Abstract
Chemically defined serum-free medium has been shown to maintain the mechanical integrity of articular cartilage explants better than serum-supplemented medium during long-term in vitro culture, but little is known about its effect on cellular mechanisms. We hypothesized that the chemically defined culture medium can regulate the spontaneous calcium signaling of in situ chondrocytes, which may modulate the cellular metabolic activities. Bovine cartilage explants were cultured in chemically defined serum-free or serum-supplemented medium for four weeks. The spontaneous intracellular calcium ([Ca2+]i) signaling of in situ chondrocytes was longitudinally measured together along with the biomechanical properties of the explants. The spontaneous [Ca2+]i oscillations in chondrocytes were enhanced at the initial exposure of serum-supplemented medium, but were significantly dampened afterwards. In contrast, cartilage explants in chemically defined medium preserved the level of calcium signaling, and showed more responsive cells with higher and more frequent [Ca2+]i peaks after one to four week culture in comparison to those in serum medium. Regardless of the culture medium that the explants were exposed, a positive correlation was detected between the [Ca2+]i responsive rate and the stiffness of cartilage (Spearman's rank correlation coefficient = 0.762). A stable pattern of [Ca2+]i peaks was revealed for each chondrocyte, i.e., the spatiotemporal features of [Ca2+]i peaks from a cell were highly consistent during the observation period (15 minutes). This study showed that the beneficial effect of chemically defined culture of cartilage explants is associated with the spontaneous [Ca2+]i signaling of chondrocytes in cartilage.
Keywords: Articular Cartilage, Serum, Chemically Defined Medium, Spontaneous, Calcium Signaling
Introduction
Autologous tissue transplantation and tissue engineering-based techniques are often used for clinical cartilage lesion repairs (Guilak et al., 2014; Hung et al., 2004; Hunziker, 2002; Peterson et al., 2002). To increase the in-shelf time of osteochondral explants and to promote the extracellular matrix deposition of chondrocytes, the medium and supplements used for long-term in vitro culture are critical for the mechanical integrity of cartilage tissue and the viability, phenotype and metabolic activities of chondrocytes. Serum, a common supplement in culture medium, is used as a major source of nutrients during the in vitro culture of chondrocytes and cartilage. However, many recent studies have shown that exposing cartilage to blood or serum can induce extracellular matrix damage. The adverse effect was partially attributed to cytotoxic oxygen metabolites (Roosendaal et al., 1999; Valentino et al., 2007). Cytokines in serum, such as COMP, IL-1, TNF-α, may also induce the inflammatory processes (Isomäki and Punnonen, 1997; Schuerwegh et al., 2003), which could further produce metalloproteinase and precipitate the degradation of cartilage matrix (Kapoor et al., 2011). Therefore, serum-supplemented medium may not always be an optimal choice for the in vitro culture of chondrocytes. Several studies showed that chemically defined serum-free medium can significantly benefit the biomechanical and biochemical properties of cartilage explants during long-term in vitro culture in comparison to traditional serum-supplemented medium (Bian et al., 2008; Bian et al., 2010; Byers et al., 2008; Garrity et al., 2012). Using the chemically defined medium, the mechanical properties and proteoglycan content of cartilage explants were increased after 2-week culture with minor loss of cell viability (Bian et al., 2008). However, little knowledge is available about the beneficial mechanisms of chemically defined medium at the cellular or molecular level.
Intracellular calcium ([Ca2+]i) signaling is one of the earliest responses in chondrocytes under many physical stimuli (Kono et al., 2006; O'Conor et al., 2014; Sanchez-Adams et al., 2014b). As an essential regulator of mechanotransduction process, [Ca2+]i signaling is the upstream of multiple signaling pathways in chondrocytes, which are ultimately involved in the regulation of various physiological processes such as secretion and gene expression (Grandolfo et al., 1998; Hung et al., 1997; Pritchard and Guilak, 2006; Pritchard et al., 2008; Sanchez-Adams et al., 2014a). Since chondrocytes are isolated in cartilage and lack direct cell-to-cell connection, the calcium wave propagation facilitated by the diffusion of messengers can be an essential intercellular communication pathway in cartilage (Kono et al., 2006). Besides the calcium signaling triggered by physical stimulation, both isolated and in situ chondrocytes have been found to release spontaneous [Ca2+]i signaling (Fodor et al., 2013; Kono et al., 2006; O'Conor et al., 2014), i.e., the [Ca2+]i concentration oscillates in chondrocytes without the presence of any extraneous mechanical or chemical stimuli.
Due to the critical role of [Ca2+]i signaling in chondrocyte mechanotransduction and cartilage remodeling, we hypothesize that the beneficial mechanisms of chemically defined medium during the in vitro culture of cartilage explants is associated with the spontaneous [Ca2+]i signaling in chondrocytes. The objectives of this study are 1) to characterize the spatiotemporal features of spontaneous [Ca2+]i signaling of in situ chondrocytes, and 2) to compare the longitudinal [Ca2+]i signaling of in situ chondrocytes cultured in two types of medium, a medium supplemented with serum and a chemically defined serum-free medium.
