Abstract
Early life experience differentially shapes later stress reactivity, as evidenced by both animal and human studies. However, early experience-related changes in the function of central visceral neural circuits that control stress responses have not been well characterized, particularly in humans. The paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST), amygdala (Amyg) and subgenual anterior cingulate cortex (sgACC) form a core visceral stress-responsive circuit. The goal of this study is to examine how childhood emotional and physical abuse relates to adulthood stressor-evoked activity within these visceral brain regions. To evoke acute states of mental stress, participants (n = 155) performed functional magnetic resonance imaging (fMRI)-adapted versions of the multi-source interference task (MSIT) and the Stroop task with simultaneous monitoring of mean arterial pressure (MAP) and heart rate. Regression analyses revealed that childhood physical abuse correlated positively with stressor-evoked changes in MAP, and negatively with unbiased, a priori extractions of fMRI blood-oxygen level-dependent signal change values within the sgACC, BNST, PVN and Amyg (n = 138). Abuse-related changes in the function of visceral neural circuits may reflect neurobiological vulnerability to adverse health outcomes conferred by early adversity.
Keywords: abuse, stress, hypothalamus, bed nucleus of the stria terminalis, amygdala
INTRODUCTION
Early life experiences have long-ranging effects, from alterations in molecular and cellular processes (Miller et al., 2011) to systems-level neural circuitries (Card et al., 2005). Importantly, such early life effects shape trajectories of risk for physical and mental health outcomes (Heim et al., 2010). The animal literature has demonstrated that early life experiences can specifically shape later-life and long-term patterns of stress reactivity. Animal studies utilizing various maternal separation paradigms, as well as studies examining naturally occurring variations in maternal care, have shown differential behavioral and physiological stress reactivity in the offspring (Meaney, 2001). Studies in humans also demonstrate that early life stress, adversity and/or maltreatment associate with alterations in stress reactivity later in life (Heim et al., 2008; Lovallo et al., 2011). However, early experience-related changes in the structure and function of stress-related visceral neural circuits have not been well characterized.
Central visceral circuits encompass descending preautonomic circuits that orchestrate physiological outflow, and ascending viscerosensory circuits that relay information regarding body state from the brainstem to higher order brain areas (Saper, 2002). Essential to these circuits are hypothalamic and limbic forebrain regions that reciprocally give rise to preautonomic projections and directly receive viscerosensory innervation (Card and Sved, 2011). These brain regions include the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST), the central nucleus of the amygdala (CeA) and medial prefrontal cortex (mPFC) [the rat homologue of the human subgenual anterior cingulate cortex (sgACC)] (Card et al., 1993). The PVN is uniquely capable of controlling both the neuroendocrine and autonomic components of the stress response and both the sympathetic and parasympathetic limbs of the autonomic nervous system (Herman et al., 2002). Thus, regions that influence PVN activity are also important regulators of stress responses. The BNST directly and densely innervates the PVN (Dong et al., 2001b) and has an excitatory influence on the PVN and stress responses (Crane et al., 2003a). The CeA does not innervate the PVN directly; however, it is heavily interconnected with the BNST (Dong et al., 2001a), suggesting that it exerts its influence on PVN activity and stress responses via its connection to the BNST (Cullinan and Herman, 1993). The mPFC directly innervates the BNST (Vertes, 2004) and has an inhibitory influence on BNST and PVN activity, as well as stress responses (Spencer et al., 2005). Thus, these brain regions form an important visceral, stress-response network with the PVN as a proximal controller of stress responses and the BNST as the ‘hub’ region through which the CeA and mPFC communicate with the PVN (Herman et al., 2003).
Several animal studies have examined the influence of early life experience on the structure and function of these hypothalamic and limbic forebrain regions. Some studies have manipulated early experience using various postnatal maternal separation paradigms that increase or decrease the quality and/or duration of maternal care (Meaney, 2001; Macrí et al., 2004). Two studies have demonstrated that maternal separation paradigms affect the circuit strength of preautonomic circuits originating within these regions of interest (ROIs) in neonatal and juvenile rats (Card et al., 2005; Banihashemi and Rinaman, 2010). Further, other reports show that maternal separation paradigms relate to changes in neural activation within the CeA, BNST and PVN in response to both interoceptive and psychological stressors (Abrahám and Kovacs, 2000; Koehnle and Rinaman, 2010; Banihashemi et al., 2011).
The goal of this study is to translate such findings from animals to humans using neuroimaging techniques. Here we examine how childhood abuse (i.e. emotional and physical) relates to adulthood stressor-evoked activity within the PVN, BNST, amygdala (Amyg) and sgACC. Stressor tasks used in this study are cognitive conflict tasks that reliably evoke neural changes in the human brain (Sheu et al., 2012), simultaneously increase heart rate (HR) and blood pressure, induce arousal, and decrease the perception of control (Gianaros et al., 2008; Gianaros et al., 2009a). We hypothesize that childhood abuse (i.e. emotional and physical) is associated with altered cardiovascular stress reactivity and stressor-evoked activity within our ROIs in adulthood. We further hypothesize that childhood abuse will be associated with stressor-evoked connectivity between our ROIs and the hub, BNST.
METHODS AND MATERIALS
Participants
Approximately 36 100 mass mailings were sent to residents of Allegheny County, PA, USA, with a 2% response rate. Of the 2%, 155 met all exclusion criteria and completed all protocols. Thus, participants were 155 adults, 78 men (mean ± s.d., mean age = 40.18 ± 6.27) and 77 women (mean age = 41.23 ± 6.03). Respondents to mailings were screened to exclude those with (i) a history of cardiovascular disease (including treatment for or diagnoses of hypertension, stroke, myocardial infarction, congestive heart failure and atrial or ventricular arrhythmias); (ii) prior cardiovascular surgery (including coronary bypass, carotid artery or peripheral vascular surgery); (iii) chronic kidney or liver conditions, Type I or II diabetes or any pulmonary or respiratory diseases; (iv) current psychiatric diagnoses of a substance abuse or mood disorder (including alcohol dependence, a somatization disorder, major depression or a subclinical depressive syndrome and panic or other anxiety disorders); (v) prior cerebrovascular trauma involving loss of consciousness; (vi) prior neurosurgery or any neurological condition; (vii) being pregnant (verified by urine test in females); (viii) having claustrophobia or metallic implants; or (ix) taking psychotropic, lipid lowering or cardiovascular medications.
Assessment of current psychiatric diagnoses of a substance abuse or mood disorder was confirmed on interview using the Patient Health Questionnaire (PHQ; Spitzer et al., 1999), an inventory validated in outpatient (Spitzer et al., 1999; Kroenke et al., 2001; Lowe et al., 2004a) and community samples (Martin et al., 2006) for sensitivity and specificity against the Diagnostic and Statistical Manual of Mental Disorders IV (Lowe et al., 2004b). The PHQ assesses depressive symptoms over the last 2 weeks, symptoms of anxiety over the last 4 weeks and signs of alcohol dependence over the last 6 months. Participants also completed inventories to characterize depressive symptoms, dispositional anxiety and core dimensions of personality. These inventories included the Beck Depression Inventory (BDI-II; Beck et al., 1996), the trait versions of the Spielberger State-Trait Anxiety Inventory (STAI-T; Spielberger et al., 1970) and the NEO personality inventory (Costa and McCrae, 1992).
The BDI-II assesses depressive symptoms; it is a 21-item questionnaire assessing the presence and severity of depressive symptoms over the past two weeks. Items are rated on a 0–3 scale, with higher scores indicating greater severity of symptoms. The BDI-II has high internal consistency (∼0.9) and validity in both psychiatric and general populations (Lasa et al., 2000; Steer et al., 2000; Storch et al., 2004). The STAI-I assesses anxiety; it is a 20-item instrument measuring the severity of anxiety experienced habitually (Spielberger et al., 1970). Participants respond to each item by rating on a 4-point frequency scale ranging from almost never (1) to always (4). The STAI-T has high internal consistency and validity in both psychiatric and general populations (Rule and Traver, 1983; Oei et al., 1990; Usala and Hertzog, 1991). The NEO is a 240-item questionnaire used to assess the following personality domains: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness. Items are scored on a 5-point Likert-type scale ranging from strongly agree to strongly disagree. Each personality dimension comprises six sub-dimensions or ‘facets’. Individual facet scores are obtained by summing the items corresponding to each facet whereas domain scores are derived from summing the appropriate individual facet scores. (See Table 1 for participant information for these scales.).
