Abstract
PI(4,5)P2 participates directly in priming and possibly fusion steps of Ca2+-triggered vesicle exocytosis. High concentration nanodomains of PI(4,5)P2 reside on the plasma membrane of neuroendocrine cells. A subset of vesicles that co-localize with PI(4,5)P2 domains appear to undergo preferential exocytosis in stimulated cells. PI(4,5)P2 directly regulates vesicle exocytosis by recruiting and activating PI(4,5)P2-binding proteins that regulate SNARE protein function including CAPS, Munc13-1/2, synaptotagmin-1, and other C2 domain-containing proteins. These PI(4,5)P2 effector proteins are coincidence detectors that engage in multiple interactions at vesicle exocytic sites. The SNARE protein syntaxin-1 also binds to PI(4,5)P2, which promotes clustering, but an activating role for PI(4,5)P2 in syntaxin-1 function remains to be fully characterized. Similar principles underlie polarized constitutive vesicle fusion mediated in part by the PI(4,5)P2-binding subunits of the exocyst complex (Sec3, Exo70). Overall, focal vesicle exocytosis occurs at sites landmarked by PI(4,5)P2, which serves to recruit and/or activate multifunctional PI(4,5)P2-binding proteins.
Keywords: phosphatidylinositol(4, 5)bisphosphate; vesicle exocytosis; CAPS/CADPS; Munc13; synaptotagmin; SNARE proteins
1. Introduction
Early studies showed that Ca2+-triggered vesicle exocytosis in permeable neuroendocrine cells requires MgATP for a priming step that precedes Ca2+-triggered fusion [1, 2]. The MgATP-dependent priming step involves phosphoinositides [3] and requires cytosolic factors (phosphatidylinositol transfer protein and phosphatidylinositol(4)phosphate 5-kinase, PI(4)P 5-kinase)[4, 5], which indicates that PI(4)P* phosphorylation to PI(4,5)P2 is essential for maintaining regulated exocytosis. This was further shown with a high affinity PI(4,5)P2-binding pleckstrin homology domain (PH) from PLCδ1 [6] and by the enzyme-catalyzed hydrolysis of PI(4,5)P2 [5, 7, 8], which inhibit evoked vesicle exocytosis in neuroendocrine cells. Evoked exocytosis was also shown to be inhibited by the small HIV-1 Tat protein, which directly enters cells and binds PI(4,5)P2 with ~20-fold greater affinity than the PLCδ1 PH domain [9, 10]. Similarly, a mouse knockout for PI(4)P 5-kinase Iγ caused a reduction in the priming of neuronal dense-core vesicles [11]. Conversely, increased synthesis of PI(4,5)P2 by PI(4)P 5-kinase activation increases sustained rates of evoked secretion [8, 12]. Phospholipase C-catalyzed inhibition of exocytosis in permeable cells [5] suggested there was no major role for PI(3,4,5)P3 (but see below), which was reinforced by the lack of inhibition of ATP-dependent priming by LY294002, a PI 3-kinase inhibitor, [13]; however, inhibition of evoked exocytosis by LY294002 was reported in other studies [14] but this compound also inhibits type III PI 4-kinases [15]. Electrophysiological studies of evoked vesicle exocytosis in neuroendocrine cells showed that PI(4,5)P2 is required for the generation of a primed pool of ready-releasable vesicles as well as for sustained secretion, which represents priming of recruited vesicles [8, 16]. These studies strongly indicate an essential role for PI(4,5)P2 in priming reactions for vesicle exocytosis but they do not exclude additional roles for PI(4,5)P2 at later steps following priming (e.g., fusion). Moreover, this work did not elucidate the precise roles for PI(4,5)P2, which has been the major focus of more recent research.
PI(4,5)P2 as a signaling molecule is abundant in the inner leaflet of the plasma membrane (2 mol%) but much sparser in intracellular membrane compartments. The intact phosphoinositide PI(4,5)P2 plays a critical role in most if not all cellular events associated with the plasma membrane including regulated vesicle exocytosis [17], constitutive vesicle exocytosis [18], endocytosis [19], F-actin assembly [20], cell adhesion [21], phagocytosis [22], viral budding [23], enzyme activation [24], ion channel regulation [25] and cytokinesis [26]. PI(4,5)P2 serves as a marker for plasma membrane identity and establishes a landmark for plasma membrane-associated cellular events [27]. The landmark role for PI(4,5)P2 is interpreted through the interactions of PI(4,5)P2 with proteins that are involved in each of the above cellular processes. Protein binding to PI(4,5)P2 occurs either through structured domains that have basic charge regions such as PH or C2 domains, or through contiguous or non-contiguous basic charge clusters on proteins [28–30]. PI(4,5)P2-binding proteins are the effectors for the biological roles of PI(4,5)P2 where PI(4,5)P2 either functions as a co-factor to activate membrane proteins or to recruit proteins to the plasma membrane for function. PI(4,5)P2 effector proteins are commonly multi-domain coincidence detectors that exhibit interactions with PI(4,5)P2 and with other membrane constituents. Membrane-binding energies sum from multiple low affinity interactions to drive high affinity membrane binding. Especially for Ca2+-dependent membrane binding, there is a marked mutual synergy among interaction partners. For vesicle fusion at the plasma membrane, there are now several examples of PI(4,5)P2-binding proteins that interact with SNARE proteins to promote their assembly for membrane fusion. Multivalent protein-lipid and protein-protein interactions result in higher affinity binding to the plasma membrane and provide orientation of the recruited protein relative to membrane sites landmarked by PI(4,5)P2.
Recent work has characterized the unique distribution of PI(4,5)P2 in the inner leaflet of the plasma membrane in neuroendocrine cells that contributes to establishing sites for vesicle exocytosis. Increasingly, the PI(4,5)P2 effector proteins involved in vesicle exocytosis have been characterized for their recruitment to or activation at sites of exocytosis. TIRF (total internal reflectance fluorescence) and super-resolution microscopies have played an increasing role in establishing the lipid and protein distribution at sites of exocytosis. A number of recent reviews have summarized the proteins and lipids involved in vesicle exocytosis [31–40].
2. PI(4,5)P2 localizes to sites of vesicle exocytosis
While a role for PI(4,5)P2 in vesicle exocytosis has been established, it is important to determine whether regulation by PI(4,5)P2 is local or distant, and whether PI(4,5)P2 localizes to sites of exocytosis. Early studies [3, 5] indicated that PI(4,5)P2 hydrolysis to diffusible products did not mediate the essential role of PI(4,5)P2 in vesicle exocytosis suggesting that regulation was local. Current technology is limited for localizing and perturbing PI(4,5)P2 although this is improving [41–43]. Several studies revealed a heterogeneous distribution of PI(4,5)P2 in the plasma membrane of neuroendocrine and other cells. Caroni and co-workers reported that immunoreactive clusters of PI(4,5)P2 were evident in fixed PC12 cells [44] using a PI(4,5)P2 antibody [45]. Because fixation does not immobilize lipids, it was possible that bivalent antibodies induce PI(4,5)P2 clustering, but a number of cellular conditions were found that alter the size of the clusters (e.g., MARCKS overexpression) suggesting the clusters were physiological. Several studies showed that diffraction-limited puncta of PI(4,5)P2 could be imaged on plasma membrane sheets derived from PC12 or chromaffin cells [8, 46, 47]. These PI(4,5)P2 puncta were similarly imaged either with PI(4,5)P2 antibody [8, 46] or with a GFP fusion of the PH domain of PLCδ1 [46, 47]. Immuno-EM studies localized PI(4,5)P2 close to chromaffin granules [48]. In the studies of Aoyagi et al., ~20% of the dense-core vesicles on membrane sheets co-localized with PI(4,5)P2 with about half of these also co-localizing with syntaxin-1 [46]. In the studies of James et al., ~20% of the dense-core vesicles on membrane sheets co-localized with the priming factor CAPS/CADPS and with PI(4,5)P2 [47]. These studies indicate that PI(4,5)P2 is distributed in microdomains on the plasma membrane of neuroendocrine cells. A subset of PI(4,5)P2 microdomains co-localize with vesicles and with proteins essential for vesicle exocytosis, which indicates that PI(4,5)P2 is present at sites of vesicle exocytosis (as well as elsewhere) and likely exerts local regulation.
