Abstract
Purpose
The aim of our study was to evaluate the risk of malignancy and to determine which clinical variables differentiate between benign and malignant focal breast lesions found incidentally on 18F-flourodeoxyglucose positron emission tomography and computed tomography (FDG PET/CT).
Methods
From March 2005 to October 2011, 21,224 women with no history of breast cancer underwent FDG PET/CT at three university-affiliated hospitals. We retrospectively identified 214 patients with incidental focal hypermetabolic breast lesions and grouped them into benign and malignant lesion groups. Of the 214 patients, 82 patients with 91 lesions were included in this study. All lesions were confirmed histologically or were assessed by follow-up imaging for greater than 2 years. The patient age, maximum standardized uptake value (SUVmax), lesion size on ultrasonography (US), and Breast Imaging-Reporting and Data System (BI-RADS) category on US in conjunction with mammography were compared between the groups. Multivariate logistic regression analysis was used to identify independent factors associated with malignancy.
Results
The risk of malignancy was 29.7% (27/91) in breast incidentalomas detected by FDG PET/CT. The univariate analysis showed that the patient age, SUVmax, tumor size, and BI-RADS category differed significantly between the malignant and benign groups. The multivariate analysis showed that the BI-RADS category was the only significant factor differentiating benign from malignant lesions (p=0.002).
Conclusion
BIRADS category based on US in conjunction with mammography was the only useful tool to differentiate between malignant and benign lesions in breast incidentalomas on FDG PET/CT.
Keywords: Breast neoplasms, Mammography, Positron-emission tomography, Ultrasonography
INTRODUCTION
18F-fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/CT) is widely used in the initial staging, evaluation of the therapeutic response, and detection of recurrent disease [1,2,3]. However, with the increasing use of FDG PET/CT, sites of increased activity have been occasionally discovered in unexpected locations, which may not correlate with the patient's clinical history or the expected spread of the primary malignancy. Most incidental malignant lesions are located in the lung, colon, thyroid, and breast. The prevalence rate of incidental primary malignancies diagnosed by FDG PET/CT is reportedly 1.0% to 1.8% [4,5,6]. Unexpectedly diffuse or focal areas of FDG uptake in the breast have been described recently. Breast incidentalomas with focal increased FDG uptake on PET/CT were found in 0.36% to 1.12% of patients and healthy subjects in multiple studies [7,8,9,10,11,12,13]. Previous reports have indicate that focal breast incidentalomas found on FDG PET/CT have variable malignancy rates (27.3%-83.3%), and additional imaging modalities such as ultrasound (US) and computed tomography (CT) have been described as clinically useful. However, there is disagreement on which clinical variables among the patient's age, lesion size, maximum standardized uptake value (SUVmax), and Breast Imaging-Reporting and Data System (BI-RADS) category on US and mammography could improve the discrimination between malignant and benign lesions [8,9,10,11,12,13]. Therefore, the aims of the present study were to establish the prevalence and clinical significance of incidental focal FDG uptake in the breast on consecutive PET/CT scans in women and to determine the significant clinical variables differentiating benign and malignant focal breast lesions, found incidentally on FDG PET/CT by using multivariate analysis.
METHODS
Ethics statement
The Institutional Review Board at Kyungpook National University Medical Center approved this retrospective study and provided all necessary ethical permissions. The requirement for informed consent was waived (IRB number: 12-1032).
