Abstract
Prior research indicates a role for the gustatory insular cortex (GC) in taste neophobia. Rats with lesions of the GC show much weaker avoidance to a novel and potentially dangerous taste than do neurologically intact animals. The current study used the retention of conditioned taste aversion (CTA) as a tool to determine whether the GC modulates neophobia by processing taste novelty or taste danger. The results show that GC lesions attenuate CTA retention (Experiment 1) and impair taste neophobia (Experiment 2). Given that normal CTA retention does not involve the processing of taste novelty, the pattern of results suggests that the GC is involved in taste neophobia via its function in processing the danger conveyed by a taste stimulus.
Keywords: Conditioned taste aversion, Retention, Taste neophobia, Gustatory insular cortex, Rat
1. Introduction
For the hungry animal, eating a familiar, nutritious food is life sustaining and pleasurable. Eating a new food, however, is a risky undertaking because of the absence of knowledge about post-ingestive consequences - is this new food safe or is it toxic? It is assumed that fear of the life-threatening potential of a new food limits intake on the initial encounter, a phenomenon known as taste neophobia. If no harmful internal consequences follow ingestion, the initial fear reaction dissipates and consumption increases as the taste of the food becomes viewed as safe and thus more pleasurable (i.e., recovery from neophobia occurs; Barnett, 1963, Corey, 1978; Domjan, 1977; Lin, Amodeo, Arthurs, & Reilly, 2012). However, if consumption is followed by aversive consequences (e.g., gastrointestinal malaise; GIM) then the food will be avoided on later encounters, a phenomenon termed conditioned taste aversion (CTA; Barker, Best, & Domjan, 1977; Braveman & Bronstein, 1985; Milgram, Krames & Alloway, 1977; Reilly & Schachtman, 2009). CTA is an example of Pavlovian learning in which the taste (conditioned stimulus; CS) of that food is associated with the aversive post-ingestive consequence (unconditioned stimulus; US). After as few as one taste-GIM pairing CS intake is suppressed as a consequence of a conditioned downshift in taste palatability (Garcia, Kovner & Green, 1970; Lin, Arthurs & Reilly, 2014). Given the critical roles of taste neophobia and CTA in survival, research has been conducted to unravel their neural substrates. Although this work has implicated the insular cortex, the nature of this involvement is not fully understood.
The insular cortex is not homogeneous and only a portion of this structure participatesin taste processing. As defined by Kosar and colleagues (1986), the gustatory insular cortex (GC) is located in the anterior region that receives taste afferents from the gustatory thalamus. With regard to taste learning, most of the studies involving the GC are concerned with its role in CTA. Thus, lesions of the GC were reported to disrupt CTA acquisition (e.g., Bermúdez-Rattoni & McGaugh, 1991; Braun, Slick, & Lorden, 1972;Cubero, Thiele, & Bernstein, 1999; Fresquet, Angst, & Sandner, 2004; Gallo, Roldan, & Bures, 1992; Nerad, Ramirez-Amaya, Ormsby, & Bermudez-Rattoni, 1996). However, as indicated by recent findings, these deficits seem not to be related to an impairment in associating the CS with the US. For example, Roman, Nibieridze, Sastre and Reilly (2006) found that GC-lesioned (GCX) rats were able to acquire CTAs with a comparable strength as neurologically intact animals after repeated conditioning trials, suggesting that the lesions do not prevent taste-GIM associative learning. The lesion-induced deficits were most profound on the first conditioning trial (i.e., before the US was experienced) when the GCX rats consumed nearly twice as much of the taste CS as the non-lesioned (SHAM) control subjects. This pattern of results indicates that the GC has a critical role in processing taste-related information prior to the engagement of the associative mechanism responsible for CTA acquisition. That is, it appears that GCX rats fail to recognize or react to a novel taste and, instead, they drink the taste solution as if it were known to be safe and familiar.
Given that CTA acquisition occurs most readily when the taste is novel than when it is familiar (a general phenomenon termed latent inhibition effect; Lubow, 1989, 2009), we subsequently conducted a latent inhibition experiment to verify the taste novelty hypothesis of the GC function. Roman and Reilly (2007) found that for a preexposed (i.e., familiar and safe) taste, GCX rats acquired CTA at the same slow rate as CS preexposed SHAM animals; this normal CTA acquisition is contrary to the notion that the GC is involved in the taste-GIM associative mechanism. Furthermore, when a novel taste served as the CS, GCX rats showed impaired performance and acquired the CTA at a rate comparable to that shown by SHAM or GCX animals conditioned with a familiar taste (see also Roman, Lin, & Reilly, 2009). This latent inhibition-like effect in GCX rats indicates that this cortical region is critical for the detection and/or responsivity to taste novelty. Thus, we proposed that GC lesions delay CTA acquisition because such lesioned animals misperceive a genuinely novel taste as if it were familiar and safe. This analysis receives further support from a later finding from the neophobia study of Lin, Roman, St Andre, and Reilly (2009). In that study, rats were allowed to freely consume a novel taste solution (0.5% saccharin) over four 15-min trials. GCX rats were found to consume significantly more of the neophobia-inducing taste than SHAM animals on first exposure. It is important to note that both the SHAM and GCX groups reached the same level at asymptote following repeated benign exposures (i.e., showed the same attenuation of taste neophobia), indicating that the neophobia deficits could not be attributed to either a lesion-induced increase in appetite or an insensitivity to the value of the taste stimulus.
