Abstract
Informational cascades can occur when rationally acting individuals decide independently of their private information and follow the decisions of preceding decision-makers. In the process of updating beliefs, differences in the weighting of private and publicly available social information may modulate the probability that a cascade starts in a decisive way. By using functional magnetic resonance imaging, we examined neural activity while participants updated their beliefs based on the decisions of two fictitious stock market traders and their own private information, which led to a final decision of buying one of two stocks. Computational modeling of the behavioral data showed that a majority of participants overweighted private information. Overweighting was negatively correlated with the probability of starting an informational cascade in trials especially prone to conformity. Belief updating by private information was related to activity in the inferior frontal gyrus/anterior insula, the dorsolateral prefrontal cortex and the parietal cortex; the more a participant overweighted private information, the higher the activity in the inferior frontal gyrus/anterior insula and the lower in the parietal-temporal cortex. This study explores the neural correlates of overweighting of private information, which underlies the tendency to start an informational cascade.
Keywords: social influence, informational cascades, fMRI, parietal cortex, insular cortex, belief updating
INTRODUCTION
Research in the social sciences has reliably demonstrated that individuals are influenced by the behavior of others (e.g. Cialdini and Goldstein, 2004; Raafat, Chater, and Frith, 2009). Stock market bubbles, for example, can emerge when traders start to follow misleading decisions made by their colleagues, disregarding their own private information. Interestingly, theoretical and empirical work in economics has shown that initial decisions of others can create an environment in which it is even rational for subsequent decision-makers to disregard their own private information and to follow others. Such a pattern of conforming decisions is called an informational cascade (Banerjee, 1992; Bikhchandani et al., 1992; Anderson and Holt, 1997). Usually, informational cascades lead to a desired outcome. However, a ‘reverse’ cascade can arise if a substantial number of initial decision-makers receive an incorrect private signal and therefore make incorrect decisions. In such situations, all subsequent decision-makers would rationally follow the initial decisions and ignore their own private signals. The theory of informational cascades can explain numerous real-life phenomenon, such as nonemployment in the labor market (Oberholzer-Gee, 2008), revolutionary regime transitions (Ellis and Fender, 2011), and financial crises (Chari and Kehoe, 2004). The probability that a cascade starts strongly depends on how people weight and integrate their own private as compared with publicly available social information (Bernardo and Welch, 2001; Nöth and Weber, 2003; Goeree et al., 2007). In the present work we define social information broadly as information that is inferred from the behavior of other people without necessarily interacting with them face-to-face. In the experiment we used a hypothetical decision scenario. In contrast to social information, private information is directly accessible to a person. Weizsäcker's (2010) meta-analysis suggests that people tend to overweight private as compared with social information, even in situations in which following others is beneficial. Due to overweighting of private information, cascades might occur less often as predicted by the theory of informational cascades. Here, we combine neurobiological, economic and computational approaches to investigate the neural mechanism of (biased) belief updating during financial decisions and to explore individual differences in the weighting and processing of private information, which can modulate the frequency of starting a cascade.
The neural underpinnings of decision-making in a social context and the impact of social information has received increasing attention in the neuroscience literature (Behrens et al., 2008; Klucharev et al., 2009; Berns et al., 2010; Campbell-Meiklejohn et al., 2010; Izuma and Adolphs, 2013). From a cognitive perspective, informational cascades are based on a process of sequential belief updating of social and private information, on which a final decision under uncertainty rests. Recent studies in the field of decision neuroscience provide evidence for the involvement of the anterior insula (Preuschoff et al., 2006, 2008), the anterior insula in combination with the inferior frontal gyrus (Paulus et al., 2003), the posterior fronto-median cortex (Volz et al., 2003, 2004) and the parietal cortex, often in combination with the dorsolateral prefrontal cortex (DLPFC) (Huettel et al., 2005; Vickery and Jiang, 2009; Mohr et al., 2010; Stern et al., 2010; Symmonds et al., 2011; Wright et al., 2012), in belief updating and decision-making under uncertainty [see Bach et al., 2011 for an overview]. Whereas the inferior parietal lobule (angular gyrus) seems to have a special role in tracking observed relative frequencies of events, activity within a region of the inferior frontal gyrus has been found to be negatively correlated with Bayesian posterior probability (d’Acremont et al., 2013).
Contrary to other paradigms exploring belief updating (e.g. the evidence accumulation task; Stern et al., 2010 or the ball/bin betting task by d’Acremont et al., 2013), informational cascades require people not only to update a belief on the basis of (private) information, but additionally to derive social information from the observed decisions of others. A better understanding of the differences in updating private as compared with social information is crucial for the theory of informational cascades, because overweighting of private information can result in fewer cascades than predicted by the theory. Here we explored the neural mechanism of biased belief updating of private as compared with social information.