Table 1.
Summary of participant characteristics
| Characteristic | Mean | s.d. | Range |
|---|---|---|---|
| Age (years) | 40.7 | 6.16 | 30–50 |
| Resting HR (bpm) | 67.25 | 10.43 | 40–97 |
| Resting MAP (mmHg) | 90.11 | 9.76 | 70–122 |
| CTQ, emotional abuse | 7.24 | 3.19 | 5–25 |
| CTQ, physical abuse | 6.11 | 1.61 | 5–13 |
| Perceived stress | 1.35 | 0.60 | 0.1–3.2 |
| Parental education | 5.48 | 2.24 | 1–9 |
| Education level | 3.90 | 1.43 | 1–6 |
| BDI total score | 3.65 | 3.64 | 0–22 |
| STAI: Trait Anxiety | 33.19 | 7.66 | 20–61 |
| STAI: AES Anger In | 15.18 | 3.22 | 10–25 |
| STAI: AES Anger Out | 13.18 | 2.64 | 8–20 |
| STAI: AIS Anger Control | 24.13 | 3.60 | 13–32 |
| NEO: Neuroticism (%) | 46.34 | 29.27 | |
| NEO: Extraversion (%) | 56.39 | 28.88 | |
| NEO: Openness (%) | 59.67 | 28.37 | |
| NEO: Agreeableness (%) | 46.16 | 28.96 | |
| NEO: Conscientious (%) | 44.60 | 30.66 |
Notes: N = 155 (for all except CTQ physical abuse, which is N = 154). Mean parental education corresponds to ‘some college, no degree’ or an ‘associate’s degree’, and mean education level corresponds to ‘technical training’ (see Methods and Materials for information regarding scales of measurement).
Of the 155 participants meeting inclusion criteria, 70% (n = 109) self-reported their ethnicity as Caucasian, 22% (n = 34) as African-American, 6% (n = 9) as Asian, 1% (n = 2) as multi-racial and 1% (n = 1) of the sample did not endorse any category of ethnicity. Participants’ average, seated resting systolic blood pressure/diastolic blood pressure was 121.44 ± 9.48/73.25 ± 8.80 mmHg (mean ± SD). This was determined by the mean of the last two of three seated clinic blood pressure readings obtained with an oscillometric device (Critikon Dinamap 8100, Johnson & Johnson, Tampa, FL) and taken 2 min apart after a ∼ 20-minute acclimation period. All participants provided informed consent after receiving an explanation of study protocols. They were also tested in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and with the approval of the University of Pittsburgh Institutional Review Board.
Overview
Participants were tested in separate sessions. In one session, participants provided informed consent, demographic and medical histories and anthropometric measures. Participants also completed additional questionnaires to characterize psychosocial measures including childhood socioeconomic status (SES), as indicated by the highest level of parental education (9-point education scale; 1 = no high school diploma, 9 = doctorate), and participant SES, as indicated by the educational level achieved by the participant. Participants also completed the Perceived Stress Scale (PSS, 10-item version; Cohen et al., 1983), which assesses recent levels of life stress experienced in the past month. The PSS contains items such as, ‘In the last month, how often have you felt nervous and stressed’, that participants respond to on a scale of 0 (never) to 4 (very often). In another session, participants underwent a functional neuroimaging protocol consisting of two functional magnetic resonance imaging (fMRI)-adapted cognitive stressor tasks, the Stroop and Multi-source interference tasks (MSIT; Bush and Shin, 2006; Gianaros et al., 2009b). Each fMRI task was accompanied by simultaneous monitoring of HR and blood pressure (described below). For further descriptive information about participant characteristics, see Table 1.
Childhood abuse assessment
Childhood abuse was assessed using the Childhood Trauma Questionnaire (CTQ), which has been demonstrated to have high test-retest reliability over time and high validity (Bernstein et al., 1994, 1997). In this study, the subscales for physical (5 items) and emotional abuse (5 items) were used. Of the five physical abuse items, four are objective statements (e.g. ‘I got hit or beaten so badly that it was noticed by someone like a teacher, neighbor, or doctor’), whereas the remaining statement is subjective (e.g. ‘I believe that I was physically abused.’) Of the five emotional abuse items, only two are objective statements (e.g. ‘People in my family called me things like stupid, lazy, or ugly.’), whereas the remaining three statements are subjective (e.g. ‘I felt that someone in my family hated me.’) Participants respond to statements regarding childhood experiences on a 5-point Likert-type scales ranging from 1 (never true) to 5 (very often true). Thus, the range of scores for each subscale is from 5 to 25, with 5 indicating no maltreatment.
For the physical abuse subscale, scores are classified as four groups indicating the severity of the maltreatment: 5–7, none-minimal; 8–9, low-moderate; 10–12, moderate-severe; and ≥13, severe-extreme. Classification groups for the emotional abuse subscale are more stringent, with higher cutoffs to increase sensitivity and specificity (Bernstein et al., 1994): 5–8, none-minimal; 9–12, low-moderate; 13–15, moderate-severe; and ≥16, severe-extreme. One participant with a physical abuse score indicating extreme physical abuse (=24) was considered a statistical outlier (8.27 s.d. from the original mean) and was excluded from further physical abuse analyses. In our sample, the range of scores is 5–13 for the physical abuse subscale (mean ± SE, 6.11 ± 0.13) and 5–21 for the emotional abuse subscale (7.12 ± 0.23). The majority of our participants fell in the ‘none or minimal’ range for physical abuse (83%, n = 128/154), while 12% (n = 18) were in the low to moderate range, 5% (n = 7) were in the moderate to severe range, and 1% (n = 1) were in the severe to extreme range. There was a similar trend for emotional abuse with the majority of our participants falling in the none or minimal range (80%, n = 124/155), while 14% (n = 21) were in the low to moderate range, 3% (n = 5) were in the moderate to severe range and 3% (n = 5) were in the severe to extreme range. As physical and emotional abuse were only moderately correlated (r = 0.33), data from these subscales were treated separately.
fMRI stressor tasks
For both the Stroop task and the MSIT, participants respond by pressing one of four buttons on a response glove. Both the Stroop task and the MSIT contain a congruent condition that serves as a control and a more demanding incongruent condition. The goal of both conditions of the Stroop task is to identify the color in which target words are shown by selecting one of four identifier words that name the color of the target word. In the congruent condition trials: (i) the target word is shown in a color that is congruent with the target word, and (ii) all identifiers are displayed in the same color as the target. In the incongruent condition: (i) the target word is displayed in a color that is incongruent with the color that the target word names, and (ii) all of the identifiers are displayed in colors that are incongruent with the colors that the identifier words name (Gianaros et al., 2008).
The goal of the MSIT is to identify the number that is different from two other numbers in a visual display by pressing one of the three buttons on the response glove. In this task, the buttons on the glove correspond to a specific number in the display (thumb button = number 1, index finger button = number 2 and middle finger button = number 3). For all trials in the congruent condition, the target number in the display appeared in a location that was compatible with its position on the response glove. For all trials in the incongruent condition, the target number appeared in a position that was incompatible with its spatial position on the response glove.
During the incongruent condition of both tasks, each participant’s accuracy at target word (Stroop) and number (MSIT) identification was titrated to and maintained at ∼50% by adjusting the inter-trial intervals (ITI). Thus, more accurate performance within a given incongruent condition prompted shorter ITIs and a shorter time in which to respond. During a practice session prior to the fMRI tasks, participants’ performance was not titrated, and participants were not informed that their performance would be titrated in the incongruent condition during the MRI protocol. When participants gave an incorrect response, they were presented with a red ‘X’ on the display, providing them with negative feedback, and if they failed to respond, they were presented with the prompt, ‘TOO LATE!’. To control for motor response differences between the incongruent and congruent conditions in both tasks, the number of trials presented in the congruent condition were matched to the number of trials completed in the incongruent condition. The two conditions are thus similar in stimulus and motor response characteristics, but different in demand.
