Abstract
Microorganisms are vulnerable to elevated levels of intracellular reactive oxygen species (ROS). This situation has led to proposals that many natural stresses might be toxic specifically because they accelerate endogenous ROS formation. Such a mechanism has been convincingly demonstrated for redox-cycling compounds. However, the evidence is much weaker for most other stressors. The hypothesis that clinical antibiotics generate lethal ROS stress has attracted much attention, and the author discusses some aspects of evidence that support or oppose this idea. Importantly, even if all cellular electron flow were somehow diverted to ROS formation, the resultant doses of H2O2 and O2− would more likely be bacteriostatic than bacteriocidal unless key defense mechanisms were simultaneously blocked.
Introduction
Life evolved in an anoxic world, and so contemporary organisms have inherited biochemical features that are substantially incompatible with the presence of oxygen. In fact, both calculations and experiments indicate that microbes have acquired just enough defensive measures to avoid overt poisoning by endogenous reactive oxygen species (ROS) [1]. Any elevation in the intracellular levels of these oxidants—notably, superoxide (O2−) and hydrogen peroxide (H2O2)—produces enough enzyme damage that growth stalls and enough DNA damage that mutagenesis accelerates.
Since life is poised on this knife’s edge, investigators often wonder whether various stressors might exert their toxic effects by amplifying the natural rate of ROS production (Table 1). A variety of experimental approaches have been used to test these ideas. The results do not always provide a consensus, and the purpose of this review is to explore why seemingly straightforward analyses can produce data that are ambiguous or contradictory.
Table 1.
The list is incomplete. Where possible, literature was cited that employs E. coli as a model system. The involvement of ROS in toxicity is generally accepted in some cases but is less settled in others.
| Some proposed triggers of ROS stress | Sample reference1 |
|---|---|
| Ionizing radiation | [52] |
| Hyperoxia | [53] |
| Quinones | [25] |
| Viologens | [24] |
| Phenazines | [26] |
| Hydroxyurea | [54] |
| Tannins | [55] |
| Clinical antibiotics | [3] |
| Amoebae | [56] |
| Plant wound response | [57] |
| Phagocytic oxidative burst | [58] |
| Lactic acid bacteria | [59] |
| Heat | [60] |
| Hydrostatic pressure | [61] |
| Salinity | [62] |
| Near-UV radiation | [63] |
| Photosynthesis | [64] |
| Alcohol | [65] |
| Iron overloading | [21] |
| Silver | [66] |
| Copper | [67] |
| Chromate | [68] |
| Cobalt | [69] |
| Tellurite | [70] |
| Nanoparticles | [71] |
| Senescence | [72] |
| Phosphate starvation | [73] |
| Peptidoglycan recognition proteins | [74] |
| Cytotoxic proteases | [75] |
| Addiction cassettes | [76] |
A particular example of this problem is the debate over clinical antibiotics. Work from many groups, spearheaded by the Collins and Walker labs [2–6], has provided evidence that aminoglycosides, β-lactams, and fluoroquinolones owe some of their lethal effects to the generation of ROS. Other groups are unconvinced and cite contrary data [7–12]. The author is mainly in the latter camp and will describe the nature of key elements of the evidence. Due to length restrictions, this discussion is not comprehensive. Readers are encouraged to read a recent review to learn an opposing viewpoint [13].
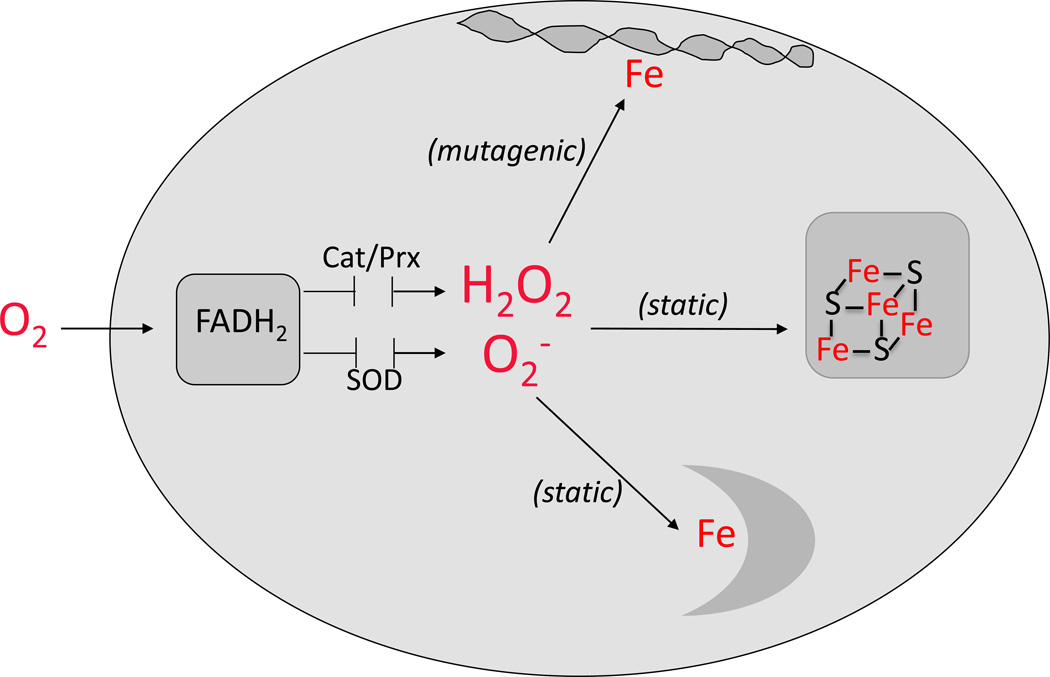