MATERIALS AND METHODS
Participants
Thirty-two people recruited from the subject pool of the University of Basel participated in our experiment. Five participants were excluded from the final data analysis (two because of technical problems during the functional magnetic resonance imaging (fMRI) data acquisition, one because of a technical error in the experimental script, one because of misuse of the response device, and one because of left-handedness). The final sample consisted of 27 healthy right-handed participants with normal or corrected-to-normal vision (mean age = 22.4 years, ± 2.0 years s.d., 20–29 years, 9 females). The study was approved by the local ethics committee and participants gave written informed consent. Participation in the study was reimbursed with a fixed amount of 30 Swiss franc (CHF) and a variable bonus (mean bonus = 3.99 CHF, ± 0.42 CHF s.d., 2.90–4.60 CHF). The variable bonus was performance contingent, so that deviations from the correct probability estimate led to a lower bonus following a nonlinear quadratic scoring rule (Selten, 1998).
Experimental design
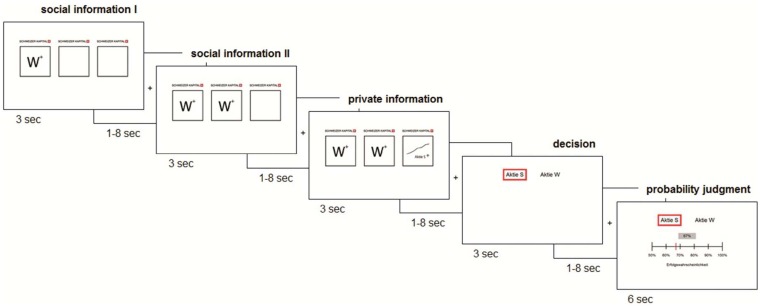
We used a hypothetical decision scenario representing an adapted version of the classical informational cascades paradigm (Anderson and Holt, 1997). In our study, participants acting as stock market traders were required to repeatedly choose the profitable (‘’) of two stocks (W or S) given some evidence e. Participants were told that stock markets are very volatile and fast-moving and that every week (trial) only one stock is profitable. At the end of each trial, participants reported the posterior probability that the chosen stock was ‘’ (Figure 1). In the 32 experimental trials, participants sequentially received three different pieces of evidence. At the beginning of a trial, two decisions made by other fictitious traders (trader I and II) in the ‘Swiss Capital Bank’ were shown, representing ‘social information I’ and ‘social information II’. The ‘social information’ was followed by ‘private information’ in the form of a personal recommendation from a rating agency. Participants were informed that all other traders also received their own personal recommendation from an independent rating agency. The likelihood of receiving a correct recommendation from a rating agency was 2/3 (indicated by the visual cue: ‘+’) or 4/5 (visual cue: ‘++’) for all traders and for the participant. The quality (‘+’ or ‘++’) of the recommendations received was indicated on the screen above the decisions of the other traders (social information I and II) or above the private information for the participant. The posterior probability that one of the two stocks was profitable (‘’) given the received and perceived evidence can be determined following Bayes theorem as:
| (1) |
where t refers to the three different points in time in the belief updating process (Figure 1). At t = 0 without a participant having received any information = 0.50. Based on the assumption that other traders incorporated all available evidence, participants could derive the recommendation received by other traders. Because trader I always received low (‘+’) quality recommendations, her decision (social information I) signaled the correct stock with a likelihood of 0.67 [i.e. = 0.67]. Next, trader II was confronted with a recommendation of either low (‘+’) quality [i.e. = 0.67] or high (‘++’) quality [i.e. = 0.80]. This evidence could then be combined with the information inferred from the decision of the first trader, which led to four possible posterior probabilities of the chosen stock by trader II (i.e. 0.50; 0.67; 0.80; 0.89). After receiving a personal recommendation (private information) participants could update their belief, which should correspond to six different posterior probabilities (i.e. 0.50; 0.67; 0.80; 0.89; 0.94; and 0.97). Importantly, by using all different combinations of decisions and private information (2 × 4 × 4 = 32 trials of interest), we created a design matrix in which the different pieces of evidence are independent, that is, seeing one piece of evidence did not allow the prediction of the next piece of evidence. To force participants to pay equal attention to social and private information and to update their probability estimate at every point in time (t), we included six filler trials in the task. In these trials, subjects had to make a decision with only one (social information I) or two (social information I & II) pieces of evidence and no private information. To familiarize themselves with the task, participants completed 11 training trials outside of the scanner before the fMRI session. To further boost their attention, filler trials were overrepresented in these training trials. The randomized sequence of trials was identical for all subjects. Trials were separated with fixation crosses, as were the different events within a trial (Figure 1). The interstimulus intervals between the time windows were varied according to a left truncated Poisson distribution [mean (λ) = 3172.78 ms, min = 1000 ms, max = 8000 ms]. Importantly, from a normative Bayesian perspective, the first two decision-makers can create a situation in which the third decision-maker (and all subsequent decision-makers) should ignore private information and just follow the decisions of others. Thus, the decision of the third decision-maker is crucial, as it can start or prematurely end an informational cascade. Therefore, in our paradigm we investigated the cognitive and neural mechanisms underlying the process of belief updating and decision-making of the third decision-maker, who can initiate or end an informational cascade.