Materials and Methods
Sample preparation and tissue culture
Cylindrical cartilage explants were harvested from the central region of femoral condyle heads of four 3-6 month-old fresh calf knee joints (Green Village, NJ) using a 3 mm biopsy punch. The superficial-to-middle zone of the cartilage (2 mm in thickness) was obtained with a custom designed cutting tool. Cartilage explants were randomly assigned to two groups and cultured at 37 °C and 100% humidity in either serum-supplemented medium (DMEM, 10% FBS, 1% P/S) or chemically defined medium (DMEM, 1% ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, 50 μg/ml ascorbate 2-phosphate) for four weeks (Bian et al., 2008). Samples used for calcium signaling were cultured in 6-well plates with 8 explants in each well. The explants used for mechanical testing were cultured individually, one sample per well, in 24-well plates, so that their mechanical properties could be measured repeatedly at various time points. Culture medium was changed every other day for both groups, and the plate was changed every week to avoid the proliferation of migrating chondrocytes at plate bottom.
Calcium signaling of in-situ chondrocytes
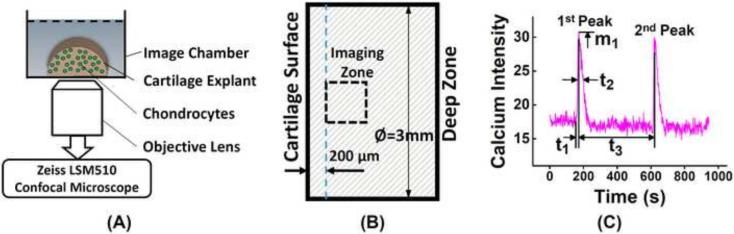
Spontaneous [Ca2+]i signaling of chondrocytes were recorded on day 2, 8, 15, and 29. Cartilage explant was halved axially with a cutting tool (ASI-Instruments, MI) and dyed with 5 μM Fluo-8 AM (AAT Bioquest, CA) and incubated for 40 min (Jing et al., 2014). The dyed cartilage was then washed three times with pure DMEM for five minutes each at 37 °C. Each half cylindrical cartilage sample, with the cutting surface facing down, was placed in an imaging chamber mounted on a confocal microscope (Zeiss LSM510) (Fig 1A). The sample was allowed a 15 min resting period for the cells to recover from any agitation during preparation (Godin et al., 2007). The focal plane of the fluorescent image was 30 μm deep below the cutting surface to avoid viewing damaged cells. The imaging area was located 200 μm below the articular surface and on the center axis of the sample, as indicated in Fig 1B. Fluorescent images of chondrocytes were recorded every 1.5 seconds for 15 minutes while the sample was undisturbed. [Ca2+]i signaling of each individual cell was analyzed with an image processing technique as described previously (Lu et al., 2012). Due to the large number of cells in each video, only a band in the center of image across the thickness direction is processed, and 150-200 cells were analyzed in each sample. Oscillation of [Ca2+]i concentration was measured by the average image intensity of each cell. A cell was defined as responsive if it shows a calcium peak with a magnitude four times higher than its maximum fluctuation along the baseline (Donahue et al., 2003). The responsive rate was defined as the fraction of responsive cells over total processed cells. For all responsive cells, the number of [Ca2+]i peaks during 15-minute period were counted. To further compare the calcium signaling between two groups, spatiotemporal features of the [Ca2+]i peaks, including the magnitude of peaks, time to reach a peak, relaxation time from a peak and time interval between two neighboring peaks, were extracted (Fig 1C) (Huo et al., 2010a; Huo et al., 2010b; Huo et al., 2008; Jing et al., 2014; Lu et al., 2012). All spatiotemporal parameters are defined in Fig 1C using a typical curve of [Ca2+]i signaling. Five cartilage explants were used for calcium signaling from each group at each time point. The number of total responding cells in the chemically defined group was 243, 169, 136, 146 at Day 2, 8, 15, 29, respectively; and the number in serum group was 262, 120, 114, 51 at Day 2, 8, 15, 29, respectively. Cell density of the cartilage samples at each time point, defined as the number of dyed cells divided by the image area, was also counted using the full calcium images of each sample.
Figure 1.
(A) Illustration of calcium imaging for in situ chondrocytes in a cartilage explant. Half cylindrical cartilage explant (3mm in diameter and 2 mm in thickness) in a glass-slide imaging chamber is mounted on a confocal microscope (Zeiss LSM510) and imaged with 20x objectives. (B) Imaging area is chose to be 200 [.proportional]m below the explant's articular surface and on the center axis of explant. (C) A typical [Ca2+]i intensity oscillation curve of a chondrocyte over 15 minutes and the definitions of spatiotemporal parameters, including the number of [Ca2+]i peaks, magnitude of 1st peak m1, time to 1st peak t1, relaxation time of 1st peak t2, and time interval between two neighboring peaks t3.
