Abstract
Enhanced external counterpulsation (EECP) therapy decreases angina episodes and improves quality of life in patients with left ventricular dysfunction (LVD). However, the underlying mechanisms relative to the benefits of EECP therapy in patients with LVD have not been fully elucidated. The purpose of this study was to investigate the effects of EECP on indices of central hemodynamics, aortic pressure wave reflection characteristics and estimates of LV load and myocardial oxygen demand in patients with LVD. Patients with chronic stable angina and left ventricular ejection fraction (LVEF) <40%, but > 30%, were randomized to either an EECP (LVEF=35.1±4.6%; n=10) or sham-EECP (LVEF=34.3±4.2%; n=7) group. Pulse wave analysis (PWA) of the central aortic pressure waveform (AoPW) and LV function were evaluated by applanation tonometry before and after 35 1-hr sessions of EECP or Sham EECP. EECP therapy was effective in reducing indices of left ventricular wasted energy (LVEw) and myocardial oxygen demand (TTI) by 25% and 19%, respectively. In addition, indices of coronary perfusion pressure (DTI) and subendocardial perfusion (SEVR) were increased by 9% and 30% after EECP, respectively. Our data indicate that EECP may be useful as adjuvant therapy for improving functional classification in heart failure patients through reductions in central blood pressure, aortic pulse pressure, wasted left ventricular energy, and myocardial oxygen demand which suggests improvements in ventricular-vascular interactions.
Keywords: central blood pressure, coronary artery disease, enhanced external counterpulsation, left ventricular dysfunction, pulse wave analysis
INTRODUCTION
Presently, there is no single pharmacologic treatment capable of concomitantly increasing cardiac contractility and reducing vascular resistance in patients with left ventricular dysfunction (LVD).(1) However, Soran and colleagues demonstrated that enhanced external counterpulsation (EECP) therapy reduced systemic vascular resistance by 20%–30% and increased cardiac output by more than 75% in optimally medicated patients with LVD.(2) Further, EECP therapy has been shown to invoke changes in aortic pressure wave reflection resulting in decreased measurements of myocardial oxygen demand, wasted LV energy, and LV afterload in patients with coronary artery disease and chronic angina pectoris.(3)
EECP is a U.S. Food and Drug Administration approved, non-invasive outpatient therapy for the treatment of patients with coronary artery disease (CAD) and refractory angina pectoris who fail to respond to standard medical management. EECP uses a series of three cuffs placed on the calves, lower thighs, and upper thighs/buttocks. The cuffs receive sequential distal-to-proximal pneumatic inflation upon onset of diastole and simultaneous release of pressure at end-diastole.(4) Sequential cuff inflation during EECP treatment produces two opposite blood flow patterns, antegrade flow in the brachial artery and retrograde flow in the femoral artery.(5) Further, sequential cuff deflation produces systolic hyperemia.(6) Thus, each session of EECP may be thought of as providing a direct dose of vascular medicine via the significant increases in pulsatile and oscillatory blood flow.(4, 6) Indeed, during EECP treatment, shear stress in the brachial and femoral arteries increase by 75% and 402%, respectively, and may provide a form of ‘massage’ on the endothelium improving its function and reducing central pressures and improving pressure wave reflection properties.(4, 5)
We reasoned that EECP may represent an effective non-invasive adjuvant therapy for the treatment of patients with mild to moderate LVD and symptomatic or refractory angina by improving central hemodynamics and decreasing LV afterload.(7) Indeed, EECP has been shown to reduce central blood pressure, wasted LV energy (LVEw), myocardial oxygen demand and improve conduit artery endothelial function in CAD patients with preserved LV function.(3, 8) Recently, we reported that conduit artery endothelial function is improved similarly in CAD patients with moderate LVD when compared to those with preserved LV function after EECP therapy.(9) To date, however, studies have not fully elucidated the mechanisms of action and the effects of EECP therapy in patients with LVD. For instance, effects of EECP on central hemodynamics have not been studied in LVD, despite evidence that central pressures may be a stronger predictor of cardiovascular risk and mortality than standard brachial artery sphygmomanometry.(10) Further, the effects of mild to moderate systolic LVD on arterial stiffness and the central aortic pressure waveform (AoPW) have been fairly well described and show an increase in aortic stiffness, pulse wave velocity (PWV), characteristic impedance, and APP compared with normal subjects.(11)
Accordingly, the purpose of this study was to investigate the effects of EECP on AoPW and indices of central hemodynamics, LV afterload and myocardial oxygen demand in patients with moderate LVD. We hypothesized that decreases in aortic wave reflection are a therapeutic target for EECP treatment in patients with moderate systolic LVD and that EECP therapy would improve indices of LV load and myocardial oxygen demand.