Normal taste neophobia, as aforementioned, requires detection of taste novelty as well as a reaction to the potential danger (typically seen as a reduction in the amount consumed). How are the deficits found in rats with GC lesions to be explained? We see two possibilities. It could be that the lesioned animals fail to recognize taste novelty (i.e., they misperceive the stimulus to be a familiar taste). Or, alternatively, the GCX rats might fail to react to the potential danger (i.e., they misperceive the stimulus to be a safe taste). Experiment 1 sought to unravel the nature of this deficit by examining whether GC lesions induced after CTA acquisition influence how an animal reacts to a now familiar but unsafe taste. If the GC is involved in the process of novelty detection, we expect to find normal performance because CTA retention would appear not to involve taste novelty detection. On the other hand, if the GC mediates the reaction to a dangerous taste, GCX rats would be expected to show a retention deficit (i.e., drinking more of the devalued CS than the SHAM animals) because of an impaired ability to perceive/react to the danger conveyed by the taste.
Research into whether GC lesions influence CTA retention can be divided into two broad categories. In the early work on this issue rats with GC damage were reported to show profound deficits in the retrieval of the aversive taste memory (Braun, Kiefer, & Ouellet, 1981; Cubero et al., 1999; Kiefer, Lawrence, & Metzler, 1986; Kiefer, Leach, & Braun, 1984; Kiefer, Metzler, & Lawrence, 1985; Yamamoto, Azuma, & Kawamura, 1981; Yamamoto, Matsuo, & Kawamura, 1980). However, due to the induction technique (i.e., ablation or electrolytic lesion), the lesions in all cases damaged neural pathways passing through the GC as well as in surrounding areas. Using excitotoxic lesions, which selectively damage cell body, Stehberg and Simon (2011) found that GCX rats showed some CTA retention, albeit weaker, than the SHAM control subjects. This latter study also found that the retention intake deficits never exceeded the level of neophobia initially showed to the taste stimulus prior to the induction of GC lesions. That is, GCX rats consumed the same amount of the saccharin CS on the retention test as they drank of the novel saccharin on the first CTA trial (see also Stehberg, Moraga-Amaro, & Simon, 2011). This latter finding prompts an immediate issue concerning how to explain the CTA retention deficit. That is, is the attenuation of CTA retention a primary deficit of the GC lesions or is it a secondary consequence of lesion-induced failure to retain a taste memory (since they did not recover from neophobia in the retention test)?1. This issue, unfortunately, cannot be answered because a critical control group (which received GC lesions but no lithium chloride [LiCl] injections) was not included to assess the function of taste memory in the studies by Stehberg and colleagues. Furthermore, in the absence of this control group, the nature of the observed GC lesion-induced CTA deficit (attenuation or prevention) cannot be determined.
Mindful of the concerns raised from prior research, the design of the present study included several features of note. First, GC lesions were induced with a neurotoxin (NMDA) using the parameters of our previous studies (e.g., Lin et al., 2009; Roman et al., 2009), which were found to attenuate taste neophobia and to delay, but not prevent, CTA acquisition. Second, in addition to the CTA group, we also included a control group given the taste CS but not the LiCl US. As noted before, this control group allows a determination of whether GC lesions prevent the formation of taste memory. The current design also will allow us to determine the magnitude of the CTA deficit (attenuation or elimination) in GCX rats. To prevent floor effects in intake from diminishing sensitivity to the detection of group differences, the dose of LiCl (0.037 M at 5 ml/kg) was chosen from Lin, Arthurs and Reilly (2013) to produce a moderate aversion to the taste (0.1% saccharin) used in the current study. Finally, after the completion of CTA retention tests, a taste neophobia experiment was conducted as a behavioral verification to confirm that the same lesion disrupts both phenomena.
2. Material and methods
2.1. Experiment 1: GC lesions and CTA retention
2.1.1. Animals
The experimental subjects were naïve male Sprague-Dawley rats purchased from Charles River Laboratory (Wilmington, MA). The experiment was conducted in two replications, each n = 24. Upon arrival, the rats were individually housed in hanging steel cages in an animal holding room with a 12-hour lightdark cycle (light on at 7:00 am) and constant temperature (∼22 °C). Food and water were given ad libitum until the experiment commenced, during which water access was limited to two daily 15-min exposures. The Animal Care and Users Committee of the University of Illinois at Chicago approved all experimental procedures Animal care was in accord with guidelines recommended by American Psychological Association (1996) and the National Institutes of Health (1996).
2.1.2. Apparatus
Experiment 1 was conducted in the home cages. All fluids were presented in bottles equipped with silicone stoppers and stainless drinking spouts that could be attached to the front panel of the cage. The amount consumed was measured to the nearest 0.5 ml.
2.1.3. CTA Acquisition
One week after their arrival, the rats were water deprived by giving two 15-min fluid access periods, spaced 4 hr apart, each day. Once morning water intake stabilized, the rats were divided into two groups (counterbalanced by baseline water intake) according to the US to be given during CTA conditioning. On the single acquisition trial, each rat was allowed to drink 0.1% saccharin (w/v) for 15 min and, 15 min later, given an IP injection of either physiological saline (Group Saline) or LiCl (0.075 M at 5 ml/kg; Group LiCl). Thereafter, the rats were given ad libitum food and water for three days before receiving surgery.
2.1.4. Surgery
To prevent the occurrence of any potential between-group differences prior to the induction of GC lesions, group assignment was counterbalanced according to saccharin intake on the acquisition trial. Half of the rats in each US condition were assigned to receive bilateral excitotoxic lesions of the GC (Groups GCX-Saline and GCX-LiCl). The rats were first anesthetized with intraperitoneal sodium pentobarbital (∼65 mg/kg) and then fixed in a stereotaxic instrument (David Kopf Instruments; Tujunga, CA) with blunt earbars. A midline incision was made to expose the skull and a trephine hole (∼3 mm diameter) was drilled above the GC in each hemisphere. Thereafter, the neurotoxin, NMDA (0.15 M; Sigma-Aldrich, St. Louis, MO), backfilled into a glass pipette (tip ∼70 μm) was iontophoretically infused into the GC at the following coordinates (relative to bregma): Site 1, AP +1.2 mm, ML ±5.2 mm, DV -5.0 mm for 10 min; Site 2, AP +1.2 mm, ML ±5.2 mm, DV -4.3 mm for 6 min. During surgery the body temperature was monitored and maintained at ∼37 °C by a heating pad. After the final infusion, the incision was closed with wound clippers. The rat was placed under a heating lamp during recovery from anesthesia before being returned to the home cage. The remaining rats (n = 24) served as SHAM control subjects: half of them received the same surgical procedures except that no NMDA was infused and the other half was given only pentobarbital anesthesia.