Mechanical properties and GAG content
Mechanical properties of cartilage explants (N=12) were measured longitudinally by unconfined compression tests on day 2, 8, 15, and 29 of culture using a loading device in a biological safety cabinet (Lu and Mow, 2008). The original thickness of the cartilage explant was measured as the distance between the upper and lower loading platens with a 5-gram compression force. During the test, 10% strain was applied on the tissue at a constant speed of 2 μm/s followed by a 15-minute relaxation period. After the tissue reaction force reached an equilibrium state, sinusoidal dynamic loading was applied at 0.5 Hz with a magnitude of ±1% strain. Equilibrium Young's modulus and dynamic modulus of the samples were determined from the recorded force history during the test.
At the end of culture, a quarter of each cartilage explant was weighed wet, and digested using papain solution at 60 °C for 16 hours (Lu et al., 2004). The glycosaminoglycan (GAG) content was measured using dimethylmethylene blue (Biocolor, Life Science Assays) dye-binding assay with chondroitin 4-sulfate as standard (Lu et al., 2004).
Statistical analysis
Student's t-test was used to compare the mechanical properties between the two groups, and the data are shown as mean ± standard deviation. For the parameters related to [Ca2+]i signaling, nonparametric Mann–Whitney U test was utilized to detect the significant difference between two groups, and the data are shown as mean ± SEM. P-values less than 0.05 are considered significant. Chi-square test was utilized to compare the responsive rate of [Ca2+]i signaling in two groups. Mann-Kendall test was performed to detect the temporal trend for parameters, confidence factor larger than 95% was considered significant. Spearman's rank correlation was performed to detect the correlation between the responsive rate and the dynamic modulus of cartilage explants. Spearman's rank correlations of <0.35, 0.35-0.5 and >0.5 are considered as weak, moderate and strong, respectively (Bekkers et al., 2009).
Results
Calcium signaling
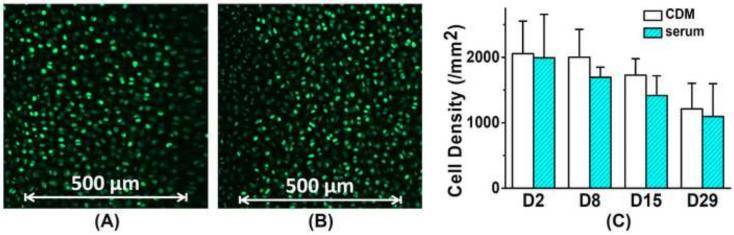
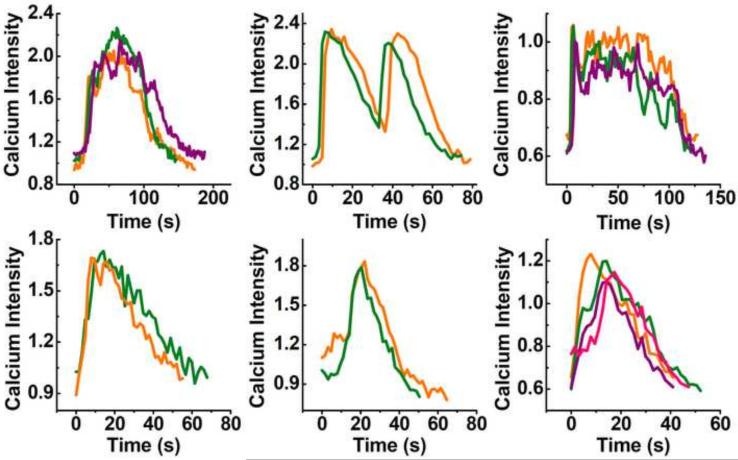
Typical calcium images of in situ chondrocytes in chemically defined and serum medium groups are shown in Fig 2. Cell density in cartilage explants decreased over time (Mann-Kendall test, confidence factor > 95%) and decreased ~40% for both groups after 4-week culture. No significant difference was detected in cell density between the two groups at any same time point (Fig 2C). In both groups, up to 20% of chondrocytes demonstrated robust spontaneous [Ca2+]i signaling, i.e., the [Ca2+]i concentration oscillated vigorously with one or more peaks (Fig 1C). The pattern of [Ca2+]i peaks varies in each individual cells, including the waveform, area, magnitude, and duration. The patterns of [Ca2+]i peaks from a specific cell were highly stable and consistent during the 15 minute observation time (Fig 3). Multiple [Ca2+]i peaks at different time points from the same cell matched well with each other in terms of the waveform. It is clear that the spontaneous [Ca2+]i wave of each in situ chondrocyte has a stable pattern.
Figure 2.
Fluorescent image of chondrocytes dyed with Fluo-8 AM in cartilage explant cultured in (A) chemically defined serum-free medium and (B) serum-supplemented medium. (C) Cell density (/mm2) in two different medium groups based on fluorescent calcium images of all samples. No significant difference was detected between two groups at the same time point. Cell density in both groups decreased with culture time (Mann-Kendall test, confidence factor > 95%).
Figure 3.
Multiple [Ca2+]i peaks released by the same chondrocyte within the 15 min recording period were overlapped to show their consistent spatiotemporal features. Each plot represents the waveform of an individual chondrocyte.