In the studies of James et al., the PLCδ1-PH-GFP probe was calibrated on supported bilayer membranes to assess membrane PI(4,5)P2 concentrations [47]. PI(4,5)P2 was present in domains at ≥ 6 mol% although this was an underestimate limited by lack of knowledge of the actual size of the diffraction-limited domain. A subsequent study [49] estimated the size of PI(4,5)P2 domains using STED microscopy. The PI(4,5)P2 domains had an average diameter (FWHM) of 73 ± 42nm (s.d.) enabling the authors to calculate that PI(4,5)P2 concentrations at the peak of the domain may reach ~82 mol%. This may also be an underestimate based on possible crowding of the PLCδ1-PH domain interacting 1:1 with PI(4,5)P2 headgroups. Nonetheless, the nanodomains of PI(4,5)P2 imaged in this study [49] appear to consist of very high concentrations of PI(4,5)P2 approaching 100 mol%. A study utilizing dSTORM with directly-labeled monoclonal antibodies to PI(4,5)P2 in PC12 cells confirmed the small size of PI(4,5)P2 nanodomains (~65 nm). From the high signal-to-noise ratio of dSTORM, the authors concluded that the majority of plasma membrane inner leaflet PI(4,5)P2 was detected in nanodomain clusters [50].
As noted, PI(4,5)P2 domains co-localize with only a subset of docked vesicles (~20% in membrane sheets; but see below); however, these could represent a primed subset of vesicles. This was suggested by studies in which the co-localization of PI(4,5)P2 with vesicles was reduced by briefly evoking exocytosis with Ca2+ influx [46]. Whether evoked vesicle fusion occurs preferentially at PI(4,5)P2-rich plasma membrane sites was addressed in a recent study [51]. Kabachinski et al. used a lower affinity PI(4,5)P2-binding PH domain probe derived from PLCδ4 rather than PLCδ1 to image PI(4,5)P2 microdomains in live PC12 cells by TIRF microscopy. As was the case for membrane lawn studies, there were a much larger number of PI(4,5)P2 microdomains than membrane-proximal vesicles, which is consistent with a role for PI(4,5)P2 in many membrane-linked events. However, ~50% of the dense-core vesicles in the TIRF field of live cells co-localized with the PLCδ4-PH-GFP probe. Ca2+-induced vesicle exocytosis was found to occur at membrane sites enriched for PI(4,5)P2 based on the PLCδ4-PH-GFP fluorescence. A PKC-C1-GFP probe detected no PI(4,5)P2 hydrolysis to DAG at sites of vesicle exocytosis under Ca2+ influx conditions optimal for exocytosis. Greater Ca2+ influx did generate DAG (see below). As anticipated for the lower affinity of the PLCδ4-PH domain for PI(4,5)P2 [15] as compared to a PLCδ1-PH domain probe, the PLCδ4-PH domain probe exhibited reduced partitioning onto the plasma membrane and only partially inhibited vesicle exocytosis [51]. These studies indicate that vesicle exocytosis can occur at membrane sites highly enriched for PI(4,5)P2. It will be of interest to use super-resolution microscopy to determine whether PI(4,5)P2 nanodomains disperse or merge with the vesicle membrane at sites of fusion. Diffusion of PI(4,5)P2 onto the vesicle membrane during fusion, as shown to occur in Xenopus egg cortical granules [52], would promote re-organization of the actin cytoskeleton that could alter vesicle fusion modes or vesicle retrieval in endocytosis.
3. Basis for PI(4,5)P2 cluster formation
An important but unresolved question is the basis for PI(4,5)P2 micro/nanodomain formation. Early studies suggested that cellular PI(4,5)P2 co-purifies with cholesterol-rich, detergent-resistant membrane domains [44, 53] but other studies provided some evidence against this [46, 49]. Recent super-resolution microscopy studies provide support for the concept that PI(4,5)P2 clusters in the cytoplasmic leaflet align with cholesterol- and sphingomyelin(SM)-rich regions in the ectoplasmic leaflet at least at some plasma membrane sites. Kobayashi and co-workers expressed a fluorescent PLCδ1-PH domain probe to label the cytoplasmic leaflet, and used an SM-binding protein to label the ectoplasmic leaflet in PALM/dSTORM studies. They detected aligned PI(4,5)P2/SM clusters that were on average ~250 nm [26]. SM clustering appeared to be required for PI(4,5)P2 domain formation [26]. These studies provide evidence that cytoplasmic leaflet PI(4,5)P2 domains may correspond in part to cholesterol/SM-rich raft domains.
Substantial experimental work suggests that PI(4,5)P2 in synthetic membranes can self-associate in microdomains via H-bonding interactions especially when electrostatic repulsive interactions are neutralized with cations such as Ca2+ [54, 55]. PI(4,5)P2 clustering can also be achieved by electrostatic interactions between proteins with basic charge regions and highly anionic PI(4,5)P2 (−4 charge at pH 7.0) [56]. The charge cluster on MARCKS peptide (KKKKKRFSFKKSFKLSGFSFKKNKK) can sequester three PI(4,5)P2 molecules whereas the much larger PH domains bind one PI(4,5)P2 molecule. The overexpression of proteins with highly basic charge domains such as MARCKS or GAP43 was shown to increase the size of PI(4,5)P2 clusters [44]. These proteins are also acylated and may play a role in raft localization. PI(4,5)P2 also binds to the basic membrane-proximal domain of the SNARE protein syntaxin-1 (see below). However, not all PI(4,5)P2 microdomains localize with syntaxin-1 clusters [46, 49] indicating that PI(4,5)P2 interactions with syntaxin-1 in neuroendocrine cells cannot account for PI(4,5)P2 clustering. However, there are many other proteins involved in exocytosis that interact with PI(4,5)P2 (see below) and these could play a role in clustering. In addition, most intrinsic membrane proteins exhibit basic regions at the cytoplasmic face of their membrane-spanning domains [57]. Thus, PI(4,5)P2 clustering may be mediated by a variety of proteins to form PI(4,5)P2 microdomains that exhibit functions characteristic of the proteins involved. The association of proteins with SM and cholesterol could localize the PI(4,5)P2 into lipid raft domains [58].
PI(4)P 5-kinase, when overexpressed in cells or applied to membrane lawns, increases the intensity of PI(4,5)P2 microdomains, and the enzyme attains a punctate distribution on the membrane [8, 12, 46, 47]. This indicates that PI(4)P phosphorylation by PI(4)P 5-kinases provides a local source of PI(4,5)P2 for microdomain formation but little is understood about the PI(4)P substrate or enzyme targeting [59]. The membrane association of PI(4)P 5-kinases is under active investigation [60, 61] but the basis for PI(4)P 5-kinase targeting to sites of exocytosis is currently unknown. PI(4)P 5-kinases interact with anionic phospholipids including PI(4)P and PI(4,5)P2, which could be the basis for recruitment [62]. ARF6 may also play a role in the recruitment and activation of PI(4)P 5-kinase at exocytic sites [12] as promoted by ARF nucleotide binding site opener (ARNO) [63]. Type Iγ isoforms of PI(4)P 5-kinase can interact with proteins that are themselves PI(4,5)P2 effectors [60]. The type Iγ isoform of PI(4)P 5-kinase is highly enriched in brain synapses and co-localizes with proteins involved in synaptic vesicle exocytosis and endocytosis [64]. Interactions between PI(4)P 5-kinase Iγ and talin and AP2 stimulate PI(4,5)P2 synthesis for actin cytoskeletal regulation and endocytosis [65–68]. PI(4)P 5-kinase Iγ has also been shown to interact with Exo70, a PI(4,5)P2-binding subunit of the octomeric exocyst complex required for cell polarity and constitutive vesicle exocytosis [69]. A PI(4)P 5-kinase β isoform co-localized with PI(4,5)P2/SM raft domains possibly through associations with RhoA [26]. While it is clear that PI(4,5)P2 concentrations in microdomains can be modulated by kinases, phosphatases, and PLCs [8, 47, 51], additional studies are needed to identify turnover rates and the basis for enzyme access into high concentration clusters of PI(4,5)P2.
4. Protein effectors of PI(4,5)P2 utilized in the secretory pathway
PI(4,5)P2 in membrane domains at high concentrations is expected to have a pronounced effect on membrane phase behavior, the occurrence of hydrophobic defects, and local membrane curvature [17, 54, 70]. However the impact of direct lipid effects on vesicle exocytosis has been difficult to assess. Most progress has been made in efforts to understand the role of PI(4,5)P2-binding effector proteins in exocytosis as discussed in the following sections (Figure 1).
Figure 1.