Escherichia coli is the model system in which the details of oxidative stress are best understood. ROS are continuously formed in oxic E. coli through the adventitious autoxidation of its redox enzymes; their accumulation is held in check by the superoxide dismutases that degrade O2− and the peroxidases and catalases that degrade H2O2 (Fig. 1). Mutants that lack either set of enzymes suffer damage to specific iron enzymes and cannot grow under conditions in which their activities are necessary [14–19]. DNA damage also increases due to reactions between H2O2 and the intracellular pool of labile iron [20–22]:
Fe2+ + H2O2 → [FeO2+] + H+ + OH− → Fe3+ + OH− + HO.
The hydroxyl radicals thus formed react avidly with virtually all biomolecules. Their reactions with DNA produce some lesions that are misread by DNA polymerase and others that block its progress. If enough of the latter lesions are generated, replication never recovers, and the cell dies. This scenario has been proposed to explain some part of the lethal action of antibiotics [13].
Figure 1. Targets of ROS in E. coli.
Autoxidation of redox enzymes generates O2− and H2O2 that are kept at low concentrations by scavenging enzymes such as catalases (Cat), peroxidases (Prx), and superoxide dismutases (SOD). Any boost in H2O2 concentration accelerates reactions with the pool of free iron; DNA damage and mutagenesis ensue. Excess O2− and H2O2 inactivate Fe/S-dependent dehydratases and mononuclear Fe enzymes, thereby disabling pathways and blocking growth.
Do redox-cycling drugs generate oxidative stress? Theory and evidence
It is useful to consider the example of redox-cycling compounds—paraquat, menadione, phenazines, and others—in thinking about how oxidative stress might arise and be detected inside bacteria. These agents include natural compounds that are released by plants and bacteria in an effort to suppress the growth of competitors. The chemical foundation for their action is well-understood. These compounds are facile acceptors and donors of single electrons, and in vitro it can be shown that they non-specifically oxidize many redox enzymes by abstracting electrons from their exposed flavin and metal centers [23]. In fact, the rate of electron transfer from enzymes to these agents can match or exceed the rate of transfer to physiological acceptors. The semiquinone species thus formed then rapidly pass the electrons to molecular oxygen. Superoxide is thereby produced—and, through its dismutation, H2O2 is generated as well [24–26].
These redox-cycling compounds can penetrate into cells like E. coli, and they easily impair the growth of aerobic but not anoxic bacteria [24,26], which suggests that they are poisonous precisely because they drive ROS production. Intracellular ROS formation has been confirmed in several ways. The exposed cells continue to consume oxygen even when cyanide is added to block cytochrome oxidases; this “cyanide-resistant respiration” is a marker of illicit oxygen reduction by the drugs [25,26]. Strains that lack catalases and peroxidases naturally release H2O2 into growth medium—and this rate is greatly augmented when redox-cycling compounds are added [7]. The OxyR regulatory protein, which directly senses H2O2, becomes activated [27]. ROS-sensitive dehydratases lose activity [15,28]. Mutants that lack SOD are hypersensitive [14,28]. Mutation rates rise [20,29]. In fact, the cell features a regulon (SoxRS) whose purpose is to directly sense these redox agents; it responds by inducing the synthesis of superoxide dismutase and of endonuclease IV, an enzyme that repairs oxidative DNA damage [30,31].
Thus a battery of measurements provide a consensus: these compounds amplify ROS formation and thereby debilitate the cell. These techniques are therefore the obvious choice to test other suspected sources of ROS stress. Interestingly, the amount of DNA damage that redox-active compounds cause is usually not sufficient to kill cells—a point that will be germane to the antibiotic issue.