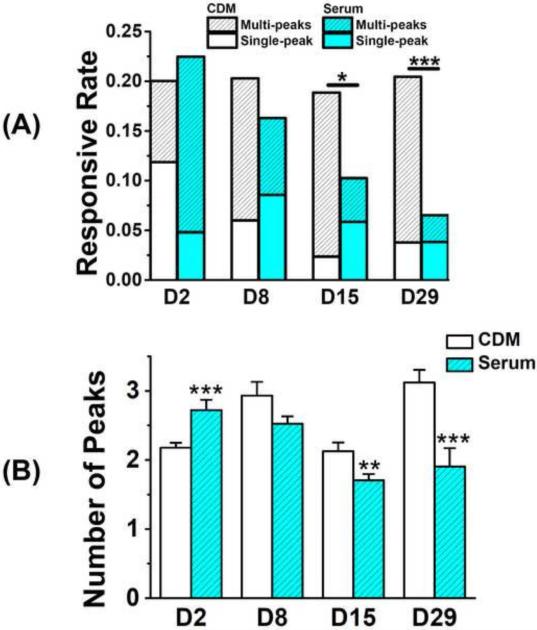
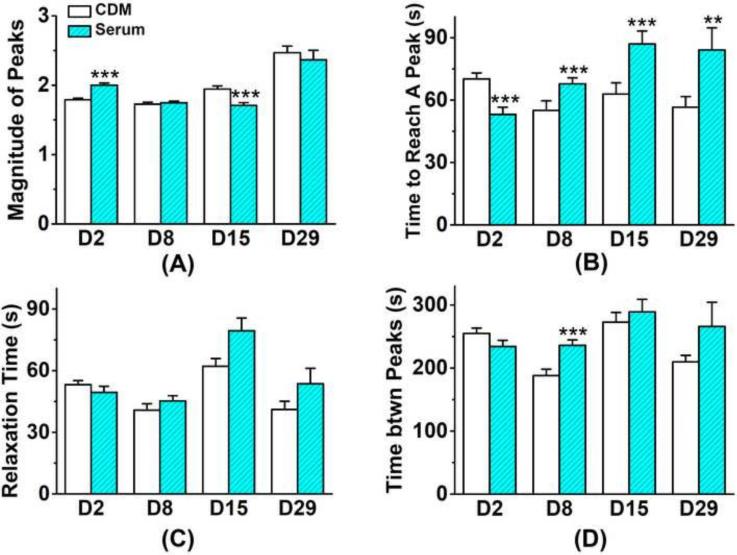
On the second day of culture, the responsive rate of cells in chemically defined group (20.0%) had no significant difference compared to the serum group (22.5%). After day 2, the calcium responsive rate in the serum group decreased with time (Mann-Kendall test, confidence factor > 95%) and dropped to 6.5% on day 29 (Fig 4A), but those in the chemically defined medium group kept relatively constant (18~20%) after day 8 and showed no dependence on culture time. After 2-week culture, the responsive rate in chemically defined serum group was significantly higher than that in serum group. On day 2, 40.7% of responsive cells showed more than one peak in 15 minutes in chemically defined medium group; while so did 78.6% of responsive cells in the serum group (Fig 4A). However, this trend reversed on day 8, 70.4% and 47.5% responsive cells showed multiple peaks in chemically defined and serum groups, respectively. Most spatiotemporal parameters of [Ca2+]i peaks in chondrocytes showed a similar trend, with vigorous oscillations in serum group on day 2 but a reversed trend afterwards. The average number of [Ca2+]i peaks during 15 minutes (Fig 4B) in the chemically defined group was significantly lower than the serum group on day 2 (2.18 ± 0.07 vs. 2.72 ± 0.15), but significantly higher on day 15 (2.13 ± 0.13 vs. 1.71 ± 0.09) and day 29 (3.12 ± 0.18 vs. 1.90 ± 0.27) according to Mann–Whitney U test. The time to reach a [Ca2+]i peak from baseline (Fig 5B) in two groups showed the same trend. On day 2, cells in serum group reached the peak significantly faster than those in the chemically defined medium group, and this difference between two groups reversed after day 8. The magnitude of [Ca2+]i peaks also showed a similar trend, but there was no difference on day 29 (Fig 5A). The two groups showed no significant difference in the peak relaxation time (Fig 5C) and the time interval between two neighboring peaks (Fig 5D) at all time points, except the time interval between neighboring peaks on day 8. Both parameters had no detectable trend over the culture time of cartilage explants.
Figure 4.
Comparison of spontaneous [Ca2+]i signaling between the serum medium and chemically defined medium groups. (A) Rate of cells in cartilage showed [Ca2+]i signaling in 15 min. Cells with single [Ca2+]i peak are separated from those with multiple peaks. (Number of total analyzed cells in chemically defined group: Day 2 = 1214 cells; Day 8 = 833 cells; Day 15 = 721 cells; Day 29 = 720 cells. Number of analyzed cells in serum group: Day 2 = 1166 cells; Day 8 = 736 cells; Day 15 = 1112 cells; Day 29 = 784 cells.) (B) Average number of [Ca2+]i peaks in responsive chondrocytes. (* p<0.05; ** p<0.01; *** p<0.001.)