2.1.5. CTA Retention Test
Following one week of recovery from surgery, the rats were returned to the water deprivation schedule. When their morning water intake stabilized, two CTA retention test trials were conducted. Each test trial was identical to the acquisition trial except that no injections were involved. The tests were spaced 72 hr apart.
2.2. Experiment 2: GCX and Taste Neophobia
Taste neophobia is not simply an intake avoidance response to a novel taste because of the potentially dangerous post-ingestive consequence. Rather, the neophobic reaction also involves an affective component. That is, the hedonic value of a taste is reduced during initial consumption and gradually increased following benign exposures (Lin, Amodeo et al., 2012). As evidenced by the amount consumed, GC lesions have been consistently found to impair taste neophobia. However, it is unclear whether this GC lesion-induced deficit is accompanied by any changes in taste palatability. Experiment 2, therefore, served two important purposes. First, it functioned as a behavioral verification for assessing whether the GC lesions induced in Experiment 1 are producing equivalent behavioral deficits to our prior neophobia work (e.g., Lin et al., 2009; Lin, Arthurs, & Reilly, 2011). Secondly, this experiment recorded both intake and palatability (using lick pattern analysis) so that we can determine whether the GC plays a role in modulating taste palatability during the course of taste neophobia.
During voluntary drinking, rats produce runs of licks (termed clusters) separated by pauses. Cluster size (number of licks in a cluster) reflects the hedonic value of the tastant being consumed (for reviews see Dwyer, 2012; Lin et al., 2014). For instance, for a non-preferred taste like quinine, cluster size decreases as the concentration increases (Hsiao & Fan, 1993; Spector & St. John, 1998). On the other hand, cluster size increases as concentration increases for a preferred taste such as sucrose (Davis & Smith, 1992; Spector, Klumpp, & Kaplan, 1998). Importantly, cluster size does not vary with the amount of sucrose consumed (which bears an inverted-U shaped function with concentration; Davis, 1998). In addition to innate value, lick cluster size also detects conditioned changes in taste palatability: it becomes smaller (i.e., palatability decreases) following CTA learning (e.g., Arthurs, Lin, Amodeo & Reilly, 2012; Lin, Arthurs, Amodeo, & Reilly, 2012; Baird, St John, & Nguyen, 2005; Dwyer, Boakes, & Hayward, 2008; Lin et al., 2013) and becomes larger (i.e., palatability increases) following recovery from taste neophobia (Lin, Amodeo et al., 2012).
2.2.1. Subjects and Apparatus
The subjects were those employed in Experiment 1. The behavioral testing occurred in eight identical drinking chambers (Med-Associate Inc., St. Albans, VT; 30.5 cm × 24.0 cm × 29.0 cm). Located in a sound-attenuated cubicle, each chamber was equipped with a house light and a white noise generator (80dB). Fluids were available through a retractable spout; in the extended position the tip of the spout was located 3 mm outside an oval hole (1.3 cm × 2.6 cm) in an end wall of the chamber. A lickometer circuit, with a temporal resolution of 10 ms, was used to record the time of occurrence of each lick. A computer, located in an adjacent room, controlled all events in, and collected data from, the drinking chambers using Med-PC software.
2.2.2. Taste Neophobia Test
After the completion of Experiment 1, the rats were given ad libitum food and water for at least 3 days before being returned to the water deprivation schedule used in Experiment 1, except the morning access period was conducted in the drinking chambers. Once morning water intake stabilized (in terms of both amount consumed and lick cluster size; ∼14 days), Experiment 2 was initiated. Two taste neophobia trials, spaced 72 hr apart, were administered. On each trial, rats were allowed to consume 0.0001 M quinine HCl (w/v; Sigma) for 15 min. During the intervened days water was presented for 15 min each morning in the drinking chambers and, 4 hr later, for 15-min in the home cages.
2.2.3. Histology
Following completion of all behavioral testing, the rats were overdosed with sodium pentobarbital (100 mg/kg) and perfused transcardially with physiological saline and then 10% formalin. The brains were then extracted and stored in 4% buffered formalin for 2 days and 20% sucrose solution for additional 2 days. Thereafter, the brains were frozen and sliced on a cryostat at the thickness of 50 μm. After staining with cresyl violet, the slices were mounted on slides and later examined for the presence of lesions using a Zeiss Axioskop 40 light microscope.
2.2.4. Data analysis
In each experiment, amount consumed served as a dependent measure. In addition, in Experiment 2 lick cluster size (calculated by dividing the total number of licks occurring in clusters by the total numbers of clusters) was employed as a second dependent measure. A cluster was defined as a run of licks separated by interlick intervals of less than 0.5 second to afford comparability with our prior work (e.g., Lin et al., 2012; Lin, Amodeo et al., 2012). Data were evaluated with analysis of variance (ANOVA); the between-subject variables were Group and US, and Trial was the withinsubject variable. Significant main effects or interactions were further analyzed with post hoc comparisons (i.e., Fisher's LSD) to determine the nature of the differences. All analyses were conducted with the help of Statistica software (6.0; StatSoft, Tulsa, OK).