Do clinical antibiotics impose oxidative stress? Evidence from ROS measurements
The oxidative-stress hypothesis of antibiotic action is representative of ROS-overproduction theories; it is an especially useful example because its proponents have employed a range of creative approaches to test the idea. The notion that standard antibiotics might create ROS stress arose initially from microarray analyses [2]. The data showed that the SoxRS, Fur, and IscR regulons were partially activated when E. coli was exposed to barely toxic doses of norfloxacin. At the time of this work it was not recognized that SoxR directly senses drugs rather than O2− [32], and so these data were interpreted to mean that both ROS and labile iron pools might somehow be perturbed. It seemed logical that the sequelae might include an increase in DNA lesions, potentially contributing to the loss of viability.
To test this idea, the investigators used redox-sensing dyes as a means of appraising oxidative stress inside living cells. They checked whether chemical antioxidants and cell-permeable iron chelators would slow cell death. They also tested whether cells would be protected by the overproduction of ROS scavenging enzymes and DNA repair enzymes. All of these approaches generated data that appeared to support the ROS hypothesis [3].
However, most of the conventional markers that had successfully detected oxidative stress in the case of redox-cycling compounds did not give any such indication in the case of clinical antibiotics. Both the original microarray experiment and subsequent analyses by RT-PCR and gene fusions did not show significant activation of the OxyR regulon [2,3,7]. This outcome was surprising, because OxyR is the natural mechanism by which the cell senses threatening levels of H2O2. The rate of H2O2 effusion from catalase/peroxidase mutants was unchanged [7]. ROS-sensitive dehydratases did not lose activity, and labile iron pools, which typically swell from oxidative damage to iron enzymes, did not grow [7]. Mutants that lack SOD or catalase/peroxidase were not particularly sensitive [7,10]. The same was true of oxyR, recA, and polA mutants, all of which are rapidly killed by authentic H2O2 [7,10]. (One exception: the DNA repair mutants were hypersensitive to norfloxacin, in accord with its direct action upon topoisomerase.)
Thus the genetic and biochemical markers that worked so well for redox-cycling compounds all came up negative for classical antibiotics. How can we account for the discrepancy?
Do dyes work?
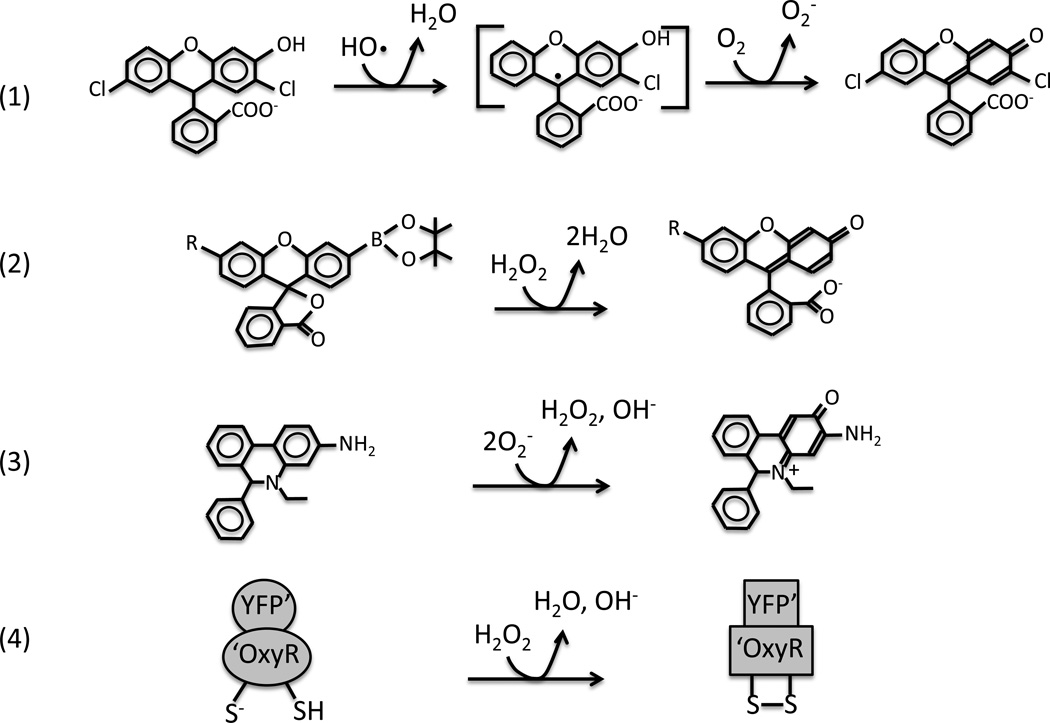
Reduced fluorescein and rhodamine dyes can be oxidized by hydroxyl radicals in vitro (Fig. 2) [33,34]. When these dyes are loaded into cells, the subsequent application of exogenous H2O2 enhances fluorescence, confirming that these indicators can detect rapid Fenton chemistry in vivo as well. Because the oxidation process involves a radical intermediate, there is an opportunity for unrelated chemical events to affect the product yield, and experts caution that perturbations of CO2, glutathione, and SOD levels, for example, can be misinterpreted as a change in the rate of hydroxyl formation [33,34].