Fig. 1.
Informational cascades task trial structure. The decisions of trader 1 (social information I) and trader 2 (social information II) were followed by a buying recommendation of a rating agency for one or the other stock (private information). At the end of every trial, participants decided which stock (W or S) provided the higher revenue and indicated the probability of the correct outcome (probability judgment). The different windows were separated with fixation crosses (see ‘experimental design’ section for details).
Behavioral data analysis
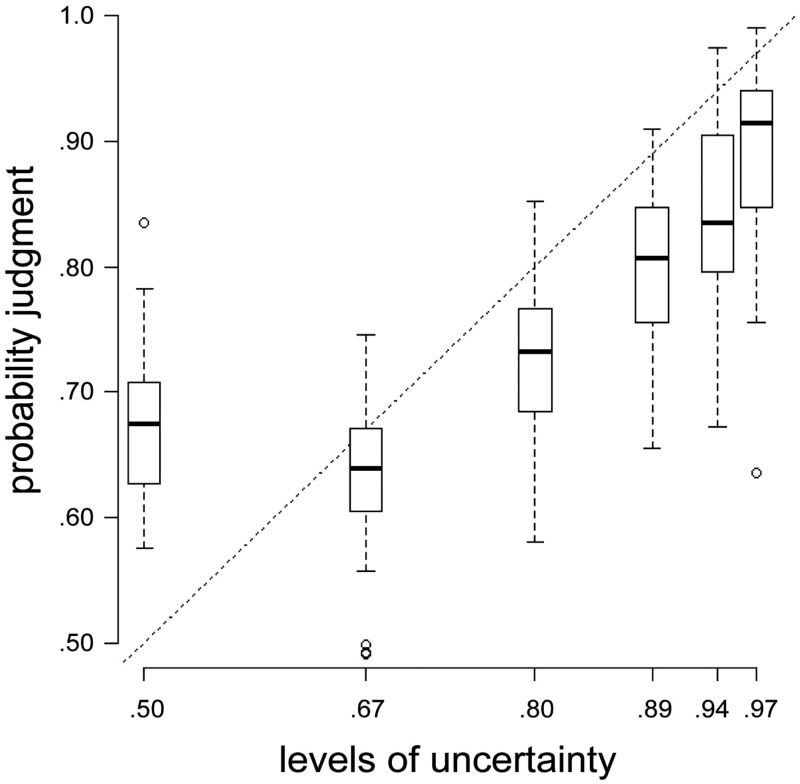
To examine whether participants differentiated between the six different posterior probabilities (i.e. = 0.50, 0.67, 0.80, 0.89, 0.94 and 0.97), we performed a one-way repeated measures analysis of variance with the six levels of uncertainty as within-subject factor and the average probability judgments as the dependent variable. The same analysis was conducted with the logarithm of the reaction times as dependent measure.
Conformity index
The experimental design matrix included six ‘conflicting’ trials in which the two pieces of social information suggested buying the same stock, whereas the private information suggested buying the other stock and where the normatively correct decision was consistent with the social information and opposite to the private information. Therefore, we calculated a ‘conformity index’ for every participant, defined as the percentage of decisions in line with the decision of the others in these specific trials.
Computational models
To explain the cognitive process underlying belief updating, we constructed an ‘Evidence Model’ that represents a modification of the model proposed by Hung and Plott (2001). According to the normative Bayesian solution (Equation 1), a participant is required to update her prior belief with every new piece of evidenceet presented at t. To simplify the Bayesian solution, Equation 1 can be transformed by computing the log odds ratio of the posterior probabilities of which of the two stocks being the profitable one (‘’) assuming equal priors (e.g. Dieckmann and Rieskamp, 2007), that is,
| (2) |
However, people might not follow the Bayesian solution and might weight their private information more heavily than the socially inferred information. To identify how people weight the different pieces of information, we extended Equation 2 by allowing pieces of information to be weighted differently, that is,
| (3) |
where represents a bias for one of the two stocks at t = 0 and refers to the weight given to the different pieces of information. If all weights are equal to 1 and = 0 then Equation 3 is identical to Equation 2, that is, the normative solution is nested within the Evidence Model specified by Equation 3.