3. Results
3.1. Histology
The size and location of NMDA-induced lesions were determined by the loss or shriveling of cells as well as the occurrence of gliosis. Figure 1A shows schematic representations of the smallest (black) and largest (light gray) GC lesions. The lesions were primarily located in the insular cortex ranging from Bregma (0.00 mm) to (anteriorly) 2.00 mm. In some cases, lesions extended into surrounding areas such as somatosensory cortex, claustrum, and piriform cortex. The majority of the lesions were located anterior to Bregma, and thus damage encroachment into the visceral insular cortex was limited. Rats with unilateral or undersized lesions were excluded from further analysis. These lesions not only are comparable in size and location, but they also produced similar neophobia deficits (see below) to our previous GC lesions induced with the same parameters (Lin et al., 2009; Roman et al., 2009). The number of subjects included in the final data analyses was: SHAM-Saline (n = 12), SHAM-LiCl (n = 12), GCX-Saline (n = 8), and GCX-LiCl (n = 9).
Fig. 1.
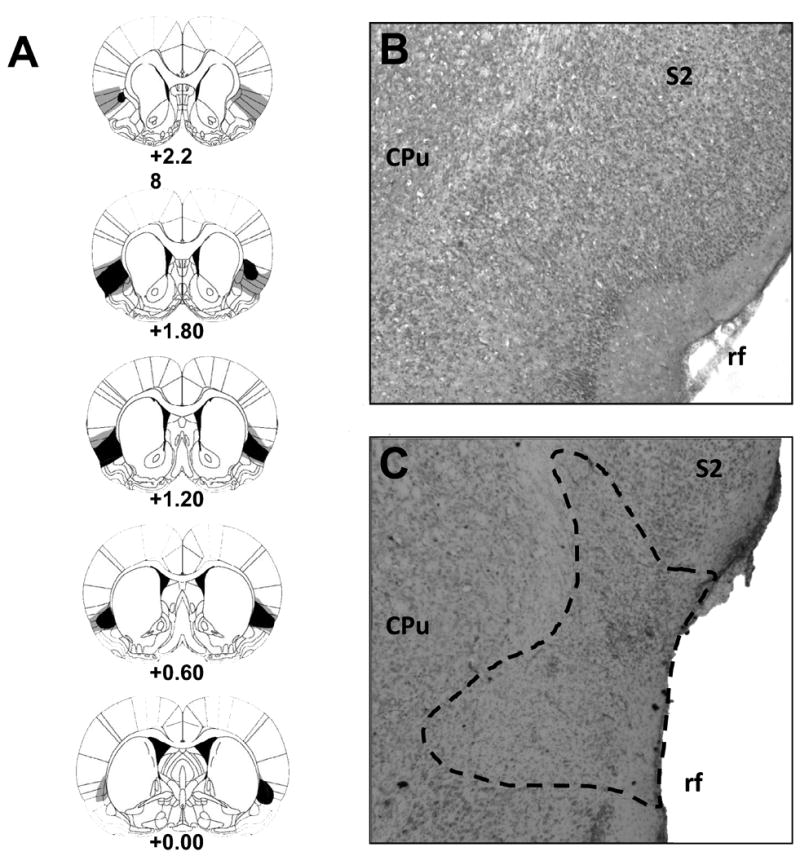
Histological results. Panel A displays a series of schematic reconstructions of gustatory insular cortex (GC) lesions at 5 coronal levels (+2.28 mm, +1.80 mm, +1.20 mm, +0.60 mm and 0.00 mm relative to bregma) from rats with the smallest (black) and largest (light gray) GC lesions. Panels B and C are representative digital photomicrographs of the GC from a neurologically intact (SHAM) subject and a rat with GC lesions, respectively. Dashed line indicates the perimeter of the lesions. CPu = caudate putamen; rf = rhinal fissure; S2 = secondary somatosensory cortex. The diagrams in Panel A were adapted from “The Rat Brain in Stereotaxic Coordinates,” by Paxinos and Watson, 2005, 5th ed. Copyright 2005 by Elsevier. Reprinted with permission.
3.2. Experiment 1: GC lesions and CTA retention
Baseline water consumption
Water intake (ml) data for each group on the morning prior the acquisition trial and the first retention trial are summarized in Table 1. Two separate 2-way ANOVAs (Lesion [a pseudofactor prior to the lesions] × US) were conducted on the Pre-acquisition and Pre-retention data and revealed no significant main effects or interactions (largest F = 1.13, ps > .05). Thus, any group differences that occur during acquisition or retention trials cannot be attributed to a general effect of GC lesions on fluid consumption.
Table 1.
Baseline water consumption (mean ± SE) from Experiment 1. The intake data were obtained from the day prior to the CTA acquisition trial (Pre-Acquisition) and the three trials before the first retention trial (Pre-Retention).
| Group | Pre-Acquisition | Pre-Retention |
|---|---|---|
| SHAM-Saline | 13.50 (±0.85) | 14.08 (±0.88) |
| SHAM-LiCl | 13.04 (±0.69) | 13.46 (±0.93) |
| GCX-Saline | 13.75 (±0.55) | 13.63 (±0.62) |
| GCX-LiCl | 14.83 (±1.51) | 13.33 (±1.11) |
CTA acquisition and retention
Figure 2 shows saccharin intake by the SHAM subjects and GCX rats across the acquisition (A1) and retention (R1 and R2) trials in Experiment 1. Inspection of the figure indicates all groups drank comparable amount of the taste CS during the pre-lesion acquisition trial (Figure 2, left panel). On the other hand, betweengroup differences in saccharin consumption emerged on the two post-lesion CTA retention trials (Figure 2, right panel). For saline-treated rats, irrespective of whether their GC was lesioned or intact, saccharin intake increased across the retention trials. That is, taste neophobia habituated. GC lesions markedly influenced the CTA retention performance of the LiCl-treated rats. Compared to Trial A1, the SHAM-LiCl subjects suppressed saccharin intake on Trial R1 whereas the GCX-LiCl rats consumed a comparable amount of saccharin on Trials A1 and R1; both LiCl-treated groups increased their saccharin intake on Trial 2 relative to Trial 1.