Patient selection
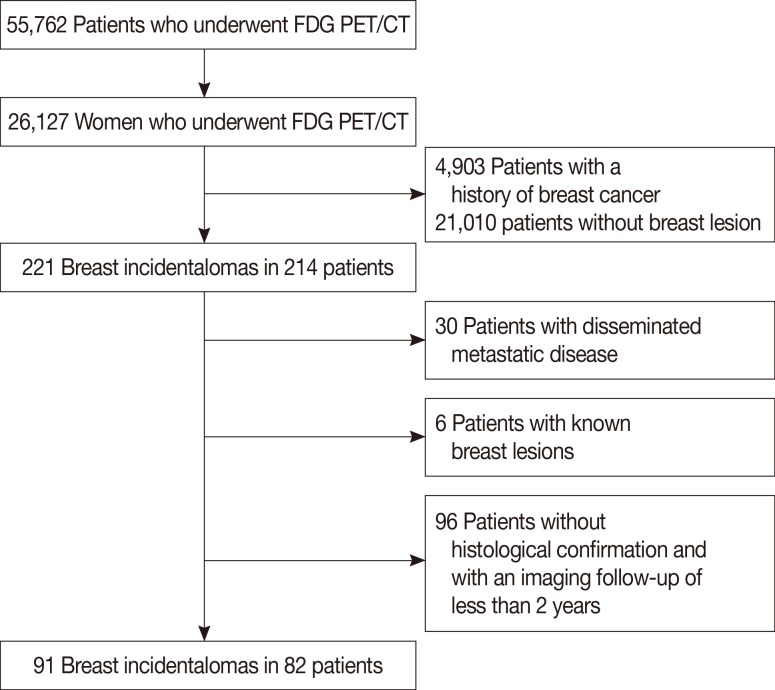
A total of 55,762 patients underwent FDG PET/CT scans for cancer evaluation or health checkup from March 2005 to October 2011 at three university-affiliated hospitals (Chonnam National University Hwasun Hospital, Kyungpook National University Hospital, and Kyungpook National University Medical Center). Of the 55,762 patients, 26,127 were female. After excluding 4,903 women with a known history of breast malignancy, we retrospectively reviewed data from 21,224 women to identify incidental focal hypermetabolic lesions in the breast of a woman with no history of breast cancer. Incidental focal FDG uptake in the breast was defined as a focally increased FDG accumulation in the breast a woman without a history of breast cancer. A review of the radiology information system database revealed 221 lesions of focal FDG uptake in the breast 214 patients. Patients with disseminated metastatic disease of known malignancy (n=30), known breast lesions (n=6), and without a histological confirmation or less than 2 years of follow-up (n=96) were excluded. Finally, 91 lesions in 82 patients were included in this study (Figure 1). Nine patients had two breast incidentalomas on FDG PET/CT. Imaging was used for staging or posttreatment follow-up of a known primary malignancy in 70.7% (58/82) of participants, for cancer screening in healthy subjects in 15.9% (13/82), and for detecting primary malignant tumors when metastatic lesions were suspected in 13.4% (11/82). The primary malignant tumors included thyroid cancer (n=24), cervical cancer (n=12), lymphoma (n=5), gastric cancer (n=4), lung cancer (n=3), colon cancer (n=2), rectal cancer (n=2), uterine leiomyosarcoma (n=2), endometrial cancer (n=1), melanoma (n=1), squamous cell cancer of the skin (n=1), and hemangiopericytoma in the skull (n=1).
Figure 1. Consort diagram. There were 55,762 patients who underwent 18F-fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/CT) between March 2005 and October 2011. Data of 82 patients met the inclusion criteria and were used in this study.
Image acquisition
All patients underwent whole-body FDG PET/CT with a Reveal RT-HiREZ 6-slice CT apparatus® (CTIMI, Knoxville, USA) and a 16-slice CT Discovery STE apparatus® (GE Medical System, Milwaukee, USA). All patients were fasted for at least 6 hours, and the blood glucose concentration was assessed before the FDG PET/CT study. Patients with elevated blood glucose concentration had their examinations rescheduled. The blood glucose concentration was managed to a target of <150 mg/dL in all subjects. Approximately 8.1 MBq of FDG/kg of body weight was injected intravenously, and the patients were advised to rest for 1 hour before image acquisition. Prior to PET, the CT was performed from the skull vertex to the knee with the patient in the supine position and breathing quietly at the following settings: 120 KeV voltage; 80 mA tube current; 1.5 pitch; 15 mm/rotation table speed; and a 2.5 mm slice thickness during tidal breathing. PET was performed with a maximum spatial resolution of 6.5 mm (Reveal PET/CT) and 5.5 mm (Discovery PET/CT) at 3 minute per bed position. PET images from both scanners were reconstructed with a 128×128 matrix, ordered-subset expectation maximum (OSEM) iterative reconstruction algorithm (four iterations, eight subsets), 5.0 mm Gaussian filter and a 3.0 mm (Reveal PET/CT) or 3.27 mm (Discovery PET/CT) slice thickness. The PET/CT data sets were reconstructed using OSEM with CT attenuation correction.