RESULTS
All subjects completed the entire EECP treatment protocol without adverse events. Resting participant descriptive and hemodynamic characteristics are presented in Table 1. Table 2 contains cardiac intervention history and drug regimens. There were no differences between the EECP and Sham groups at study entry with respect to blood pressure, drug therapy, previous cardiovascular history, cardiovascular risk factors, revascularization procedures and metabolic profile and there were no changes observed in any dependent variable in the Sham group after ‘treatment’ (P>0.05).
Table 1.
Resting patient descriptive and hemodynamic characteristics.
| EECP (n = 10; 8 male, 2 female)
|
Sham (n = 7; 5 male, 2 female)
|
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Age (y) | 64.2 ± 2.6 | - | 66.2 ± 3.5 | - |
| Height (cm) | 169 ± 4 | - | 172 ± 5 | - |
| Weight (kg) | 94 ± 5 | 92 ± 6 | 98 ± 6 | 95 ± 6 |
| BMI (kg/m2) | 31.5 ± 2.3 | 30.7 ± 2.3 | 33.3 ± 3.1 | 33.0 ± 3.3 |
| EF (%) | 35.1 ± 4.6 | - | 34.3 ± 4.2 | - |
| HR (bpm) | 78 ± 3 | 76 ± 4 | 77 ± 4 | 79 ± 5 |
| PSBP (mmHg) | 132 ± 5 | 125 ± 6*† | 131 ± 6 | 134 ± 7 |
| PDBP (mmHg) | 78 ± 3 | 71 ± 4*† | 77 ± 3 | 78 ± 2 |
| PMAP (mmHg) | 96 ± 3 | 89 ± 3*† | 97 ± 3 | 97 ± 2 |
| PPP (mmHg) | 53 ± 6 | 56 ± 5 | 59 ± 4 | 59 ± 4 |
| ASBP (mmHg) | 125 ± 4 | 116 ± 3*† | 127 ± 3 | 130 ± 4 |
| ADBP (mmHg) | 76 ± 2 | 73 ± 2 | 78 ± 3 | 78 ± 4 |
| AMAP (mmHg) | 93 ± 3 | 86 ± 4*† | 95 ± 4 | 96 ± 5 |
| APP (mmHg) | 53 ± 3 | 47 ± 4*† | 55 ± 3 | 57 ± 2 |
| AgBP (mmHg) | 15 ± 2 | 10 ± 3*† | 14 ± 3 | 16 ± 4 |
| AIx (%) | 29.4 ± 3.3 | 19.3 ± 3.7**† | 26.8 ± 4.5 | 28.2 ± 4.8 |
| AIx@75 (%) | 31.2 ± 3.6 | 21.7 ± 4.0*† | 28.7 ± 4.8 | 30.5 ± 5.2 |
| CCS (a.u.) | 3.2 ± 0.3 | 1.1 ± 0.5*† | 3.1 ± 0.3 | 3.2 ± 0.5 |
Values are mean ± SEM. Significant values are reported from between-group and between-timepoint repeated measures analysis of variance and Tukey post hoc analysis. BMI indicates body mass index; EF, ejection fraction, HR, heart rate; PSBP, peripheral systolic blood pressure; PDBP, peripheral diastolic blood pressure; PMAP, peripheral mean arterial pressure; PPP, peripheral pulse pressure; ASBP, aortic systolic blood pressure; ADBP, aortic diastolic blood pressure; AMAP, aortic mean arterial pressure; APP, aortic pulse pressure; AIx, augmentation index; AIx@75, augmentation index normalized to 75 beats per minute; CCS, Canadian Cardiovascular Society angina classification.
P<0.05 vs. pretreatment values,
P<0.01 vs. pretreatment values,
P<0.05 between groups at same timepoint.
Table 2.
