Abstract
Alzheimer’s disease (AD) is a devastating disorder that strikes 1 in 10 Americans over the age of 65, and almost half of all Americans over 85 years old. The odds of an individual developing Alzheimer’s disease double every five years after the age of 65. While it has become increasingly common to meet heart attack or cancer survivors, there are no Alzheimer’s disease survivors. There is mounting evidence that dietary polyphenols, including resveratrol, may beneficially influence Alzheimer’s disease (AD). Based on this consideration, several studies reported in the last few years were designed to validate sensitive and reliable translational tools to mechanistically characterize brain bioavailable polyphenols as disease-modifying agents to help prevent the onset of AD dementia and other neurodegenerative disorders. Several research groups worldwide with expertise in AD, plant biology, nutritional sciences, and botanical sciences have reported very high quality studies that ultimately provided the necessary information showing that polyphenols and their metabolites, which come from several dietary sources, including grapes, cocoa etc., are capable of preventing AD. The ultimate goal of these studies was to provide novel strategies to prevent the disease even before the onset of clinical symptoms. The studies discussed in this review article provide support that the information gathered in the last few years of research will have a major impact on AD prevention by providing vital knowledge on the protective roles of polyphenols, including resveratrol.
Keywords: resveratrol, Alzheimer’s disease, polyphenols, SIRT1
Polyphenols as novel “natural drug” agents in the prevention and possible therapy of Alzheimer’s disease
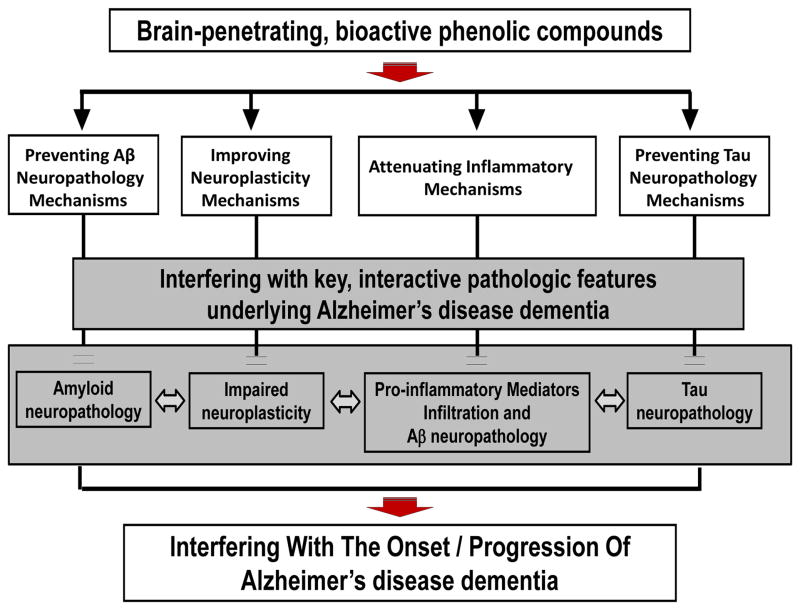
A notable example of one of the first achievements from ongoing investigations on the role of polyphenols in Alzheimer’s disease (AD) is the demonstration that polyphenols from select red wines, including resveratrol, may help attenuate AD dementia by modulating β-amyloid (Aβ) neuropathology through the inhibition of both Aβ generation and abnormal Aβ oligomerization and through the promotion of Aβ clearance, and by modulating tau neuropathology through the inhibition of abnormal tau phosphorylation and tau aggregation (Wang, et al., 2006; Ho, et al., 2009; Ono, et al., 2008; Ono, et al., 2006; Marambaud, et al., 2005; Vingtdeux, et al., 2010; Wang, et al., 2008; Ho, et al., 2009; Wang, et al., 2010). All of these Aβ and tau mechanisms are key therapeutic targets for AD. While polyphenols from certain red wines inhibit Aβ aggregation, others do not (Ho, et al., 2009). These studies were conducted a few years ago, and provided, for the first time, the basis for subfractionation of complex grape-derived polyphenol preparations into increasingly less complex isolates for use in bioactivity studies, in vitro and in vivo (Wang, et al., 2012; Ho, et al., 2013). Recent fractionation studies have also revealed that a grape seed polyphenolic extract (GSPE) is capable of significantly attenuating AD-type phenotypes in transgenic AD mice, primarily due to its ability to increase the bioavailability of flavan-3-ol molecules (e.g., catechin, epicatechin, etc.) in the brains (Wang, et al., 2012; Ferruzzi, et al., 2009; Wang, et al., 2008). Interestingly, it was also reported that quercetin-3-O-glucuronide, from red wines and Concord grape juice, is capable of reaching the brain and contributes to protection against AD by modulating multiple mechanisms, including by: reducing Aβ generation, reducing Aβ oligomerization, and promoting neuroplasticity processes (Ho, et al., 2013). Notably, other studies revealed that resveratrol may promote intracellular Aβ clearance, in part by activating autophagy and AMPK signaling in vivo (Vingtdeux, et al., 2011). Overall, outcomes from these studies support the notion that autophagy and inflammation work in concert with respect to the anti-amyloidogenic effect of resveratrol. Moreover, recent studies suggest that polyphenols may also reduce abnormal tau hyperphorylation and tau aggregation (Ho, et al., 2009; Wang, et al., 2010). A major achievement in the search for the role of polyphenols in AD prevention and therapies is the finding that multiple polyphenol metabolites, derived from dietary polyphenols, are able to cross the blood-brain barrier (BBB) and to penetrate and accumulate in the brain at pharmacologically relevant sub-μM to μM concentration (Wang, et al., 2013; Ferruzzi, et al., 2009; Ho, et al., 2013). Moreover, we found that certain brain-penetrating polyphenols are capable of modulating AD neuropathogenic mechanisms. For example, we found that one of the brain-penetrating polyphenol metabolites, quercetin-3-O-glucoside, is capable of modulating Aβ neuropathogenic mechanisms (Ho, et al., 2013). Moreover, we found that another brain-penetrating polyphenol metabolite, 3′-O-methyl-epicatechin-5-O-β-glucuronide, is capable of directly modulating synaptic plasticity by promoting cAMP response element-binding protein (CREB) signal transduction, which is involved in mechanisms associated with learning and memory functions (Wang, et al., 2012; Ho, et al., 2013). Based on these findings, we proposed that the dietary polyphenol preparations that we studied are able to modulate AD through the activities of their brain-penetrating polyphenol preparations, which modulate multiple pathogenic processes such as Aβ and tau neuropathogenic mechanisms, neuroplasticity, and inflammation (see Figure 1).