Patients with an incidental hypermetabolic lesion in the breast underwent diagnostic mammography and US. Mammography was performed with one of two full-field digital mammography units (Senographe 2000D, GE Healthcare, Milwaukee, USA; Lorad Selenia, Hologic, Danbury, USA). Patients under 35 years of age did not undergo mammography routinely. Mammograms performed by outside clinics were reviewed before breast US examination. Breast US examinations were performed by two experienced breast radiologists by using high-resolution instruments (HDI 5000, Philips ATL, Bothell, USA; Acuson Sequoia®, Siemens Medical Solutions, Mountain View, USA; LOGIQ9 System, GE Healthcare, Milwaukee, USA; iU22 system, Philips Healthcare, Bothell, USA) with linear 5 to 12 MHz or compact linear 8 to 15 MHz transducers.
Image interpretation
All FDG PET/CT images were analyzed by one of six experienced nuclear medicine physicians. Based on the physiologic distribution of FDG, any focal uptake greater than the background uptake in the breast tissue was considered abnormal. After breast incidentalomas were identified, the SUVmax was obtained for semiquantitative analysis.
One of two experienced breast radiologists (H.J.K. and H.S.L. with 9 years of breast imaging experience) interpreted the US results in conjunction with the mammography results according to BI-RADS criteria [14,15]. US was performed within 2 months of identifying breast incidentalomas. The BIRADS assessment was determined based on the US results and adopted the higher mammography and US categories. In three patients (<35 years) who did not undergo mammography, the BI-RADS category was determined by US alone. The BIRADS categories were reclassified as follows for statistical analysis: categories 1 and 2, category 3, category 4a, category 4b, and category 4c and 5. To evaluate the diagnostic performance of breast US, the patients were divided into two groups: those with a BI-RADS category 1 to 3 (negative group) and those with a BI-RADS category 4 or higher (positive group). The longest diameter of the mass on US was also recorded. During the US exam, the incidentalomas on FDG PET/CT were correlated based on the lesion size, depth, surrounding tissue, and location [16].
Final diagnosis
The final assessment of breast incidentalomas was based on the histological diagnosis or imaging follow-up of more than 2 years. Eighty-two lesions in 73 patients were investigated by breast US and nine lesions in nine patients were investigated by follow-up FDG PET/CT only. Sixty lesions were histopathologically confirmed by US-guided core needle biopsy and/or surgery. In the remaining 31 lesions, the final diagnosis was based on follow-up correlative conventional imaging (mammography and breast US) and FDG PET/CT. If no missed cancer was detected during the follow-up period, then the lesions were considered to be benign.
Data analysis
After testing the parameters for normality using the Kolmogorov-Smirnov test, the study variables were compared between the malignant and benign groups through univariate analysis using the Mann-Whitney U-test for the numerical values (age, SUVmax, and lesion size) and the Fisher exact test with linear-by-linear association for the categorical values (BIRADS categories). Continuous data are presented as the median and interquartile range (IQR, range from the 25th to the 75th percentiles). Multivariate logistic regression analysis was performed to identify independent factors associated with malignancy. In all statistical analyses, p-values less than 0.05 were considered significant. The statistical analysis was performed using SPSS version 21 for Windows, statistical package (SPSS Inc., Armonk, USA) and MedCalc version 12.2.1.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
Of 91 lesions, 27 lesions (29.7%) were confirmed as malignant and 64 lesions (70.3%) were benign. Among the 60 pathologically diagnosed lesions, the malignant lesions included invasive ductal carcinoma (IDC) not otherwise specified (n=21), medullary carcinoma (n=1), metaplastic carcinoma (n=1), ductal carcinoma in situ (DCIS) (n=1), invasive lobular carcinoma (n=1), secondary lymphoma (n=1), and metastatic melanoma (n=1). Representative images of the malignant lesions are shown in Figure 2. The diagnoses for the 33 benign lesions were as follows: fibroadenoma (n=10), intraductal papilloma (n=7), fibrocystic change (n=5), sclerosing adenosis (n=3), chronic inflammation (n=2), abscess (n=1), diabetic mastopathy (n=1), hamartoma (n=1), angiolipoma in (n=1), angiomyoepithelioma (n=1), and columnar cell hyperplasia (n=1). Representative images of the benign lesions are shown in Figure 3.