Figure 2. Intracellular fluorescent probes of ROS.
(A) Reduced fluorescein derivatives (here, dihydrodichlorofluorescein) can be oxidized in a two-step process initiated by HO·. Carbonate radicals, high-valence metals, and other strong univalent oxidants can also initiate the reaction [33]. (B) Boronate-caged probes developed by the Chang group [39] react directly with H2O2. These probes have detected an influx of 100 µM intracellular H2O2 into eukaryotic cells. Ratiometric methods can correct for loading discrepancies. (C) Hydroethidine can be oxidized by O2− to 2-hydroxyethidium. Oxidized metal centers can oxidize the probe to a distinct ethidium derivative that can be distinguished by its fluorescence spectrum [77]. The detection limit in vivo has not been determined. (D) HyPer [38] is an artificial protein in which the H2O2-sensing domain of OxyR is fused to the fluorescent domain of YFP. Oxidation shifts the fluorescence spectrum. This probe is reversible in vivo, like OxyR itself, and has detected an infusion of 5–25 µM H2O2 into E. coli.
However, three other issues potentially complicate the interpretation of bacterial experiments. First, the dyes can be oxidized not only by hydroxyl radicals but also by high-valence metal centers, including the compound I form of heme enzymes and even cytochrome c [7,33–36]. Thus metabolic effects that alter the redox states of such enzymes can, at least in principle, generate a read-out that could be misconstrued as evidence of ROS stress. Ideally the involvement of Fenton chemistry in dye oxidation would be confirmed by checking whether the fluorescence yield depends upon the titer of catalase/peroxidase.
Second, disruption of the membrane barrier can affect the amount of the dye that penetrates into cells. This is true not only because an intact lipid bilayer sterically excludes such compounds but also because pmf-dependent pumps may actively excrete them [33]. Aminoglycoside antibiotics in particular disrupt the cytoplasmic membrane and increase the infusion of small molecules, including dyes [37]. The proper control is to normalize the fluorescent signal to the amount of dye inside the cells. Such ratiometry is common to the probe methods that measure other metabolites, and it is standard for the use of peroxidase-based and boronate-dependent H2O2 sensors [38,39]. However, it has not yet become the usual practice in fluorescent-dye experiments.
Finally, bacterial cell dimensions can change during protracted stress. If cells become large, their fluorescence rises proportionately, even if there is no difference in internal fluorophore concentration. Two labs independently demonstrated that this artifact undermined initial cell-sorting experiments that appeared to show high ROS after antibiotic treatment, as both ampicillin and norfloxacin triggered cell filamentation [11,12]. Measurements of side- and back-scatter, or of the natural fluorescence of dye-free cells, reveal the problem. The better approach is to normalize the fluorescence of the total cell population to its biomass; alternatively, if cell sorting is desired, the data must be normalized to side-scatter or to simultaneous measurements of a GFP-tagged housekeeping protein.
Dye-based ROS methods have found a broad clientele because the manipulations are straightforward. However, interpretation is ambiguous unless proper controls are performed. Interested readers are advised to review expert literature [33,34].
Can chemical “antioxidants” suppress oxidative damage?
Glutathione, ascorbate, and vitamin E have frequently been added to bacterial cultures in an effort to suppress ROS phenotypes. These compounds are natural antioxidants in mammalian systems. Glutathione is the reductive substrate for glutathione peroxidase, the primary scavenger of H2O2, and ascorbate plays the same role for ascorbate peroxidase. However, neither enzyme is present in E. coli, and by themselves these chemicals do not directly degrade H2O2 or O2− at a useful rate [40]. Vitamin E suppresses lipid peroxidation in eukaryotic organisms by reducing lipid radicals. However, the absence of polyunsaturated fatty acids means that lipid peroxidation is not likely to happen in most bacteria [41,42]. Thus there is no rationale for the use of any of these compounds as diagnostic antioxidants in bacteria.
Cell-penetrating iron chelators like dipyridyl and desferrioxamine quickly block Fenton chemistry and thereby prevent ROS from damaging targets such as DNA [43]. However, when bacteria are incubated with these compounds for extended periods of time, the resultant iron starvation triggers adjustments in metabolism with wide-ranging effects on growth and energy charge. Dipyridyl was found to protect E. coli from aminoglycoside antibiotics not because it prevented hydroxyl radical production but because the diminished membrane potential inhibited antibiotic import [10].
Can enzyme overproduction identify the damage mechanism?