Figure 1.
Brain-penetrating polyphenol metabolites derived from certain bioactive dietary polyphenol preparation may attenuate AD dementia by modulating Aβ and tau neuropathogenic mechanisms, neuroplasticity, and inflammatory mechanisms.
These scientific achievements are indicators of the widespread success of research in polyphenols in AD. Most excitingly, for the first time, these studies provided the basis for translational investigations into clinical studies exploring the feasibility of developing select polyphenols for preventative strategies in AD. As discussed further below, this increasing interest in the field of polyphenols is reflected by 85 currently listed clinical trials in the NIH clinicaltrials.gov registry exploring the role of resveratrol in several conditions, including 5 studies in AD and 29 on the role of type 2 diabetes (T2D) in cognitive functions associated with aging. This evidence strongly supports the widespread mounting interest in the role polyphenols, including the use of resveratrol for prevention and treatment of AD and age-related cognitive deterioration.
Resveratrol, inflammation, and type 2 diabetes: implications in metabolic disturbances associated with the onset and progression of AD
A large body of literature has shown that resveratrol, a naturally occurring polyphenol (trans-3,4′,5-trihydroxystilbene), exerts beneficial effects on AD, an age-related neurodegenerative condition that in some cases is also comorbid with certain metabolic disorders, such as type 2 diabetes and obesity (Baur, et al., 2006; Lagouge, et al., 2006). Resveratrol mimics caloric restriction by extending the lifespan of several small organisms (Baur & Sinclair, 2006; Greet & Brunet, 2009), and by delaying specific age-related phenotypes, e.g., abnormal glucose metabolism (Poulsen, et al., 2013). Resveratrol is also thought to beneficially influence cognitive deterioration (Ranney & Petro, 2009; Abraham & Johnson, 2009). Clinical studies are underway to explore the benefits of resveratrol for treating individuals with dementia, particularly those characterized by mild cognitive impairment (MCI), a clinical condition that eventually progresses to AD.
The direct molecular targets of resveratrol, in vitro and in vivo, are unknown. The compound has been suggested to modulate cellular processes by activating key metabolic sensor/effector proteins, including AMP-activated protein kinase (AMPK), sirtuin 1 (SIRT1), and peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) (Cantó & Auwerx, 2009; Um, et al., 2010; Vingtdeux, et al., 2011). It was initially proposed that resveratrol binds in vitro to SIRT1 and activates the deacetylase activity of this enzyme (Howitz, et al., 2003). However, recent studies have challenged these data by showing that the reported direct interaction between resveratrol and SIRT1 in vitro was likely an artificial observation, implying that resveratrol might act in vivo by targeting other proteins (Borra, et al., 2005; Pacholec, et al., 2010). Nevertheless, SIRT1 appears to be required for resveratrol metabolic functions in vivo by contributing to an energy sensing network involving AMPK and PGC-1α (Cantó & Auwerx, 2009; Ruderman, et al., 2010).
Resveratrol has been shown to have beneficial effects in in vitro models of epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and nerve injury (Rocha-González, et al., 2008). AMPK, SIRT1, and PGC-1α were all thought to be involved in the etiology of these neurological disorders. Based on evidence that resveratrol modulates these proteins, it was proposed that resveratrol has therapeutic potential in the above-mentioned neurodegenerative diseases. The therapeutic potential of resveratrol in neurodegenerative disorders is supported by multiple studies demonstrating neuroprotective effects of resveratrol in different cell culture and in vitro systems (Vingtdeux, et al., 2008).