Figure 5.
Comparison of spatiotemporal features of the spontaneous [Ca2+]i peaks in the serum medium and chemically defined medium groups. (A) Average magnitude of [Ca2+]i peaks normalized over the baseline; (B) Average time to reach a peak from baseline; (C) The time for a peak to relax to 50% of its magnitude; (D) Time between two neighboring peaks. (* p<0.05; ** p<0.01; *** p<0.001.)
Mechanical properties and biochemical content
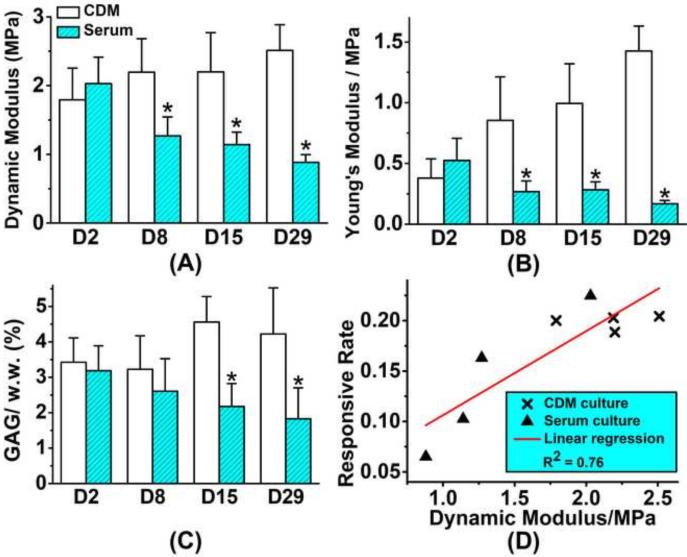
The dynamic modulus and equilibrium Young's modulus of cartilage explants in the chemically defined medium group were significantly higher than those in the serum group after day 8, and Mann-Kendall test showed the difference gap between two groups increased with time (Fig 6A&B, confidence factor > 95%). After 4-week culture, dynamic modulus in chemically defined medium group increased 40.2%, and the Young's modulus increased 276% in comparison to day 2; while in the serum group, dynamic modulus decreased 56.7%, and the Young's modulus decreased 67.3%. GAG content of chemically defined group was more than twice of that in corresponding serum group after 2-week culture (Fig 6C). Spearman's rank correlation coefficient between the [Ca2+]i responsive rate and the dynamic modulus of cartilage explants in both groups is 0.762, showing a significant positive correlation between the two parameters (Fig 6D).
Figure 6.
Mechanical properties and GAG content of cartilage explants (12 explants in each group). (A) Dynamic modulus and (B) equilibrium Young's modulus are determined by unconfined compression tests longitudinally during in vitro culture. (C) GAG content of the cartilage explants. (* p<0.05) (D) A strong correlation is detected between the [Ca2+]i responsive rate of chondrocytes and the dynamic modulus of cartilage explants (Spearman's rank correlation coefficient = 0.762).
Discussion
Calcium signaling has been observed in chondrocytes in response to hydrostatic pressure, compressive loading (Browning et al., 2004; Roberts et al., 2001), fluid flow (Degala et al., 2011), mechanical stimulation (Guilak et al., 1999), extracellular ATP (D'Andrea and Vittur, 1996), and osmotic stress (Chao et al., 2006; O'Conor et al., 2014). In this study, we showed that bovine chondrocytes residing in their natural solid matrix can release spontaneous [Ca2+]i signaling without any physical stimuli. For both isolated and in situ chondrocytes, spontaneous [Ca2+]i signaling have been noticed (Fodor et al., 2013; Kono et al., 2006; O'Conor et al., 2014). Using chondrocytes seeded in agarose constructs, O'Conor et al. found that ~20% of cells had spontaneous calcium signaling (O'Conor et al., 2014), which is in the same range with our finding. Spontaneous calcium signaling has also been noticed in other types of cells, such as neurons (Charles and Hales, 1995), bone cells (Ishihara et al., 2012) and mammary epithelial cells (Furuya et al., 1993). Although the mechanism of spontaneous calcium signaling in chondrocytes is not fully understood or confirmed, a few studies proposed several potential candidate pathways. The underlying oscillation in membrane potential driven by K+ channels can activate the action potential and the influx of Ca2+ which initiates the spontaneous calcium signaling (Charles and Hales, 1995). Calcium concentration in extracellular space is usually in the range of 10−3 M in cartilage (Parvizi et al., 2002), while cytosol calcium concentration can be as low as 10−7 M (Matta and Zakany, 2012). The 10,000 fold gradient of Ca2+ could also result in the calcium influx into cytosol to evoke calcium responses (Machaca, 2011). Another potential stimulus for spontaneous response is the oxidative stress, which can cause rapid increase in calcium concentration in the cytoplasm (Rooney et al., 1991; Roveri et al., 1992). In cartilage solid matrix, fixed negative charges on GAG chains results in an unique ionic environment for chondrocytes, which may also influence the resting membrane potential of chondrocytes and contribute to the spontaneous calcium signaling (Matta and Zakany, 2012).