Baseline patient cardiac intervention history and drug regimens.
| EECP (n = 10) (8 male, 2 female) | Sham (n = 7) (5 male, 2 female) | |
|---|---|---|
| Prior CABG | 7 (70) | 6 (86) |
| Prior PTCA | 7 (70) | 5 (67) |
| Prior Myocardial Infarction | 5 (50) | 4 (56) |
| Multivessel CAD | 9 (90) | 6 (89) |
| Diabetes Mellitus | 5 (50) | 3 (43) |
| Hypertension | 9 (90) | 6 (86) |
| Hyperlipidemia | 9 (90) | 6 (86) |
| Lipid-lowering drug | 9 (90) | 6 (86) |
| β-blocker | 8 (80) | 6 (86) |
| Calcium channel blocker | 3 (30) | 2 (22) |
| Long-lasting nitrates | 8 (80) | 5 (78) |
| ACE inhibition or ARB | 9 (90) | 6 (86) |
| Insulin | 2 (20) | 2 (22) |
Values are presented as the number of patients per group and the percentage within each group (in parentheses). There were no significant differences (P>0.05) in baseline characteristics, drug regimens, and cardiac intervention history between CAD and LVD groups at baseline. CAD indicates coronary artery disease with normal left ventricular function; LVD, left ventricular dysfunction (ejection fraction <40%, but >30%); CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty; ACE, angiotensin-converting enzyme; and ARB, angiotensin receptor blocker.
Average number of anginal episodes per day was reduced (2.3±1.24 to 0.9±0.87 and 2.5±1.59 to 2.3±1.62 in EECP and sham, respectively; P<0.05, treatment versus sham), and daily nitrate usage decreased (1.9±1.27 to 0.4±0.83 and 1.8±1.42 to 2.1±1.31 in EECP and sham, respectively; P<0.05, treatment versus sham) in the EECP group. Concomitantly, EECP led to a ~2 class decrease in Canadian Cardiovascular Society (CCS) angina classification (Table 1). There were no changes in any measure of angina symptom reduction in the sham group.
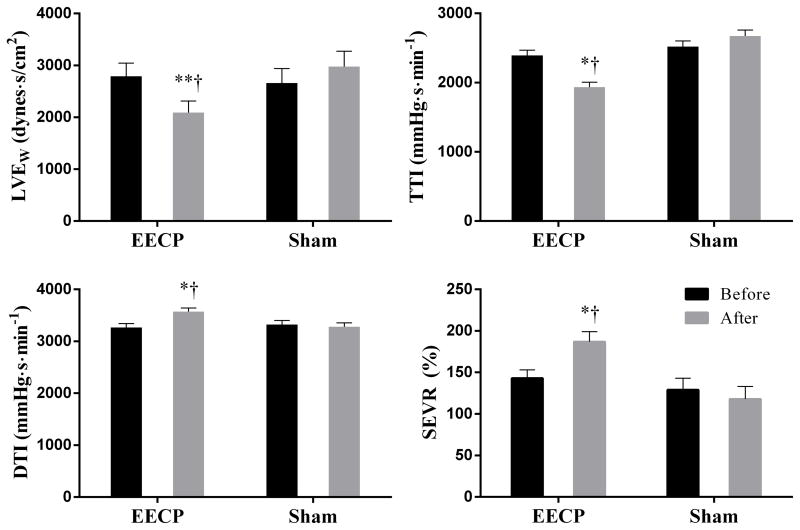
Peripheral and aortic systolic blood pressures (PSBP and ASBP) were significantly reduced after EECP therapy by 7±5 and 9±4 mmHg, respectively; P<0.05 (Table 1). EECP was effective in reducing augmentation index (AIx) by 34%, augmented blood pressure (AgBP) by 50%, aortic mean arterial pressure (AMAP) by 7.5% and aortic pulse pressure (APP) by 11%; P<0.05 (Table 1). In addition, aortic tension time index (TTI), an index of myocardial oxygen demand, and wasted left ventricular pressure energy (LVEw) were reduced by 19% and 25%, respectively; P<0.05. Further, indices of coronary perfusion pressure, diastolic pressure time index (DTI) and subendocardial perfusion (SEVR) were increased after EECP by 9% and 30%, respectively; P<0.05. (Figure 1).
Figure 1.
Data are absolute values from within group repeated measures ANOVA and Tukey post hoc analysis of between-group and between-timepoint differences in absolute values. Absolute values for LVEw, wasted left ventricular energy; TTI, tension time index; DTI, diastolic time index; SEVR, sub-endocardial viability ratio are presented. *P<0.05 vs. pretreatment values, **P<0.01 vs. pretreatment values, †P<0.01 between groups at same timepoint. Values are mean ± SEM.