Bioavailability and clinical trials of resveratrol in Alzheimer’s disease and other neurodegenerative diseases
The bioavailability of resveratrol has received considerable attention (Walle, 2011). The oral bioavailability of resveratrol is rather low; less than 1%. Micronized resveratrol also has low systemic availability, although it can reach levels 3.6 times higher than that of the standard form (Howells, et al., 2011). Although in vivo evidence is emerging in animal models that resveratrol is bioavailable (Vingtdeux, et al., 2010) and bioactive (Karuppagounder, et al., 2009; Vingtdeux, et al., 2010) in the brain, conclusive results in human trials are still lacking. Due to resveratrol’s very low bioavailability and how rapidly it is metabolized, it has been suggested that some of its metabolites may be responsible for its biological activity. In particular, reservatrol-3-sulfate and resveratrol-3-O-glucuronide are the major metabolites of resveratrol, and di- and tri-sulfated derivatives have been also detected in substantial concentrations in vivo (Wenzel, et al., 2005).
There are currently as many as 85 clinical trials listed in the NIH clinicaltrials.gov registry that investigate the effect of resveratrol on diverse conditions including: 5 are involving AD, are there are also others involving several conditions associated with AD, for example, type 2 diabetes and cognitive performance (memory) in the elderly, among others (see Table 1 for a list of selected clinical trials). Interestingly, a phase II study of 119 individuals with mild to moderate AD, although underpowered to detect clinical benefits, found that resveratrol was safe and generally well-tolerated at doses up to 1 gram orally twice daily. While minor side effects were reported, including gastrointestinal nausea and diarrhea, the data analyses are now underway. The available information at this point is that there are alterations in some cerebrospinal fluid and plasma proteins, as well as in volumetric imaging outcomes, in the resveratrol-treated group compared to the placebo-treated group (clinicaltrials.gov identifier NCT01504854). Furthermore, we note that there are also 29 clinical trials, some of which are listed in Table 1, related to the potential therapeutic role of resveratrol in T2D and other features of metabolic syndrome. As we discuss below, recent studies are testing the hypothesis that resveratrol and other polyphenols may improve cognitive performance in neurological controls and possibly delay the onset of MCI, and eventually its progression to AD, through control of peripheral glucose metabolism.
Table 1.
Selected clinical trials from the NIH clinicaltrials.gov registry that investigate the effects of resveratrol on Alzheimer’s disease and diabetes and/or metabolic syndrome.
| Status | Study | Conditions |
|---|---|---|
| Active, not recruiting | Resveratrol for AD | AD |
| Completed | Randomized Trial of a Nutritional Supplement in AD | AD |
| Unknown | Pilot Study of the Effects of Resveratrol Supplement in Mild-to-Moderate AD | AD |
| Recruiting | Short Term Efficacy and Safety of Perispinal Administration of Etanercept in Mild to Moderate AD | AD |
| Recruiting | Effects of Dietary Interventions on the Brain in Mild Cognitive Impairment (MCI) | Mild Cognitive Impairment |
| Completed | Resveratrol-Leucine Metabolite Synergy in Pre-diabetes | Impaired Glucose Tolerance |
| Completed | Effects of Resveratrol in Patients With T2D | T2D |
| Active, not recruiting | Resveratrol in T2D and Obesity | T2D; Obesity; Insulin Resistance |
| Active, not recruiting | Resveratrol and T2D | T2D |
| Recruiting | Effect of Resveratrol on Age-related Insulin Resistance and Inflammation in Humans | T2D Mellitus; Insulin Resistance |
| Recruiting | Resveratrol and the Metabolic Syndrome | Obesity; Insulin Resistance; Metabolic Syndrome |
| Completed | Dietary Polyphenols and Lipid Oxidation | Obesity; Insulin Sensitivity; T2D Mellitus |
| Unknown | Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance | Impaired Glucose Tolerance |
| Completed | Effect of Resveratrol Administration on Metabolic Syndrome, Insulin Sensitivity and Insulin Secretion | Metabolic Syndrome X |
| Recruiting | Resveratrol and the Metabolic Syndrome | Obesity; Insulin Resistance; Metabolic Syndrome |
| Recruiting | Resveratrol in Metabolic Syndrome | Metabolic Syndrome |
| Withdrawn | Mechanisms of Metabolic Regulation of Resveratrol on Humans With Metabolic Syndrome | Insulin Resistance |
| Completed | Long-term Investigation of Resveratrol in Obesity | Obesity; Inflammation; Insulin Sensitivity; Osteoporosis |
| Completed | Potential Beneficial Effects of Resveratrol | Metabolic Syndrome; Obesity |
| Recruiting | The Effects of Red Wine Polyphenols on Microvascular Dysfunction | Obesity |
Abbreviations: AD=Alzheimer’s disease; T2D=type 2 diabetes.
Does resveratrol work through activation of SIRT1?