When estimating the Evidence Model (see Supplementary Methods), we also imposed three different constraints on the model parameters. First, in the full model (FM) we estimated one bias parameter and three different weights for each piece of information at the three points in time (social information I, social information II and private information), providing four parameters. Second, for the social model (SM), we assumed no bias (i.e. = 0) and one single weight for social information (i.e. = ) and one weight for private information (i.e. ), leading to a total of two free parameters. Third, we also determined the goodness-of-fit of the normative Bayesian model (BM) by setting = 0 and all other weights to 1 (i.e. = 1). Whereas the BM has no flexibility in weighting information differently, the FM allows weighting each piece of information in a different way. The SM assumes that people do not have a bias for one of the options, treat both pieces of social information equally but weight their private information differently. The SM is more complex than the BM but less complex than the FM.
Information weighting index
A decision-maker following Bayesian principles should weight the social and private information equally. To examine to what extent participants deviated from the Bayesian approach, we determined an information weighting index for the SM by dividing the estimated weight for the private information (, i.e. using the mode of the marginal posterior distribution as a point estimate) by the sum of the estimated weights for the private and social information (i.e. + ). An information weighting index >0.50 indicates overweighting of private as compared with social information, whereas values of <0.50 indicate overweighting of social as compared with private information.
Functional imaging data analyses
To study the neural underpinnings of belief updating with social and private information, two first level models were calculated in the context of a Generalized linear model (GLM) (SPM8, Wellcome Trust Center for Neuroimaging, University College London). Our experimental design is characterized by three updating stages (Figure 1). In every trial, participants were forced to update their belief after the decisions of two traders (social information I & II) and after they had received their own private information.
We computed how much a signal given at t = 2 increased/decreased the belief in the option that was more probable at stage t = 1 following the Bayesian solution (i.e. Equation 1). Likewise, we determined the difference of the posterior probability between t = 2 and t = 3. Please note that as the decision of trader 1 was always based on a low (+) quality signal for either stock W or S. Belief updating from t = 0 (i.e. the beginning of a trial) to t = 1 was the same for every trial and therefore not explicitly modeled.
First level analysis
In the ‘first level model 1’, belief updating at the social information II (belief updating by social information) and at the private information (belief updating by private information) stages was modeled with a single parametric regressor to account for general effects of belief updating at both stages (i.e. independent of the social or private nature of the information). Brain activity at the time of the decision and at the time of the probability judgment was modeled with separate parametric regressors tracking the log odds of the probability judgments and the decision for either stock W or S. We also included parametric regressors coding for the stock with the highest posterior probability (at t = 1 and t = 2 and 3 combined) and for the quality of the private information [low (+) or high (++)] at t = 2 and 3 combined. Decision and/or probability judgment time windows in which participants gave no answer and filler stimuli were included in the GLM as regressors of no interest.
In the ‘first level model 2’, the second (social information II) and third (private information) belief updating stages were modeled separately using parametric regressors to account for the specific effects of belief updating by social and private information. The quality of the private information [low (+) or high (++)] was included as a parametric regressor for the belief updating stage at t = 3. In all other respects, first level models 1 and 2 were similar. To account for head movements, both first level models included motion parameters.
Second level analysis
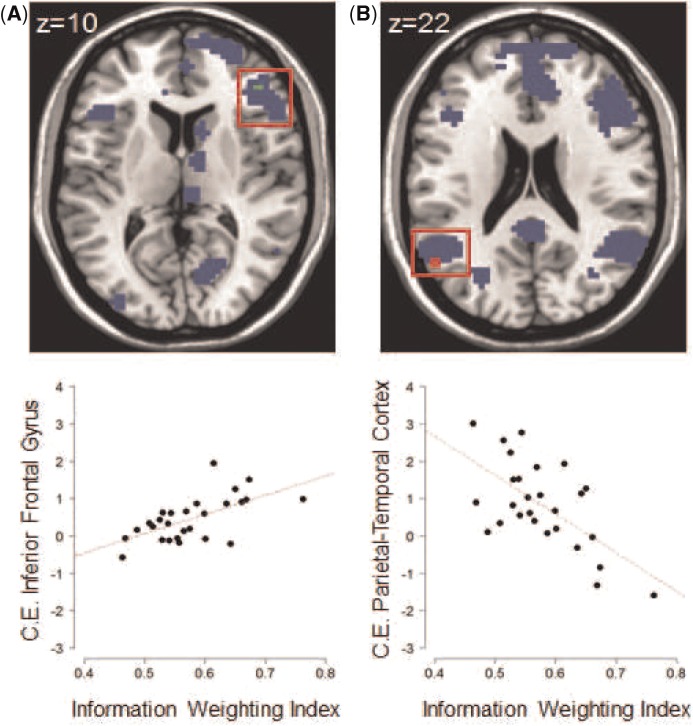
To test for the general (first level model 1) and specific (first level model 2) effects of belief updating as well as for the effects of an increase in subjective uncertainty during decision-making (first level model 2 — see supplementary fMRI results) we used one-sample t-tests on the group level (P < 0.001, uncorrected with a minimum cluster size of 20 voxels). To test how belief updating by private information was modulated by interindividual differences in information weighting, we used a multiple regression design (P < 0.001 or 0.005, uncorrected) with the ‘information weighting index’ as a covariate. To restrict the search volume only to brain regions involved in belief updating by private information we used the results of the respective second level analysis as an explicit mask (P < 0.005, uncorrected with a minimum cluster size of 20 voxels). To further illustrate these findings, we extracted the contrast estimates within two Region of interest (ROI)s (Figure 5) and plotted them against the information weighting index. The ROIs were defined with the MarsBaR toolbox for Statistical parametric mapping (SPM) (Brett et al., 2002).