DISCUSSION
The International EECP Patient Registry reported that EECP treatment decreases angina episodes and improves quality of life in patients with severe left ventricular dysfunction (LVD) (ejection fraction ≤ 35%) and these improvements can last up to 5 years.(12) However, previous studies have not elucidated the mechanism of action and the overall effects of EECP therapy in patients with LVD. Interactions between the LV and peripheral arterial system (ventricular-vascular coupling) are essential determinants of cardiovascular function.(13) Aortic reflection waves arriving during mid to late systole are an important determinant of LV afterload and interfere significantly with LV mechanics.(11, 14) The present, single-blinded, prospective study demonstrates that EECP has beneficial effects on the ventricular-vascular interaction via improvements in central hemodynamics, aortic wave reflection characteristics, and indices of myocardial oxygen demand in CAD patients with refractory angina and moderate systolic LVD.
Central blood pressures are more strongly related to vascular disease than peripheral pressures (10) and central hemodynamic indices are independent predictors of future cardiovascular events and all-cause mortality.(10, 15–17) Further, central blood pressures offer a more direct measure of LV load and arterial stiffness providing useful clinical information independent of peripheral BP.(10, 18) This has led to international consensus regarding the clinical importance and potential application of central blood pressures.(19) In the present study, we observed significant reductions in estimates of resting aortic pulse (APP), mean arterial (AMAP), and systolic blood pressures (ASBP) after EECP therapy. In addition, augmented blood pressure (AgBP), the aortic pressure augmented by the early reflection of the pulse pressure wave returning to the aorta during systole, was reduced following EECP. The observed differences indicate a significant reduction in pulsatile LV afterload and together, these changes in central hemodynamics and the AoPW, may be responsible, in part, for the improvements in LV ejection fraction previously observed in patients with ischemic heart failure (LVEF; 30±13 %) following EECP treatment.(20)
AIx has been shown to be a better independent predictor of future cardiovascular events than peripheral blood pressure.(10) Importantly, these non-invasively determined indices of central pressure and pulse pressure waveforms have proven to be reliable and valid measures when compared with data acquired from invasive catheter procedures.(21) In the present study, EECP improved the timing and amplitude of aortic pressure wave reflection in patients with moderate LVD as evidenced by the ~10% reductions in both AIx and AIx normalized to a heart rate of 75 beats per minute. Moreover, the observed beneficial effects of EECP on AoPW characteristics resulted in reductions in clinically important measures of wasted left ventricular energy (LVEw) and estimated myocardial oxygen demand (TTI). LVEW is an index of the extra myocardial oxygen requirement, directly associated with left ventricular remodeling, and is dependent on the amplitude of aortic pressure augmentation and systolic duration of the pressure wave.(22) In the current study, a decrease in both LVEw and TTI contributed to reduced wasted left ventricular work. Further, significant decreases in APP and LVEw suggest reductions in LV afterload and improved ventricular-vascular coupling after EECP. Indeed, noninvasive estimates of both APP and LVEw are associated with LVEF in patients with LVD.(23) Importantly, reductions in left ventricular work and myocardial oxygen demand were manifested in the concurrent improvement in Canadian Cardiovascular Society angina functional classification (CCS) and reduction in anginal symptoms and nitrate usage in patients with moderate LVD after EECP therapy.
Experimental Considerations
Limitations of the present study include the small sample size and radial artery pressure waveforms were calibrated using peripheral blood pressures measured by standard brachial cuff sphygmomanometry. It should be noted that this method of calibration may result in an overestimation of the central aortic pressures and remains the ‘achilles heel’ of PWA of the AoPW.(24, 25) In this case, the overestimation would result in a systematic error and therefore affect all results proportionally. In addition, left ventricular ejection fraction was not measured after EECP or Sham-EECP treatment. However, Kozdag et al. recently reported that LVEF was significantly improved by EECP treatment in a cohort of patients with ischemic LVD that closely resembled the patients in the present study.(20) Further, Paglia and colleagues recently suggested that non-invasively estimated measures of central pulse pressure and LVEw, similar to the methods used in the current study, are strongly associated with LVEF in patients with LVD.(23) Therefore, we can only speculate that the patients in the current study responded similarly to those in the previous studies.