Bacteria respond to threats by inducing defensive enzymes, and the identities of those enzymes can point to the damage mechanism. For example, the protective effect of superoxide dismutase induction helped to confirm that O2− is central to the mechanism by which redox-cycling compounds poison cells [44]. A complementary approach is to artificially overexpress defensive enzymes to see whether they suppress injuries.
A potential problem with this strategy is that the excessive synthesis of any enzyme can debilitate cells and hinder growth. Slow-growing cells are generally less vulnerable to killing mechanisms, so misinterpretations can ensue. A clever control is the parallel overproduction of inactive mutant forms of the defensive protein [6]. For example, overproduction of superoxide dismutase, NADH peroxidase, catalase, MutT, and MutS all diminished the rates at which classical antibiotics killed E. coli, and mutant forms of these enzymes were less protective. However, in those experiments a powerful plasmid/promoter system was used, and even the mutant proteins provided notable protection. Constitutive induction of defensive proteins from their native promoters, through use of a modified form of OxyR, did not provide any protection [10].
The reciprocal experiment is to diminish the levels of putatively protective enzymes. In the antibiotic example, mutants lacking the ROS scavenging enzymes—superoxide dismutase, NADH peroxidase, and catalase—were no more sensitive than wild-type cells [7,10].
Is it plausible that E. coli could make enough H2O2 to kill itself?
Some oxidative stress models posit that stressors accelerate the rate of H2O2 production simply by elevating flux through the respiratory chain [3,6]. This seems unlikely, since the chain appears not to be the primary site of basal ROS formation [45]. A derivative model is that enzymes within the chain are partially disabled by stress, thereby enhancing their tendency to directly leak electrons to oxygen. Could this generate enough ROS to kill cells?
It is hard to kill cells with achievable levels of ROS. The awareness that HO· is potentially lethal came from ionizing radiation experiments. In retrospect, this was a misleading example, since ionizing radiation creates spurs of radicals that generate multiple lesions close together on DNA. Double-stranded breaks result and are often fatal. Fenton chemistry appears not to work in that way: lesions are dispersed, and DNA-repair processes are generally successful.
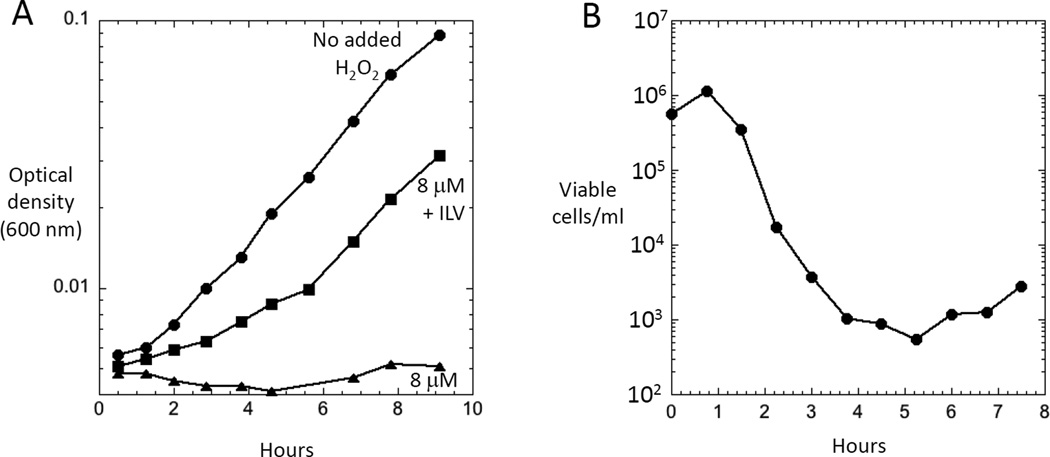
Experiments confirm that oxidative stress is much more likely to inhibit growth than to kill cells. Based on direct measurements of H2O2 production and catalase/peroxidase levels, the steady-state level of H2O2 inside unstressed aerobic E. coli has been projected to be ~ 50 nM [1]. Studies have used catalase/peroxidase mutants to conveniently establish higher steady-state levels of H2O2 within cells. As little as 0.5 µM intracellular H2O2 can block growth in a defined medium by inactivating mononuclear iron enzymes [46], and higher doses inactivate iron-sulfur dehydratases such as those involved in amino-acid synthesis (Fig. 3A). However, even 8 µM intracellular H2O2 does not produce enough DNA damage to cause death, as evidenced by the resumption of growth when amino acids are provided (Fig. 3) [17]. Since the steady-state level of H2O2 in wild-type cells is proportionate to the rate of its formation, a > 150-fold increase in endogenous H2O2 production—which would be needed to establish an 8 µM intracellular level—would apparently still be non-lethal. Under standard conditions about 0.3% of the oxygen consumption leads to H2O2 formation, and so a 150-fold increase would represent an unrealistic 45% of oxygen consumption. Thus for any stressor to impose lethal ROS damage, it must do more than simply accelerate H2O2 formation. Indeed, in our studies redox-cycling compounds like paraquat have been bacteriostatic but not bacteriocidal.