Resveratrol and SIRT1 are best known for their pro-longevity properties. Studies in S. cerevisiae, C. elegans, and Drosophila have established SIRT1 orthologs as key determinants of longevity (Finkel, et al., 2009). In several models, the effects of calorie restriction on lifespan extension were proposed to be SIRT1-dependent (Finkel, et al., 2009). Resveratrol was also found to mimic calorie restriction at the molecular level and to extend longevity in both yeast and in worms (Baur & Sinclair, 2006; Greet & Brunet, 2009). SIRT1 was thought to be the molecular target of resveratrol, based on in vitro studies showing that the polyphenol can enhance SIRT1 deacetylase activity (Howitz, et al., 2003). Concerns have been raised, however, about the validity of the enzymatic assay used in this study. Indeed, consecutive studies have shown that resveratrol does not activate SIRT1 in vitro, and thus is likely to work through direct interaction with one or several other proteins in vivo (Beher, et al., 2009; Borra, et al., 2005; Pacholec, et al., 2010). Resveratrol is a relatively potent activator of AMPK in cell cultures and in vivo in mice (Cantó & Auwerx, 2009; Vingtdeux, et al., 2010; Dasgupta & Milbrandt, 2007; Hawley, et al., 2010). Resveratrol’s effect on AMPK activity was proposed to be indirect as well, either by increasing cytosolic calcium levels (Vingtdeux, et al., 2010) or by facilitating AMP-dependent activation of AMPK (Hawley, et al., 2010). Nevertheless, AMPK activation by resveratrol is dependent on sirtuin function. Also, SIRT1 appears to be required for resveratrol metabolic functions in skeletal muscle in vivo, by contributing to the activation of AMPK as well as PGC-1α, which is a downstream substrate of SIRT1 (Cantó & Auwerx, 2009; Cantó & Auwerx, 2009; Ruderman, et al., 2010). In spite of evidence implicating resveratrol as having the potential to modulate the AMPK/SIRT1/PGC-1α signaling network, the direct in vivo molecular target(s) of resveratrol remain(s) elusive.
Polyphenols and resveratrol in AD and other neurodegenerative disorders
Resveratrol is found naturally in low concentrations among certain food sources such as peanuts, some berries, red grapes, and grape by-products such as red wines and red grape juices (Burns, et al., 2002; Sanders, et al., 2000; Rimando, et al., 2004). Prospective studies have shown that moderate red wine consumption is associated with lower relative risk of dementia (Orgogozo, et al., 1997). More recently, James Joseph and colleagues demonstrated that dietary supplementation with a Concord purple grape juice significantly improves memory function in subjects with mild cognitive impairment (Krikorian, et al., 2010). Consistent with these observations, we demonstrated that moderate red wine consumption beneficially modulates AD-type cognitive deterioration in the Tg2576 transgenic mouse model of AD by attenuating Aβ neuropathology (Wang, et al., 2006; Ho, et al., 2009). Moreover, we demonstrated that total polyphenolics isolated from red wine significantly attenuate the generation of Aβ peptide in primary cortico-hippocampal neuron cultures generated from Tg2576 mice (Wang, et al., 2006). While red wine and grape juice are among some of the richest dietary sources of resveratrol, we found that the content of resveratrol in red wines or grape juices cannot account for the neurological benefits derived from normal consumption of red wine (or grape juice). For example, while resveratrol has been found to promote Aβ clearance (Marambaud, et al., 2005), the content of resveratrol in the red wine we used in the Wang et al. (2006) study is approximately 10-fold lower than the minimal effective concentration shown to promote Aβ clearance in vitro (Marambaud, et al., 2005). Thus, these studies suggest that grape-derived polyphenolic components other than resveratrol may also exert neurological health benefits. Consistent with this, we demonstrated that a red wine polyphenolic preparation containing no detectable contents of resveratrol significantly attenuates the development of β-amyloid neuropathology and cognitive deterioration in Tg2576 AD mice (Ho, et al., 2009). Collectively, our observations raise an intriguing possibility that a combination of grape-derived polyphenolics may modulate AD by simultaneously reducing Aβ generation, interfering with aggregation of Aβ peptides, and/or promoting molecular mechanisms involved in memory consolidation.
Our observation that certain red wine polyphenolic preparations potently interfere with aberrant aggregation of Aβ peptides also has implications for other neurodegenerative disorders. Abnormal aggregation of certain proteins in susceptible brain cells is a common feature of a number of neurodegenerative disorders, including AD, PD, ALS, and HD. Recent evidence indicates that mutant huntingtin (htt) aggregation in susceptible brain cells may be responsible for the onset and progression of the HD-type phenotype (Yamamoto, et al., 2000; MacDonald, et al., 1992), implicating abnormal htt protein aggregation as a novel target for HD treatment. We recently found that a grape-derived polyphenolic preparation interferes with abnormal aggregation of htt protein in an in vitro cell model of HD degeneration (Wang, et al., 2010). Collectively, accumulating evidence from our group and others provides the motivation for further investigations to explore, pre-clinically, the suitability of alternative grape-derived polyphenols as potential novel agents for the treatment of AD and other neurodegenerative conditions also caused by abnormal protein aggregation.
A recent study (Pasinetti, et al., 2011) examined the therapeutic potential of a micronized proprietary resveratrol formulation, SRT501, in the N171-82Q transgenic mouse model of HD. HD is a progressive and devastating genetic neurodegenerative disorder that is associated with downregulation of PGC-1α activity. The study found that SRT501 treatment did not lead to significant improvement in weight loss, motor performance, survival, or striatal atrophy. However, other studies have reported neuroprotective effects of resveratrol and a distantly related polyphenol, fisetin, in HD models. An interesting connected is that HD has been associated with diabetes mellitus. Interestingly, evidence from the Ho et al. study suggests that the resveratrol formulation induced beneficial anti-diabetic effects in N171-82Q mice. The observation that oral SRT501-M treatment induced biological responses in the cortex but not in the striatum is consistent with previous studies that found that orally administered resveratrol can cross the BBB and accumulate in the cerebral cortex, but not in the hippocampus (Karuppagounder, et al., 2009; Vingtdeux, et al., 2010). This selective accumulation of resveratrol in different brain regions remains unexplained, but may be due to the diffusion rate of the compound or to its specific metabolism in the brain. Nevertheless, the (Pasinetti, et al., 2011) study indicates that resveratrol should be tested further in HD, and that improved administration and formulation methods must be implemented and more potent resveratrol metabolites or analogues should be identified prior to evaluation in animal models.