The Stroop and MSIT tasks were separated by a 10- to 12-min recovery period. Each task lasted 9 min, 20 s and was composed of trials alternating between congruent and incongruent conditions. The congruent and incongruent conditions lasted 52 to 60 s and were preceded by a 10 - to 17-s period during which participants fixated on a cross-hair. The two tasks were counterbalanced across individuals. Thus, both tasks involve processing cognitive conflict, receiving negative feedback and responding under time pressure to unpredictable stimuli. Previous research with these tasks has indicated that the incongruent conditions of both tasks elicit subjective distress in that participants report experiencing significantly more ‘unpleasantness’, significantly increased arousal and significantly less control during the incongruent condition compared with baseline. Further, participants reliably exhibit significant increases on average in HR, as well as systolic and diastolic blood pressure during the incongruent condition compared with baseline (Gianaros et al., 2008, 2009a; Sheu et al., 2012).
Heart rate and blood pressure
During fMRI, cardiovascular measures were taken intermittently using oscillometric methods used in our prior studies (Gianaros et al., 2009b, 2011). Oscillometric recordings of HR and mean arterial blood pressure (MAP) were taken from the brachial artery of the left arm (not used for task responding) with an automated monitor (model 3155MVS; In-Vivo Research, Orlando, FL). Resting HR and MAP were recorded every 2 min during an 8-min baseline during which structural brain images were acquired before the task. HR and MAP were recorded every 1 min during the task, such that cuff inflation coincided with the fixation period initiating each condition (this ensured that the conditions were matched for procedural effects caused by cuff inflation; Gianaros et al., 2005a,b). Resting HR and MAP were computed as the average of the last two baseline recordings. Task-related blood pressure was computed as the average of the four recordings taken approximately at the end of each incongruent condition. Stressor-evoked cardiovascular reactivity, defined as a change from rest to the incongruent conditions of both tasks, was measured by subtracting baseline values from the incongruent condition values for both HR and MAP (Kamarck and Lovallo, 2003).
Abuse and cardiovascular reactivity analyses
The change in HR during the Stroop task and the change in HR during the MSIT were significantly correlated (r = 0.72, P < 0.01). Further, the change in MAP between the two tasks was also significantly correlated (r = 0.79, P < 0.01). To increase the reliability of the following analyses, stressor-evoked changes in HR and MAP were averaged across tasks (MSIT and Stroop). To investigate how well abuse predicts stressor-evoked cardiovascular reactivity, four hierarchical linear regressions were computed using the raw, uncategorized CTQ scores for either physical or emotional abuse as independent variables and either average change in HR or average change in MAP as dependent variables. As demographic (e.g. gender and race) and psychosocial variables (e.g. childhood and adulthood SES and chronic stress) can influence cardiovascular reactivity (Gump et al., 1999; Barnes et al., 2000; Matthews et al., 2001), the following variables were entered together as covariates: age, gender, race, perceived stress, highest parental education, participant educational level and either resting HR or MAP.
fMRI protocol and data acquisition
fMRI data were acquired while participants performed the Stroop task and the MSIT within a 3-Tesla Trio TIM whole-body MRI scanner (Siemens, Erlangen, Germany), equipped with a 12-channel phased-array head coil. Blood-oxygen level-dependent (BOLD) images were acquired over the task periods with a T2*-weighted gradient-echo EPI sequence using the following parameters: field-of-view (FOV) = 205 × 205 mm (matrix size = 64 × 64), time-to-repetition (TR) = 2000 ms, time-to-echo (TE) = 28 ms and flip angle (FA) = 90°. Thirty-nine slices (3 mm thick, no gap) were obtained in an interleaved sequence in an inferior-to-superior direction for each TR. For each task, three initial TRs were discarded, allowing for magnetic equilibration, yielding 280 BOLD images. For spatial normalization of BOLD images to standard Montreal Neurological Institute (MNI) space, T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) neuroanatomical images were acquired over 7 min 17 s with these parameters: FOV = 256 × 208 mm (matrix size = 256 × 208), TR = 2100 ms (192 slices, 1 mm thick, no gap), time-to-inversion (TI) = 1100 ms, TE = 3.29 ms and FA = 8°.
fMRI data preprocessing
fMRI data were preprocessed and analyzed with statistical parametric mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Before analyses, BOLD images for each task were realigned to the first image of the series by 6-parameter rigid-body transformation. Realigned BOLD images were normalized to standard MNI space by the registration of each participant’s T1-weighted structural image to an MNI template in SPM. In this process, the T1-weighted grey image was segmented from the MPRAGE and co-registered to the mean realigned BOLD images. The T1-weighted image was then registered to the SPM MNI grey template by nonlinear affine transformation; the registration was hence applied to normalize all BOLD images to the MNI template and rescaled to a voxel size of 2 × 2 × 2 mm. Finally, normalized images were smoothed with a 6 mm full-width-at-half-maximum Gaussian kernel.
Neuroimaging analyses
After preprocessing, a contrast image reflecting relative task-related brain activity (i.e. BOLD signal change between congruent and incongruent task conditions) was estimated for each participant with the general linear model (GLM). To this end, task conditions as the regressors were modeled with rectangular waveforms convolved with the default SPM hemodynamic response function (HRF). The GLM-estimated statistical parameter maps were then used to calculate contrast images. Before estimation, low-frequency BOLD signal noise related to physiological artifact was removed by high-pass filtering (187-s cutoff). Serial BOLD signal autocorrelations were also accounted for by a first-order autoregressive model. Additionally, regression vectors derived from the realignment preprocessing step were included in the GLMs to account for the variance of BOLD signal changes attributable to head movement.
Data from 138 of the 155 participants were used for the following neuroimaging analyses, as these subjects had high quality images (i.e. free of movement-related or other artifacts) for both tasks. Parallel to the approach used in the cardiovascular reactivity analyses (see above), all fMRI analyses were performed with contrast maps that were averaged across the tasks (i.e. MSIT and Stroop task). This approach is supported by a recent study showing that the test-retest reliability and power in detecting activity is improved by aggregating data from both tasks (Sheu et al., 2012).
Conjunction analysis
A conjunction analysis was performed to examine the extent of overlap of relative deactivation (Congruent>Incongruent) evoked by each task, thereby providing additional support for an averaged contrast map approach.
ROI analyses
To create regions consistent with the sgACC, BNST and PVN, ROIs were hand drawn using MRIcron on the ch2better template. The BNST and PVN ROIs were based on the depictions of these structures in Atlas of the Human Brain (Mai et al., 2008). The BNST ROI was based on plates 18 (using Talairach reference systems, y = −2.7 mm) through 24 (y = + 2.7 mm) and encompassed the central, medial, lateral and ventral divisions. The PVN ROI was based on plates 20 (y = −1.3 mm) through 28 (y = 8.0 mm) and included parvocellular, magnocelluar, dorsal and posterior subnuclei. The sgACC ROI was based on its depiction in Cingulate Neurobiology and Disease (Vogt, 2009). The Amyg ROI was derived from the SPM Anatomy toolbox using the 50% probabilistic map (Amunts et al., 2005; Eickhoff et al., 2005). None of the four ROIs overlapped (Figure 1). Further details regarding hand-drawn ROI characteristics are displayed in Table 2.
Fig. 1.
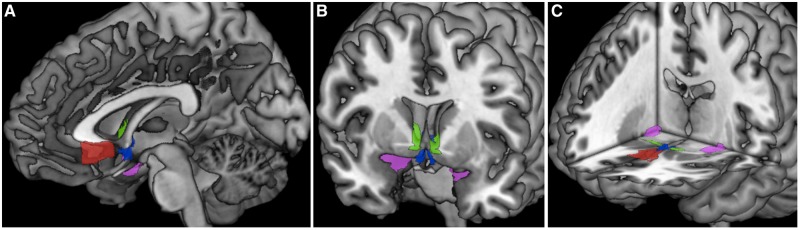
ROI masks. Images showing sagittal (A), coronal (B) and axial (C) views of all four ROIs: sgACC (in red), BNST (in green), PVN (in blue) and Amyg (in magenta). The sgACC, BNST and PVN ROI masks were hand-drawn using MRIcron, and the Amyg mask was derived from the SPM anatomy toolbox. (See Table 2 for ROI characteristics).
