminal microbiome and local inflammatory cells under physiologic and pathophysiologic influences affect many aspects of intestinal function. The “NutriChip” microfluidic prototype initially explored macroscopic co-culture of Caco-2 cells with U937 cells, of which the latter differentiate into macrophages and can mimic an intestinal immune system.51 However, culture conditions for the intestinal epithelial cells and the immune cells were incompatible, so the platform had to be re-designed such that the two cell populations were separated by a permeable membrane.
It is not known whether there is a microbiome associated with enteroid cultures or how to incorporate study of enteroids with the microbiome, which is a complex and individual-specific mixture of both aerobic and anaerobic species. Most cultures are not overtly contaminated but whether noncultivatable microbes are present remains to be determined. The microbiome may be important for functional maturation of the intestine but appears to be less important for architectural maturation based on normal intestinal development and maturation occurring in utero as well as maturation of either organoids or enteroids in vitro or transplanted organoids or enteroids that are maintained in sterile environments.
The intestine also absorbs nutrients from the intestinal lumen, which then are removed to the rest of the body via the basolateral blood flow. In the absence of vasculature, the intestinal cells will not be using/removing this material normally. The epithelium in the crypt–villus axis experiences variable sheer stress that changes based not only on where the cells are in the vertical axis, but also the time period (post- or pre-prandial state) during which a study is performed. Both luminal flow and blood flow occur with intermittent changes due to oral intake, intestinal secretion, and peristaltic contractions. We are far away from being able to mimic these forces and their influences on gene and protein expression in the enteroids.
(e) Building enteroid-based intestinal disease profiles
To study enteric diseases using enteroids, multiple controls must be in place. Ideally, this would be established in a population of enteroids from healthy control subjects that is extensively phenotyped and genotyped to serve as a defined control for consideration of biologic diversity and polymorphisms and be used for comparison in the study of human diseases. The number of subjects and degree of phenotypic and genotypic diversity can only be speculated upon given that functional similarity across individuals, or even groups of similar individuals (twins), has not yet been characterized.
(f) From laboratory model to pharmaceutical standard
To use organoids/enteroids for drug testing, thought will need to be given to regulatory oversight requirements and standardization of culture procedures, including Food and Drug Administration (FDA) requirements, which also may need to evolve to consider these mini-intestines. Enteroid cultures can potentially be established from any individual, providing a platform for developing individualized medicine related to the GI tract. Additionally, large collections can be made into an intestinal epithelial array to serve as a platform to determine drug efficacy and toxicity profiles to separate the population into responders and non-responders.
Conclusion
Development of human organoids/enteroids as tools to study ISCs, cellular differentiation and fate determination, physiology, pathophysiology, drug development, nutrition, intestinal repair, and regenerative medicine, among many other uses, is in its infancy and many of the most fundamental questions concerning their use have not yet been addressed. However, there is potential that these new mini-intestines will provide a powerful ex-vivo model of human intestine that will further understand both normal intestinal physiology and digestive diseases, ultimately accelerating drug development for many human diseases. The importance of intestinal responses is emphasized because of the desirability of having a model for oral drug delivery as well as to study the role of the intestine as a portal for systemic drug delivery.
Acknowledgments
We acknowledge use of the Kudsi Imaging Core Facility and the Mouse Physiology Core of the Conte Hopkins Digestive Disease Basic & Translational Research Core Center (Hopkins) and the Cellular and Molecular Morphology and Flow Cytometry Cores of the Texas Medical Center Digestive Diseases Center and the Dan L. Duncan Cancer Center (Baylor). This study was approved by the Johns Hopkins University School of Medicine and the Baylor College of Medicine Institutional Review Boards.
This work was supported in part by National Institutes of Health Grants U18-TR000552, T32-DK007632, K01-DK088950, R01-DK026523, R01-DK061765, P01-DK072084, R01-AI080656, P30-DK056338 (Baylor), and P30-DK089502 (Hopkins).
Footnotes
Author contributions: All authors participated in the design, interpretation, and analysis of the data and review of the manuscript; JFA, JI, OK, NCZ, KE, SEB, and SEC performed the experiments, JRB provided microscopy technical assistance, and JFA, JI, OK, MKE, and MD wrote the manuscript.