In this study, the spontaneous [Ca2+]i signaling of each individual chondrocytes were found to have a stable spatiotemporal pattern. Similar phenomenon was reported in bone osteoblasts (Godin et al., 2007) and a few other types of cells (Prentki et al., 1988), in which the stable profile was termed as a “fingerprint” in cell calcium signaling. Such calcium “fingerprint” of cells could be related to numerous cell-specific parameters, such as the morphology and topology of cell body and nucleus, the distribution of calcium stores, and the location of the inositol (1,4,5)- trisphosphate (IP3)-generating apparatus (Godin et al., 2007; Kraus et al., 1996). The unaltered stable profile in [Ca2+]i signaling of each chondrocyte found in this study could be a critical indicator of the phenotype and capabilities of the cells, about which there is little knowledge available.
Serum-supplemented medium and chemically defined medium are both widely used in the in vitro culture of chondrocytes and neocartilage. In this study, exposure to serum supplemented medium significantly altered the spontaneous calcium signaling in chondrocytes and showed a biphasic nature during the in vitro culture process, promoting the calcium signaling at the initial stage but dampening it after prolonged exposure. Many components in serum could be responsible for this biphasic effect. Cytokines in serum, such as proinflammatory mediator interleukin-1 (IL-1), can induce transient increase of [Ca2+]i via the PLC-IP3 pathway (Pritchard and Guilak, 2006). This mechanism may contribute to the initial boost of calcium signaling of in situ chondrocytes exposed to serum. Indeed many other enzymes, hormones, cytokines, and electrolytes in serum could induce transient [Ca2+]i oscillations in cells and be responsible for the high responsive rate in the serum group on day 2 (Petersen et al., 2005). However, most of these chemicals in serum are not available to chondrocytes at physiological condition. Long term exposure to these foreign components may induce complicated changes in chondrocytes, including dedifferentiation and apoptosis of chondrocytes (Stewart et al., 2000). It has been shown that pre-exposure to IL-1 can significantly inhibit the calcium responses of chondrocytes under osmotic stress (Pritchard et al., 2008). Dedifferentiation of chondrocytes leads to the increased production of proteolytic enzymes including matrix metallopeptidase, which in turn damages the extracellular matrix as demonstrated by our mechanical testing data. Mechanical compromise of solid matrix can further regulate the cell fate and metabolisms. All the above factors may contribute to the dampened [Ca2+]i activities in chondrocytes after long-term serum exposure.
In the mechanotransduction activities of chondrocytes, [Ca2+]i signaling is often regarded as one of the earliest events and the upstream of many anabolic and catabolic pathways (Guilak et al., 2014). Disturbance in the Ca2+ homeostatic mechanism during long term in vitro culture can significantly affect tissue repair and growth. It has been shown that up-regulation of aggrecan gene in response to mechanical loading was suppressed by the chelation of [Ca2+]i (Fitzgerald et al., 2004), and chelation of extracellular Ca2+ completely abolished the production of cartilage matrix by preventing the proliferation of chondrocytes (Matta and Zakany, 2012). Therefore disturbance in chondrocyte [Ca2+]i signaling can affect the remodeling of solid matrix and its mechanical properties during long term culture. In this study, the intensity of spontaneous calcium signaling was dampened in serum group but maintained in the chemically defined medium group, while the mechanical properties of explants exhibited a matching trend in the corresponding groups. Thus proper Ca2+ homeostasis, represented by the spontaneous [Ca2+]i signaling in chondrocytes, might reflect the normal metabolic activities of in situ chondrocytes. Moreover, spatiotemporal features of [Ca2+]i signaling is a direct indicator of the chondrocytes capability to maintain the [Ca2+]i level under a dynamic environment with oscillating extracellular ion concentration, electric potential, mechanical loading, osmotic and oxidative stress. Statistical analysis showed that the [Ca2+]i responsive rate of chondrocytes has a positive correlation to the dynamic modulus of cartilage explants after long-term culture. Although such statistical correlation cannot prove the immediate dependency of these two parameters, it is consistent with the findings in literature, i.e., interrupted calcium signaling can affect the mechanical integrity of cartilage. Furthermore, many studies showed that the mechanical stiffness of ECM or substrate has a profound effect on the fate and metabolic activities of cells (Engler et al., 2006; Wilusz et al., 2014). Substrate rigidity influences the spontaneous [Ca2+]i oscillations in human mesenchymal stem cells (Kim et al., 2009). Lowering the substrate stiffness significantly inhibited both the magnitudes and frequencies of the cytoplasmic Ca2+ oscillation in comparison to rigid substrate.