Fig. 5.
Interindividual differences in belief updating by private information. Blue color indicates brain regions whose activity increased with increasing uncertainty during belief updating by private information. Results of the regression analysis (red boxes) represent activity of the subregions within the inferior frontal gyrus (A) and the parietal-temporal cortex (B) that was significantly correlated with overweighting of private information (information weighting index): the green color indicates a positive correlation, whereas the red color indicates a negative correlation. The two scatterplots display the average contrast estimates per subject within the respective cluster plotted against the information weighting index. The dashed red line displays a linear regression model. Note: P < 0.001, uncorrected with no minimum cluster size; brain regions in blue color (P < 0.005, uncorrected with a minimum cluster size of 20 voxels) display the explicit mask. Clusters are overlayed on a chi2better.nii.gz template provided by MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/).
RESULTS
Behavioral results
Overall, participants performed the task consistent with the Bayesian solution: in 93.18% of all trials in which participants (N = 27) made a decision, they decided in accordance with the Bayesian solution, with seven participants always choosing the more profitable stock. The six different levels of uncertainty significantly modulated participants’ probability judgments, F(3.64, 94.51) = 70.28, P < 0.001 (see Figure 2 for details), with the probability judgments as dependent variable and the six levels of uncertainty as independent variable. The reaction times did not differ significantly between the six levels of uncertainty, F(2.59, 67.44) = 1.57, P = 0.21.
Fig. 2.
The effect of the different levels of uncertainty signaled by social and private information on participants’ probability judgments. An increase in objective certainty (x-axis) led to increased probability judgments (y-axis).
Note: the dotted line indicates the prediction of the normative Bayesian model (BM) (cf. Equation 1). The boxes range from the lower quartile to the upper quartile of the distribution. The black band in the middle of the box represents the median. The whiskers represent the minimum and the maximum of the distribution as long as these estimates are not further away from the median than ±1.5 × Interquartile range (IQR). Circles represent outliers.
Model comparison and parameter estimation
To further explore how participants weighted the different types of information in belief updating, we compared the three different models described above according to their Deviance information criterion (DIC) values (see Supplementary Methods for details on model estimation and model comparison). The SM, which assumes a differential weighting of social as compared with private information, performed best ( = 10.4; = 1590.4). This result was further supported by an analysis at the individual level: the Bayes factors favored the SM as compared with the FM for 24 of all 27 participants.
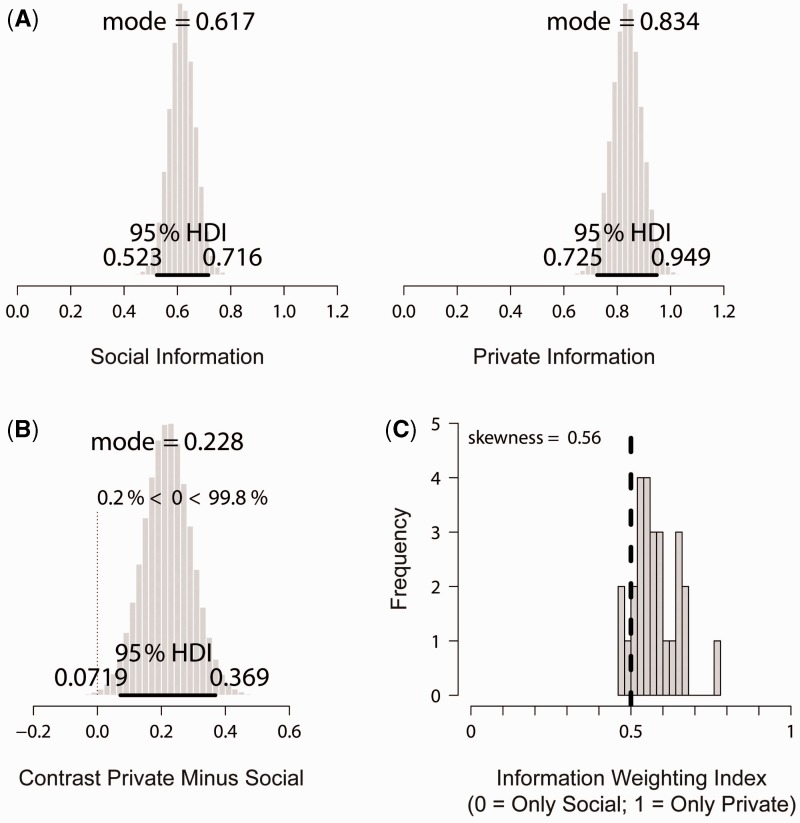
Figure 3 illustrates the difference in weighting of social and private information (SM) in belief updating. The weights given to social information ( — Figure 3A, left) were credibly smaller than the weights given to private information ( — Figure 3A, right). This is further illustrated by the contrast − (Figure 3B). Thus, during belief updating, participants substantially overweighted private as compared with social information. We also calculated the ‘information weighting index’ on the basis of the estimated parameters of the SM for each participant. The information weighting index (Figure 3C) was significantly negatively correlated with the ‘conformity index’, Pearson’s product moment correlation r(25) = −0.83, P < 0.001, suggesting that the more people overweighted private as compared with social information, the less often they started a cascade in the trials of interest.