Table 2.
ROI characteristics
| ROIs | Center of Mass | X-extent (mm) | Y-extent (mm) | Z-extent (mm) | Volume (mm3) |
|---|---|---|---|---|---|
| SgACC | −0.661, 24, −8.2 | −11, 8 | 12.5, 31.5 | −16, −0.5 | 1764.75 |
| Left BNST | −6.6, 5.15, −3.19 | −19.5, −2 | 3, 8 | −10.5, 7.5 | 223.5 |
| Right BNST | 7.55, 5.1, −2.79 | 3, 23.5 | 3, 8 | −9, 8 | 234 |
| PVN | 0.72, 4.49, −9.36 | −5.5, 7.5 | 0, 8 | −15.5, 2 | 225.25 |
Notes: Characteristics, including center of mass, extent in X, Y and Z directions and volume, are presented.
In order to acquire estimates of activity (activation or deactivation) from each of the four ROIs, a random effects analysis was conducted with an averaged contrast map of all participants. Clusters in each of the ROIs that displayed significant BOLD signal changes were identified by a t-test with a false discovery rate (FDR) of 0.05 for multiple comparison correction and a cluster extent threshold of 20 voxels. The individual BOLD signal change in each ROI was then described by the first eigenvariate of the adjusted contrast (adjusted for mean and covariates) in the cluster. Only significant deactivation (i.e. lower BOLD signal in the incongruent condition compared to the congruent condition), not activation, was detected in these four ROIs. The deactivation displayed within each ROI was bilateral except for that within the sgACC. Extracted parameter estimates of deactivation were significantly correlated across hemispheres of each ROI (left and right BNST, r = 0.92; left and right PVN, r = 0.96; left and right Amyg r = 0.78; P < 0.01 for all ROIs) and were thus, averaged across hemispheres.
To investigate how well abuse predicts activity within our ROIs, eight hierarchical linear regressions were computed using the raw, uncategorized CTQ scores for either physical or emotional abuse as independent variables and activity within each of the four ROIs (sgACC, BNST, Amyg and PVN) as dependent variables. Consistent with abuse and reactivity analyses, the following variables were entered together as covariates: age, gender, race, perceived stress, highest parental education and participant educational level.
Whole-brain analyses
Main effects of the Stroop and MSIT tasks were reported previously in separate samples and sub-samples (Gianaros et al., 2008, 2011). Accordingly, results from whole-brain analyses will not be described in detail in the present report, as whole-brain analyses and voxel-wise regressions were performed only to test whether our ROIs displayed significant stressor-evoked activity and correlate with abuse. To this end, contrast images (Incongruent vs Congruent) were submitted to a regression model in SPM8 in which either physical or emotional abuse was entered as a covariate of interest along with the same set of covariates previously entered in the ROI analyses. Main effects maps were used as ROIs in voxel-wise regressions in order to limit the analysis to areas of significant task activity. All whole-brain analyses were FDR corrected for multiple comparisons at 0.05.
Psychophysiological interaction analysis
For functional connectivity analyses, the first eigenvariate timeseries representing the BOLD signal for the BNST seed was extracted for each participant, for each task. Specifically, each time-series was extracted from the first principal component of BOLD signal activity of voxels within the BNST seed. Next, each BOLD signal time-series was mean-centered and submitted to a deconvolution algorithm using the canonical SPM8 HRF. Following deconvolution, an interaction vector was created, which represented the product of the deconvolved BOLD signal time-series and a vector coding for task condition (1 for incongruent, −1 for congruent). The interaction vector was subsequently re-convolved with the SPM8 HRF, creating a so-called psychophysiological interaction (PPI) vector. Finally, all three vectors, corresponding to task condition (convolved with HRF), seed BOLD activity and the PPI task-by-seed BOLD activity term, were entered as regressors in orthogonal individual GLM design matrices, wherein one PPI GLM was executed for each participant, task and seed region. Individual GLMs were then estimated, and PPI contrast maps were generated. Hence, individual GLMs testing for PPIs would demonstrate that inter-regional and time-dependent covariation (functional connectivity) with our seed region was greater during the incongruent than during the congruent condition of the tasks (Stroop or MSIT). The PPI contrast maps for the Stroop task and MSIT were averaged for each participant and submitted to a random effects analysis to locate the cluster in each ROI that showed a significant PPI effect. The first eigenvariate of the PPI in each cluster was then extracted to examine the relationship between childhood abuse and stressor-evoked functional connectivity.
Whole-brain PPI analysis
Main effects maps were calculated using averaged task maps as an ROI, in order to limit the whole-brain PPI analysis to task-related activity only (i.e. to examine task-related functional connectivity within the context of task-related activity). Whole-brain voxel-wise regressions were performed with PPI data using the PPI main effects map as an ROI, in order to examine the relationship between childhood abuse and functional connectivity. All whole-brain PPI analyses were FDR corrected for multiple comparisons at 0.05. (See ‘Abuse and functional connectivity’ in ‘Results’ section for more details.)
RESULTS
Relationship between childhood abuse and cardiovascular reactivity
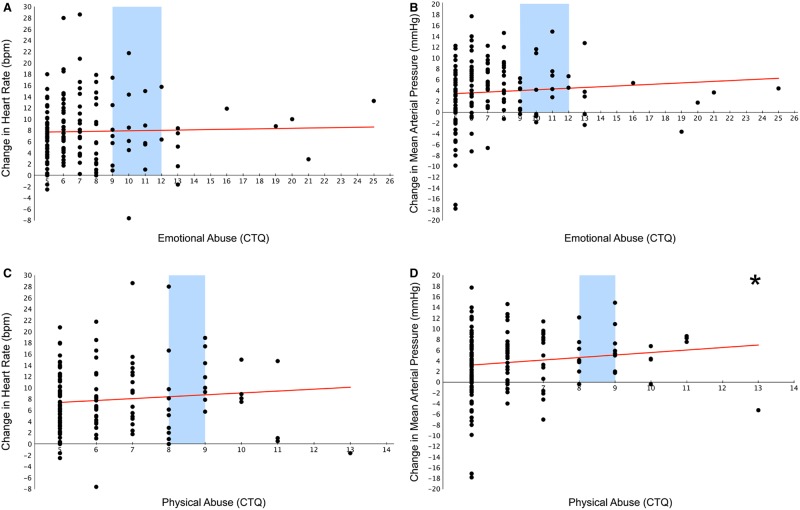
To test whether abuse predicts the change in HR or MAP from baseline to the incongruent conditions of the stress tasks above and beyond potential confounders (i.e. age, gender, race, perceived stress, highest parental education, participant educational level and resting HR or MAP), hierarchical linear regressions were performed (Figure 2). Emotional abuse (EA) was not a significant predictor of either change in HR (β = 0.063, P = 0.48, R2 change = 0.003; Figure 2A) or change in MAP (β = 0.13, P = 0.12, R2 change = 0.01; Figure 2B). However, although not a significant predictor of change in HR (β = 0.104, P = 0.22, R2 change = 0.01; Figure 2C), physical abuse (PA) did significantly predict the change in MAP (β = 0.172, P = 0.03, R2 change = 0.028; Figure 2D).
Fig. 2.
Abuse and stressor-evoked cardiovascular reactivity. Scatterplots displaying the relationship between emotional (A and B) and physical (C and D) abuse and cardiovascular reactivity [i.e. the change in HR (bpm = beats per minute; A and C) or MAP (mmHg = millimeters of mercury; B and D) from baseline to the incongruent, or stress-inducing condition of the tasks]. Childhood physical abuse significantly predicts the stressor-evoked change in MAP in adulthood. Blue bars indicate the range considered low to moderate abuse (A–D). All scatterplots show raw, uncorrected data. *P < 0.05.