References
- 1.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 2.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, Clevers H, Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–7. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adeno-carcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–49. [PubMed] [Google Scholar]
- 6.Hilgers A, Conradi R, Burton P. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res. 1990;7:902–10. doi: 10.1023/a:1015937605100. [DOI] [PubMed] [Google Scholar]
- 7.Hayeshi R, Hilgendorf C, Artursson P, Augustijns P, Brodin B, Dehertogh P, Fisher K, Fossati L, Hovenkamp E, Korjamo T, Masungi C, Maubon N, Mols R, Müllertz A, Mönkkönen J, O’Driscoll C, Oppers-Tiemissen HM, Ragnarsson EGE, Rooseboom M, Ungell A-L. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur J Pharmaceut Sci. 2008;35:383–96. doi: 10.1016/j.ejps.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Sambuy Y, Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 9.Papapietro O, Teatero S, Thanabalasuriar A, Yuki KE, Diez E, Zhu L, Kang E, Dhillon S, Muise AM, Durocher Y, Marcinkiewicz MM, Malo D, Gruenheid S. R-Spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nat Commun. 2013;4:1898. doi: 10.1038/ncomms2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–6. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 13.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–60. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–4. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furstenberg RJv, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G409–17. doi: 10.1152/ajpgi.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JCY, Magness ST, Wong MH, Martin MG, Helmrath M, Li L. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology. 2013;145:383–95. e21. doi: 10.1053/j.gastro.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JCY, Martin MG, Magness ST. Brief report: CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 2013;31:2024–30. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk MEG, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buczacki SJA, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–9. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 23.Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, Fodde R. Paneth cells in intestinal homeostasis and tissue injury. PLoS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe C, Kljavin Noelyn M, Ybarra R, de Sauvage Frederic J. Lgr5+ Stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–59. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Watson CL, Howell JC, Schweitzer JI, Munera J, Mahe MM, Sundaram N, Vallance J, Shroyer NF, Wells JM, Helmrath MA. 925b Murine model for studying human intestine: human intestinal organoids (HIOs) engrafted in vivo develop into mature epithelial and mesenchymal intestinal tissue. Gastroenterology. 2013;144:S161. [Google Scholar]
- 26.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng. 2011;17:1841–50. doi: 10.1089/ten.tea.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, Vacanti JP. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg. 2003;238:35–41. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–12. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Walker NM, Cook MT, Ootani A, Clarke LL. Functional Cftr in crypt epithelium of organotypic enteroid cultures from murine small intestine. Am J Physiol Cell Physiol. 2012;302:C1492–503. doi: 10.1152/ajpcell.00392.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NWM, Bijvelds MJC, Scholte BJ, Nieuwenhuis EES, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–45. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 31.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 32.Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 2012;3:e00159-12–e-12. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovbasnjuk O, Zachos N, In J, Foulke-Abel J, Ettayebi K, Hyser J, Broughman J, Zeng X-L, Middendorp S, de Jonge H, Estes M, Donowitz M. Human enteroids: preclinical models of non-inflammatory diarrhea. Stem Cell Res Ther. 2013;4:S3. doi: 10.1186/scrt364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford SE, Hyser JM, Utama B, Estes MK. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc Natl Acad Sci U S A. 2012;109:E3405–13. doi: 10.1073/pnas.1216539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, Lewis M, Stelzner M, Martín MG, Dunn JCY. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Meth. 2013;19:961–9. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domansky K, Leslie DC, McKinney J, Fraser JP, Sliz JD, Hamkins-Indik T, Hamilton GA, Bahinski A, Ingber DE. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab Chip. 2013;13:3956–64. doi: 10.1039/c3lc50558h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo B-K, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H. Controlled gene expression in primary Lgr5 organoid cultures. Nat Meth. 2011;9:81–3. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]
- 38.Schwank G, Andersson-Rolf A, Koo B-K, Sasaki N, Clevers H. Generation of BAC transgenic epithelial organoids. PLoS One. 2013;8:e768–71. doi: 10.1371/journal.pone.0076871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwank G, Koo B-K, Sasselli V, Dekkers Johanna F, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent Cornelis K, Nieuwenhuis Edward ES, Beekman Jeffrey M, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martín MG, Dunn JCY. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6:e268–98. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue Y, Grant JP, Snyder PJ. Effect of glutamine-supplemented total parenteral nutrition on recovery of the small intestine after starvation atrophy. J Parenter Enteral Nutr. 1993;17:165–70. doi: 10.1177/0148607193017002165. [DOI] [PubMed] [Google Scholar]
- 42.Basson MD, Di Li G, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168:476–88. doi: 10.1002/(SICI)1097-4652(199608)168:2<476::AID-JCP26>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Bercik P, Armstrong D, Fraser R, Dutoit P, Emde C, Primi MP, Blum AL, Kucera P. Origins of motility patterns in isolated arterially perfused rat intestine. Gastroenterology. 1994;106:649–57. doi: 10.1016/0016-5085(94)90698-x. [DOI] [PubMed] [Google Scholar]
- 44.Chaturvedi LS, Gayer CP, Marsh HM, Basson MD. Repetitive deformation activates Src-independent FAK-dependent ERK motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2008;294:C1350–61. doi: 10.1152/ajpcell.00027.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926–8. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–74. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Ingber DE. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol. 2013;5:1130–40. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 48.Cassuto J, Jodal M, Tuttle R, Lundgren O. On the role of intramural nerves in the pathogenesis of cholera toxin-induced intestinal secretion. Scand J Gastroenterol. 1981;16:377–84. doi: 10.3109/00365528109181984. [DOI] [PubMed] [Google Scholar]
- 49.Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–5. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 50.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109–20. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- 51.Ramadan Q, Jafarpoorchekab H, Huang C, Silacci P, Carrara S, Koklu G, Ghaye J, Ramsden J, Ruffert C, Vergeres G, Gijs MAM. NutriChip: nutrition analysis meets microfluidics. Lab Chip. 2013;13:196–203. doi: 10.1039/c2lc40845g. [DOI] [PubMed] [Google Scholar]