Fig. 2.
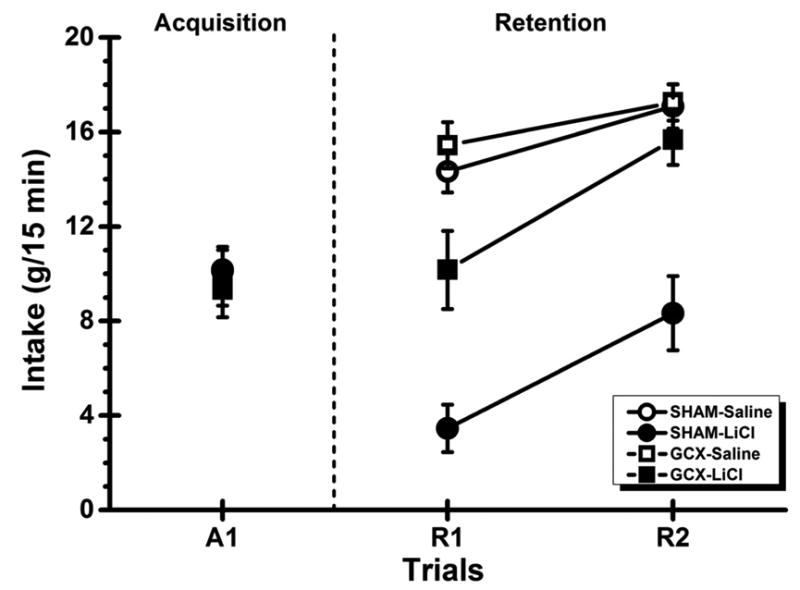
Experiment 1: Mean (±SE) saccharin intake for the normal (SHAM) subjects and rats with bilateral gustatory insular cortex lesions (GCX) on the CTA acquisition trial (A1) and the two retention trials (R1 and R2). In each lesion group, the rats received either saline or LiCl administration following the taste presentation on the acquisition trial. The vertical dashed line shows when brain surgery occurred.
These impressions about the data were substantiated by statistical analyses. The 3-way ANOVA found significant main effects of Lesion, F(1, 37) = 6.35, p < .05, US, F(1, 37) = 23.65, p < .001, and Trial, F(2, 74) = 38.06, p < .001. There were also significant interactions of Lesion × US, F(1, 37) = 5.39, p < .05, Lesion × Trial, F(2, 74) = 10.46, p < .001, US × Trial, F(2, 74) = 24.42, p < .001, and, more importantly, Lesion × US × Trial, F(2, 74) = 5.39, p < .01. To determine the nature of the significant 3-way interaction, post hoc comparisons (i.e., LSD tests) were conducted and found that the rats in each group consumed comparable amount of the saccharin CS on the acquisition trial (Fs < 1). Furthermore, the SHAM-Saline and GCX-Saline groups each showed an increase in saccharin intake from A1 to R1 and from R1 to R2 (ps < .05), and no significant group differences occurred on any of the three trials (ps > .05). Importantly, additional followup analyses further revealed that LiCl administrations significantly suppressed intake in Group SHAM-LiCl (p < .05) but not in Group GCX-LiCl (p > .05) from A1 to R1. Moreover, the saccharin CS intake of Group GCX-LiCl was significantly lower than Group GCX-Saline (p < .05) and significantly higher than Group SHAM-LiCl on Trial R1 (p < .05). This pattern of results suggests that GCX rats retained a weaker CTA than the neurologically intact animals following the damage in the GC. In other words, GC lesions do not erase CTA memory but only attenuate the retrieval of the memory. Finally, both SHAM-LiCl and GCX-LiCl groups showed extinction of CTA learning as evidenced by the significant increase in saccharin intake from R1 to R2 (ps < .05).
3.3. Experiment 2: GC lesions and taste neophobia
Baseline water consumption
Table 2 shows the water baseline data prior to the taste neophobia trials for Groups SHAM and GCX. Two separate one-way ANOVAs were conducted on intake and cluster size, respectively, and revealed no significant group effects (Fs < 1).
Table 2.
Baseline water consumption (mean ± SE) from Experiment 2 was assessed using intake and lick cluster size. The data were collected on the day prior to the first neophobia trial.
| Group | Intake | Lick Cluster Size |
|---|---|---|
| SHAM | 12.11 (±0.57) | 126.91 (±17.23). |
| GCX | 13.05 (±0.84) | 114.19 (±13.89) |
Taste neophobia
For both dependent measures (intake and cluster size), independent 3-way ANOVAs were first conducted to determine whether experience in Experiment 1 had any influence on performance in Experiment 2. Indicating no such carry-over effects, these analyses revealed neither significant Lesion × CTA nor Lesion × CTA × Trials interactions (ps >.05). Accordingly, the taste neophobia data were collapsed across prior learning experience and analyzed using separate 2-way ANOVAs with Lesion and Trials as, respectively, the between-subjects and within-subjects factors.
Quinine intake over the two neophobia trials is summarized in Figure 3A. As is readily evident, the SHAM subjects consumed less of the novel quinine solution than the GCX rats on Trial 1 and increased their intake on Trial 2 after they experienced the benign post-ingestive consequence of Trial 1. On the other hand, the GCX rats consumed almost twice as much quinine solution on Trial 1 as the SHAM subjects and maintained the same level of intake on Trial 2. This view was supported by statistical analyses which found a significant main effect of Trial, F(1, 39) = 35.00, p < .001; the main effect of Lesion did not achieve significance, F(1, 39) = 3.31, p = 0.076. This analysis, more importantly, revealed a significant Lesion × Trial interaction, F(1, 39) = 14.62, p < .001. Post hoc comparisons indicated that Group GCX drank significantly more of the novel quinine solution than Group SHAM on Trial 1 (p < .05). Group SHAM showed an increase in intake from Trials 1 to 2 (p < .05); on Trial 2 both groups consumed comparable amount of the taste (p > .05). Figure 3B shows lick cluster size data. A separate ANOVA conducted on these data revealed a significant Trial effect, F(1, 39) = 5.78, p < .05, but not a Lesion effect, F(1, 39) = 1.45, p > .05. This analysis also found a significant interaction, F(1, 39) = 12.93, p < .001. Post hoc analyses further revealed that relative to Group SHAM, Group GCX produced clusters that were significantly larger in size when drinking the novel quinine solution on Trial 1 (p < .05). Group SHAM then showed an increase in cluster size from Trials 1 to 2, (p < .05). On Trial 2, the average size of lick clusters did not differ between Groups SHAM and GCX (p > .05).