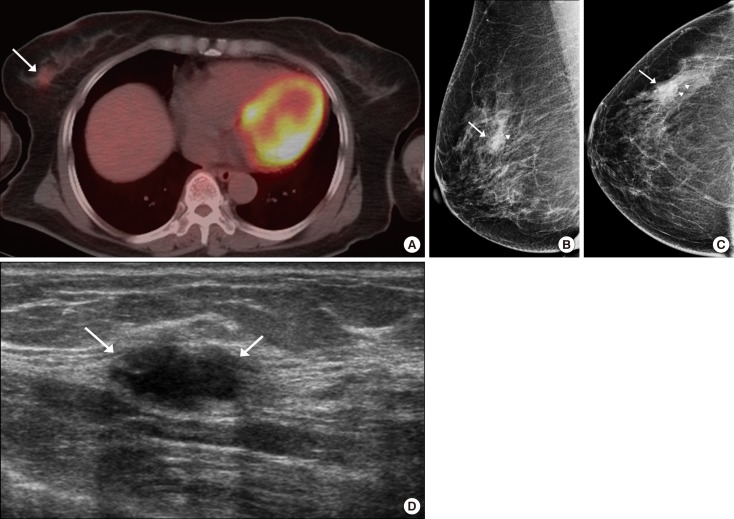
Figure 2. Breast incidentaloma in a 61-year-old woman with thyroid cancer. (A) Axial 18F-fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/CT) image shows focally increased uptake (maximum standardized uptake value, 2.6) (arrow) in the right upper outer breast. (B, C) Mammography shows a focal asymmetry (arrows) with suspicious microcalcifications (arrowheads). (D) Ultrasonography (US) image shows a 1.8-cm hypoechoic mass (arrows) with microlobulated margin and an oval shape in the right upper outer breast, correlating with the focal uptake on the PET/CT scan. Final assessment of the mass on US showed that it was of category 4c. The lesion was confirmed to be an invasive ductal carcinoma by USguided core biopsy.
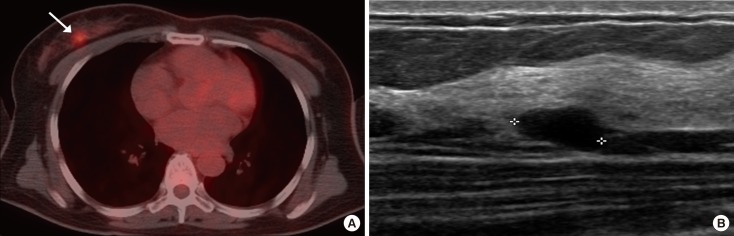
Figure 3. Breast incidentaloma in a 48-year-old woman with lymphoma. (A) Axial 18F-fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/CT) image shows focally increased uptake (maximum standardized uptake value, 5.7) (arrow) in the right upper outer breast. (B) Ultrasonography (US) image shows a 1.0-cm hypoechoic mass with circumscribed margin and an oval shape (asterisk) in the right upper outer breast, correlating with the focal uptake on the PET scan. Final assessment of the mass on US showed that it was of category 3. The lesion was confirmed to be an intraductal papilloma by excision.
The median age (40.3 years; IQR, 42.0-50.5 years) of the benign group was lower than that (55.0 years; IQR, 46.3-68.0 years) for the malignant group (p=0.002). The median SUVmax in the malignant group was 4.2 (range, 1.3-16.0; IQR, 2.5-6.7), which was significantly higher than that in the benign group (median, 2.3; range, 1.0-5.7; IQR, 1.7-2.9; p<0.001).
On breast US, the median size of lesions detected on US was 11.0 mm (range, 3.0-80.0 mm; IQR, 9.0-19.8). Seventeen lesions initially identified on PET/CT did not show any focal lesions on US, and the absence of malignancy was confirmed in follow-up FDG PET/CT or US over a 2-year period. The size of the malignant lesions on US (median, 16 mm; IQR, 12.5-20.0 mm) was statistically larger than that of the benign lesions (median, 8 mm; IQR, 0.0-14.0 mm; p<0.001) (Table 1). The rate of malignancy according to the combined BIRADS categories was 0% (0/20) for category 1 or 2, 0% (0/14) for category 3, 10.5% (2/19) for category 4a, 55.6% (5/9) for category 4b, and 100.0% (20/20) for category 4c or 5. The BIRADS categories on US in conjunction with mammography differed significantly between the malignant and benign lesion groups according to the Fisher exact test, and the malignancy rate of tended to increase as the BI-RADS category increased on the linear-by-linear correlation (p<0.001) (Table 1).