Fig. 3.
Different weighting of social and private information (SM). (A) Marginal posterior distributions for the weight of the social information () and for the weight of the private information (). (B) The contrast private information minus social information ( − ) indicates a strong difference of weighting of social and private information. (C) The distribution of the information weighting index shows that the majority of subjects overweight private as compared with social information. Note: The 95% Highest Density Interval (95% HDI) spans 95% of the distribution. The vertical dashed line indicates hypothetical unbiased information weighting (i.e. equal weighting of social and private information).
fMRI results
To investigate the neural processing of social and private information increasing uncertainty, we analyzed neural activity associated with belief updating.
General effects of belief updating
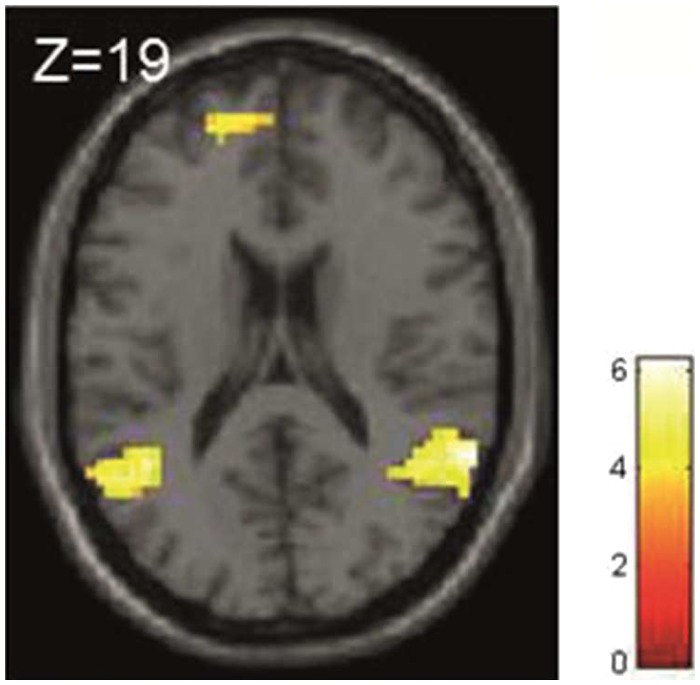
To correctly estimate the probability of choosing the better stock, a participant had to update her (prior) belief with every piece of information received (social information I & II and private information). Therefore, for the initial analysis we used a single parametric regressor that tracked the belief updating process independent of the social or private nature of the information (at t = 2 and 3 combined). Besides others, we found significant activity in fronto-parietal brain regions and in the precuneus during belief updating; that is, the activity of these regions increased with an increase in uncertainty (see Table 1, Figure 4 for further details).
Table 1.
Neural correlates of belief updating
| Contrast | Region | Montreal Neurological Institute centroid |
||||
|---|---|---|---|---|---|---|
| x | y | z | Number of voxel | Z value | ||
| General effects of belief updating (independent of social and private information) | Superior temporal gyrus/inferior parietal cortex | 63 | −49 | 19 | 221 | 4.83 |
| Precuneus/posterior cingulate | 3 | −61 | 34 | 222 | 4.79 | |
| Superior/middle frontal gyrus (DLPFC) | −15 | 29 | 52 | 104 | 4.77 | |
| Superior temporal gyrus/inferior parietal cortex | −42 | −61 | 28 | 229 | 4.54 | |
| Superior/middle frontal gyrus | 21 | 26 | 46 | 59 | 3.99 | |
| Superior/medial frontal gyrus | −18 | 53 | 19 | 35 | 3.87 | |
| Belief updating by social information | Middle temporal gyrus | −42 | −58 | 22 | 28 | 3.79 |
| Belief updating by private information | Superior/middle frontal gyrus (DLPFC)/DMPFC | 48 | 32 | 19 | 1807 | 5.84 |
| Precuneus/posterior cingulate | 6 | −58 | 40 | 309 | 5.69 | |
| Inferior frontal gyrus/anterior insula | 48 | 41 | −14 | 205 | 5.36 | |
| Inferior parietal lobe | 33 | −64 | 40 | 524 | 4.83 | |
| Inferior Parietal Lobe | −48 | −64 | 43 | 372 | 4.52 | |
| Middle occipital gyrus | 27 | −88 | −5 | 145 | 4.45 | |
| Middle temporal gyrus | 42 | −52 | −11 | 161 | 4.41 | |
| Cerebellum | −33 | −73 | −38 | 298 | 4.24 | |
| Inferior frontal gyrus/anterior insula | −33 | 20 | −2 | 108 | 4.17 | |
| Middle/inferior frontal gyrus | −39 | 41 | −8 | 49 | 4.13 | |
| Middle occipital gyrus | −36 | −64 | −11 | 120 | 4.07 | |
| Parahippocampal gyrus | 21 | −28 | −11 | 20 | 3.95 | |
| Dorsal striatum | 12 | 14 | 7 | 20 | 3.85 | |
Note: P < 0.001, uncorrected with a minimum cluster size of 20 voxels.