Fig. 3.
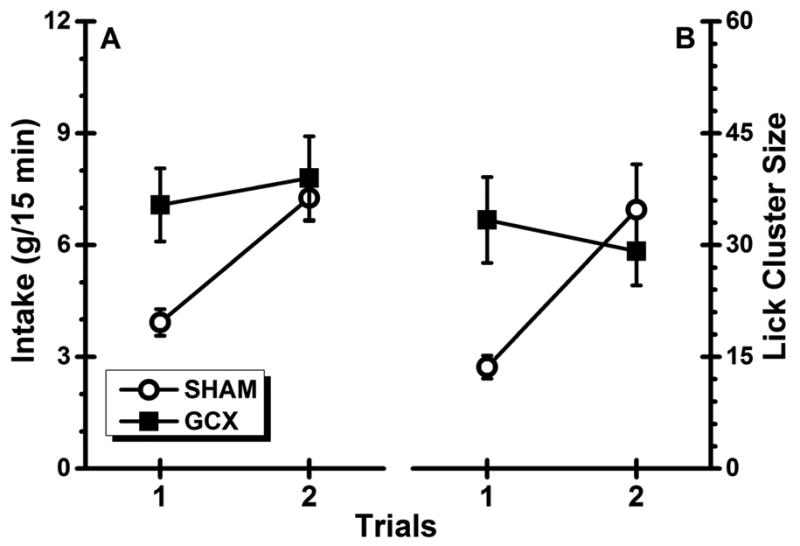
Experiment 2: Mean (±SE) quinine-directed performance (Panel A: intake; Panel B: lick cluster size) for the normal (SHAM) subjects and rats with bilateral gustatory insular cortex lesions (GCX) across the two taste neophobia trials.
4. Discussion
As revealed in Experiment 1, the GCX rats did not fully retain the CTA memory acquired prior to surgery. This CTA retention deficit is in accord with the results obtained in prior studies which employed non-selective lesions that damaged intrinsic neurons as well as fibers passing through the GC. With the necessary control groups we also found that excitotoxic lesions do not abolish the CTA memory. Rather, neuronal loss only attenuated CTA retention, as evidenced by the finding that on Retention Trial 1 GCX-LiCl rats consumed significantly less saccharin than the GCX-Saline control subjects. Experiment 2 used our standard taste neophobia procedure and found that the same GC lesions that caused CTA retention deficits in Experiment 1 significantly elevated intake and lick cluster size (i.e., palatability) of a novel, fear-inducing taste, a result that replicates the established finding that GC lesions attenuate taste neophobia (e.g., Lin et al., 2009, Lin et al., 2011). How does this pattern of deficits help us to understand the behavioral function of the GC? Before discussing this issue, we will first comment on the recent CTA retention study of Hashimoto and Spector (2014).
In contrast to the majority of the results in the literature and the data obtained in the current study, Hashimoto and Spector (2014) found little evidence of CTA retention deficits consequent to GC lesions (see also Schier, Hashimoto, Bales, Blonde, & Spector, 2014). Hashimoto and Spector argued that the previously reported retention deficits are to be explained as the consequence of the GC lesions extending into the visceral portion of the insular cortex. By their analysis, neurons in the visceral cortex not the GC are critical for CTA retention. Although this might be the case for some of the reported results, for two reasons this argument cannot explain the results reported herein. First, our histological examination found little indication that the lesions in the GCX rats consistently extended into the visceral cortex. Nonetheless, Group GCX-LiCl still showed a significant deficit in CTA retention. Second, although there are many between-study procedure differences that may account for the supposed inconsistent results reported in CTA retention studies, we suggest that the magnitude of CTA acquired prior to surgery must also be considered when interpreting the retention data reported by Hashimoto and Spector. Their rats received two CS-US (NaCl-LiCl) acquisition trials in the home cage. Intake of the CS in the LiCl-treated rats that would be given GC lesions fell from ∼26.0 ml on the first acquisition trial to ∼1.0 ml on the second acquisition trial. The first post-lesion retention test was conducted in a drinking chamber and involved repeated brief access trials to three concentration of NaCl randomly intermixed with brief access trials to three concentrations of KCl and water. The second retention test involved a 48-hr continuous access preference test (CS versus water) in the home cage. No lesion-induced differences were reported on either type of assessment of CTA retention. Indeed, on the second retention test it was reported that the GCX-LiCl rats like the SHAM-LiCl subjects “displayed near complete avoidance” of the CS, each group showing a preference score that was virtually 0 (see their Figure 6). By any standard this can be considered a profound CTA. Thus, we are inclined to the view that the apparent absence of a GC lesion-induced CTA retention deficit in the Hashimoto and Spector (2014) study may be a consequence of a floor effect in CS consumption that obscured detection of group differences. As our current results show, CTA retention deficits are detectable in rats with lesions of the GC when floor effects in CS intake are avoided.