Table 1. Clinical and imaging characteristics of the 91 breast lesions in 82 patients.
| Characteristic | Benign No. (%) | Malignant No. (%) | p-value |
|---|---|---|---|
| No. of lesions | 64 (70.3) | 27 (29.7) | - |
| Age (yr)* | 40.3 (42.0-50.5) | 55.0 (46.3-68.0) | 0.002 |
| SUVmax* | 2.3 (1.7-2.9) | 4.2 (1.3-16.0) | <0.001 |
| BI-RADS US category† | 55 (67.1) | 27 (32.9) | <0.001 |
| 1 | 17 (26.6) | 0 | |
| 2 | 3 (4.6) | 0 | |
| 3 | 14 (21.9) | 0 | |
| 4a | 17 (26.6) | 2 (7.4) | |
| 4b | 4 (6.3) | 5 (18.5) | |
| 4c | 0 | 8 (29.6) | |
| 5 | 0 | 12 (44.5) | |
| Nodule size (mm)* | 8 (0.0-14.0) | 16 (12.5-20.0) | <0.001 |
SUVmax=maximum standardized uptake value; BI-RADS=Breast Imaging-Reporting and Data System; US=ultrasonography.
*Median (interquartile range); †Eighty-two lesions in 73 patients were investigated by breast US.
On multivariate analysis by logistic regression analysis, the BI-RADS category was the only significant factor that differented between malignant and benign lesions (p=0.002). The diagnostic performance of the BI-RADS categories was also analyzed. The sensitivity, specificity, positive predictive value, negative predictive value (NPV), and accuracy were 100% (27/27), 61.8% (34/55), 56.3% (27/48), 100.0% (34/34), and 74.4% (61/82), respectively
DISCUSSION
FDG PET/CT is widely used in the staging, restaging, and monitoring for tumor recurrence and therapeutic response in cancer patients, as well as in cancer screening in healthy people. FDG PET/CT has undergone explosive growth in its clinical application, and the incidence of breast incidentalomas detected by FDG PET/CT has increased in tandem. In this study, the prevalence of focal breast uptake on FDG PET/CT was 1.0% (214/21,224), and the prevalence of clinically significant breast incidentaloma was 0.84% (178/21,224), excluding those patients with disseminated metastatic disease (n=30) and known breast lesions (n=6). This finding is consistent with that of a meta-analysis study evaluating the prevalence and clinical significance of incidental breast uptake on PET (prevalence, 0.82%; 95% confidence interval [CI], 0.51%-1.2%) [17].
The malignancy risk determined in our study was 29.7% (27/91), which is similar to the reported risk in the most recent study (27.3%), but lower than the risk in other previous reports (48%, 95% CI, 35%-58%) [7,9,10,11,12,13,17,18,19]. This may be attributable to the more careful and comprehensive diagnostic workup in the most recent studies. Nuclear medicine physicians and clinicians have increasingly raised concern about incidental lesions on FDG PET/CT because many studies have shown high malignancy rates of incidental FDG uptake in other organs as well as in the breast, even without CT correlates [20]. In addition, the current study included more cases (9.9%, 9/91) with follow-up FDG PET/CT alone without other imaging modalities and excluded cases of suspected metastasis to the breasts.
Prior studies disagree on which clinical variables are significantly associated with malignancy. These studies did not determine the independent factors that could improve the discrimination between malignant and benign lesions [8,10,11,12,13]. In the present study, although age, SUVmax, lesion size, and BI-RADS category were statistically significant factors in the univariate analysis, in multivariate analysis, the BI-RADS category was the only significant factor differentiating between malignant and benign breast incidentalomas. These results corroborate the findings of the previous several studies that BI-RADS category is a common significant factor [9,11,13]. Previous studies revealed that the SUVmax or BI-RADS category was significantly associated with malignancy [9,10,11,12,13], but the independent significance of these two factors has not been evaluated in multivariate analysis. Furthermore, BI-RADS had a 100% sensitivity and 100% NPV in the present report, results consistent with those of a recent study [13]. In this study, none of the malignant lesions was detected on mammography alone, but US detected one DCIS (9 mm) and one IDC (9 mm) lesion that were not found on mammography because of dense breast tissue. This may be because malignancies detected on mammography with no concurrent US abnormality are usually DCIS, which is known to generate false-negative results on FDG PET/CT [21,22,23]. Breast cancer detected by US only is usually IDC, which is more likely to be detected by PET/CT than DCIS is [24].