Fig. 4.
Neural correlates of belief updating by social and private information. Neural activity of the frontal and parietal cortices increased with increasing uncertainty of the decision. Note: P < 0.001, uncorrected with a minimum cluster size of 20 voxels.
Specific effects of belief updating by social or private information
Because our behavioral results indicated a differential processing of private and social information, we analyzed the two main belief updating stages (social information II and private information) independently. The left middle temporal gyrus/inferior parietal lobule was active during belief updating when subjects processed social information II (Table 1), whereas activity of the anterior insula, the DLPFC and the parietal cortex, besides others (Table 1 and Figure 5), correlated with belief updating by private information.
Modulation of belief updating by individual differences in overweighting private information
The probability of an informational cascade starting depends on the differential weighting of private and social information. Therefore, we used the information weighting index to analyze how the process of belief updating (at t = 3) is modulated by interindividual differences in information weighting. The regression analysis showed a positive correlation of the belief updating activity in the inferior frontal gyrus with the information weighting index: a similar positive correlation was observed in the anterior insula using a more liberal threshold (P < 0.005; uncorrected). Overall, the more participants overweighted private as compared with social information, the more active the inferior frontal gyrus/anterior insula were during belief updating of private information (Figure 5A and Table 2). An opposite effect was found in the parietal-temporal cortex: the more participants overweighted private as compared with social information, the less active the parietal-temporal cortex was during belief updating of private information (Figure 5B and Table 2).
Table 2.
Neural correlates of interindividual differences in overweighting private information
| Contrast | Region | Montreal Neurological Institute centroid |
Number of voxel | Z value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlation with Information Weighting Index | Inferior frontal gyrus | 45 | 38 | 10 | 3 (11) | 3.35 |
| Inferior frontal gyrus/anterior Insula | 39 | 17 | −5 | (3) | (2.90) | |
| Negative correlation with Information Weighting Index | Middle temporal gyrus | −51 | −64 | 22 | 8 (36) | 3.46 |
| Midbrain | −3 | −10 | −11 | 1 (3) | 3.45 | |
| Middle temporal gyrus | −54 | 2 | −23 | 1 (8) | 3.38 | |
| Middle temporal gyrus | −48 | 11 | −29 | 1 | 3.35 | |
| Midbrain | −6 | −13 | −8 | 1 | 3.24 | |
| Middle temporal gyrus | −63 | −31 | −8 | 1 (20) | 3.13 | |
| Middle temporal gyrus | −51 | 2 | −29 | 1 | 3.11 | |
| Precuneus | −3 | −52 | 40 | 1 (14) | 3.11 | |
| Middle temporal gyrus | −57 | −31 | −11 | 1 | 3.10 | |
| Cerebellum | −33 | −85 | −38 | (8) | (3.01) | |
| Middle frontal gyrus | −39 | 17 | 52 | (3) | (2.86) | |
| Cerebellum | −15 | −88 | −38 | (3) | (2.81) | |
| Medial frontal gyrus | −6 | 50 | 46 | (1) | (2.64) | |
| Cerebellum | −18 | −82 | −29 | (1) | (2.63) | |
Note: All results uncorrected. Two different uncorrected thresholds are reported: Z values in brackets are significant at P < 0.005 (uncorrected), whereas Z values without brackets represent results significant at P < 0.001 (uncorrected). The number of voxels reported in brackets is significant at P < 0.005 (uncorrected), whereas the number of voxels reported without brackets is significant at P < 0.001 (uncorrected).
DISCUSSION
By combining neurobiological, economic and computational approaches, we were able to show that people who tend to overweight private as compared with social information show a decreased activity in the parietal-temporal cortex and an increased activity in the inferior frontal gyrus/anterior insula while updating their beliefs by private information. This study illuminates the neural underpinnings of biased belief updating by private information — the cognitive process that is decisive for the emergence and stability of informational cascades.