Summary
EECP treatment reduces indices of aortic blood pressures, augmentation index and myocardial oxygen demand in CAD patients with refractory angina and moderate systolic LVD. Our data indicate that EECP may be useful as adjuvant therapy for improving functional classification in heart failure patients through reductions in central blood pressure, aortic pulse pressure, wasted left ventricular energy, and myocardial oxygen demand which suggests improvements in ventricular-vascular interactions.
MATERIALS and METHODS
Baseline status of subjects
Seventeen (n=17) consecutive patients with chronic stable angina and moderate left ventricular systolic dysfunction (EF <40%, but > 30%) referred for EECP treatment, were enrolled in this study and randomized to either an EECP (n=10) or sham-EECP (n=7) group. Patients were referred for EECP therapy due to the presence of chronic angina for greater than 3 months secondary to myocardial ischemia in the presence of angiographic multi-vessel CAD that could not be controlled by a combination of medical therapy, angioplasty/stent, and/or coronary artery bypass graft (CABG) surgery. All patients were receiving, and continued to receive, optimal pharmacologic therapy as determined by their cardiovascular physician during participation in this study. Further, no changes in drug regimen were observed in any patient during their study participation. This study was approved by the Institutional Review Board of the University of Florida and written informed consent was obtained from all subjects.
Exclusion criteria
Patients were excluded from the study if they met any of the following criteria: absence of ST segment depression (1 mm minimum) during graded exercise testing; > 75 years of age; CABG within past 3 months or percutaneous coronary intervention in past 6 months; cardiac catheterization for any reason within past 2 weeks; cardiac arrhythmia that would significantly interfere with triggering of the EECP device; LV ejection fraction (LVEF) > 40%; symptomatic heart failure and/or LV ejection fraction < 30%; decompensated heart failure; severe aortic regurgitation; valvular heart disease; implantable cardioverter-defibrillator if triggered within past 6 months, history of deep vein thrombosis; uncontrolled hypertension; pregnancy; pulmonary congestion; or systemic hypotension.
EECP or Sham treatment
Patients in the EECP (n = 10) and Sham (n = 7) groups received 35 1-hour daily sessions of EECP for 7 consecutive weeks using cuff inflation pressures of 300 and 70 mm Hg, respectively. Specifically, eligible participants were assigned at random to receive either active EECP (300 mmHg) or placebo treatment delivered as sham therapy at a significantly lower pneumatic pressure (70 mmHg). It was previously determined that 70 mmHg inflation pressure is adequate to preserve the appearance and feel of EECP application but insufficient to alter blood pressure (BP).(26) To prevent study participants from recognizing any observable differences between Sham and EECP treatment, appointments were scheduled to minimize opportunities for study subjects in one group to interact or discuss their experience with other patients undergoing EECP or Sham treatment. Study personnel administering treatment, involved in data collection and/or processing of data did not discuss aspects of the treatment such that participants would be able to determine their group assignment. An EECP (Vasomedical, Westbury, New York) therapeutic system consists of an air compressor, a treatment table, a control console and an integrated set of pneumatic cuffs, and has been described previously.(4) Briefly, cuffs that are positioned on the calves, thighs and upper thighs/buttocks are inflated with compressed air to sequentially from distal to proximal in early diastole and rapidly deflated immediately prior to the onset of systole. Inflation and deflation of the cuffs is triggered by events in the cardiac cycle via microprocessor-interpreted electrocardiogram signals. LVD groups were studied before and after a standard course of EECP or Sham treatment consisting of 35 1-hr sessions 5 days a week for 7 weeks.
Pulse wave analysis (PWA)
Prior to and following 35 sessions of EECP therapy or sham, subjects reported to the laboratory for testing within 48 hours of their first and last sessions. After 15 minutes of supine rest in a temperature controlled room, heart rate and brachial BP measurements were performed in triplicate in the left arm using an automated noninvasive BP cuff (Omron, Inc., Bannockburn, IL, USA). An average of 3 heart rate and BP measurements were used for resting values. Thereafter, the assessment of arterial wave reflection characteristics were performed non-invasively using the SphygmoCor Pulse Wave Analysis Px system and SCOR-2000 Version 6.31 software (AtCor Medical, Sydney, Australia) as described previously.(27) The SphygmoCor system of pulse wave analysis (PWA) (AtCor Medical, Inc.) is a U.S. Food and Drug Administration approved device for the non-invasive measurement of central blood pressures, augmentation index (AIx), and pulse wave velocity (PWV).