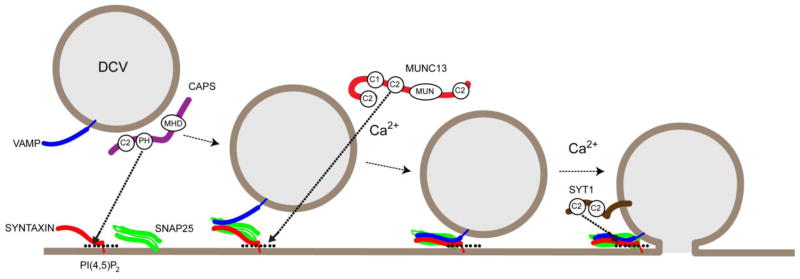
Schematic of proposed roles for PI(4,5)P2 (black circles) in regulated vesicle exocytosis. Dense-core vesicles (DCV) are depicted in a linear scheme undergoing docking, priming and fusion. Three PI(4,5)P2- and SNARE-binding proteins are shown: CAPS (purple) as a DCV-bound protein that undergoes activation at PI(4,5)P2 domains via a central PH domain; Munc13-1 (or ubMunc13-2, red) as a cytosolic protein recruited to PI(4,5)P2 domains via a central C2 domain; and synaptotagmin-1 (brown) as a DCV protein directed to fusion sites by PI(4,5)P2 via its membrane-distal C2 domain. The SNARE proteins (syntaxin-1, red; SNAP-25, green; VAMP-2/synaptobrevin-2, blue) are shown progressively assembling into complexes with syntaxin-1 shown interacting with PI(4,5)P2.
4.1. PI(4,5)P2 interactions with SNARE proteins
Syntaxin-1 plays a central role in the regulated exocytosis of vesicles as a partner for SNAP-25 on the plasma membrane that assembles into a four helix bundle with VAMP-2 (aka synaptobrevin-2) on the vesicle to mediate bilayer fusion (Figure 1). Evidence that syntaxin-1 interacts with PI(4,5)P2 (see below) prompted studies to determine whether PI(4,5)P2 regulates syntaxin-1 function in exocytosis. Syntaxin-1 as well as SNAP-25 are present in the plasma membrane mainly distributed in high copy number clusters in equilibrium with smaller pools of monomers [71–82]. Initial studies on PC12 cell membrane preparations suggested that there was some overlap between syntaxin-1 and SNAP-25 clusters as well as some degree of colocalization (30–40%) of syntaxin-1 clusters with docked vesicles [71]. Ca2+-triggered dense-core vesicle exocytosis appeared to occur near syntaxin-1 clusters in these membrane preparations suggesting a role for syntaxin-1 clusters in vesicle docking and fusion [71].
Studies in synthetic bilayer membranes showed that PI(4,5)P2 reduces the mobility of syntaxin-1/SNAP-25 heterodimers possibly indicating a role for PI(4,5)P2 in SNARE protein clustering [83]. It was subsequently suggested that fusion-competent vesicles in PC12 cells localize preferentially to plasma membrane sites that contain either PI(4,5)P2 domains or PI(4,5)P2 domains co-localized with syntaxin-1 clusters [46]. Studies in live PC12 cells revealed that a subset of docked vesicles are indeed present at PI(4,5)P2-enriched domains where exocytosis occurs without hydrolysis of PI(4,5)P2 under optimal Ca2+ influx conditions [51]. Other studies in PC12 cells showed that syntaxin-1 clusters are present at sites of vesicle docking but the syntaxin-1 clusters disappear with exocytosis [75]. PI(4,5)P2 domains co-localize with detected syntaxin-1 clusters to only a very limited extent (5–30%), and co-localizing PI(4,5)P2 and syntaxin-1 clusters occupy only a small fraction of vesicle docking sites [46, 47, 49]. Such results could indicate that sites for vesicle exocytosis are highly specialized in consisting of PI(4,5)P2 domains and syntaxin-1 clusters. However, the strongest colocalization is with vesicles and PI(4,5)P2 domains (30–50%) rather than with syntaxin-1 clusters. Additional live cell studies monitoring PI(4,5)P2, syntaxin-1 and vesicle exocytosis are needed to assess this especially since there are reports that secretory vesicles localize to sites of low SNARE density [84]. The exact function of syntaxin-1 clusters in vesicle exocytosis is currently unclear [85].
Syntaxin-1 clusters consist of 50–90 copies of syntaxin-1 occupying 50–90 nm diameter regions of the plasma membrane [73, 75, 79]. Syntaxin-1 clusters may partially or fully overlap with SNAP-25 clusters with some indication for the presence of syntaxin-1/SNAP-25 heterodimers as well as Munc18-1 [71, 74, 76, 82, 86]. Syntaxin-1 clusters may exhibit a gradient of protein density with possible protein or phospholipid interactions and monomer exchange at the periphery of the cluster [79]. Clustering is largely maintained by homophilic interactions by the SNARE domain of syntaxin-1 [72] and interactions with cholesterol [49, 87, 88]. Recent studies indicate that PI(4,5)P2 and PI(3,4,5)P3 promote syntaxin-1 clustering [49, 81]. Syntaxin-1 interactions with phosphoinositides are mediated by electrostatic interactions with a membrane-proximal sequence of basic residues K260ARRKK265 [47, 89]. Giant unilamellar vesicles containing cholesterol and 1.5 mol% (but not 5 mol%) PI(4,5)P2 or 1.5 mol% PI(3,4,5)P3 formed large (micron-sized) clusters of syntaxin-1 but cluster formation was abrogated by mutation in the basic cluster (to KARRAA) [49, 81]. Importantly, treatment of PC12 cell membranes with the 5-phosphatase synaptojanin-1 eliminated syntaxin-1 clusters [49]. Because the coincidence of high concentration PI(4,5)P2 microdomains and syntaxin-1 clusters is low (see above), these results indicate that syntaxin-1 clusters are promoted by the moderate (1–2 mol%) concentrations of PI(4,5)P2 found in membrane regions between high concentration PI(4,5)P2 microdomains [47]. The high concentrations of PI(4,5)P2 found in the microdomains at sites of vesicle exocytosis would not be required for syntaxin-1 clustering but may be important for other functions (see below).
Several studies indicate that PI(4,5)P2 or PI(3,4,5)P3 interactions with syntaxin-1 significantly affect syntaxin-1 function in membrane fusion. PI(4,5)P2 at 1–10 mol% inhibits SNARE-dependent liposome fusion in a manner similar to lysophosphatidylcholine, an inverted cone-shaped phospholipid that alters membrane curvature [47]. K264A, K265A and K252A, K253A mutations in syntaxin-1 markedly increased the inhibition, which led to the suggestion that syntaxin-1 sequesters PI(4,5)P2 to enable membrane curvature favorable for fusion [47]. A K260Q, K265L syntaxin-1 mutant was found to partially inhibit CAPS stimulation of fusion suggesting that CAPS interacts with syntaxin-1 near its PI(4,5)P2 binding site [90]. In cellular studies, the replacement of wild-type syntaxin-1 with K260AAAKK or A260AAAAA mutants reduced evoked vesicle fusion consistent with an overall positive action in fusion for syntaxin interactions with acidic phospholipids [89]. Syntaxin-1 generally binds acidic phospholipids such as PA and PI(4,5)P2 so that the actual lipid bound in cells is difficult to determine [89]. Lam et al. [89] showed that PLD1 overexpression compensated for the inhibitory effect of syntaxin-1 mutants in evoked exocytosis, which suggested that PA may regulate syntaxin-1. By contrast, PI(3,4,5)P3 interactions with syntaxin-1 were inferred to be important for neurotransmitter secretion at the Drosophila neuromuscular junction by replacing syntaxin-1 with the KARRAA mutant, which reduced synaptic syntaxin-1 clustering and evoked transmitter release [81].
The membrane-proximal basic charge region of syntaxin-1 inserts deeply into the phospholipid headgroup layer [91]. Such interactions might alter the conformation of syntaxin-1 in the bilayer possibly opening its SNARE domain for self-interactions (for clustering) or for interactions with other SNARE proteins. Charge neutralization mutants in the juxtamembrane domain syntaxin-1 could affect its conformation and protein interactions. The current work indicates that PI(4,5)P2 and/or PI(3,4,5)P3 (or PA) regulates syntaxin-1, but it will be important to establish whether it is clustering or another property (conformation) of syntaxin-1 required for regulated exocytosis that is affected by acidic phospholipids. This may be a highly-conserved property of syntaxins as indicated by the conservation of membrane-proximal charge clusters and the essential nature of the juxtamembrane domain of the yeast syntaxin proteins Sso1/2p [92].