Neuroimaging analyses
Conjunction
The conjunction analysis revealed that the Stroop task and the MSIT elicited significant activity within all of our ROIs (Figure 3A). Whole-brain analyses of the averaged task maps confirmed that our ROIs overlap with areas of significant deactivation (Figure 3B).
Fig. 3.
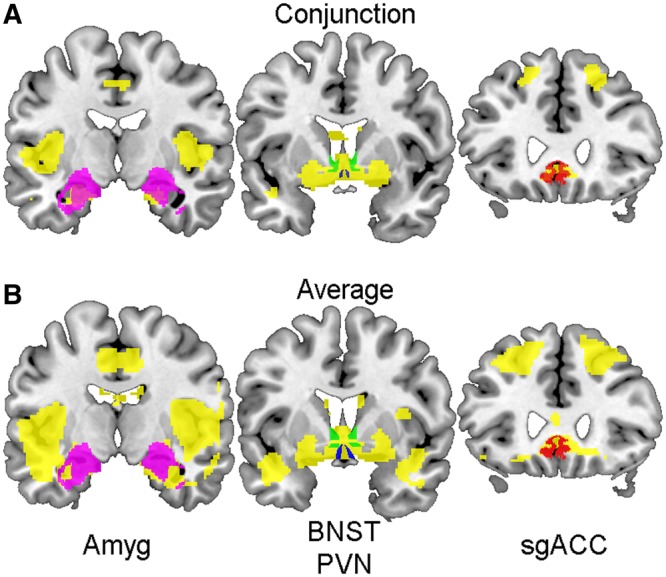
Stressor tasks main effects maps. Coronal sections (posterior to anterior from left to right) displaying significant voxels (in yellow) derived from a conjunction analysis of both the MSIT and the Stroop task (A), and significant task-related activity (in yellow) averaged across both tasks (B). ROIs are overlying main effects maps (A and B): Amyg (left, in magenta), BNST (middle, in green), PVN (middle, in blue) and sgACC (right, in red). Areas of significant conjunction between both tasks and significant task-related activity overlap with all four ROIs.
Abuse and activity within ROIs
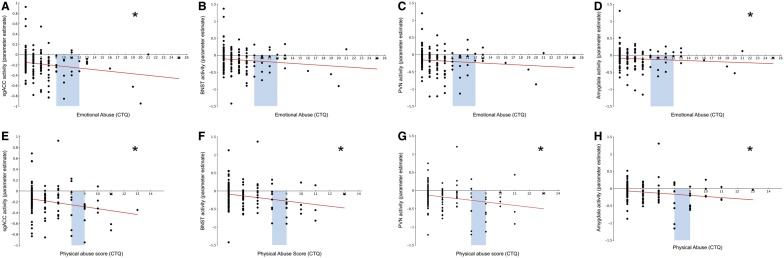
Each ROI displays significant task-related deactivation (Figure 4); extracted parameter estimates were used to perform hierarchical regression analyses examining whether abuse predicted activity changes within each ROI (Figure 5). EA was a significant predictor of activity within the sgACC (β = −0.206, P = 0.026, R2 change = 0.036) and the Amyg (β = −0.178, P = 0.050, R2 change = 0.027), but not within the BNST (β = −0.151, P = 0.103, R2 change = 0.019) or PVN (β = −0.175, P = 0.055, R2 change = 0.026; Figure 5A–D). Whole-brain voxel-wise regression revealed similar results with significant voxels being located within both the sgACC and Amyg, but not within the BNST and PVN (Figure 6A).
Fig. 4.
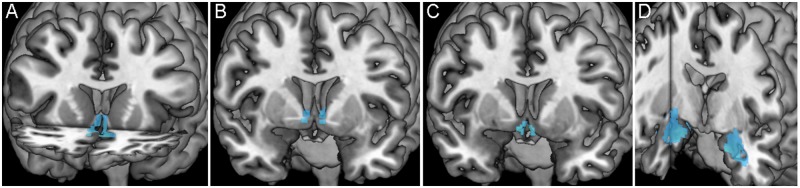
Averaged stressor-evoked deactivation within each ROI. Images display significant stressor-evoked deactivation (in blue) within each ROI [sgACC (A, in coronal and axial planes), BNST (B, coronal plane), PVN (C, coronal plane) and Amyg (D, coronal and sagittal planes)] from which parameter estimates were extracted for subsequent analyses.
Fig. 5.
Abuse and stressor-evoked activity within ROIs. Scatterplots displaying the relationship between emotional (A–D) and physical (E–H) abuse and stressor-evoked activity within each ROI. Childhood emotional abuse significantly predicts stressor-evoked activity within the sgACC (A) and the Amyg (D) but not the BNST (B) or PVN (C). Childhood physical abuse significantly predicts the stressor-evoked change in activity within all four ROIs: sgACC (E), BNST (F), PVN (G) and Amyg (H). Blue bars indicate the range considered low to moderate abuse (A–H). All scatterplots show raw, uncorrected data. *P < 0.05.
Fig. 6.
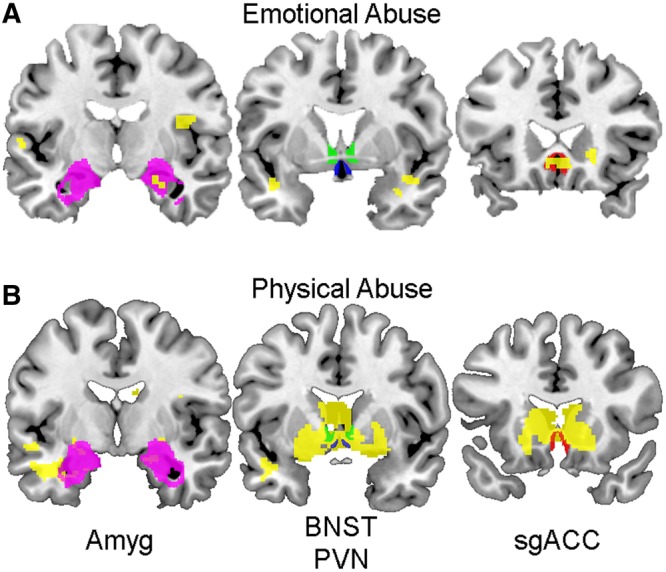
Whole-brain regressions of abuse and stressor-evoked activity. Coronal sections (posterior to anterior from left to right) displaying voxels that significantly describe the relationship between emotional (A) and physical (B) abuse with stressor-evoked activity (in yellow). ROIs are overlaid to display the extent of overlap between ROIs and significant voxels: Amyg (left, in magenta), BNST (middle, in green), PVN (middle, in blue) and sgACC (right, in red). Whole-brain voxel-wise regression revealed that emotional abuse predicts activity within both the sgACC and Amyg, but not the BNST and PVN (A). Also, whole-brain voxel-wise regression revealed that physical abuse predicts activity located within all four ROIs (B).
In contrast to EA, PA was a significant predictor of activity changes within all of our ROIs: the sgACC (β = −0.264, P = 0.003, R2 change = 0.065), BNST (β = −0.278, P = 0.001, R2 change = 0.072), PVN (β = −0.278, P = 0.001, R2 change = 0.072) and Amyg (β = −0.221, P = 0.010, R2 change = 0.046; Figure 5E–H). With increasing PA scores, all ROIs showed less stressor-evoked activity. Whole-brain voxel-wise regression with PA revealed similar results with significant voxels being located within all ROIs (Figure 6B).
Abuse and functional connectivity
Whole-brain PPI maps with BNST as the seed revealed significant connectivity with sgACC, PVN and Amyg ROIs (Figure 7). Using connectivity coefficients extracted from each ROI, multiple hierarchical regression analyses were performed that included all of the covariates. Results showed that neither EA nor PA significantly predicted connectivity estimates derived from the BNST seed.
Fig. 7.