Figure 3. Impact of elevated H2O2 upon cell fitness.
(A) Catalase/peroxidase mutants that are suffused with 8 µM H2O2 exhibit an auxotrophy for branched-chain amino acids due to dehydratase inactivation. The figure indicates growth curves in defined glucose medium to which exogenous H2O2 was added. If branched-chain amino acids (ILV) are supplied, cells can grow. (All media also contained aromatic amino acids to counteract inhibition of that biosynthetic pathway. Data are from Jang and Imlay [17].) (B) Substantial cell death from H2O2 stress occurs only if catalase/peroxidase mutants additionally carry a Δdps mutation that blocks the sequestration of intracellular iron by this mini-ferritin. Anoxic cells growing in liquid medium were aerated at time zero, and at intervals the number of surviving cells was determined by plating on anoxic plates. This phenotype was evident in LB medium containing ~ 10 µM Fe and ~ 5 µM H2O2. Data are from Park et al. [47].
Micromolar H2O2 has been observed to kill E. coli cultures in the lab only when the cells were aerated in iron-rich LB medium and carried mutations in oxyR, dps, recA, polA, or xthA [47] (Fig. 3B). The OxyR protein induces Dps, a ferritin-like protein that sequesters iron. The latter three genes encode key enzymes in recombinational (recA) or excision (polA, xthA) DNA-repair pathways. These observations still allow the possibility that exogenous stressors might impose ROS- driven death, but to do so those stressors would need to disable defenses as well as elevate ROS formation. There is no evidence yet that antibiotics are able to do this. For example, iron levels did not rise during antibiotic treatment [7].
Conclusions
When workers propose that oxidative damage is responsible for the lethal effects of an exogenous stress, they face two challenges. The first is to unambiguously demonstrate that a stress truly does raise the level of intracellular ROS. Unfortunately, the experimental techniques that are most direct are not always easy or widely applicable. The second challenge is that only special, contrived circumstances have been found in which micromolar H2O2 can be lethal. Mechanisms that simultaneously disable key oxidative defenses would be required. This may surprise people: even the doses of H2O2 that accumulate in phagosomes (< 10 µM [48–51]) may not be directly cidal.
The quest for new and better antibiotic therapies is an urgent one, and the examination of cell physiology during antibiotic action may provide helpful ideas. However, the author hesitates to accept the notion that oxidative stress is an important contributor to antibiotic lethality. A simple model that antibiotics generate lethal doses of oxidative damage would require that a very large fraction of oxygen consumption be diverted towards H2O2 formation; this prediction was not supported by a battery of assays that have easily detected other ROS stresses. This outcome conflicts with the data from experiments with ROS probes. Potential problems with those probes have been repeatedly highlighted in expert reviews, and cell-sorting analysis may introduce additional complications. Other approaches that have led workers to the oxidative-stress hypothesis—protection by chemical antioxidants and by protein overproduction—also have complications that must be considered. At this point one hopes that new measures of oxidative stress might be developed that can resolve the mixed results obtained thus far.
Highlights.
Workers have hypothesized that many different stresses accelerate ROS formation.
Common measures of ROS formation can be subverted by artifacts.
ROS are typically bacteriostatic rather than bacteriocidal.
The evidence that antibiotics impose oxidative stress is mixed.
Acknowledgments
Work in the author’s lab on this topic is supported by grant GM49640 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Kohanski MA, Dwyer DJ, Wierzbowski J, Cotarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA. 2014;111:E2100–E2109. doi: 10.1073/pnas.1401876111. A recent study that argues that ROS are an important component of antibiotic action.
- 7.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahoney TF, Silhavy TJ. The Cpx stress response confers resistance to some, but not all bactericidal antibiotics. J Bacteriol. 2013;195:1869–1874. doi: 10.1128/JB.02197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. One of several papers that present evidence against the ROS theory of antibiotic toxicity.
- 10. Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, Su SY, Espinosa L, Loiseau L, Py B, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013;340:1583–1587. doi: 10.1126/science.1238328. This study demonstrated that indirect effects confound some experiments that were thought to show a role for ROS in antibiotic action.
- 11. Renggli S, Keck W, Jenal U, Ritz D. Role of autofluorescence in flow cytometric analysis of Escherichia coli treated with bactericidal antibiotics. J Bacteriol. 2013;195:4067–4073. doi: 10.1128/JB.00393-13. One of two papers that showed that morphological changes can compromise analyses that employ fluorescent markers of ROS stress.