4.2. PI(3,4,5)P3 as a regulator of synaptic vesicle exocytosis
As noted in the preceding section, studies at the Drosophila neuromuscular junction implicated PI(3,4,5)P2 as a regulator of syntaxin-1 and neurotransmitter release [81]. These studies, which used RNAi to PI 3-kinase to reduce pre-synaptic PI(3,4,5)P3 levels and a targeted GRP1 PH domain to block PI(3,4,5)P3 function, found that neurotransmitter release was moderately (~50%) reduced whereas endocytosis was not affected [81]. The results indicated that PI(3,4,5)P3 rather than PI(4,5)P2 plays an important regulatory role in synaptic vesicle exocytosis at Drosophila neuromuscular junctions. Studies are needed to determine if this result is specific to invertebrates or to synapses, and whether it extrapolates to regulated dense-core vesicle exocytosis in vertebrate neuroendocrine cells. Previous studies in vertebrate neuroendocrine cells had assessed a role for PI(3,4,5)P3 in evoked vesicle exocytosis using LY294002, a broad spectrum inhibitor of PI 3-kinase, resulting in no effect [13] or an inhibitory effect at high concentrations [14, 93]. More recent studies revealed that LY294002 as well as IC87114, an isoform-specific inhibitor of PI 3-kinaseδ, actually stimulated vesicle exocytosis because of a transient enhancement of PI(4,5)P2 levels [8, 40, 94]. While some of the previous studies perturbing PI(4,5)P2 in vertebrate neuroendocrine cells (e.g., 5-phosphatase overexpression) can be interpreted as also affecting PI(3,4,5)P3, other studies (inhibition by PLCδ1-PH domain and PLC overexpression) are more difficult to re-interpret as a role for PI(3,4,5)P3 unless functional pools of PI(3,4,5)P3 are in rapid exchange with PI(4,5)P2. High concentration ~100 nm domains of PI(3,4,5)P3 and ~60 nm domains of PI(4,5)P2 appear to be clearly segregated on the plasma membrane of PC12 cells [50]. Because PI(4,5)P2 domains have been shown to activate and recruit proteins in the regulation of dense-core vesicle exocytosis [46, 51], it will be of interest to localize PI(3,4,5)P3 and PI(4,5)P2 domains with exocytosis in both vertebrate neuroendocrine cells and synapses. Most PI(4,5)P2-binding effector proteins for regulated vesicle exocytosis utilize mainly electrostatic interactions with little selectivity that may exhibit higher affinity binding to PI(3,4,5)P3. A central role for PI(3,4,5)P3 in synaptic vesicle exocytosis could represent a synaptic specialization that increases the efficiency of synaptic transmission. It could also provide a means to independently regulate exocytosis and endocytosis in the synapse.
4.3. CAPS as an effector for PI(4,5)P2
CAPS (Calcium-dependent activator protein for secretion, aka CADPS) was originally identified as a required factor for Ca2+-triggered vesicle exocytosis that was essential for a priming step following PI(4)P phosphorylation [2, 95, 96]. It was named for the Ca2+-dependent process it functions in but, in spite of very low affinity Ca2+ binding [97], does not have a known Ca2+-dependent activity. CAPS does interact with phosphoinositides and its role as a regulator of vesicle exocytosis is PI(4,5)P2-dependent (Figure 1). The central PH domain in CAPS interacts with PI(4,5)P2 and to a lesser extent with PI(4)P [7, 47, 98, 99]. However, dependent on the assay, the CAPS PH domain also interacts with other highly-charged inositides [47]. PH domain mutants of CAPS abrogated for PI(4,5)P2 binding fail to function in regulated exocytosis in permeable [98] or intact cells [51] indicating that PI(4,5)P2 is an essential co-factor for activating CAPS via binding to its PH domain. Because of low affinity interactions, it is likely that CAPS is activated by PI(4,5)P2, the dominant phosphoinositide in the plasma membrane. CAPS was also characterized as a SNARE-binding protein that interacts with each of the three neuronal SNARE proteins, and with syntaxin-1 at its membrane-proximal, PI(4,5)P2-binding domain [90]. As a SNARE- and PI(4,5)P2-binding protein, CAPS was found to accelerate SNARE-dependent liposome fusion in a PI(4,5)P2-dependent manner [47, 100]. CAPS with PH domain mutations was strongly impaired in its activation of SNARE-dependent liposome fusion [47, 100].
Recent studies show that CAPS is distributed in the cytoplasm but is also bound to dense-core vesicles in neuroendocrine cells [51, 98]. C-terminal truncations of CAPS fail to associate with vesicles and fail to rescue evoked exocytosis in cells depleted for CAPS, which indicates that CAPS likely functions from the vesicle, although it is unclear what mediates CAPS binding to dense-core vesicles. CAPS may promote SNARE complex assembly when vesicles are near the plasma membrane to enable CAPS interactions with PI(4,5)P2 domains in trans. Because the PH domain of CAPS binds PI(4,5)P2 with low affinity, close proximity of vesicles to high concentration domains of PI(4,5)P2 may be essential for CAPS activation [47]. Previous studies showed that PI(4,5)P2 binding to purified CAPS alters its protease sensitivity in vitro [99]. It will be important to determine the PI(4,5)P2-dependent molecular transitions in CAPS responsible for altered protease sensitivity and how they relate to CAPS protein activation for SNARE complex assembly. CAPS may function as a co-incidence detector to signal vesicle arrival at PI(4,5)P2 domains in proximity to plasma membrane SNARE proteins (Figure 1).
Recent studies showed that the hydrolysis of PI(4,5)P2 at Ca2+ levels that activate PLCη2 attenuated CAPS function in evoked vesicle exocytosis [51]. This was interpreted to indicate that local PI(4,5)P2 concentrations in microdomains were reduced to below a threshold required for CAPS activation. By contrast, the DAG generated by PLCη2-catalyzed hydrolysis activated Munc13 [51]. These studies indicated that PLCη2 is a Ca2+-dependent regulator of vesicle exocytosis that can shift the function of PI(4,5)P2-dependent effectors.
4.4. Munc13-1/2 as effectors for PI(4,5)P2
Recent studies indicate that Munc13 proteins are effectors for PI(4,5)P2 in evoked vesicle exocytosis (Figure 1). Munc13-1 and ubMunc13-2 contain three distinct C2 domains but only one of these, the central or C2B domain, exhibits canonical Ca2+-binding sequences and Ca2+-dependent phospholipid binding [39]. The crystal structure of C2B revealed a short amphipathic alpha helix in loop 3 that was proposed to bind phospholipids [101]. Phospholipid binding studies showed that the C2B domain of Munc13-1 or ubMunc13-2 exhibited Ca2+-dependent binding to PI(4)P and PI(4,5)P2. Asp to Asn mutations (DN) in the Ca2+-binding sites of C2B were shown to affect the properties of ubMunc13-2 in supporting evoked synaptic vesicle exocytosis [101]. DN mutations in ubMunc13-2 did not affect vesicle exocytosis in response to single action potentials, but markedly reduced synaptic facilitation in response to multiple action potentials. Synaptic facilitation results from accumulated synaptic Ca2+ in response to trains of action potentials. Previous studies indicated that Munc13 activation at its C1 domain by DAG [102] and at a more N-terminal site by Ca2+-calmodulin [103] contribute to short-term synaptic plasticity. Thus, the Ca2+-dependent activation at C2B represents a third mechanism for facilitation. If there was also increased PI(4)P and PI(4,5)P2 synthesis promoted by elevated Ca2+ [104, 105], this would further enable Munc13 binding to the plasma membrane via its C2B domain [101]. However, Munc13 proteins localize to the cytomatrix of the active zone [106, 107] and are not obviously translocated to the presynaptic membrane in response to Ca2+ elevations [108]. Ca2+-dependent PI(4,5)P2 binding by Munc13 may instead alter molecular interactions (e.g., SNARE complex assembly)[109] that promote synaptic vesicle exocytosis. A somewhat different mechanism involving the translocation of Munc13 to PI(4,5)P2 domains was recently described for neuroendocrine cells [51].
In neuroendocrine PC12 and chromaffin cells, Munc13-1/2 proteins are cytosolic but function at plasma membrane sites of docked vesicles to regulate SNARE complex formation [102, 110]. Recent studies revealed that Ca2+ influx promotes the translocation of Munc13-1 to the plasma membrane in PC12 cells [51, 111]. The translocation of Munc13-1-GFP into punctate domains on the plasma membrane was extremely rapid (<10s) in response to depolarization and Ca2+ influx [51, 111]. Neutralization of the Ca2+-binding sites of the C2B domain was found to inhibit evoked vesicle exocytosis and to prevent the Ca2+-dependent translocation of Munc13-1, which indicated that PI(4,5)P2 binding by C2B may mediate translocation [51]. Consistent with this, overexpression of the high affinity PI(4,5)P2-binding PH domain of PLCδ1 blocked translocation of Munc13-1-GFP to microdomains [51]. Mutation in the C1 domain to compromise DAG binding had little effect on the initial Ca2+-induced translocation of Munc13-1 [51]. However, the overexpression of PLCη2, a Ca2+-activated PLC, enhanced and stabilized the translocation of Munc13-1 due to increased DAG generation at PI(4,5)P2 microdomains (G. Kabachinski, M. Yamaga, T. Martin, unpublished). The translocation of Munc13-1 was very similar to the activation mechanism for PKC with initial Ca2+-dependent interactions with PI(4,5)P2 mediated by the C2 domain and subsequent membrane stabilization by DAG interactions with the C1 domain [112]. Interestingly, Ashery and co-workers found that Ca2+-dependent Munc13-1 translocation was markedly enhanced by or dependent upon the overexpression of Doc2b [111], which interacts with Munc13-1 [113]. The C2 domains of Doc2b also exhibit Ca2+-dependent interactions with PI(4,5)P2 [114] so Doc2b and Munc13-1 may be co-recruited to PI(4,5)P2 domains as a complex. Munc13-1 dissociated more slowly than Doc2b from the membrane upon Ca2+ level decreases consistent with a distinct mechanism for Munc13-1 stabilization at the membrane [111]. Munc13-1 is proposed to function at the membrane by promoting SNARE protein complex assembly by binding to syntaxin-1 [39, 102, 109, 110]. Multiple ligands (PI(4,5)P2, syntaxin-1, Doc2b, RIM) for Munc13-1 recruitment would provide a high density of interactions for coincidence detection at sites of vesicle exocytosis.