Consecutive measurements were then performed in the left arm by a highly trained technician possessing extensive experience in non-invasive vascular assessment techniques and the average of the first three high quality recordings at the radial artery per subject were captured for analysis. Optimal recording of the pressure wave was obtained when the hold-down force of the transducers on the artery was such that the resulting waveform had a stable baseline for at least 10 cardiac cycles and resulted in a quality index (QI) of >90%. QI is an internal measure derived from an algorithm which includes average pulse height variation, diastolic variation and maximum rate of rise of the peripheral waveform and accounts for variation in tonometer hold down pressure and waveform capture.
The SphygmoCor systems contain AtCor Medical/Millar tipped pressure tonometer (Millar Instruments, Houston, TX, USA) and use a validated generalized mathematical transfer function to synthesize a central aortic pressure waveform and correct for pressure wave amplification in the upper limb.(28) The generalized transfer function has been validated using both intra-arterially and noninvasively obtained radial pressure waves.(29) Central pulse pressure (APP) was recorded as an estimate of afterload and the augmentation index (AIx) as a measure of the relative contribution of reflected pulse waves to central blood pressure. This technique has been shown to be highly reproducible.(21) Further, in our laboratory, reproducibility has been established in young, healthy men with a mean coefficient of variation of 6.5%, 2.1%, 2.4% and 2.4% for aortic augmentation index (AIx), round trip travel time of the reflected pressure wave (Δtp), central systolic and diastolic blood pressure, respectively.(30)
The following PWA parameters, related to the amplification and temporal characteristics of the reflecting wave, were used as dependent variables in the present study: central aortic SBP (ASBP), central aortic DBP (ADBP), mean arterial pressure (MAP), end systolic pressure (ESP), ejection duration (ED), AIx, AIx normalized to an HR of 75 bpm (AIx@75) and Δtp. ED is a measure of time, in milliseconds, of the duration of each cardiac systole.(29) MAP was obtained from an integration of the waveform. ESP is defined as the pressure at the end of systole, which is the pressure at the end of ED.(27) AIx, expressed as a percentage, characterizes augmentation of central pressures and is defined as reflected wave amplitude divided by pulse pressure.(31, 32) Δtp is the round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back and is measured from the foot of the forward traveling pressure wave to the foot of the reflected wave.(27)
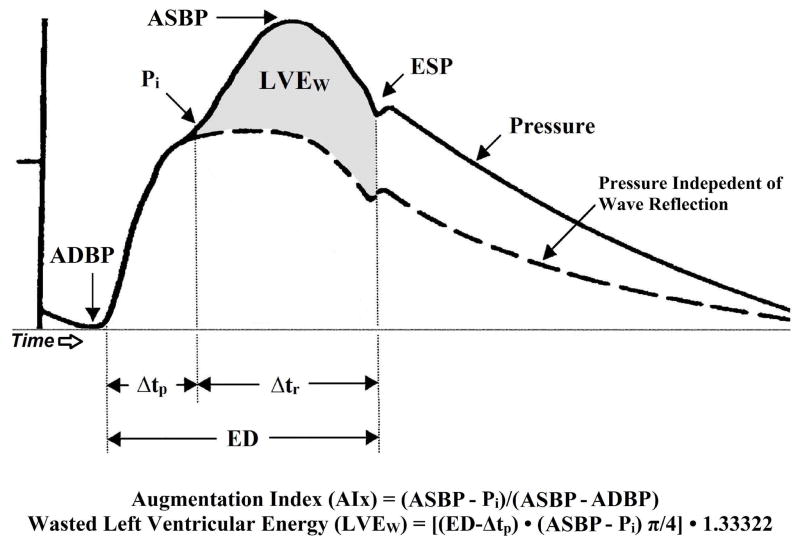
The measured central aortic pressure waveform (AoPW) is the summation of the forward-travelling waveform (incident) wave generated by the left ventricular (LV) ejection and a backward-traveling wave caused by reflection of the forward wave from sites of change in impedance within the peripheral arterial system.(33–35) The central aortic pressure wave (Ps−Pd) is composed of a forward traveling wave with amplitude (Pi−Pd), generated by left ventricular ejection and a reflected wave with amplitude (Ps−Pi) that is returning to the ascending aorta from the periphery (Figure 2).(30) The contribution or amplitude of the reflected wave to ascending aortic pulse pressure can be estimated by AIx. AIx is defined as reflected wave amplitude divided by pulse pressure and expressed as a percentage [AIx = (Ps−Pi)/(Ps−Pd) × 100].