A significant difference in the SUVmax has been reported between benign and malignant lesions in several studies of unexpected hypermetabolic lesions on FDG PET/CT [9,10,11,18]. Kang et al. [9] reported that the SUVmax and US findings were useful for differentiating benign from malignant lesions based on a multiple regression analysis. By contrast, the other studies [8,13], suggested that SUVmax was not significantly different between malignant and benign lesions. SUVmax is affected by several factors related to the patient as well as by technical and procedural factors; as a result, the SUVmax varies among individual tumors and greatly depends on tumor size. Limited sensitivity was shown for the detection of tumors measuring less than 1 cm and for low-grade malignancies. Invasive lobular carcinomas more often received a false-negative diagnosis (65.2%) than invasive ductal carcinomas (23.7%) did [25,26]. Moreover, several benign lesions, including inflammation, physiologic lactation, fibroadenoma, fat necrosis, and fibrocystic change, are well known to exhibit high FDG uptake on PET/CT [23,27]. In our study, several benign lesions, including fibroadenoma, intradutal papilloma, fibrocystic changes, and sclerosing adenosis, showed increased uptake on FDG PET/CT. In addition, the range of SUVmax (1.3-16.0) in these lesions overlapped with the range observed in the malignant lesions (1.0-5.7). Therefore, SUVmax alone should not be used to differentiate between malignant and benign breast incidentalomas on FDG PET/CT.
Our study has some limitations. First, the sample size was relatively small. However, the number of enrolled patients in our study was higher than in any previous study on this topic. Second, the final diagnosis of several benign lesions were not based on pathological confirm, but on imaging follow-up studies. However, only cases with a follow-up of over 2 years were included, as in other studies. Last, there may be a bias caused by the different PET/CT units and radiologists. However, a similar methodology was used during all PET/CT examinations and the interobserver agreement of the BI-RADS lexicon for US and mammography is reportedly sufficient to enable accurate and consistent assessment of breast images [28,29].
In conclusion, the BI-RADS category based on combined US and mammography was the only useful tool to differentiate between malignant and benign lesions in breast incidentalomas on FDG PET/CT.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Kostakoglu L, Agress H, Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–340. doi: 10.1148/rg.232025705. [DOI] [PubMed] [Google Scholar]
- 2.Bohuslavizki KH, Klutmann S, Kröger S, Sonnemann U, Buchert R, Werner JA, et al. FDG PET detection of unknown primary tumors. J Nucl Med. 2000;41:816–822. [PubMed] [Google Scholar]
- 3.Bar-Shalom R, Valdivia AY, Blaufox MD. PET imaging in oncology. Semin Nucl Med. 2000;30:150–185. doi: 10.1053/snuc.2000.7439. [DOI] [PubMed] [Google Scholar]
- 4.Beatty JS, Williams HT, Aldridge BA, Hughes MP, Vasudeva VS, Gucwa AL, et al. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery. 2009;146:274–281. doi: 10.1016/j.surg.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46:752–757. [PubMed] [Google Scholar]
- 6.Agress H, Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230:417–422. doi: 10.1148/radiol.2302021685. [DOI] [PubMed] [Google Scholar]
- 7.Korn RL, Yost AM, May CC, Kovalsky ER, Orth KM, Layton TA, et al. Unexpected focal hypermetabolic activity in the breast: significance in patients undergoing 18F-FDG PET/CT. AJR Am J Roentgenol. 2006;187:81–85. doi: 10.2214/AJR.05.0548. [DOI] [PubMed] [Google Scholar]
- 8.Chung A, Schoder H, Sampson M, Morrow M, Port E. Incidental breast lesions identified by 18F-fluorodeoxyglucose-positron emission tomography. Ann Surg Oncol. 2010;17:2119–2125. doi: 10.1245/s10434-010-0950-2. [DOI] [PubMed] [Google Scholar]
- 9.Kang BJ, Lee JH, Yoo IR, Kim SH, Choi JJ, Jeong SH, et al. Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am J Roentgenol. 2011;197:341–347. doi: 10.2214/AJR.10.6126. [DOI] [PubMed] [Google Scholar]