In summary, this study confirmed the spontaneous calcium signaling of in situ chondrocytes in articular cartilage, and the spatiotemporal features of the [Ca2+]i oscillations were analyzed. It was found that the waveform of multiple [Ca2+]i peaks from the same chondrocyte possess a consistent profile. The spontaneous calcium signaling is also influenced by the supplements in culture medium. Chemically defined medium maintained the [Ca2+]i oscillations in chondrocytes over long-term culture and benefits the mechanical integrity of cartilage explants when compared to the serum-supplemented medium.
Acknowledgement
NIH 2P20-RR16458, Musculoskeletal Transplant Foundation, and DOD W81XWH-13-1-0148.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors state that they have no conflicts of interest.
References
- Bekkers J, de Windt TS, Raijmakers N, Dhert W, Saris D. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthritis and Cartilage. 2009;17:1434–1439. doi: 10.1016/j.joca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bian L, Lima EG, Angione SL, Ng KW, Williams DY, Xu D, Stoker AM, Cook JL, Ateshian GA, Hung CT. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41:1153–1159. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Stoker AM, Marberry KM, Ateshian GA, Cook JL, Hung CT. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. The American journal of sports medicine. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J, Saunders K, Urban J, Wilkins R. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41:299–308. [PubMed] [Google Scholar]
- Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Engineering Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao P.-h.G., West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. American Journal of Physiology-Cell Physiology. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Charles AC, Hales T. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. Journal of neurophysiology. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- D'Andrea R, Vittur F. Ca2+ oscillations and intercellular Ca2+ waves in ATP - stimulated articular chondrocytes. Journal of Bone and Mineral Research. 1996;11:946–954. doi: 10.1002/jbmr.5650110711. [DOI] [PubMed] [Google Scholar]
- Degala S, Zipfel WR, Bonassar LJ. Chondrocyte calcium signaling in response to fluid flow is regulated by matrix adhesion in 3-D alginate scaffolds. Archives of biochemistry and biophysics. 2011;505:112–117. doi: 10.1016/j.abb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Donahue HJ, Jacobs CR. Osteoblastic cells have refractory periods for fluid-flow-induced intracellular calcium oscillations for short bouts of flow and display multiple low-magnitude oscillations during long-term flow. J Biomech. 2003;36:35–43. doi: 10.1016/s0021-9290(02)00318-4. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. Journal of Biological Chemistry. 2004;279:19502–19511. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- Fodor J, Matta C, Oláh T, Juhász T, Takács R, Tóth A, Dienes B, Csernoch L, Zákány R. Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell calcium. 2013;54:1–16. doi: 10.1016/j.ceca.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Furuya K, Enomoto K.-i., Yamagishi S. Spontaneous calcium oscillations and mechanically and chemically induced calcium responses in mammary epithelial cells. Pflügers Archiv. 1993;422:295–304. doi: 10.1007/BF00374284. [DOI] [PubMed] [Google Scholar]
- Garrity JT, Stoker AM, Sims HJ, Cook JL. Improved osteochondral allograft preservation using serum-free media at body temperature. The American journal of sports medicine. 2012;40:2542–2548. doi: 10.1177/0363546512458575. [DOI] [PubMed] [Google Scholar]
- Godin LM, Suzuki S, Jacobs CR, Donahue HJ, Donahue SW. Mechanically induced intracellular calcium waves in osteoblasts demonstrate calcium fingerprints in bone cell mechanotransduction. Biomechanics and modeling in mechanobiology. 2007;6:391–398. doi: 10.1007/s10237-006-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandolfo M, Calabrese A, D'andrea P. Mechanism of Mechanically Induced Intercellular Calcium Waves in Rabbit Articular Chondrocytes and in HIG 82 Synovial Cells. Journal of Bone and Mineral Research. 1998;13:443–453. doi: 10.1359/jbmr.1998.13.3.443. [DOI] [PubMed] [Google Scholar]
- Guilak F, Butler DL, Goldstein SA, Baaijens FP. Biomechanics and mechanobiology in functional tissue engineering. J Biomech. 2014;47:1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Zell RA, Erickson GR, Grande DA, Rubin CT, McLeod KJ, Donahue HJ. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. Journal of orthopaedic research. 1999;17:421–429. doi: 10.1002/jor.1100170319. [DOI] [PubMed] [Google Scholar]
- Hung CT, Allen FD, Mansfield KD, Shapiro IM. Extracellular ATP modulates [Ca2+] i in retinoic acid-treated embryonic chondrocytes. American Journal of Physiology-Cell Physiology. 1997;272:C1611–C1617. doi: 10.1152/ajpcell.1997.272.5.C1611. [DOI] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Hunziker E. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell calcium. 2010a;47:234–241. doi: 10.1016/j.ceca.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo B, Lu XL, Guo XE. Intercellular calcium wave propagation in linear and circuit-like bone cell networks. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2010b;368:617–633. doi: 10.1098/rsta.2009.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo B, Lu XL, Hung CT, Costa KD, Xu Q, Whitesides GM, Guo XE. Fluid flow induced calcium response in bone cell network. Cellular and molecular bioengineering. 