The current study found that GC lesions attenuated CTA retention. Detailed examination of this behavioral deficit (see Figure 2) revealed that Group GCX-LiCl consumed the saccharin CS on the first retention test as if it was a novel tastant; the lesioned rats neither suppressed their saccharin intake, as did Group SHAM-LiCl (which acquired a CTA), nor increased intake, as did Group SHAM-Saline (which showed habituation of neophobia; see Stehberg et al. [2011] for a similar observation). One explanation for this pattern of results is that the lesions disrupted taste memory so that the GCX-LiCl rats acted as if they had never been exposed to the tastant (i.e., displaying no evidence of CTA and showing the same level of neophobia post-lesion as they demonstrated pre-lesion). However, such an explanation should also apply to the GCX-Saline subjects and, as demonstrated by the post-lesion elevation in saccharin intake, it clearly does not. Nor can a loss of taste memory in GCX rats explain the attenuation of taste neophobia in Experiment 2 because, of course, there was no memory of the quinine tastant on the first neophobia trial. How, then, is the pattern of GC lesion-induced taste deficits to be explained?
As noted in the Introduction, neophobia requires the detection of taste novelty and an immediate behavioral response that serves to limit the potential life-threatening consequences of the consumption of an unknown food. On the other hand, CTA retention does not involve taste novelty detection but depends on the acquired knowledge that the food is toxic. The fact that rats with GC lesions can neither express normal taste neophobia nor retrieve a CTA memory suggests that this cortical area may be critically involved in the detection/responsivity to the danger conveyed by taste stimuli. Thus, GCX rats show little avoidance of a novel or toxic food because they treat that taste as if it is safe (or less dangerous). Finally, we note that the GC appears only to be involved in taste-related behaviors because lesions of this cortical area have little impacts on behaviors guided by non-gustatory events (e.g., odor or trigeminal stimulus; Kiefer et al., 1984; Lin et al., 2009).
Somewhat similar to our proposed analysis, Stehberg and colleagues have argued “the IC [GC in our terminology] is involved in general taste rejection, including the first neophobic response and CTA” (Stehberg et al., 2011). According to this “taste rejection” hypothesis, GC lesions completely block CTA retention. However, this claim receives little support from the current results (i.e., GC lesions attenuated but did not prevent CTA retention). Furthermore, if the GC is responsible for taste rejection responses, it is not immediately clear why rats lacking a functional GC are able to avoid/reject a taste after additional CS-US pairings (e.g., Roman et al., 2006). We, therefore, favor the view that the GC has a role in processing the danger/safety property of the taste rather than a general rejection function.
Using lick pattern analysis, we recently demonstrated that taste palatability changes as a function of taste danger/safety. That is, relative to when the tastant is novel and potentially dangerous, that stimulus becomes more palatable as it become more familiar and safe during the habituation of neophobia (Lin, Amodeo et al., 2012). On the other hand, a tastant becomes unpalatable or disgusting when it is paired with toxins, drugs of abuse or internal pain (Arthurs et al., 2012; Lin, Arthurs et al., 2012; Lin et al., 2013; see Lin et al., 2014 for a review). If our analysis is correct, then GCX rats would be expected to show elevated palatability to a novel fear-inducing tastant and, post-lesion, to a taste CS previously associated with GIM because the lesion reduces the perception of, or responsivity to, the danger conveyed by such stimuli. This is exactly what was found in Experiment 2, in which the GCX rats perceived the novel, potentially dangerous quinine solution as more palatable (indicated by larger lick cluster sizes) than the SHAM animals at the initial contact with the taste (i.e., on the first neophobia trial). Furthermore, Kiefer and Orr (1992) also found that using cortical ablation, GCX rats, relative to control subjects, showed fewer aversive taste reactivity responses when a LiCl-paired sucrose CS was infused into the mouths. These data suggest that GC lesions disrupt a process involved in the detection of information concerning the danger conveyed by a taste stimulus and, consequently, elevate the perceived hedonic value of that taste. Accordingly, a comprehensive understanding of how the GC modulates taste-related learning or, more generally, ingestive behavior can be achieved only when both the danger/safety and palatability components of a tastant are taken into consideration.
5. Conclusion
The present results show that the same GC lesions that disrupt taste neophobia also attenuate CTA retention. Although it might be argued that these deficits represent independent functions, we are inclined to the parsimonious interpretation that both deficits have a common underlying cause. That is, we propose that the GC is critically involved in the processing of taste danger/safety. Essentially, this account states that on the first post-lesion encounter with a specific tastant the GCX rat fails to perceive and/or respond to the danger conveyed by that stimulus. This analysis not only encompasses the two behavioral deficits reported herein but also explains why post-lesion CTA acquisition is delayed, but not prevented, by GC lesions (e.g., Lin et al., 2011; Roman et al., 2006).
Excitatory GC lesions were found to attenuate, but not eliminate, CTA retention.
The same GC lesions also disrupted taste neophobia.
The GC may have little involvement in the processing of taste novelty/familiarity.
Instead, the GC is critically involved in the processing of taste danger/safety.
Acknowledgments
This work was supported by grants DC06456 from the National Institute of Deafness and Other Communication Disorders.