- 10.Chae EY, Cha JH, Kim HH, Shin HJ, Kim HJ, Oh HY, et al. Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18FFDG PET/CT: clinical significance and differential diagnosis. Acta Radiol. 2012;53:530–535. doi: 10.1258/ar.2012.120015. [DOI] [PubMed] [Google Scholar]
- 11.Kim MY, Cho N, Chang JM, Yun BL, Bae MS, Kang KW, et al. Mammography and ultrasonography evaluation of unexpected focal 18FFDG uptakes in breast on PET/CT. Acta Radiol. 2012;53:249–254. doi: 10.1258/ar.2011.110495. [DOI] [PubMed] [Google Scholar]
- 12.Dunne RM, O'Mahony D, Wilson G, McDermott R, O’Keeffe SA. The role of the breast radiologist in evaluation of breast incidentalomas detected on 18-fludeoxyglucose positron emission tomography/CT. Br J Radiol. 2013;86:20130034. doi: 10.1259/bjr.20130034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Lee EH, Park JM, Chang YW, Kim HH, Jeong SH. Role of combined BI-RADS assessment using mammography and sonography for evaluation of incidental hypermetabolic lesions in the breast on 18FFDG PET-CT. Acta Radiol. 2013;54:1117–1124. doi: 10.1177/0284185113492453. [DOI] [PubMed] [Google Scholar]
- 14.Mendelson EB, Bohn-Velez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. American College of Radiology, BI-RADS Committee, editors. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013. ACR BI-RADS ultrasound. [Google Scholar]
- 15.Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol. 2008;190:1209–1215. doi: 10.2214/AJR.07.3259. [DOI] [PubMed] [Google Scholar]
- 16.Park VY, Kim MJ, Kim EK, Moon HJ. Second-look US: how to find breast lesions with a suspicious MR imaging appearance. Radiographics. 2013;33:1361–1375. doi: 10.1148/rg.335125109. [DOI] [PubMed] [Google Scholar]
- 17.Bertagna F, Treglia G, Orlando E, Dognini L, Giovanella L, Sadeghi R, et al. Prevalence and clinical significance of incidental F18-FDG breast uptake: a systematic review and meta-analysis. Jpn J Radiol. 2014;32:59–68. doi: 10.1007/s11604-013-0270-0. [DOI] [PubMed] [Google Scholar]
- 18.Litmanovich D, Gourevich K, Israel O, Gallimidi Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: incidence and clinical significance. Eur J Nucl Med Mol Imaging. 2009;36:1558–1564. doi: 10.1007/s00259-009-1147-4. [DOI] [PubMed] [Google Scholar]
- 19.Beatty JS, Williams HT, Gucwa AL, Hughes MP, Vasudeva VS, Aldridge BA, et al. The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am J Surg. 2009;198:495–499. doi: 10.1016/j.amjsurg.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Hawkins RA, Yeh BM, Wang ZJ. Focal fluorine-18 fluorodeoxyglucose-avid lesions without computed tomography correlate at whole-body positron emission tomography-computed tomography in oncology patients: how often are they malignant. Nucl Med Commun. 2011;32:802–807. doi: 10.1097/MNM.0b013e3283483e04. [DOI] [PubMed] [Google Scholar]
- 21.Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003;180:941–948. doi: 10.2214/ajr.180.4.1800941. [DOI] [PubMed] [Google Scholar]
- 22.Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of mammographically detected clustered microcalcifications. Radiology. 2000;217:849–854. doi: 10.1148/radiology.217.3.r00nv27849. [DOI] [PubMed] [Google Scholar]
- 23.Lim HS, Yoon W, Chung TW, Kim JK, Park JG, Kang HK, et al. FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics. 2007;27(Suppl 1):S197–S213. doi: 10.1148/rg.27si075507. [DOI] [PubMed] [Google Scholar]
- 24.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avril N, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–3502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98:267–274. doi: 10.1007/s10549-006-9159-2. [DOI] [PubMed] [Google Scholar]
- 27.Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198:W304–W314. doi: 10.2214/AJR.11.7130. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Kim EK, Kim MJ, Youk JH, Lee JY, Kang DR, et al. Observer variability of Breast Imaging Reporting and Data System (BI-RADS) for breast ultrasound. Eur J Radiol. 2008;65:293–298. doi: 10.1016/j.ejrad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Adibelli ZH, Ergenc R, Oztekin O, Ecevit S, Unal G, Abal Y. Observer variability of the Breast Imaging Reporting and Data System (BIRADS) lexicon for mammography. Breast Care (Basel) 2010;5:11–16. doi: 10.1159/000272277. [DOI] [PMC free article] [PubMed] [Google Scholar]