A recent study by (Maher, et al., 2011) in HD models reported neuroprotective effects of two polyphenols: resveratrol, in vitro, and fisetin, in vivo. Resveratrol was found to be ineffective in the N171-82Q HD mouse model, whereas fisetin was neuroprotective in vivo in the R6/2 mouse model of HD. However, fisetin is rather distantly related to resveratrol, and thus the in vivo fisetin data cannot be extrapolated towards resveratrol. It remains to be established whether other formulations or dosing regimens of the compound would have efficacy in animal models and human clinical trials for HD.
SIRT1 and Alzheimer disease
A main pathogenic event in AD is the cerebral aggregation of the neurotoxic peptide Aβ (Citron, 2010). Aβ is generated from the sequential endoproteolysis of a longer precursor, amyloid precursor protein (APP), by the action of two enzymes, β-secretase and γ-secretase. Another enzyme, α-secretase, precludes Aβ production by cleaving APP at the Aβ sequence (Selkoe, 2001). Several proteases of the A Disintegrin And Metalloproteinase (ADAM) family, such as ADAM10, have been implicated in the α-secretase cleavage of APP. In 2006, we showed that calorie restriction attenuates Aβ production, in part by promoting α-secretase through mechanisms involving activation of SIRT1 (Qin, et al., 2006). Calorie restriction was found to increase SIRT1 expression and NAD+ levels in the brain of the Tg2576 transgenic mouse model of AD. Furthermore, we found that SIRT1 expression in primary Tg2576 neurons and in APP-transfected Chinese hamster overy (CHO) cells reduces the generation of Aβ from APP by facilitating α-secretase activity (Qin, et al., 2006). Additional work demonstrated that expression of SIRT1 in the brain results in decreased Rho-associated protein kinase ROCK1 expression and elevated secretase activity in vivo, implicating ROCK1 in SIRT1-mediated α-secretase activity (Qin, et al., 2006). Moreover, studies in squirrel monkeys demonstrated that calorie restriction in primates also effectively reduces the contents of cortical Aβ peptides, and that this decrease is inversely correlated with SIRT1 protein concentrations in the same brain region (Qin, et al., 2006).
These results by Qin et al. were recently confirmed in SIRT1 transgenic and knockout mice (Donmez, et al., 2010). The authors found that Aβ production and the resulting amyloid deposition is decreased in the brains of SIRT1 transgenics. In contrast, both Aβ production and amyloid deposition in the brain are elevated in SIRT1 knockouts when crossed with APP-transgenic mice. Mice overexpressing SIRT1 also showed a reduction in brain inflammation, tau phosphorylation (another hallmark of AD), and in cognitive defects specific to the APP-transgenic mice. Conversely, SIRT1 deficiency exacerbated Aβ-linked pathology in the mice. In this study, SIRT1 was proposed to activate α-secretase by directly activating ADAM10 expression. The authors further demonstrated that SIRT1 can deacetylate and coactivate the retinoic acid receptor β, a known modulator of ADAM10 transcription (Donmez, et al., 2010). Collectively, the original discoveries Qin et al. (2006a and b), and the confirmatory studies from Donmez et al. (2010), suggest that pharmacological activation of SIRT1 may represent a promising approach to preventing amyloid deposition and neurodegeneration in AD (Wang, et al., 2010; Wang, et al., 2010).
A novel possible pathway for resveratrol-mediated responses in the brain via AMPK activation and increased protein clearance
Our laboratory and others have recently found that resveratrol controls Aβ accumulation by facilitating its proteolytic clearance in neuronal cells (Marambaud, et al., 2005; Vingtdeux, et al., 2008; Vingtdeux, et al., 2010) and reduces cerebral amyloid deposition in vivo in APP transgenic mice (Karuppagounder, et al., 2009; Vingtdeux, et al., 2010). We demonstrated, for the first time, that AMPK activation is responsible for the anti-amyloidogenic effects of resveratrol (Vingtdeux, et al., 2010). AMPK is a heterotrimeric Ser/Thr protein kinase that is activated by different upstream kinases such as liver kinase B1 (LKB1) and Ca(2+)/CaM-dependent protein kinase kinase β (CaMKKβ), a kinase predominantly expressed in neural tissue (Carling, et al., 2008; Fogarty, et al., 2010; Viollet, et al., 2009). AMPK controls protein degradation by inhibiting the protein kinase mechanistic target of rapamycin (mTOR) and by activating autophagy (Hardie, 2007). Our work revealed that resveratrol can activate AMPK by increasing CaMKKβ-dependent phosphorylation of AMPK. Activation of AMPK by resveratrol led to mTOR inhibition, autophagy induction, and proteolytic clearance of Aβ (Vingtdeux, et al., 2010). Importantly, we also demonstrated that orally administered resveratrol in mice can cross the BBB to activate brain AMPK and to reduce Aβ levels and deposition in the cerebral cortex (Vingtdeux, et al., 2010). These studies highlight the therapeutic potential of AMPK activation as an anti-amyloidogenic strategy in AD, but also motivate the search for resveratrol metabolites/analogues or other polyphenolic compounds (or polyphenolic preparations) with improved potency and brain penetration properties as anti-neurodegenerative molecules (Vingtdeux, et al., 2008; Wang, et al., 2010; Wang, et al., 2010).