(27, 34) Ps indicates peak systolic pressure, Pi is an inflection point that indicates the beginning upstroke of the reflected pressure wave, and Pd is the minimum diastolic pressure. The forward and reflected waves travel in opposite directions along the artery at the same velocity. The round-trip travel time (Δtp) of the forward traveling wave from the ascending aorta to the major reflection site and back is measured from the foot of the forward traveling pressure wave to Pi. Δtp is inversely related to arterial pulse wave velocity and arterial stiffness, and directly related to the distance to the reflecting site.(27) Additional calculations derived from PWA included left ventricular wasted pressure energy (LVEW) and the tension–time index (TTI). LVEW can be estimated in dynes·s/cm2 as [(ED − Δtp)(ASBP − Pi)π/4]1.33322, where Pi is the first inflection point marking the onset of reflected aortic pressure wave return from the periphery (Figure 2).(30) The TTI, a marker of left ventricular work and myocardial oxygen demand, was calculated as (ED × HR × mean aortic SBP)/1000, and is expressed in mmHg s/min. Arterial tonometry measurements took approximately 30 minutes per laboratory visit. The assessment of central pressure waves is further described in detail by Nichols and Singh.(27)
Figure 2.
A typical central aortic pressure waveform synthesized from the radial artery pressure waveform using applanation tonometry, with superimposed waveform of aortic blood flow. The dotted line is representative of the theoretical aortic pressure waveform independent of wave reflection. The line labeled flow is a representative waveform of aortic blood flow. Augmentation index is the ratio of augmented pressure (ASBP − Pi) and central aortic pulse pressure (ASBP - ADBP). Wasted left ventricular pressure energy is directly related to augmented pressure (ASBP − Pi) and to the time duration of the reflected aortic pressure wave, (ED − Δtp). ASBP, central aortic systolic blood pressure; Pi, pressure at the first inflection point marking the onset of reflected aortic pressure wave return from the periphery; ADBP, central aortic diastolic blood pressure; Δtp, round trip travel time of the reflected pressure wave to the major peripheral reflecting site and back to the aorta; Δtr, systolic duration of the reflected aortic pressure wave; ED, ejection duration; ESP, end systolic pressure; LVEW, wasted left ventricular pressure energy; AIx, augmentation index.
Statistical analysis
All statistical analyses were performed using SPSS version 21.0 for Windows (SPSS, Chicago, IL, USA). Continuous variable data are presented as mean ± SD. All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of P<0.05 was required for statistical significance. Repeated measures analysis of variance (ANOVA) was used to evaluate all continuous dependent variables. When a significant group-by-time interaction was observed, within-group comparisons between time points and between-group comparisons at each time point were performed using Tukey post hoc analysis.
Acknowledgments
Source of Funding: This project was funded by the National Institutes of Health, NIH/HLB Grant #R01 HL077571 to Randy W. Braith, PhD.
Footnotes
Disclosure
The authors declare no conflicts of interest.
Contributor Information
Darren T. Beck, Email: darrentbeck@mail.uri.edu.
Darren P. Casey, Email: darren-casey@uiowa.edu.
Jeffrey S. Martin, Email: jmartin@auburn.vcom.edu.
Paloma D. Sardina, Email: psardina@hhp.ufl.edu.
Randy W. Braith, Email: rbraith@hhp.ufl.edu.