Footnotes
We have previously demonstrated that rats with GC lesions can form a taste memory because they show normal recovery from taste neophobia (e.g., Lin et al., 2009). However, we are not sure whether this conclusion can be applied to the Stehberg and Simon (2011) results. Inspection of the two sets of histology suggests that the GC lesions in the Stehberg and Simon study extended, relative to the GC lesions in our study, into the more posterior portions of the insular cortex that may have included non-gustatory areas.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Guidelines for ethical conduct in the care and use of animals. Washington DC: American Psychological Association; 1996. [Google Scholar]
- Arthurs J, Lin JY, Amodeo LR, Reilly S. Reduced palatability in druginduced taste aversion: II. Aversive and rewarding unconditioned stimuli. Behavioral Neuroscience. 2012;126:433–444. doi: 10.1037/a0027676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EAN. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behavioral Neuroscience. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco, Texas: Baylor University Press; 1977. [Google Scholar]
- Barnett SA. The rat: A study in behavior. Chicago: Aldine; 1963. [Google Scholar]
- Braveman NS, Bronstein P, editors. Experimental assessments and clinical applications of conditioned food aversions. Annals of the New York Academy of Sciences. 1985;443:1–441. [PubMed] [Google Scholar]
- Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain research. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Kiefer SW, Ouellet JV. Psychic ageusia in rats lacking gustatory neocortex. Experimental Neurology. 1981;72:711–716. doi: 10.1016/0014-4886(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Braun J, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiology & Behavior. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- Corey DT. The determinants of exploration and neophobia. Neuroscience and Biobehavioral Reviews. 1978;2:235–253. [Google Scholar]
- Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Research. 1999;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- Davis JD. A model for the control of ingestion-20 years later. Progress in Psychobiology and Physiological Psychology. 1998;17:127–173. [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106:217–228. [PubMed] [Google Scholar]
- Domjan M. Attenuation and enhancement of neophobia for edible substances. In: Barker LM, Best MR, Domjan M, editors. Learning mechanisms in food selection. Waco, Texas: Baylor University Press; 1977. pp. 151–179. [Google Scholar]
- Dwyer DM. Licking and liking: The assessment of hedonic responses in rodents. The Quarterly Journal of Experimental Psychology. 2012;65:371–394. doi: 10.1080/17470218.2011.652969. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Boakes RA, Hayward AJ. Reduced palatability in lithiumand activity-based, but not in amphetamine-based, taste aversion learning. Behavioral Neuroscience. 2008;122:1051–1060. doi: 10.1037/a0012703. [DOI] [PubMed] [Google Scholar]
- Fresquet N, Angst MJ, Sandner G. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behavioural Brain Research. 2004;153:357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Gallo M, Roldan G, Bureš J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behavioural Brain Research. 1992;52:91–97. doi: 10.1016/s0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kovner R, Green KF. Cue properties vs palatability of flavors in avoidance learning. Psychonomic Science. 1970;20:313–314. [Google Scholar]
- Hashimoto K, Spector AC. Extensive lesions in the gustatory cortex in the rat do not disrupt the retention of a presurgically conditioned taste aversion and do not impair unconditioned concentration-dependent licking of sucrose and quinine. Chemical Senses. 2014;39:57–71. doi: 10.1093/chemse/bjt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S, Fan RJ. Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behavioral Neuroscience. 1993;107:317–326. doi: 10.1037//0735-7044.107.2.317. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Lawrence GJ, Metzler CW. Learned alcohol aversions in rats: Gustatory and olfactory components. Alcohol. 1986;3:27–31. doi: 10.1016/0741-8329(86)90068-6. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Leach LR, Braun JJ. Taste agnosia following gustatory neocortex ablation: dissociation from odor and generality across taste qualities. Behavioral Neuroscience. 1984;98:590–608. doi: 10.1037//0735-7044.98.4.590. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Metzler CW, Lawrence GJ. Neocortical involvement in the acquisition and retention of learned alcohol aversions in rats. Alcohol. 1985;2:597–601. doi: 10.1016/0741-8329(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behavioral Neuroscience. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Research. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Lin JY, Amodeo LR, Arthurs J, Reilly S. Taste neophobia and palatability: The pleasure of drinking. Physiology & Behavior. 2012;106:515–519. doi: 10.1016/j.physbeh.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Amodeo LR, Reilly S. Reduced palatability in druginduced taste aversion: I. Variations in the initial value of the conditioned stimulus. Behavioral Neuroscience. 2012;126:423–432. doi: 10.1037/a0027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Role of the insular cortex in morphine-induced conditioned taste avoidance. Brain Research. 2011;1384:80–88. doi: 10.1016/j.brainres.2011.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Reduced palatability in pain-induced conditioned taste aversions. Physiology and Behavior. 2013;119:79–85. doi: 10.1016/j.physbeh.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Reilly S. Conditioned taste aversion, drugs of abuse and palatability. Neuroscience and Biobehavioral Reviews. 2014;45:28–45. doi: 10.1016/j.neubiorev.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Research. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge, England: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press, USA; 2009. pp. 37–57. [Google Scholar]
- Milgram NW, Krames L, Alloway T, editors. Food aversion learning. New York: Plenum Press; 1977. [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Nerad L, Ramírez-Amaya V, Ormsby CE, Bermúdez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiology of Learning and Memory. 1996;66:44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. San Diego, California: Academic Press; 2005. [Google Scholar]
- Reilly S, Schachtman TR, editors. Conditioned taste aversion: behavioral and neural processes. New York: Oxford University Press; 2009. [Google Scholar]
- Roman C, Lin JY, Reilly S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Research. 2009;1259:68–73. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behavioral Neuroscience. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. European Journal of Neuroscience. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. Highresolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proceedings of the National Academy of Sciences. 2014;111:1162–1167. doi: 10.1073/pnas.1315624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Spector AC, John SJ. Role of taste in the microstructure of quinine ingestion by rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1998;274:R1687–R1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- Stehberg J, Moraga-Amaro R, Simon F. The role of the insular cortex in taste function. Neurobiology of Learning and Memory. 2011;96:130–135. doi: 10.1016/j.nlm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Stehberg J, Simon F. Involvement of the insular cortex in retention of conditioned taste aversion is not time dependent. Neurobiology of Learning and Memory. 2011;95:14–18. doi: 10.1016/j.nlm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Azuma S, Kawamura Y. Significance of cortical-amygdalarhypothalamic connections in retention of conditioned taste aversion in rats. Experimental Neurology. 1981;74:758–768. doi: 10.1016/0014-4886(81)90249-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory Tarea in rats and its role in taste discrimination. Journal of Neurophysiology. 1980;44:440–455. doi: 10.1152/jn.1980.44.3.440. [DOI] [PubMed] [Google Scholar]