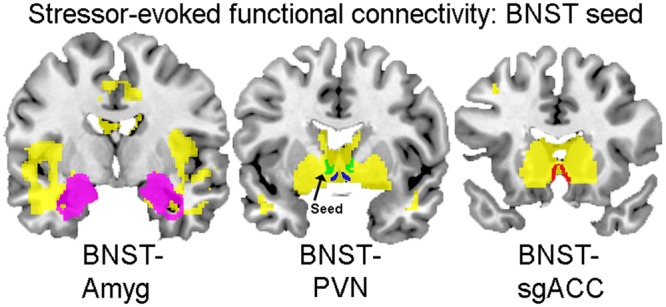
PPI main-effects map with BNST seed. Coronal sections (posterior to anterior from left to right) displaying stressor-evoked functional connectivity with the BNST seed. Voxels that are positively correlated with BNST seed activity are displayed in yellow. ROIs are overlaid to demonstrate the extent of their overlap with positively correlated voxels: Amyg (left, in magenta), BNST (middle, in green), PVN (middle, in blue) and sgACC (right, in red).
Whole-brain voxel-wise regression with EA revealed that significant voxels overlapped with the sgACC at a significance level of P = 0.01 (20 voxels), with no overlap within the PVN or Amyg. However, when the significance threshold was changed to P = 0.05 (5 voxels), overlap also appears within the PVN. Whole-brain voxel-wise regression with PA revealed no overlap within any ROIs at a significance level of P = 0.05 (5 voxels). Thus, similar to regression analyses, in which connectivity estimates were derived from ROIs within a BNST seed PPI map, the effects did not indicate a predictive relationship between either EA or PA and functional connectivity.
Brain activity within ROIs and cardiovascular stress reactivity
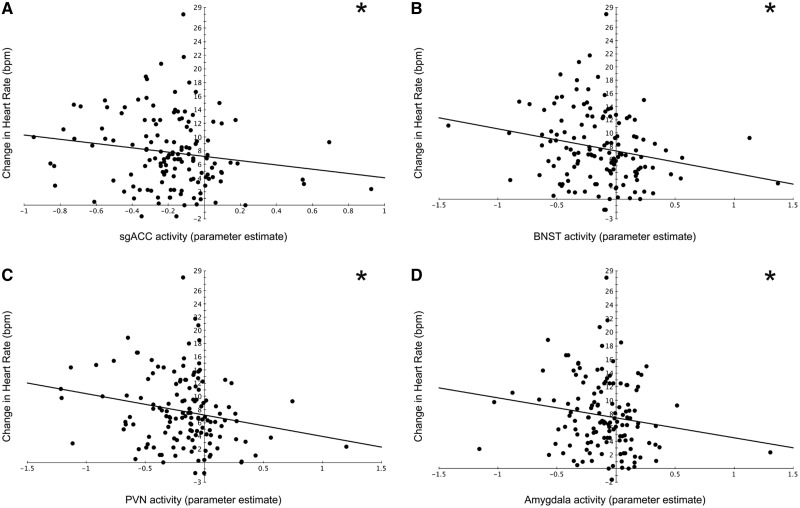
Interestingly, extracted activity from each of the ROIs significantly predicted the change in HR, but not MAP, from baseline to the incongruent condition (Figure 8): BNST (β = −0.261, P = 0.002, R2 change = 0.063), PVN (β = −0.224, P = 0.009, R2 change = 0.046), sgACC (β = −0.200, P = 0.019, R2 change = 0.038), Amyg (β = −0.183, P = 0.036, R2 change = 0.030).
Fig. 8.
Stressor-evoked ROI activity and HR reactivity. Scatterplots displaying the relationship between stressor-evoked activity within each ROI and the change in HR (bpm = beats per minute) from baseline to the incongruent, or stress-inducing condition of the tasks. Stressor-evoked activity within each ROI [sgACC (A), BNST (B), PVN (C) and the Amyg (D)] predicts HR reactivity. All scatterplots show raw, uncorrected data. *P < 0.05
DISCUSSION
Individuals with a history of childhood abuse display altered and/or dysregulated stress responses (Carpenter et al., 2007; Heim et al., 2010; Carpenter et al., 2011; Lovallo et al., 2011). However, how early experience shapes central visceral circuits that control stress responses is still unclear (for review, see Rinaman et al., 2011). The sgACC, BNST, PVN and Amyg form an integrated visceral circuit that is critical in the control of stress responses (Schwaber et al., 1982; Luiten et al., 1985; Ter Horst et al., 1989; Aston-Jones et al., 1999; Clayton and Williams, 2000; Rinaman et al., 2000; Dong et al., 2001b; Vertes, 2004). Early life experience (i.e. postnatal maternal separation) has been shown to alter the developmental assembly and later structure and stress-induced activity of visceral circuits and brain regions (Card et al., 2005; Banihashemi and Rinaman, 2010; Koehnle and Rinaman, 2010; Banihashemi et al., 2011).
Our goal was to translate these findings to human studies by determining the influence of childhood abuse on stressor-evoked activity within and communication between central visceral brain regions. We hypothesized that individuals with a self-reported history of emotional or physical abuse would display altered responses to stress within these brain regions that would co-occur with altered cardiovascular stress reactivity. We also hypothesized that the functional connectivity of our ROIs to a hub ROI, the BNST, would be associated with childhood emotional and/or physical abuse.
Our results demonstrated that childhood physical abuse correlated positively with stressor-evoked changes in MAP, and negatively with BOLD activity within the sgACC, BNST, PVN and Amyg. In contrast, emotional abuse did not predict stressor-evoked cardiovascular measures but did correlate negatively with stressor-evoked activity within the sgACC and the Amyg. Lastly, stressor-evoked activity within all four ROIs correlated negatively with changes in HR.
Abuse and activity within ROIs
A primary result from this study is that childhood physical abuse significantly predicts stressor-evoked activity within all four of our central visceral ROIs (Figure 5E–H). Interestingly, our participants fall below the ‘severe-to-extreme’ range for self-reported childhood physical abuse, indicating that stressor-evoked activity within these visceral brain regions is sensitive to childhood physical abuse even at lower levels. Our results also show that childhood emotional abuse is also associated with stressor-evoked activity within the sgACC and Amyg, but not within the BNST and PVN. It is unclear why emotional abuse did not relate to activity in these regions as physical abuse did, however, this may be due to differences in severity, duration and frequency of abuse occurrence (Thompson and Kaplan, 1996; Heim et al., 2010). As the PVN integrates control of neuroendocrine and preautonomic stress responses (Herman et al., 2002) and the BNST innervates the PVN directly (Dong et al., 2001b), these regions can be considered more proximal to controlling orchestrated stress responses. Interestingly, emotional abuse influences the regions comparatively more distal to the control of stress responses, perhaps indicating that emotional abuse shapes the modulation of BNST and PVN activity indirectly, by influencing the sgACC and Amyg. During behavioral states of stress, the BNST has an excitatory influence on the PVN (Crane et al., 2003a), and the sgACC has an inhibitory influence on PVN activity (Crane et al., 2003b; Spencer et al., 2005), presumably via its innervation of the BNST (Spencer et al., 2005). Thus, we hypothesized that abused individuals would display decreased activity in the sgACC, indicating less ability to inhibit the stress-promoting responses of the BNST and PVN, resulting in increased activity in BNST and PVN and heightened stress responses. However, the relationships between both types of abuse and ROI activity were consistently moderate and negative in magnitude, such that as emotional or physical abuse scores increased, the stressor-evoked activity within these regions decreased. Thus, our results were consistent with our hypothesis concerning sgACC, but not for the remaining ROIs. One interpretation of the decrease in ROI activity with increasing abuse scores is that childhood abuse has an inhibitory influence on the ROIs as a network. Further, limitations in the resolution of fMRI do not allow us to visualize the activity of the many distinct and heterogenous subnuclei comprising these ROIs (Dong et al., 2001b; Herman et al., 2002), which could display different trends in activity.
Our results show that physical and emotional abuse predicts less Amyg activity in response to mental stress tasks. This contrasts with studies that have shown maltreatment-related heightened Amyg reactivity to a social threat task (McCrory et al., 2011; McCrory et al., 2013). Potential explanations for this difference could be that our paradigms do not necessarily evoke threat per se and do not have a social evaluative component. Our demanding and conflict-based tasks involving time pressure and negative performance feedback also evoke HR and blood pressure responses that are unlikely to be evoked by emotional face-viewing paradigms. In addition, mental stressors do not engage the Amyg in the same ways as direct threat-based tasks, and they may also engage different amygdalar subnuclei (Kinzig et al., 2003; Wang et al., 2005). For example, the main effects of our tasks evoke a mean signal decrease in the Amyg, whereas social threat tasks and emotional face paradigms typically evoke a mean signal increase (McCrory et al., 2011; McCrory et al., 2013). Thus, task differences may account for contrasting results in this context.