Caloric restriction, resveratrol and neurodegeneration
Resveratrol is known to have beneficial metabolic effects and is considered a mimetic of dietary/caloric restriction. Since resveratrol is being considered for a treatment for HD, it is of high interest that Ho et al. found beneficial anti-diabetic effects of resveratrol in the mouse model of HD. Chaturvedi et al. recently demonstrated that administration of SRT501-M benefits metabolic impairments observed in N171-82Q mice by reducing levels of brown adipose tissue (BAT) vacuolation and blood glucose, two metabolic parameters that are abnormally elevated in this HD mouse model, although not all of the HD mice responded to SRT501-M treatment (Chaturvedi, et al., 2010). In BAT, a transcriptional response was observed in treated HD mice with an increase in the expression of PGC-1α and two of its downstream effectors, nuclear respiratory factor-1 (NRF-1) and uncoupling protein-1 (UCP-1). Impaired PGC-1α function has also previously been shown to play a role in muscle dysfunction (Chaturvedi, et al., 2009) and mitochondrial dysfunction (Weydt, et al., 2006) in HD. It is possible that resveratrol may improve the HD phenotype via metabolic benefits. However, since CNS-related indicators of HD were not improved in the resveratrol-treated N171 mice, it appears that the metabolic effects of resveratrol would not ameliorate CNS neurodegeneration. Moreover, an implication of the Ho et al. study is that caloric restriction is unlikely to ameliorate central HD symptoms. In contrast to HD, Wang et al. (2005) demonstrated that caloric restriction does attenuate β-amyloid neuropathology and improves glucose metabolism in a mouse model of AD (Wang, et al., 2005).
Mild cognitive impairment (MCI): an important target for secondary prevention with combination polyphenol treatment
MCI is defined as a syndrome of subjective and/or objective evidence for cognitive deficits with no evidence (or minimal evidence) of functional decline (Albert, et al., 2011). MCI has been subtyped into amnestic MCI (affecting memory) vs. nonamnestic MCI (affecting other cognitive domains only). Individuals with amnestic MCI are at high risk of developing incident AD (Petersen, 2004), and most have significant brain amyloid burden (Johnson, et al., 2013). Since individuals with MCI are, by definition, functioning well, it would be a substantial public health benefit if interventions could be found to prevent their progression to dementia (i.e., secondary prevention of dementia). There is currently no FDA-approved intervention for secondary prevention of MCI, and trials of current AD treatments have failed (spectacularly) in this regard (Raschetti, et al., 2007). MCI defines a risk group, not a disease state, and in fact, many individuals with MCI do not progress to dementia or AD (Mitchell & Shiri-Feshki, 2009); these individuals probably do not have a brain disease. Given these observations, it is particularly important for an intervention to be relatively safe and non-invasive. Most of the interventions currently under investigation in this field are cognitive or behavioral interventions, with relatively few drug trials, the most advanced of which is intranasal insulin, the investigation of which is based on parallel hypotheses to ours (Craft, et al., 2012; Reger, et al., 2008). Individuals with MCI frequently have metabolic risk factors including insulin resistance and prediabetes, and these individuals are at particular risk of cognitive decline.
Prediabetes and cognition in the elderly and in individuals with MCI: implications of resveratrol in the modulation of glucose metabolism and in the prevention of MCI progression into frank mild AD dementia
Prediabetes is a condition in which glucose levels are elevated, but do not meet the criteria for diabetes. In the U.S., approximately one in three adults (≥ 20 years old), or an estimated 79 million adults, have this condition (CAP, 2011). These individuals are at higher risk of developing diabetes and cardiovascular disease (CVD), and also are at higher risk of death (Abraham & Fox, 2013). Prediabetes is also a risk factor for future cognitive decline. One study found that individuals with prediabetes have worse baseline cognitive scores compared to those with normal glucose levels; their risk of developing clinically significant cognitive impairment (dementia, MCI, or very low cognitive score) in 4 years was almost two fold higher compared to those with normal glucose levels. Interestingly, the risk of future cognitive impairment in individuals with prediabetes was as high as in those with T2D in this study (Yaffe, et al., 2004). Another longitudinal study found that MCI individuals with prediabetes had an almost six fold higher risk of converting from MCI to AD compared to MCI individuals with normal glucose levels. This effect was even higher than the risk of converting to AD among MCI individuals with T2D. One of the possible reasons for this effect may be that insulin resistance and elevated glucose levels are often ignored for many years in individuals with prediabetes (Xu, et al., 2010). Therefore, it is important to recognize that individuals with prediabetes are at an insulin resistant state that is in “the continuum of metabolic disorders,” and that they will likely become diabetic in the future (Luchsinger, 2010).