- 12. Paulander W, Wang Y, Folkesson A, Charbon G, Lobner-Olesen A, Ingmer H. Bactericidal antibiotics increase hydroxyphenyl fluorescein signal by altering cell morphology. PLoS One. 2014;9:e92231. doi: 10.1371/journal.pone.0092231. One of two papers that showed that morphological changes can compromise analyses that employ fluorescent markers of ROS stress.
- 13. Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu Rev Pharmacol Toxicol. 2014;55 doi: 10.1146/annurev-pharmtox-010814-124712. Epub ahead of print. A new review that consolidates the evidence linking ROS to antibiotics.
- 14.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo CF, Mashino T, Fridovich I. α,β-dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J Biol Chem. 1987;262:4724–4727. [PubMed] [Google Scholar]
- 16.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- 17.Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci USA. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol. 2013;89:123–134. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farr SB, D'Ari R, Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci USA. 1986;83:8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massey V, Singer TP. Studies on succinic dehydrogenase: VI. The reactivity of beef heart succinic dehydrogenase with electron carriers. J Biol Chem. 1957;229:755–762. [PubMed] [Google Scholar]
- 24.Hassan HM, Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978;253:8143–8148. [PubMed] [Google Scholar]
- 25.Hassan HM, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 26.Hassan HM, Fridovich I. Mechanism of the antibiotic action of pyocyanine. J Bacteriol. 1980;141:156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomposiello PJ, Bennik MH, Demple B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol. 2001;183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991;266:1478–1483. [PubMed] [Google Scholar]
- 29.Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–1316. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsaneva IR, Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990;172:4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu M, Imlay J. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol Microbiol. 2011;79:1136–1150. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Rad Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 34. Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJR, II, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Rad Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. An analysis of the pluses and minuses of redox dyes as detectors of ROS stress.
- 35.Burkitt MJ, Wardman P. Cytochrome c is a potent catalyst of dichlorofluorescein oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem Biophys Res Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 37.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bilan DS, Pase L, Joosen L, Gorokhovatsky AY, Ermakova YG, Gadella TWJ, Grabher C, Schultz C, Lukyanov S, Belousov VV. HyPer-3: A genetically encoded H2O2 probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem Biol. 2013;8:535–542. doi: 10.1021/cb300625g. A modified version of OxyR allows imaging to detect H2O2 stress.
- 39. Lin VS, Dickinson BC, Chang CJ. Boronate-based fluorescent probes: imaging hydrogen peroxide in living systems. Meth Enzymol. 2013;526:19–43. doi: 10.1016/B978-0-12-405883-5.00002-8. Development of irreversible fluorescent probes of H2O2.
- 40.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Rad Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 41.Nichols DS, McMeekin TA. Biomarker techniques to screen for bacteria that produce polyunsaturated fatty acids. J Microbiol Methods. 2002;48:161–170. doi: 10.1016/s0167-7012(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 42.Bielski BHJ, Arudi RL, Sutherland MW. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J Biol Chem. 1983;258:4759–4761. [PubMed] [Google Scholar]
- 43.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 44.Hassan HM, Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J. Biol. Chem. 1977;252:7667–7672. [PubMed] [Google Scholar]
- 45.Seaver LC, Imlay JA. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem. 2004;279:48742–48750. doi: 10.1074/jbc.M408754200. [DOI] [PubMed] [Google Scholar]
- 46.Sobota JM, Gu M, Imlay JA. Intracellular hydrogen peroxide and superoxide poison 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J Bacteriol. 2014;196:1980–1991. doi: 10.1128/JB.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx− mutants of Escherichia coli. Proc Natl Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. Implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 49.Imlay JA. Oxidative Stress. In: Neidhardt FC, editor. EcoSal--Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; 2009. http://www.ecosal.org. [Google Scholar]
- 50.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Mol Microbiol. 2011;80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burton NA, Schurmann N, Casse O, Steeb AK, Claudi B, Zanki J, Schmidt A, Bumann D. Disparate impact of oxidative host defenses determines the fate of Salmonella during systemic infection in mice. Cell Host & Microbe. 2014;15:72–83. doi: 10.1016/j.chom.2013.12.006. Modelling indicates that H2O2 levels are modest even inside phagocytes.