2008;1:58–66. doi: 10.1007/s12195-008-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara Y, Sugawara Y, Kamioka H, Kawanabe N, Kurosaka H, Naruse K, Yamashiro T. In situ imaging of the autonomous intracellular Ca 2+ oscillations of osteoblasts and osteocytes in bone. Bone. 2012;50:842–852. doi: 10.1016/j.bone.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Isomäki P, Punnonen J. Pro-and anti-inflammatory cytokines in rheumatoid arthritis. Annals of medicine. 1997;29:499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, Luo E, Guo XE. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28:1582–1592. doi: 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature reviews. Rheumatology. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Seong J, Ouyang M, Sun J, Lu S, Hong JP, Wang N, Wang Y. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218:285–293. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T, Nishikori T, Kataoka H, Uchio Y, Ochi M, Enomoto K. Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochemistry and Function. 2006;24:103–111. doi: 10.1002/cbf.1304. [DOI] [PubMed] [Google Scholar]
- Kraus M, Wolf B, Wolf B. Crosstalk between cellular morphology and calcium oscillation patterns Insights from a stochastic computer model. Cell Calcium. 1996;19:461–472. doi: 10.1016/s0143-4160(96)90055-x. [DOI] [PubMed] [Google Scholar]
- Lu X, Mow V. Biomechanics of articular cartilage and determination of material properties. Medicine Science in Sports Exercise. 2008;40:193. doi: 10.1249/mss.0b013e31815cb1fc. [DOI] [PubMed] [Google Scholar]
- Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. Journal of Bone and Mineral Research. 2012;27:563–574. doi: 10.1002/jbmr.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XL, Sun DD, Guo XE, Chen FH, Lai WM, Mow VC. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Annals of biomedical engineering. 2004;32:370–379. doi: 10.1023/b:abme.0000017534.06921.24. [DOI] [PubMed] [Google Scholar]
- Machaca K. Ca2+ signaling, genes and the cell cycle. Cell calcium. 2011;49:323–330. doi: 10.1016/j.ceca.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Matta C, Zakany R. Calcium signalling in chondrogenesis: implications for cartilage repair. Frontiers in bioscience (Scholar edition) 2012;5:305–324. doi: 10.2741/s374. [DOI] [PubMed] [Google Scholar]
- O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proceedings of the National Academy of Sciences. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Parpura V, Greenleaf JF, Bolander ME. Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. Journal of orthopaedic research. 2002;20:51–57. doi: 10.1016/S0736-0266(01)00069-9. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell calcium. 2005;38:161–169. doi: 10.1016/j.ceca.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Peterson L, Brittberg M, Kiviranta I, Åkerlund EL, Lindahl A. Autologous chondrocyte transplantation biomechanics and long-term durability. The American journal of sports medicine. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- Prentki M, Glennon M, Thomas A, Morris RL, Matschinsky F, Corkey B. Cell-specific patterns of oscillating free Ca2+ in carbamylcholine-stimulated insulinoma cells. Journal of Biological Chemistry. 1988;263:11044–11047. [PubMed] [Google Scholar]
- Pritchard S, Guilak F. Effects of interleukin 1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis & Rheumatism. 2006;54:2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- Pritchard S, Votta BJ, Kumar S, Guilak F. Interleukin-1 inhibits osmotically induced calcium signaling and volume regulation in articular chondrocytes. Osteoarthritis and Cartilage. 2008;16:1466–1473. doi: 10.1016/j.joca.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SR, Knight MM, Lee DA, Bader DL. Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. Journal of Applied Physiology. 2001;90:1385–1391. doi: 10.1152/jappl.2001.90.4.1385. [DOI] [PubMed] [Google Scholar]
- Rooney T, Renard D, Sass E, Thomas A. Oscillatory cytosolic calcium waves independent of stimulated inositol 1, 4, 5-trisphosphate formation in hepatocytes. Journal of Biological Chemistry. 1991;266:12272–12282. [PubMed] [Google Scholar]
- Roosendaal G, MARIEKE EV, JOANNES JM, MARIJKE H, BERG FPL, Bijlsma JW. Blood-induced joint damage. Arthritis & Rheumatism. 1999;42:1025–1032. doi: 10.1002/1529-0131(199905)42:5<1025::AID-ANR23>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Roveri A, Coassin M, Maiorino M, Zamburlini A, van Amsterdam FTM, Ratti E, Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Archives of biochemistry and biophysics. 1992;297:265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- Sanchez-Adams J, Leddy HA, McNulty AL, O'Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014a;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Adams J, Leddy HA, McNulty AL, O'Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Current rheumatology reports. 2014b;16:1–9. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerwegh A, Dombrecht E, Stevens W, Van Offel J, Bridts C, De Clerck LS. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis and cartilage. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- Stewart MC, Saunders KM, Burton Wurster N, Macleod JN. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. Journal of bone and mineral research. 2000;15:166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- Valentino L, Hakobyan N, Rodriguez N, Hoots W. Pathogenesis of haemophilic synovitis: experimental studies on blood - induced joint damage. Haemophilia. 2007;13:10–13. doi: 10.1111/j.1365-2516.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]