In light of these associations between insulin resistance and cognition, the effects of intensive (HgA1C <6.0%) and standard glycemic control (HgA1C 7.0–7.9%) on longitudinal cognitive function was examined in the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND). Although higher HbA1c, an indicator of average blood sugar levels over a period of weeks/months, was associated with lower cognitive scores at baseline, there were no differences in the cognitive scores between the intensive and standard glycemic control groups at 20 and 40 month follow up times (Launer, et al., 2011). Moreover, higher mortality in the intensive glycemic control group led to early termination of the study (ACCORD Study Group, et al., 2011).
Consistent with this evidence, a recent study tested whether supplementation with resveratrol can enhance memory performance in older adults, and addressed potential mechanisms underlying this effect, in the context of glucose metabolism (Witte, et al., 2014). The investigators found that resveratrol supplementation in older adults leads to significant improvement in memory performance in association with improved glucose metabolism, as indicated decreased HbA1c, as well as increased functional connectivity in the brain.
Therefore, novel strategies to address insulin resistance and cognitive dysfunction in this population are needed. While MCI represents a high risk group for conversion to AD, there is also a great variability in the risk due to the heterogeneity of this population (Farias, et al., 2009). Hence, identifying and targeting treatment towards a high risk subgroup of MCI individuals such as those with prediabetes will allow for more effective intervention.
Polyphenol combination therapy as a novel strategy to delay the conversion of MCI into AD
BDPP(Bioactive Dietary Polyphenol Preparation) is a combination of three bioactive and commercially available polyphenol products (Concord grape juice, grape seed extract, and resveratrol), and is a novel nutraceutical combination designed to provide three selective, bioactive, polyphenol-rich dietary preparations to simultaneously target multiple AD pathogenic targets, as well as metabolic syndrome phenotypes (primarily through resveratrol action). As we describe in more detail below, each of the BDPP components are capable of exerting unique mechanisms of action against AD pathogenic mechanisms. Thus, in comparison to individual BDPP components, application of BDPP will allow for a more comprehensive coverage of AD pathogenic targets, and therefore is a more efficacious strategy for treating patients with early AD and prediabetes.
In ongoing studies in our laboratory, we found that BDPP targets amyloid load, synaptic plasticity, and cognition in mouse models of AD and metabolic syndrome (Wang, et al., 2014). Among MCI individuals, those with prediabetes are at high risk of converting to AD. The advantages of this nutraceutical include a benign adverse effect profile, oral administration, and a refreshing lack of intellectual property issues, which will keep costs down. This is particularly relevant as we enter into an era where we may be able to target prodromal and preclinical AD for secondary prevention. Since secondary prevention, by definition, involves treatment of a large population, it would be particularly useful if an intervention for secondary prevention was also inexpensive, which is unlikely with the currently studied strategies (passive immunotherapy and beta-secretase inhibition, for example). See Figure 2 for a scheme of the working hypothesis regarding the possible application of resveratrol along with other bioactive dietary polyphenols for the prevention and therapeutic attenuation of AD dementia.
Figure 2. Bioactive Dietary Polyphenol Preparation (BDPP), a novel nutraceutical combination designed to simultaneously target multiple pathogenic mechanisms underlying AD.
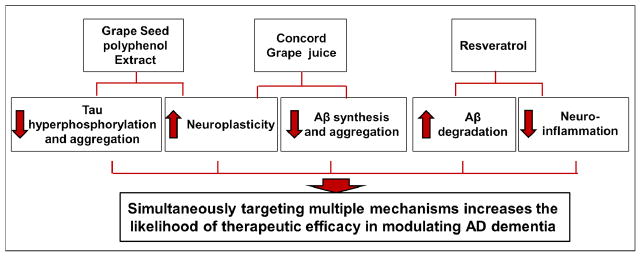
The schematic identifies the possible contribution of individual BDPP components for the prevention and therapeutic attenuation of AD dementia. Experimental evidence suggests that resveratrol may benefit AD dementia by promoting Aβ degradation and by decreasing neuroinflammation. We further proposed that application of resveratrol, along with other bioactive dietary polyphenol preparations, will modulate additional AD mechanisms (e.g., Aβ synthesis, Aβ aggregation, tau hyperphosphorylation, and tau aggregation), which will increase the likelihood of therapeutic success.
Conclusions
Although recent reports have shown that resveratrol and other grape derived polyphenols metabolites can cross the BBB and lead to some biological responses in the brain following oral dosing (Abd El-Mohsen, et al., 2006; Ho, et al., 2010; Karuppagounder, et al., 2009; Vingtdeux, et al., 2010), the important question of how to improve polyphenol, in particular resveratrol, bioavailability in the brain remains to be addressed. Indeed, should resveratrol be tested further, improved administration and formulation methods should be implemented, and more potent resveratrol metabolites or analogues should be identified prior to evaluation in animal models. Another line of research is to focus on novel, brain-targeting grape-derived polyphenols with promising anti-neurodegenerative properties (Ho & Pasinetti, 2010; Wang, et al., 2008).
Highlights.
Polyphenols and their metabolites are capable of preventing Alzheimer’s disease.
The direct molecular targets of resveratrol, in vitro and in vivo, are unknown.
Certain polyphenolic preparations interfere with aggregation of β-amyloid peptides.
Acknowledgments
Funding was provided by the NIH-NCCAM grant P01AT004511-01 as part of the Icahn School of Mount Sinai Center of Excellence for Research in Complementary and Alternative Medicine for Alzheimer’s Disease (to Pasinetti).