References
- 1.Strobeck JE. Enhanced external counterpulsation in congestive heart failure: Possibly the most potent inodilator to date. Congest Heart Fail. 2002;8:201–3. doi: 10.1111/j.1527-5299.2002.01743.x. [DOI] [PubMed] [Google Scholar]
- 2.Soran O, Kennard ED, Kelsey SF, Holubkov R, Strobeck J, Feldman AM. Enhanced external counterpulsation as treatment for chronic angina in patients with left ventricular dysfunction: A report from the international EECP patient registry (IEPR) Congest Heart Fail. 2002;8:297–302. doi: 10.1111/j.1527-5299.2002.00286.x. [DOI] [PubMed] [Google Scholar]
- 3.Casey DP, Beck DT, Nichols WW, Conti CR, Choi CY, Khuddus MA, et al. Effects of enhanced external counterpulsation on arterial stiffness and myocardial oxygen demand in patients with chronic angina pectoris. Am J Cardiol. 2011;107:1466–72. doi: 10.1016/j.amjcard.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braith RW, Casey DP, Beck DT. Enhanced external counterpulsation for ischemic heart disease: A look behind the curtain. Exerc Sport Sci Rev. 2012;40:145–52. doi: 10.1097/JES.0b013e318253de5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurovich AN, Braith RW. Enhanced external counterpulsation creates acute blood flow patterns responsible for improved flow-mediated dilation in humans. Hypertens Res. 2013;36:297–305. doi: 10.1038/hr.2012.169. [DOI] [PubMed] [Google Scholar]
- 6.Martin JS, Beck DT, Aranda JM, Jr, Braith RW. Enhanced external counterpulsation improves peripheral artery function and glucose tolerance in subjects with abnormal glucose tolerance. J Appl Physiol. 2012;112:868–76. doi: 10.1152/japplphysiol.01336.2011. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR., Jr Treatment options for angina pectoris and the future role of enhanced external counterpulsation. Clin Cardiol. 2002;25:II22–5. doi: 10.1002/clc.4960251407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, et al. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: A randomized sham-controlled study. Circulation. 2010;122:1612–20. doi: 10.1161/CIRCULATIONAHA.109.923482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck D, Martin J, Casey D, Avery J, Sardina P, Braith R. Enhanced external counterpulsation improves endothelial function and exercise capacity in patients with ischemic left ventricular dysfunction. Clin Exp Pharmacol Physiol. 2014;41:628–36. doi: 10.1111/1440-1681.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 11.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soran O, Kennard ED, Kfoury AG, Kelsey SF, Investigators I. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from the international EECP patient registry) Am J Cardiol. 2006;97:17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 13.Nichols WW, O’Rourke MF. Mcdonalds’s blood flow in arteries: Theoretical, experimental, and clinical principles. 4. 1998. [Google Scholar]
- 14.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–24. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 16.Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, et al. Central blood pressure measurements and antihypertensive therapy: A consensus document. Hypertension. 2007;50:154–60. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 17.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–8. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 18.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The strong heart study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.Kozdag G, Ertas G, Aygun F, Emre E, Kirbas A, Ural D, et al. Clinical effects of enhanced external counterpulsation treatment in patients with ischemic heart failure. Anatol J Cardiol. 2012;12:214–21. doi: 10.5152/akd.2012.064. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto J, Nichols WW, O’Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: Influence of arterial wave reflection. Am J Hypertens. 2008;21:329–33. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 23.Paglia A, Sasso L, Pirozzi F, Iannuzzi A, Carlomagno A, Abete P, et al. Arterial wave reflections and ventricular-vascular interaction in patients with left ventricular systolic dysfunction. Int Heart J. 2014 doi: 10.1536/ihj.14-159. dx.doi.org/10.1536/ihj.14–159. [DOI] [PubMed]
- 24.Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: Importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–8. doi: 10.1161/01.HYP.0000166723.07809.7e. [DOI] [PubMed] [Google Scholar]
- 25.Segers P, Mahieu D, Kips J, Rietzschel E, De Buyzere M, De Bacquer D, et al. Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension. 2009;54:414–20. doi: 10.1161/HYPERTENSIONAHA.109.133009. [DOI] [PubMed] [Google Scholar]
- 26.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): Effect of eecp on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 27.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–51. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47:1203–8. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- 29.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–7. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 30.Martin JS, Beck DT, Gurovich AN, Braith RW. The acute effects of smokeless tobacco on central aortic blood pressure and wave reflection characteristics. Exp Biol Med. 2010;235:1263–8. doi: 10.1258/ebm.2010.009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Manipulation of ascending aortic pressure and flow wave reflections with the valsalva maneuver: Relationship to input impedance. Circulation. 1981;63:122–32. doi: 10.1161/01.cir.63.1.122. [DOI] [PubMed] [Google Scholar]
- 33.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–56. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 34.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: Relationship to pressure wave forms. Circulation. 1980;62:105–16. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 35.O’Rourke MF. Time domain analysis of the arterial pulse in clinical medicine. Med Biol Eng Comput. 2009;47:119–29. doi: 10.1007/s11517-008-0370-7. [DOI] [PubMed] [Google Scholar]