Abuse and functional connectivity
To understand how the communication between our ROIs is altered by childhood abuse, we investigated the extent to which the stressor-evoked activity between the BNST and the other ROIs varied together using functional connectivity analyses. We had hypothesized particularly that BNST-PVN activity would be more tightly coupled in individuals with a self-reported history of abuse, which would be consistent with the notion that the BNST and PVN coordinate to promote the stress response (Crane et al., 2003a; Spencer et al., 2005), perhaps contributing to heightened stress reactivity in these individuals. However, we saw no such predictive effects of abuse on stressor-evoked functional connectivity, which may be due to the limitations in the sensitivity of the analyses.
Abuse and cardiovascular reactivity
We found that childhood physical abuse is associated with a greater change in MAP from baseline to the incongruent conditions of the tasks (i.e. the Stroop and MSIT), but not the change in HR. This is consistent with a rat study in which ‘handling’ or brief postnatal maternal separation alters the change in MAP, not HR, in response to a conditioned fear stimulus (Sanders and Knoepfler, 2008). Blood pressure and HR have distinct underlying autonomic influences [i.e. sympathetic and parasympathetic (vagal) innervation of the sinoatrial node controlling HR vs additional sympathetic innervation of the myocardium and vasculature controlling blood pressure]; this is supported by the moderate correlation (r = 0.39) between the change in HR and the change in MAP. Thus, under some circumstances, the influence of childhood physical abuse on later cardiovascular stress reactivity may be concentrated on neural circuits that more closely relate to blood pressure or vascular regulation, rather than HR.
The influence of early or cumulative adversity on stress reactivity later in life is an unclear issue in the literature, with some studies indicating increases in stressor-evoked cardiovascular activity and others indicating decreases (Carpenter et al., 2007; Carpenter et al., 2011; Lovallo et al., 2011). These studies vary in their inclusion of individuals with prior history and/or current affective disorders, including depression and posttraumatic stress disorder. Thus, our results are novel in demonstrating that levels of childhood physical abuse that are considered below extreme are associated with comparatively larger changes across subjects in stressor-evoked MAP in a physically and mentally healthy sample.
Interestingly, our cardiovascular reactivity results contrast with our neural results in that childhood PA predicts increased stressor-evoked MAP while predicting decreased stressor-evoked neural activity within our ROIs. However, these findings can also be interpreted as increases in childhood physical abuse being associated with greater stressor-evoked changes in MAP, as well as greater stressor-evoked changes in neural activity within our ROIs between incongruent and congruent conditions. Thus, there is consistency with physical abuse predicting greater relative reactivity changes both centrally and peripherally.
Brain activity within ROIs and cardiovascular stress reactivity
Each of our ROIs is thought to be involved in the neural control of cardiovascular activity, as evidenced by research in animal models (Tavares et al., 2004; Crestani et al., 2009; Pyner, 2009). Indeed, we found that activity within all of our ROIs significantly predicts the change in HR from baseline to the stress-inducing (i.e. incongruent) conditions. Interestingly, the same is not true for the change in MAP. One possible interpretation is that the change in MAP requires the recruitment of a network of brain regions involved in baroreflex control (Gianaros et al., 2011) or relates more closely to activity in subsets of voxels within or outside of our a priori anatomical ROIs, which would be identified with other analytical approaches (e.g. voxel-wise analyses) not used here. Thus, the extracted and aggregate activity of each ROI on its own may be unlikely to predict the change in MAP for these or other reasons.
Limitations of the study
Limitations of retrospective reporting of childhood abuse
We note that retrospective reporting of childhood adversity has limitations, namely retrospective recall, psychopathology-related memory impairment and potential false-negative results. Importantly, evidence supports the assumption that false-positive results are likely to be rare (Hardt and Rutter, 2004). This is true particularly when participants are asked to report on salient issues (e.g. the occurrence of an event, such as abuse) and less so when participants are asked to report on details regarding the timing and duration of events (Brewin et al., 1993; Hardt and Rutter, 2004; Miller et al., 2011). The reliability of retrospective reporting of childhood abuse is evidenced by good agreement between sibling retrospective reports of abuse, and prospectively collected court, clinic and research records (Bifulco et al., 1994; Bifulco et al., 1997; Hardt and Rutter, 2004).
Limitations of using a healthy sample
Our participants consisted of individuals that were not excluded for physical disorders, such as cardiovascular disease, and psychiatric diagnoses. Excluding participants with mental and physical health problems is likely to minimize the number of individuals that have experienced childhood abuse, as the occurrence of childhood abuse is associated with increased risk for poor physical health outcomes, including neurological disorders and cardiovascular disease (Danese et al., 2009; Wegman and Stetler, 2009; Fuller-Thomson et al., 2010; Midei et al., 2012; Rich-Edwards et al., 2012) and poor mental health outcomes, including depression and anxiety disorders (Hankin, 2005; Gibb et al., 2007; Springer et al., 2007; Rogosch et al., 2011). Indeed, a majority of our participants experienced none to minimal levels of emotional and physical abuse. However, this can also be viewed as a strength of this study. Our study was able to examine the relationships between abuse and central and peripheral stressor-evoked activity with minimal confounding influences from physical or mental health problems.
There is a possibility that some of our participants have mental health disorders that we did not assess. Although we did not conduct a formal clinical interview for diagnostic purposes, we did administer other scales that provide for more complete sample characteristics. These include the BDI, STAI and NEO. Participants’ scores fell within normative ranges across these inventories (Table 1) and do not suggest the presence of salient disorders, such as depression or anxiety disorders.
Although the majority of our participants’ experienced low levels of childhood physical abuse, the possibility remains that they may be either resilient to mental or physical disorders or have not undergone stressors that would unmask any latent vulnerabilities to such disorders. Participants in this study were not excluded for having a history of affective disorders; thus, it is possible they may not be representative of a psychologically resilient sample. Further, the increased MAP reactivity in individuals with childhood physical abuse would appear to be inconsistent with the notion of physiological resilience.
Limitations of examining small ROIs
The small size of our ROIs is a limitation of this study, as it influences our ability to reliably localize these structures. Normalization and smoothing techniques decrease our ability to localize with certainly small structures such as the PVN and BNST. Given the size of our smoothing kernel (6 mm) and the size of our ROIs (Table 2), the PVN’s size and location make it perhaps the most difficult to localize. Given that our ‘functional’ PVN ROI may extend past the boundaries drawn, additional subnuclei of the hypothalamus may be included in this analysis, such as medial hypothalamic areas. These regions are also preautonomic (Goren et al., 1997) and receive input from the BNST (Dong et al., 2001b). Thus, these regions are likely contributing to effects that are part of the circuit of interest. However, gaining anatomical precision in ROI analyses is a great area of interest that must be further explored in future research.
CONCLUSIONS
In conclusion, the results of this study suggest that childhood physical and emotional abuse influence stressor-evoked responses within visceral brain regions integral to the control of stress responses and that childhood physical abuse predicts greater stressor-evoked changes in MAP. This study is unique in examining physically and mentally healthy individuals, and in demonstrating that levels of physical abuse that are not considered extreme relate to stressor-evoked brain and cardiovascular activity. Further research is needed to investigate the underlying structural and phenotypic neural changes accompanying these abuse-related changes in stressor-evoked function. Thus, understanding abuse-related influences on central visceral circuits may provide insight into how early adversity heightens vulnerability to both physical and mental health problems.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank Sara Snyder, Dr. Israel Christie and Ikechukwu Onyewuenyi for assisting in data collection and reduction.
This work was funded by National Institutes of Health Heart, Lung and Blood Institute Grants R01-HL089850 and R01-HL101421 to P.J.G and by F32-HL104770 to L.B.