4.5. C2 domain proteins as effectors for PI(4,5)P2
C2 domain-containing proteins are enriched at membrane trafficking nodes. These include Munc13, CAPS, synaptotagmin, rabphilin, synaptotagmin-like proteins (e.g., Slp4a/granuphilin), extended synaptotagmins, double C2 domain (DOC2) proteins, piccolo/aczonin, Rab11 FIP, ferlins, phospholipases, protein kinases, lipid kinases, and Rho GEF and GAP proteins [35, 115–118]. Synaptotagmin-1 has been extensively studied because of its role as a Ca2+-sensor for regulated vesicle exocytosis [32, 119]. As a vesicle-localized type I membrane protein, synaptotagmin-1 consists of an N-terminal luminal domain, a transmembrane region, a linker region, and two tandem (C2A and C2B) Ca2+-binding C2 domains (Figure 1). Both C2 domains exhibit Ca2+-dependent interactions with phosphatidylserine but the C2B domain also interacts with PI(4,5)P2 via a lysine-rich polybasic domain within the concave surface of the β3-β4 region [36, 80, 118, 120-124]. PI(4,5)P2 binding to this basic patch enhances the Ca2+-sensitivity of synaptotagmin for membrane interactions [123, 125, 126]. Because PI(4,5)P2 is a dominant phospholipid in the cytoplasmic leaflet at vesicle exocytic sites [46, 47, 51], C2B interactions with PI(4,5)P2 could mediate vectorial interactions between vesicle-tethered synaptotagmin-1 and plasma membrane sites for exocytosis [80, 121]. Mutations (K326A, K327A) in the polybasic site have been shown to impair synaptic vesicle exocytosis [127] and to impair SNARE-dependent liposome fusion employing reconstituted synaptotagmin/VAMP2 donor liposomes with PI(4,5)P2/syntaxin-1/SNAP-25 acceptor liposomes [128].
At elevated Ca2+ levels, synaptotagmin-1 promotes close membrane apposition by bridging the membranes although the detailed mechanism for bridging remains uncertain [124, 129–132]. Membrane bridging would promote proximity of vesicle and plasma membrane SNARE proteins for complex assembly for fusion. In addition, synaptotagmin-1 interacts directly with SNARE protein complexes potentially promoting C-terminal zippering of SNARE complexes [133–138]. Some studies indicate that the polybasic region in C2B mediates SNARE binding by synaptotagmin-1 [139, 140] but it appears that SNARE binding and PI(4,5)P2 associations can occur simultaneously [80, 114, 119]. Dual interactions of synaptotagmin-1 with PI(4,5)P2 and plasma membrane SNARE proteins is an example of coincidence detection at the plasma membrane. Synaptotagmin-1-mediated bridging of vesicle and plasma membrane would confer vectorial properties on vesicle fusion.
Affinity chromatography of chromaffin granule membrane extracts on biotinylated PI(4,5)P2 bound to avidin-conjugated beads identified several PI(4,5)P2-binding proteins including synaptotagmin-1, synaptotagmin-7 and Slp4a/granuphilin, which are C2 domain-containing proteins [141]. There are a large number of diverse C2 domain proteins involved in the regulation of vesicle exocytosis. These are either soluble (e.g., DOC2, Munc13-1), tethered on vesicles through Rab interactions (e.g., Munc13-4, rabphilin, Slp4a/granuphilin) [35, 117, 142], or vesicle transmembrane proteins (e.g., synaptotagmins-1, -7 and -9). These proteins possess polybasic regions in their C2 domains with several demonstrated to interact with PI(4,5)P2 as well as with SNARE proteins [114, 120, 143, 144]. It will be of considerable interest to determine whether these mechanistically similar proteins function collectively, redundantly, or antagonistically to regulate vesicle exocytosis.
4.6. Protein effectors of PI(4,5)P2 utilized in constitutive secretory pathways
Analysis of late Sec genes in the secretory pathway of Saccharomyces cerevisae revealed the exocyst, an octomeric complex of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 subunits, which serves as a major hub for the actin-dependent, polarized exocytosis of post-Golgi vesicles at the bud site of yeasts [145, 146]. Homologous proteins are expressed in vertebrate cells [147] where the exocyst plays roles in polarized membrane growth and vesicle trafficking [148]. For example, exocyst is required for the insulin-stimulated trafficking of glucose transporter Glut4 vesicles to the plasma membrane [149]. Exocyst is proposed to mediate vesicle localization and tethering to target membranes and to enable assembly of SNARE complexes for fusion. A large number of protein-protein and protein-lipid interactions have been identified for the subunits of this oligomeric complex including interactions with phosphoinositides, SNAREs and GTPases [148]. Live cell imaging studies suggest that a vesicle-associated subcomplex of 6 subunits associates with plasma membrane-associated Sec3 and Exo70 subunits to complete a tethering complex [150]. The Sec6 subunit of the complex regulates SNARE complex formation in association with a member of the Sec1/Munc18 family of SNARE regulators [151]. Exocyst is a multi-protein effector for a vesicle Rab(Sec4) that communicates with PI(4,5)P2 and SNAREs at plasma membrane sites, and is functionally similar to multisubunit tethering complexes found at other membrane fusion sites [152, 153]. Several exocyst subunits (Sec15, Exo70, Exo84, Sec6) share structural similarity of tandem helical bundles also found in other tethering complexes (GARP, COG, Dsl1) [152, 154, 155] as well as in CAPS and Munc13 proteins [33, 156] suggesting an ancestral relationship.
The plasma membrane-associated Sec3 and Exo70 subunits serve as landmarks for determining sites of vesicle docking and exocytosis at the plasma membrane [150]. Each of these exocyst subunits binds PI(4,5)P2 and small GTPases [157, 158]. Sec3 contains a novel PI(4,5)P2-binding PH domain that also interacts with Rho1 and Cdc42, which serves as a coincidence detector for plasma membrane associations [159, 160]. Exo70 utilizes a surface patch of basic residues at its C terminus to mediate interactions with PI(4,5)P2 [161]. Studies with a mutant yeast PI(4)P 5-kinase (Mss4) to deplete plasma membrane PI(4,5)P2 showed that polarized secretion was impaired [18, 162]. It should be noted that polarized secretion in yeast is highly dependent on the organization of the actin cytoskeleton where several PI(4,5)P2-binding proteins regulate Rho, Rac and Cdc42 function [18]. In mammalian cells, the exocytosis of post-Golgi vesicles at the plasma membrane was blocked by replacing endogenous Exo70 with a mutant lacking PI(4,5)P2 binding [161]. Recent studies also indicate that Exo70 functions to form PI(4,5)P2-dependent scaffolds that promote plasma membrane deformation for cell migration [163, 164]. Exocyst subunits appear to be important PI(4,5)P2 effectors in yeast and mammalian cells for polarity establishment and polarized vesicle exocytosis.
5. Summary
PI(4,5)P2 is a landmark for plasma membrane-associated cellular events. A substantial fraction of PI(4,5)P2 in neuroendocrine cells segregates into numerous high concentration ~70 nm domains. Some of the PI(4,5)P2 domains localize with dense-core vesicles and with proteins essential for regulated vesicle exocytosis possibly representing preferential sites for vesicle exocytosis. CAPS on vesicles requires high concentrations of PI(4,5)P2 for its activation. Vesicle synaptotagmins utilize PI(4,5)P2 to orient to plasma membrane sites. Munc13 proteins undergo Ca2+-dependent recruitment to PI(4,5)P2 domains. These proteins function as coincidence detectors by interacting with SNARE proteins to regulate membrane fusion. A key SNARE protein, syntaxin-1, also interacts acidic phospholipids including PI(4,5)P2 to enable its clustering. Similar principles are at work in constitutive vesicle fusion relying in part on PI(4,5)P2-binding by the exocyst complex for plasma membrane localization. Live cell studies employing super-resolution microscopy with better fluorescent probes for PI(4,5)P2 are needed to clarify spatial and temporal aspects of PI(4,5)P2 function in recruiting and activating proteins for vesicle exocytosis.