Abbreviations
- AMPK
5′ adenosine monophosphate-activated protein kinase
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- BDPP
Bioactive Dietary Polyphenol Preparation
- BAT
Brown adipose tissue
- CaMKKβ
Ca(2+)/CaM-dependent protein kinase kinase β
- CREB
cAMP response element-binding protein
- CVD
Cardiovascular disease
- CHO
Chinese hamster ovary
- UCP-1
Uncoupling protein-1
- cAMP
Cyclic adenosine monophosphate
- HD
Huntington’s disease
- LKB1
Liver kinase B1
- MCI
Mild cognitive impairment
- NRF-1
Nuclear respiratory factor-1
- PD
Parkinson’s disease
- PGC-1α
Proliferator-activated receptor γ co-activator-1α
- mTOR
Protein kinase mechanistic target of rapamycin
- SIRT1
Sirtuin 1
- T2D
Type 2 diabetes
- Aβ
β-amyloid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd El-Mohsen M, et al. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96(1):62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12(6):445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham TM, Fox CS. Implications of rising prediabetes prevalence. Diabetes Care. 2013;36(8):2139–2141. doi: 10.2337/dc13-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACCORD Study Group et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Beher D, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74(6):619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Burns J, et al. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20(7):325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Prevention 2011 [Google Scholar]
- Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet. 2009;18(16):3048–3065. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, et al. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington’s disease following chronic energy deprivation. Hum Mol Genet. 2010;19(16):3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142(2):320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Farias ST, et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi MG, et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J Alzheimers Dis. 2009;18(1):113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, et al. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426(1):109–117. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greet EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DJ, et al. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Ho L, et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J Alzheimers Dis. 2009;16(1):59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27(2):769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Pasinetti GM. Polyphenolic compounds for treating neurodegenerative disorders involving protein misfolding. Expert Rev Proteomics. 2010;7(4):579–589. doi: 10.1586/epr.10.69. [DOI] [PubMed] [Google Scholar]
- Ho L, Yemul S, Wang J, Pasinetti GM. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J Alzheimers Dis. 2009;16(2):433–439. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells LM, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res. 2011;4(9):1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Johnson KA, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9(5 Suppl):S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, et al. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R, et al. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103(5):730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- Lagouge M, et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Launer LJ, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10(11):969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA. Insulin resistance, type 2 diabetes, and AD: cerebrovascular disease or neurodegeneration? Neurology. 2010;75(9):758–759. doi: 10.1212/WNL.0b013e3181eee287. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, et al. The Huntington’s disease candidate region exhibits many different haplotypes. Nat Genet. 1992;1(2):99–103. doi: 10.1038/ng0592-99. [DOI] [PubMed] [Google Scholar]
- Maher P, et al. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet. 2011;20(2):261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Ono K, et al. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283(47):32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Naiki H, Yamada M. The development of preventives and therapeutics for Alzheimer’s disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des. 2006;12(33):4357–4375. doi: 10.2174/138161206778793010. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153(3):185–192. [PubMed] [Google Scholar]
- Pacholec M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, et al. Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp Neurol. 2011;232(1):1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, et al. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp Neurol. 2011;232(1):1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, et al. Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann N Y Acad Sci. 2013:74–82. doi: 10.1111/nyas.12141. [DOI] [PubMed] [Google Scholar]
- Qin W, et al. Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10(4):417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Qin W, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ranney A, Petro MS. Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav Pharmacol. 2009;20(4):330–336. doi: 10.1097/FBP.0b013e32832f0193. [DOI] [PubMed] [Google Scholar]
- Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 2007;4(11):e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Rimando AM, et al. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52(15):4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- Rocha-González HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14(3):234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):751–760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TH, McMichael RJ, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48(4):1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Presenilin, Notch, and the genesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(20):11039–11041. doi: 10.1073/pnas.211352598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M, et al. SirT1 in muscle physiology and disease: lessons from mouse models. Dis Model Mech. 2010;3(5–6):298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, et al. Small-Molecule Activators of AMP-Activated Protein Kinase (AMPK), RSVA314 and RSVA405, Inhibit Adipogenesis. Mol Med. 2011;17(9–10):1022–1030. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2011;121(3):337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, et al. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285(12):9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, et al. Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front Biosci (Landmark Ed) 2009;14:3380–3400. doi: 10.2741/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front Aging Neurosci. 2014;6(42) doi: 10.3389/fnagi.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci. 2012;32(15):5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim Biophys Acta. 2010;1804(8):1690–1694. doi: 10.1016/j.bbapap.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19(6):659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20(13):2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. POTENTIAL APPLICATION OF GRAPE DERIVED POLYPHENOLS IN HUNTINGTON’S DISEASE. Transl Neurosci. 2010;1(2):95–100. doi: 10.2478/v10134-010-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):653–661. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. Role of standardized grape polyphenol preparation as a novel treatment to improve synaptic plasticity through attenuation of features of metabolic syndrome in a mouse model. Mol Nutr Food Res. 2013;57(12):2091–2102. doi: 10.1002/mnfr.201300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol Nutr Food Res. 2005;49(5):482–494. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- Weydt P, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4(5):349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, et al. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59(11):2928–2935. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101(1):57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]