- 52.Frey HE, Pollard EC. Ionizing radiation and bacteria: nature of the effect of irradiated medium. Radiation Res. 1966;28:668–676. [PubMed] [Google Scholar]
- 53.Boehme DE, Vincent K, Brown OR. Oxygen and toxicity: inhibition of amino acid biosynthesis. Nature. 1976;262:418–420. doi: 10.1038/262418a0. [DOI] [PubMed] [Google Scholar]
- 54.Davies BW, Kohanski MA, Simmons LA, Winkler JA, Collins JJ. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Molecular Cell. 2009;36:845–860. doi: 10.1016/j.molcel.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith AH, Imlay JA, Mackie RI. Increasing the oxidative stress response allows Escherichia coli to overcome inhibitory effects of condensed tannins. Appl Environ Micro. 2003;69:3406–3411. doi: 10.1128/AEM.69.6.3406-3411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedard K, Lardy B, Krause K-H. NOX family NADPH oxidases: Not just in mammals. Biochemie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glass GA, DeLisle DM, DeTogni P, Gabig TG, Magee BH, Markert M, Babior BM. The respiratory burst oxidase of human neutrophils. Further studies of the purified enzyme. J Biol Chem. 1986;261:13247–13251. [PubMed] [Google Scholar]
- 59.Seki M, Iida K, Saito M, Nakayama H, Yoshida S. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactase oxidase and coupling with aerobic utilization of lactate. J Bacteriol. 2004;186:2046–2051. doi: 10.1128/JB.186.7.2046-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat-shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aertsen A, Spiegeleer PD, Vanoirbeek K, Lavilla M, Michiels CW. Induction of oxidative stress by high hydrostatic pressure in Escherichia coli. Appl Environ Microbiol. 2005;71:2226–2231. doi: 10.1128/AEM.71.5.2226-2231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra Y, Chaurasia N, Rai LC. AhpC (alkyl hydroperoxide reductase) from Anabaena sp. PCC 7120 protects Escherichia coli from multiple abiotic stresses. Biochem Biophys Res Commun. 2009;381:606–611. doi: 10.1016/j.bbrc.2009.02.100. [DOI] [PubMed] [Google Scholar]
- 63.Hoerter J, Pierce A, Troupe C, Epperson J, Eisenstark A. Role of enterobactin and intracellular iron in cell lethality during near-UV irradiation in Escherichia coli. Photochem Photobiol. 1996;64:537–541. doi: 10.1111/j.1751-1097.1996.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 64.Behrendt L, Stall M, Cristescu SM, Harren FJ, Schliep M, Larkum AW, Kuhl M. Reactive oxygen production induced by near-infrared radiation in three strains of the Chl d-contining cyanobacterium Acaryochloris marina. F1000 Res. 2013;2:44. doi: 10.12688/f1000research.2-44.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutherford BJ, Dahl RH, Price RE, Szmidt HL, Benke PI, Mukhopadhyay A, Keasling JD. Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl Environ Microbiol. 2010;76:1935–1945. doi: 10.1128/AEM.02323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park HJ, Kim JY, Kim J, Lee JH, Hahn JS, Gu MB, Yoon J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009;43:1027–1032. doi: 10.1016/j.watres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Kumura T, Nishioka H. Intracellular generation of superoxide by copper sulphate in Escherichia coli. Mutat Res. 1997;389:237–242. doi: 10.1016/s1383-5718(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 68.Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A. Effect of chromate stress on Escherichia coli K-12. J Bacteriol. 2006;188:3371–3381. doi: 10.1128/JB.188.9.3371-3381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barras F, Fontecave M. Cobalt stress in Escherichia coli and Salmonella enterica: molecular bases for toxicity and resistance. Metallomics. 2011;3:1130–1134. doi: 10.1039/c1mt00099c. [DOI] [PubMed] [Google Scholar]
- 70.Perez JM, Calderon IL, Arenas FA, Fuentes DE, Pradenas GA, Fuentes EL, Sandoval JM, Castro ME, Elias AO, Vasquez CC. Bacterial toxicity of potessium tellurite: unveiling anancient enigma. PLoS One. 2007;2:e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, Banin E. Understanding the antibacterial mechanism of CuO nanoparticles: revelaing the route of induced oxidative stress. Small. 2012;8:3326–3337. doi: 10.1002/smll.201200772. [DOI] [PubMed] [Google Scholar]
- 72.Fredriksson A, Nystrom T. Conditional and replicative senescence in Escherichia coli. Curr Opin Micro. 2006;9:612–618. doi: 10.1016/j.mib.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 73.Moreau PL. Diversion of the metabolic flux from pyruvate dehydrogenase to pyruvate oxidase deccreases oxidative stress during glucose metabolism in nongrowing Escherichia coli cells incubated under aerobic, phosphate starvation conditions. J Bacteriol. 2004;186:7364–7368. doi: 10.1128/JB.186.21.7364-7368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kashyap DR, Rompca A, Gaballa A, Helmann JD, Chan J, Chang CJ, Hozo I, Gupta D, Dziarski R. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathog. 2014;10:e1004280. doi: 10.1371/journal.ppat.1004280. A recent study that implicates ROS as effectors of toxicity from an immune response.
- 75. Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi: 10.1016/j.cell.2014.03.062. Another toxic immune action which ROS are proposed to mediate.
- 76.Kolodkin-Gal I, Sat B, Keshet A, Engelberg-Kulka H. The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics. PLoS Biol. 2008;6:e319. doi: 10.1371/journal.pbio.0060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Rad Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]