Highlights.
PI(4,5)P2 is clustered into high concentration nanodomains on the plasma membrane.
Vesicle exocytosis occurs at PI(4,5)P2-rich membrane domains.
PI(4,5)P2 activates/recruits SNARE-binding proteins (CAPS, Munc13, synaptotagmin).
PI(4,5)P2 regulates the SNARE protein syntaxin-1.
PI(4,5)P2- and SNARE-binding exocyst complex mediates polarized vesicle exocytosis.
Acknowledgments
Work in the Martin lab is supported by grants from the NIH (DK025861, DK040428).
Footnotes
Abbreviations used: PI, phosphosphatidylinositol; PI(4)P, phosphatidylinositol 4-monophosphate; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; PKC, protein kinase C; TIRF, total internal reflectance fluorescence; PALM, photoactivated localization microscopy; STORM/dSTORM, stochastic optical reconstruction microscopy/direct; STED, stimulated emission depletion microscopy; FWHM, full width half maximum; MARCKS, myristoylated alanine-rich C kinase substrate; CAPS (aka CADPS), Ca2+-dependent activator protein for secretion; Munc13, mammalian Unc13 protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; VAMP-2 (aka synaptobrevin-2), vesicle-associated membrane protein-2; GRP1, general receptor of phosphoinositides.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holz RW, Bittner MA, Peppers SC, Senter RA, Eberhard DA. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. The Journal of biological chemistry. 1989;264:5412–5419. [PubMed] [Google Scholar]
- 2.Hay JC, Martin TF. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. The Journal of cell biology. 1992;119:139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. The Biochemical journal. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 5.Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 6.Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. The Journal of biological chemistry. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- 7.Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debaisieux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355–363. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 10.Tryoen-Toth P, Chasserot-Golaz S, Tu A, Gherib P, Bader MF, Beaumelle B, Vitale N. HIV-1 Tat protein inhibits neurosecretion by binding to phosphatidylinositol 4,5-bisphosphate. Journal of cell science. 2013;126:454–463. doi: 10.1242/jcs.111658. [DOI] [PubMed] [Google Scholar]
- 11.Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aikawa Y, Martin TF. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. The Journal of cell biology. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin TF, Loyet KM, Barry VA, Kowalchyk JA. The role of PtdIns(4,5)P2 in exocytotic membrane fusion. Biochemical Society transactions. 1997;25:1137–1141. doi: 10.1042/bst0251137. [DOI] [PubMed] [Google Scholar]
- 14.Chasserot-Golaz S, Hubert P, Thierse D, Dirrig S, Vlahos CJ, Aunis D, Bader MF. Possible involvement of phosphatidylinositol 3-kinase in regulated exocytosis: studies in chromaffin cells with inhibitor LY294002. Journal of neurochemistry. 1998;70:2347–2356. doi: 10.1046/j.1471-4159.1998.70062347.x. [DOI] [PubMed] [Google Scholar]
- 15.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiological reviews. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, Efanov AM, Meister B, Thams P, Yang SN, Rorsman P, Berggren PO, Gromada J. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin TF. Role of PI(4,5)P(2) in Vesicle Exocytosis and Membrane Fusion. Sub-cellular biochemistry. 2012;59:111–130. doi: 10.1007/978-94-007-3015-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakir-Tamang L, Gerst JE. Phosphoinositides, exocytosis and polarity in yeast: all about actin? Trends in cell biology. 2009;19:677–684. doi: 10.1016/j.tcb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harbor perspectives in biology. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss SE. How actin gets the PIP. Science signaling. 2012;5:pe7. doi: 10.1126/scisignal.2002839. [DOI] [PubMed] [Google Scholar]
- 21.Schill NJ, Anderson RA. Out, in and back again: PtdIns(4,5)P(2) regulates cadherin trafficking in epithelial morphogenesis. The Biochemical journal. 2009;418:247–260. doi: 10.1042/BJ20081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinstein S. Imaging signal transduction during phagocytosis: phospholipids, surface charge, and electrostatic interactions. American journal of physiology Cell physiology. 2010;299:C876–881. doi: 10.1152/ajpcell.00342.2010. [DOI] [PubMed] [Google Scholar]
- 23.Alfadhli A, Barklis E. The roles of lipids and nucleic acids in HIV-1 assembly. Frontiers in microbiology. 2014;5:253. doi: 10.3389/fmicb.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oude Weernink PA, Lopez de Jesus M, Schmidt M. Phospholipase D signaling: orchestration by PIP2 and small GTPases. Naunyn-Schmiedeberg’s archives of pharmacology. 2007;374:399–411. doi: 10.1007/s00210-007-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Current opinion in neurobiology. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Abe M, Makino A, Hullin-Matsuda F, Kamijo K, Ohno-Iwashita Y, Hanada K, Mizuno H, Miyawaki A, Kobayashi T. A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Molecular and cellular biology. 2012;32:1396–1407. doi: 10.1128/MCB.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 28.Kutateladze TG. Molecular analysis of protein-phosphoinositide interactions. Current topics in microbiology and immunology. 2012;362:111–126. doi: 10.1007/978-94-007-5025-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annual review of biophysics and biomolecular structure. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 30.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nature reviews Molecular cell biology. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 31.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harbor perspectives in biology. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James DJ, Martin TF. CAPS and Munc13: CATCHRs that SNARE Vesicles. Frontiers in endocrinology. 2013;4:187. doi: 10.3389/fendo.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammar MR, Kassas N, Chasserot-Golaz S, Bader MF, Vitale N. Lipids in Regulated Exocytosis: What are They Doing? Frontiers in endocrinology. 2013;4:125. doi: 10.3389/fendo.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14:949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 36.Koch M, Holt M. Coupling exo- and endocytosis: an essential role for PIP(2) at the synapse. Biochimica et biophysica acta. 2012;1821:1114–1132. doi: 10.1016/j.bbalip.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayinger P. Phosphoinositides and vesicular membrane traffic. Biochimica et biophysica acta. 2012;1821:1104–1113. doi: 10.1016/j.bbalip.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annual review of cell and developmental biology. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 40.Wen PJ, Osborne SL, Meunier FA. Dynamic control of neuroexocytosis by phosphoinositides in health and disease. Progress in lipid research. 2011;50:52–61. doi: 10.1016/j.plipres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P. Optogenetic control of phosphoinositide metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2316–2323. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz C. Challenges in studying phospholipid signaling. Nature chemical biology. 2010;6:473–475. doi: 10.1038/nchembio.389. [DOI] [PubMed] [Google Scholar]
- 43.Wakelam MJ. The uses and limitations of the analysis of cellular phosphoinositides by lipidomic and imaging methodologies. Biochimica et biophysica acta. 2014;1841:1102–1107. doi: 10.1016/j.bbalip.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. The Journal of cell biology. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukami K, Matsuoka K, Nakanishi O, Yamakawa A, Kawai S, Takenawa T. Antibody to phosphatidylinositol 4,5-bisphosphate inhibits oncogene-induced mitogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. The Journal of biological chemistry. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 47.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. The Journal of cell biology. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umbrecht-Jenck E, Demais V, Calco V, Bailly Y, Bader MF, Chasserot-Golaz S. S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic. 2010;11:958–971. doi: 10.1111/j.1600-0854.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 49.van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, Jahn R. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Richards DA. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biology open. 2012;1:857–862. doi: 10.1242/bio.20122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabachinski G, Yamaga M, Kielar-Grevstad DM, Bruinsma S, Martin TF. CAPS and Munc13 utilize distinct PIP2-linked mechanisms to promote vesicle exocytosis. Molecular biology of the cell. 2014;25:508–521. doi: 10.1091/mbc.E12-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Molecular biology of the cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. The Journal of biological chemistry. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 54.Wang YH, Slochower DR, Janmey PA. Counterion-mediated cluster formation by polyphosphoinositides. Chemistry and physics of lipids. 2014 doi: 10.1016/j.chemphyslip.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvemini IL, Gau DM, Reid J, Bagatolli LA, Macmillan A, Moens PD. Low PIP(2) molar fractions induce nanometer size clustering in giant unilamellar vesicles. Chemistry and physics of lipids. 2014;177:51–63. doi: 10.1016/j.chemphyslip.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson J, Persson B, von Heijne G. Comparative analysis of amino acid distributions in integral membrane proteins from 107 genomes. Proteins. 2005;60:606–616. doi: 10.1002/prot.20583. [DOI] [PubMed] [Google Scholar]