REFERENCES
- Abrahám IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. European Journal of Neuroscience. 2000;12:3003–14. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210(5):343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–98. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010;165(1):265–77. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, O'Neill EJ, Rinaman L. Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience. 2011;192:413–28. doi: 10.1016/j.neuroscience.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes V, Treiber F, Musante L, Turner J, Davis H, Strong W. Ethnicity and socioeconomic status: impact on cardiovascular activity at rest and during stress in youth with a family history of hypertension. Ethnicity & disease. 2000;10(1):4. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. Journal of personality assessment. 1996;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry. 1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Harris TO. Childhood Experience of Care and Abuse (CECA): a retrospective interview measure. Journal of Child Psychology and Psychiatry. 1994;35(8):1419–35. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown G, Lillie A, Jarvis J. Memories of childhood neglect and abuse: corroboration in a series of sisters. Journal of Child Psychology and Psychiatry. 1997;38(3):365–74. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin. 1993;113(1):82. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nature protocol. 2006;1(1):308–13. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. The Journal of Neuroscience. 2005;25(40):9102–11. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Rinaman L, Lynn RB, et al. Pseudorabies virus-infection of the rat central nervous system ultrastructural characterization of viral replication, transport, and pathogenesis. Journal of Neuroscience. 1993;13(6):2515–39. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Sved AF. Central autonomic pathways. In: Llewellyn-Smith IJ, Verberne AJ, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press, Inc; 2011. pp. 3–22. [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214(1):367–75. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EC, Williams CL. Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behavioural Brain Research. 2000;112:151–8. doi: 10.1016/s0166-4328(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. 1992 Neo PI-R professional manual. Odessa, FL: Psychological assessment resources. [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. The Journal of Comparative Neurology. 2003a;467:232–42. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic-pituitary-adrenal axis response to a physical stressor, systemic delivery of interleukin-1 beta. European Journal of Neuroscience. 2003b;17:1473–81. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves FHF, Tavares RF, Corrêa FM. Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress: The International Journal on the Biology of Stress. 2009;12(3):268–78. doi: 10.1080/10253890802331477. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 1993;332(1):1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington HL, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163(12):1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001b;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fuller-Thomson E, Brennenstuhl S, Frank J. The association between childhood physical abuse and heart disease in adulthood: Findings from a representative community sample. Child Abuse and Neglect. 2010;34(9):689–98. doi: 10.1016/j.chiabu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Derbtshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005a;42(6):627–35. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosomatic Medicine. 2005b;67(1):31–9. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biological Psychiatry. 2009a;65(11):943–50. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Remo AM, Christie IC, Crtichley HD, Wang J. Heightened resting neural activity predicts exaggerated stressor-evoked blood pressure reactivity. Hypertension. 2009b;53:819–25. doi: 10.1161/HYPERTENSIONAHA.108.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Human Brain Mapping. 2011;33(7):1700–16. doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28(4):990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depression and Anxiety. 2007;24(4):256–63. doi: 10.1002/da.20238. [DOI] [PubMed] [Google Scholar]
- Goren Z, Asian N, Berkman K, Ean T, Sule O, Onat F. Role of paraventricular and dorsomedial nuclei of the hypothalamus and central nucleus of the amygdala on muscimol-induced cardiovascular responses. Fundamental & Clinical Pharmacology. 1997;11(5):408–15. doi: 10.1111/j.1472-8206.1997.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Gump BB, Matthews KA, Räikkönen K. Modeling relationships among socioeconomic status, hostility, cardiovascular reactivity, and left ventricular mass in African American and White children. Health Psychology. 1999;18(2):140–50. doi: 10.1037//0278-6133.18.2.140. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Childhood maltreatment and psychopathology: prospective tests of attachment, cognitive vulnerability, and stress as mediating processes. Cognitive Therapy and Research. 2005;29(6):645–71. [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry. 2004;45(2):260–73. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology. 2010;52(7):671–90. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. European Journal of Neuroscience. 2002;16(3):381–5. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosomatic Medicine. 2003;65(1):9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of Neuroscience. 2003;23(15):6163–70. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehnle TJ, Rinaman L. Early experience alters limbic forebrain Fos responses to a stressful interoceptive stimulus in young adult rats. Physiology and Behavior. 2010;100:105–15. doi: 10.1016/j.physbeh.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa L, Ayuso-Mateos JL, Vazquez-Barquero JL, Dıez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. Journal of Affective Disorders. 2000;57(1–3):261–5. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry. 2011;71(4):344–9. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B, Grafe K, Zipfel S, Witte S, Loerch B, Herzog W. Diagnosing ICD-10 depressive episodes: superior criterion validity of the Patient Health Questionnaire. Psychotherapy and Psychosomatics. 2004a;73(6):386–90. doi: 10.1159/000080393. [DOI] [PubMed] [Google Scholar]
- Lowe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) Journal of Affective Disorders. 2004b;81(1):61–6. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Research. 1985;329(1–2):374–8. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Macrí S, Mason GJ, Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. European Journal of Neuroscience. 2004;20(4):1017–24. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the human brain. New York: Academic Press; 2008. 3rd Edition. [Google Scholar]
- Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. General Hospital Psychiatry. 2006;28(1):71–7. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychology. 2001;20(6):403–10. [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry. 2013;202(4):269–76. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):R947–8. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Midei AJ, Matthews KA, Chang YF, Bromberger JT. Childhood physical abuse is associated with incident metabolic syndrome in mid-life women. Health Psychology. 2012;32(2):121–7. doi: 10.1037/a0027891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;131(6):959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei TP, Evans L, Crook GM. Utility and validity of the STAI with anxiety disorder patients. British Journal of Clinical Psychology. 1990;29(Pt 4):429–32. doi: 10.1111/j.2044-8260.1990.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. Journal of Chemical Neuroanatomy. 2009;38(3):197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mason S, Rexrode K, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women/clinical perspective. Circulation. 2012;126(8):920–7. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiology and Behavior. 2011;104(4):632–40. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience. 2000;20(7):2731–41. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23(4):1107–24. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule WR, Traver MD. Test-retest reliabilities of State-Trait Anxiety Inventory in a stressful social analogue situation. Journal of Personality Assessment. 1983;47(3):276–7. doi: 10.1207/s15327752jpa4703_8. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Knoepfler J. Neonatal handling increases cardiovascular reactivity to contextual fear condiioning in borderline hypertensive rats (BHR) Physiological Behavior. 2008;95(1–2):72–6. doi: 10.1016/j.physbeh.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25(1):433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct conections with the nucleus of the solitary tract and the dorsal motor nucleus. The Journal of Neuroscience. 1982;2(10):1424–38. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu LK, Jennings JR, Gianaros PJ. Test–retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiology. 2012;49(7):873–84. doi: 10.1111/j.1469-8986.2012.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2005;481(4):363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press Inc; 1970. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: Results from a large population-based sample of men and women. Child Abuse and Neglect. 2007;31(5):517–30. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer RA, Rissmiller DJ, Beck AT. Use of Beck Depression Inventory-II with depressed geriatric inpatients. Behaviour Research and Therapy. 2000;38(3):311–8. doi: 10.1016/s0005-7967(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory—second edition in a sample of college students. Depression and Anxiety. 2004;19(3):187–9. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Antunes-Rodrigues J, de Aguiar Correa FM. Pressor effects of electrical stimulation of medial prefrontal cortex in unanesthetized rats. Journal of Neuroscience Research. 2004;77(4):613–20. doi: 10.1002/jnr.20195. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, De Boer P, Luiten PGM, Van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785–97. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Thompson AE, Kaplan CA. Childhood emotional abuse. The British Journal of Psychiatry. 1996;168(2):143–8. doi: 10.1192/bjp.168.2.143. [DOI] [PubMed] [Google Scholar]
- Usala PD, Hertzog C. Evidence of differential stability of state and trait anxiety in adults. Journal of Personality and Social Psychology. 1991;60(3):471–9. doi: 10.1037//0022-3514.60.3.471. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt B. Cingulate Neurobiology and Disease. OUP Oxford: Oxford University Press; 2009. [Google Scholar]
- Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17804–9. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71(8):805–12. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]