- 58.Surma MA, Klose C, Simons K. Lipid-dependent protein sorting at the trans-Golgi network. Biochimica et biophysica acta. 2012;1821:1059–1067. doi: 10.1016/j.bbalip.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Thapa N, Hedman AC, Anderson RA. Phosphatidylinositol 4,5-bisphosphate: targeted production and signaling. BioEssays: news and reviews in molecular cellular and developmental biology. 2013;35:513–522. doi: 10.1002/bies.201200171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwiatkowska K. One lipid, multiple functions: how various pools of PI(4,5)P(2) are created in the plasma membrane. Cellular and molecular life sciences: CMLS. 2010;67:3927–3946. doi: 10.1007/s00018-010-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fairn GD, Ogata K, Botelho RJ, Stahl PD, Anderson RA, De Camilli P, Meyer T, Wodak S, Grinstein S. An electrostatic switch displaces phosphatidylinositol phosphate kinases from the membrane during phagocytosis. The Journal of cell biology. 2009;187:701–714. doi: 10.1083/jcb.200909025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitale N, Chasserot-Golaz S, Bailly Y, Morinaga N, Frohman MA, Bader MF. Calcium-regulated exocytosis of dense-core vesicles requires the activation of ADP-ribosylation factor (ARF)6 by ARF nucleotide binding site opener at the plasma membrane. The Journal of cell biology. 2002;159:79–89. doi: 10.1083/jcb.200203027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 65.Morgan JR, Di Paolo G, Werner H, Shchedrina VA, Pypaert M, Pieribone VA, De Camilli P. A role for talin in presynaptic function. The Journal of cell biology. 2004;167:43–50. doi: 10.1083/jcb.200406020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 67.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 68.Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11934–11939. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong X, Xu Q, Huang Y, Singh RD, Anderson R, Leof E, Hu J, Ling K. An association between type Igamma PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Molecular biology of the cell. 2012;23:87–98. doi: 10.1091/mbc.E11-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carvalho K, Ramos L, Roy C, Picart C. Giant unilamellar vesicles containing phosphatidylinositol(4,5)bisphosphate: characterization and functionality. Biophysical journal. 2008;95:4348–4360. doi: 10.1529/biophysj.107.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. The EMBO journal. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sieber JJ, Willig KI, Heintzmann R, Hell SW, Lang T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophysical journal. 2006;90:2843–2851. doi: 10.1529/biophysj.105.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 74.Rickman C, Medine CN, Dun AR, Moulton DJ, Mandula O, Halemani ND, Rizzoli SO, Chamberlain LH, Duncan RR. t-SNARE protein conformations patterned by the lipid microenvironment. The Journal of biological chemistry. 2010;285:13535–13541. doi: 10.1074/jbc.M109.091058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barg S, Knowles MK, Chen X, Midorikawa M, Almers W. Syntaxin clusters assemble reversibly at sites of secretory granules in live cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20804–20809. doi: 10.1073/pnas.1014823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knowles MK, Barg S, Wan L, Midorikawa M, Chen X, Almers W. Single secretory granules of live cells recruit syntaxin-1 and synaptosomal associated protein 25 (SNAP-25) in large copy numbers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20810–20815. doi: 10.1073/pnas.1014840107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gandasi NR, Barg S. Contact-induced clustering of syntaxin and munc18 docks secretory granules at the exocytosis site. Nature communications. 2014;5:3914. doi: 10.1038/ncomms4914. [DOI] [PubMed] [Google Scholar]
- 78.Zilly FE, Halemani ND, Walrafen D, Spitta L, Schreiber A, Jahn R, Lang T. Ca2+ induces clustering of membrane proteins in the plasma membrane via electrostatic interactions. The EMBO journal. 2011;30:1209–1220. doi: 10.1038/emboj.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bar-On D, Wolter S, van de Linde S, Heilemann M, Nudelman G, Nachliel E, Gutman M, Sauer M, Ashery U. Super-resolution imaging reveals the internal architecture of nano-sized syntaxin clusters. The Journal of biological chemistry. 2012;287:27158–27167. doi: 10.1074/jbc.M112.353250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, Mullar S, Diederichsen U, Fasshauer D, Grubmuller H, Hell SW, Eggeling C, Kuhnel K, Jahn R. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nature structural & molecular biology. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khuong TM, Habets RL, Kuenen S, Witkowska A, Kasprowicz J, Swerts J, Jahn R, van den Bogaart G, Verstreken P. Synaptic PI(3,4,5)P3 is required for Syntaxin1A clustering and neurotransmitter release. Neuron. 2013;77:1097–1108. doi: 10.1016/j.neuron.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 82.Pertsinidis A, Mukherjee K, Sharma M, Pang ZP, Park SR, Zhang Y, Brunger AT, Sudhof TC, Chu S. Ultrahigh-resolution imaging reveals formation of neuronal SNARE/Munc18 complexes in situ. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2812–2820. doi: 10.1073/pnas.1310654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophysical journal. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L, Dun AR, Martin KJ, Qiu Z, Dunn A, Lord GJ, Lu W, Duncan RR, Rickman C. Secretory vesicles are preferentially targeted to areas of low molecular SNARE density. PloS one. 2012;7:e49514. doi: 10.1371/journal.pone.0049514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van den Bogaart G, Lang T, Jahn R. Microdomains of SNARE proteins in the plasma membrane. Current topics in membranes. 2013;72:193–230. doi: 10.1016/B978-0-12-417027-8.00006-4. [DOI] [PubMed] [Google Scholar]
- 86.Lopez I, Ortiz JA, Villanueva J, Torres V, Torregrosa-Hetland CJ, del Mar Frances M, Viniegra S, Gutierrez LM. Vesicle motion and fusion are altered in chromaffin cells with increased SNARE cluster dynamics. Traffic. 2009;10:172–185. doi: 10.1111/j.1600-0854.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 87.Murray DH, Tamm LK. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murray DH, Tamm LK. Molecular mechanism of cholesterol- and polyphosphoinositide-mediated syntaxin clustering. Biochemistry. 2011;50:9014–9022. doi: 10.1021/bi201307u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Molecular biology of the cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daily NJ, Boswell KL, James DJ, Martin TF. Novel interactions of CAPS (Ca2+-dependent activator protein for secretion) with the three neuronal SNARE proteins required for vesicle fusion. The Journal of biological chemistry. 2010;285:35320–35329. doi: 10.1074/jbc.M110.145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kweon DH, Kim CS, Shin YK. Insertion of the membrane-proximal region of the neuronal SNARE coiled coil into the membrane. The Journal of biological chemistry. 2003;278:12367–12373. doi: 10.1074/jbc.M211123200. [DOI] [PubMed] [Google Scholar]
- 92.Van Komen JS, Bai X, Rodkey TL, Schaub J, McNew JA. The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo. Eukaryotic cell. 2005;4:2017–2028. doi: 10.1128/EC.4.12.2017-2028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meunier FA, Osborne SL, Hammond GR, Cooke FT, Parker PJ, Domin J, Schiavo G. Phosphatidylinositol 3-kinase C2alpha is essential for ATP-dependent priming of neurosecretory granule exocytosis. Molecular biology of the cell. 2005;16:4841–4851. doi: 10.1091/mbc.E05-02-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen PJ, Osborne SL, Zanin M, Low PC, Wang HT, Schoenwaelder SM, Jackson SP, Wedlich-Soldner R, Vanhaesebroeck B, Keating DJ, Meunier FA. Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nature communications. 2011;2:491. doi: 10.1038/ncomms1500. [DOI] [PubMed] [Google Scholar]
- 95.Nishizaki T, Walent JH, Kowalchyk JA, Martin TF. A key role for a 145-kDa cytosolic protein in the stimulation of Ca(2+)-dependent secretion by protein kinase C. The Journal of biological chemistry. 1992;267:23972–23981. [PubMed] [Google Scholar]
- 96.Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 97.Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. The Journal of biological chemistry. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- 98.Grishanin RN, Klenchin VA, Loyet KM, Kowalchyk JA, Ann K, Martin TF. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. The Journal of biological chemistry. 2002;277:22025–22034. doi: 10.1074/jbc.M201614200. [DOI] [PubMed] [Google Scholar]
- 99.Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwich GD, Martin TF. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. The Journal of biological chemistry. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- 100.James DJ, Kowalchyk J, Daily N, Petrie M, Martin TF. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17308–17313. doi: 10.1073/pnas.0900755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Sudhof TC. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nature structural & molecular biology. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Current opinion in neurobiology. 2004;14:328–340. doi: 10.1016/j.conb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Lipstein N, Sakaba T, Cooper BH, Lin KH, Strenzke N, Ashery U, Rhee JS, Taschenberger H, Neher E, Brose N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13-1 signaling. Neuron. 2013;79:82–96. doi: 10.1016/j.neuron.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Frere SG, Chang-Ileto B, Di Paolo G. Role of phosphoinositides at the neuronal synapse. Sub-cellular biochemistry. 2012;59:131–175. doi: 10.1007/978-94-007-3015-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Chen X, Jia C, Geng X, Du X, Zhang H. Depolarization increases phosphatidylinositol (PI) 4,5-bisphosphate level and KCNQ currents through PI 4-kinase mechanisms. The Journal of biological chemistry. 2010;285:9402–9409. doi: 10.1074/jbc.M109.068205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C, Geitmann M, Danielson H, Kolarow R, Niemann G, Lessmann V, Kilimann MW. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. The Journal of biological chemistry. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- 108.Kalla S, Stern M, Basu J, Varoqueaux F, Reim K, Rosenmund C, Ziv NE, Brose N. Molecular dynamics of a presynaptic active zone protein studied in Munc13-1-enhanced yellow fluorescent protein knock-in mutant mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:13054–13066. doi: 10.1523/JNEUROSCI.4330-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. The EMBO journal. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedrich R, Gottfried I, Ashery U. Munc13-1 Translocates to the Plasma Membrane in a Doc2B- and Calcium-Dependent Manner. Frontiers in endocrinology. 2013;4:119. doi: 10.3389/fendo.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 113.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, Takai Y. Physical and functional interactions of Doc2 and Munc13 in Ca2+-dependent exocytotic machinery. The Journal of biological chemistry. 1997;272:16081–16084. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 114.Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein science: a publication of the Protein Society. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. The Journal of biological chemistry. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 117.Friedrich R, Yeheskel A, Ashery U. DOC2B, C2 domains, and calcium: A tale of intricate interactions. Molecular neurobiology. 2010;41:42–51. doi: 10.1007/s12035-009-8094-8. [DOI] [PubMed] [Google Scholar]
- 118.Corbalan-Garcia S, Gomez-Fernandez JC. Signaling through C2 domains: more than one lipid target. Biochimica et biophysica acta. 2014;1838:1536–1547. doi: 10.1016/j.bbamem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 119.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annual review of biochemistry. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 120.Guillen J, Ferrer-Orta C, Buxaderas M, Perez-Sanchez D, Guerrero-Valero M, Luengo-Gil G, Pous J, Guerra P, Gomez-Fernandez JC, Verdaguer N, Corbalan-Garcia S. Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20503–20508. doi: 10.1073/pnas.1316179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature structural & molecular biology. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 122.Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Radhakrishnan A, Stein A, Jahn R, Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. The Journal of biological chemistry. 2009;284:25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuo W, Herrick DZ, Cafiso DS. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry. 2011;50:2633–2641. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van den Bogaart G, Meyenberg K, Diederichsen U, Jahn R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. The Journal of biological chemistry. 2012;287:16447–16453. doi: 10.1074/jbc.M112.343418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li L, Shin OH, Rhee JS, Arac D, Rah JC, Rizo J, Sudhof T, Rosenmund C. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. The Journal of biological chemistry. 2006;281:15845–15852. doi: 10.1074/jbc.M600888200. [DOI] [PubMed] [Google Scholar]
- 127.Loewen CA, Lee SM, Shin YK, Reist NE. C2B polylysine motif of synaptotagmin facilitates a Ca2+-independent stage of synaptic vesicle priming in vivo. Molecular biology of the cell. 2006;17:5211–5226. doi: 10.1091/mbc.E06-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z, Liu H, Gu Y, Chapman ER. Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. The Journal of cell biology. 2011;195:1159–1170. doi: 10.1083/jcb.201104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seven AB, Brewer KD, Shi L, Jiang QX, Rizo J. Prevalent mechanism of membrane bridging by synaptotagmin-1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3243–3252. doi: 10.1073/pnas.1310327110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER. Mechanism and function of synaptotagmin-mediated membrane apposition. Nature structural & molecular biology. 2011;18:813–821. doi: 10.1038/nsmb.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Connell E, Giniatullina A, Lai-Kee-Him J, Tavare R, Ferrari E, Roseman A, Cojoc D, Brisson AR, Davletov B. Cross-linking of phospholipid membranes is a conserved property of calcium-sensitive synaptotagmins. Journal of molecular biology. 2008;380:42–50. doi: 10.1016/j.jmb.2008.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van den Bogaart G, Thutupalli S, Risselada JH, Meyenberg K, Holt M, Riedel D, Diederichsen U, Herminghaus S, Grubmuller H, Jahn R. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nature structural & molecular biology. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nature structural & molecular biology. 2010;17:318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai AL, Huang H, Herrick DZ, Epp N, Cafiso DS. Synaptotagmin 1 and SNAREs form a complex that is structurally heterogeneous. Journal of molecular biology. 2011;405:696–706. doi: 10.1016/j.jmb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vrljic M, Strop P, Ernst JA, Sutton RB, Chu S, Brunger AT. Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nature structural & molecular biology. 2010;17:325–331. doi: 10.1038/nsmb.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 137.Lai Y, Lou X, Wang C, Xia T, Tong J. Synaptotagmin 1 and Ca2+ drive trans SNARE zippering. Scientific reports. 2014;4:4575. doi: 10.1038/srep04575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lynch KL, Gerona RR, Larsen EC, Marcia RF, Mitchell JC, Martin TF. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Molecular biology of the cell. 2007;18:4957–4968. doi: 10.1091/mbc.E07-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rickman C, Archer DA, Meunier FA, Craxton M, Fukuda M, Burgoyne RD, Davletov B. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. The Journal of biological chemistry. 2004;279:12574–12579. doi: 10.1074/jbc.M310710200. [DOI] [PubMed] [Google Scholar]
- 140.Dai H, Shen N, Arac D, Rizo J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. Journal of molecular biology. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Osborne SL, Wallis TP, Jimenez JL, Gorman JJ, Meunier FA. Identification of secretory granule phosphatidylinositol 4,5-bisphosphate-interacting proteins using an affinity pulldown strategy. Molecular & cellular proteomics: MCP. 2007;6:1158–1169. doi: 10.1074/mcp.M600430-MCP200. [DOI] [PubMed] [Google Scholar]
- 142.Boswell KL, James DJ, Esquibel JM, Bruinsma S, Shirakawa R, Horiuchi H, Martin TF. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. The Journal of cell biology. 2012;197:301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sato M, Mori Y, Matsui T, Aoki R, Oya M, Yanagihara Y, Fukuda M, Tsuboi T. Role of the polybasic sequence in the Doc2alpha C2B domain in dense-core vesicle exocytosis in PC12 cells. Journal of neurochemistry. 2010;114:171–181. doi: 10.1111/j.1471-4159.2010.06739.x. [DOI] [PubMed] [Google Scholar]
- 144.Lyakhova TA, Knight JD. The C2 domains of granuphilin are high-affinity sensors for plasma membrane lipids. Chemistry and physics of lipids. 2014;182:29–37. doi: 10.1016/j.chemphyslip.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 146.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. The EMBO journal. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 147.Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 148.Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 150.Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. The Journal of cell biology. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morgera F, Sallah MR, Dubuke ML, Gandhi P, Brewer DN, Carr CM, Munson M. Regulation of exocytosis by the exocyst subunit Sec6 and the SM protein Sec1. Molecular biology of the cell. 2012;23:337–346. doi: 10.1091/mbc.E11-08-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annual review of cell and developmental biology. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 153.Brocker C, Engelbrecht-Vandre S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Current biology: CB. 2010;20:R943–952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 154.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nature structural & molecular biology. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 155.Sivaram MV, Furgason ML, Brewer DN, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nature structural & molecular biology. 2006;13:555–556. doi: 10.1038/nsmb1096. [DOI] [PubMed] [Google Scholar]
- 156.Li W, Ma C, Guan R, Xu Y, Tomchick DR, Rizo J. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. The Journal of cell biology. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. The EMBO journal. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baek K, Knodler A, Lee SH, Zhang X, Orlando K, Zhang J, Foskett TJ, Guo W, Dominguez R. Structure-function study of the N-terminal domain of exocyst subunit Sec3. The Journal of biological chemistry. 2010;285:10424–10433. doi: 10.1074/jbc.M109.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamashita M, Kurokawa K, Sato Y, Yamagata A, Mimura H, Yoshikawa A, Sato K, Nakano A, Fukai S. Structural basis for the Rho- and phosphoinositide-dependent localization of the exocyst subunit Sec3. Nature structural & molecular biology. 2010;17:180–186. doi: 10.1038/nsmb.1722. [DOI] [PubMed] [Google Scholar]
- 161.Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Molecular biology of the cell. 2007;18:4483–4492. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochimica et biophysica acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, Guo W. Exo70 generates membrane curvature for morphogenesis and cell migration. Developmental cell. 2013;26:266–278. doi: 10.1016/j.devcel.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thapa N, Anderson RA. PIP2 signaling, an integrator of cell polarity and vesicle trafficking in directionally migrating cells. Cell adhesion & migration. 2012;6:409–412. doi: 10.4161/cam.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]