Making an optimal decision when observing other people’s decisions and receiving personal (private) information as represented by the informational cascades paradigm requires the integration of available social and private information as described by the Bayesian solution. Deviations from the Bayesian solution (e.g. overweighting of private information) can influence subsequent decisions and therefore the occurrence of informational cascades. It is especially important for the theory of informational cascades to understand how the neural process of belief updating (of private information) is modulated by such deviations. The computational analysis of the behavioral data showed that subjects weighted private and social information differently: the majority of subjects (24 of 27 participants) overweighted private as compared with social information. This finding is consistent with recent research on informational cascades: a comprehensive meta-analysis by Weizsäcker (2010) showed that decision-makers often overweight private information even in situations in which it would be optimal to follow others. The results of our behavioral control study (see Supplementary Results) indicate that subjects specifically overweight private information, which cannot alternatively be explained by an order-effect of overweighting recent information. Importantly, previous studies have shown that overweighting of private information strongly influences the emergence and stability of informational cascades (Bernardo and Welch, 2001; Nöth and Weber, 2003; Goeree et al., 2007). We also found a strong negative correlation between the individual tendency to make conforming decisions (conformity index) and overweighting of private information (information weighting index). This clearly indicates that overweighting of private information lowers the tendency to follow others and thereby lowers the probability that an informational cascade starts or continues.
Our fMRI results showed that an increase in uncertainty during belief updating by either social or private information activated the parietal-temporal cortex — a region of the brain previously associated with number processing (Dehaene et al., 1998, 2003). Additionally, we found that an increase in uncertainty during belief updating by private information activated the DMPFC, bilateral anterior insula and DLPFC — brain regions closely linked to decision risk (for a review, see Mohr et al., 2010). Furthermore, we demonstrated that stronger individual overweighting of private information positively correlated with activity in the inferior frontal gyrus/anterior insula and negatively with activity in the parietal-temporal cortex.
It has been shown that the inferior frontal gyrus is often co-active with the anterior insula (Paulus et al., 2003; Wright et al., 2012) and may constitute the so called ‘fronto-insular junction’ (Craig, 2009). In the decision-making under risk literature, activity of the inferior frontal gyrus has been related to higher risk aversion (Christopoulos et al., 2009), an increase in positive skewness (the chance of a better than average outcome is small) (Symmonds et al., 2011), an increase in the variance of an outcome (uncertainty) for risk-seeking individuals (Tobler et al., 2007), ambiguous vs. non-ambiguous gambles, especially for ambiguity-averse individuals (Bach et al., 2011), and increasing uncertainty (Huettel et al., 2005). Interestingly, a more posterior region within the inferior frontal gyrus was recently found to be more active the more improbable an event becomes as the result of a Bayesian updating process (d’Acremont et al., 2013). Tracking of Bayesian posterior probabilities, however, has to be differentiated from belief updating of uncertainty, as these are two different processes based on two different, but related, concepts (probability of occurrence with 0 ≤ P ≤ 1 as compared with uncertainty with 0.5 ≤ P ≤ 1). How belief updating leads to adjusted representations of posterior probabilities (i.e. the outcome of the belief updating process) is not yet known.
Activity in the anterior insula has been linked to risk anticipation (Preuschoff et al., 2006; Mohr et al., 2010), prediction of risk (Preuschoff et al., 2008), risk-aversion mistakes (Kuhnen and Knutson, 2005), intolerance of uncertainty (Simmons et al., 2008), risk during the selection of the potential behavioral responses (Huettel, 2006) and to the integration of subjective risk preference (Symmonds et al., 2011). Activity of the insular cortex has also been associated with the degree of harm avoidance (Paulus et al., 2003) and choice strategies that try to minimize losses (Venkatraman et al., 2009). Thus, we can speculate that the stronger uncertainty-related activity of the inferior frontal gyrus/anterior insula during the processing of private information conflicting with social information can overcome the effects of social conformity in subjective estimates of uncertainty.
However, according to the computational model (SM) overweighting of private information changes the posterior probability and thereby uncertainty. Thus, increased uncertainty could potentially explain increased activation of the inferior frontal gyrus/anterior insula in participants who strongly overweighted private information. To examine this explanation we determined whether overweighting of private information indeed increased uncertainty. The (un-)certainty measured as the average absolute difference between the posterior probability and a pure chance prediction of 0.5 across all trials was nearly the same for the SM with 0.2627 and the standard model (BM) with 0.2602. Therefore, overweighting of private information did not on average increase uncertainty and can be ruled out as an explanation for the increased activity of the inferior frontal gyrus/anterior insula. Instead, it appears plausible that people who are very sensitive to cues associated with uncertainty as reflected in increased activity of the inferior frontal gyrus/anterior insula tend to overweight private information. Overall, our results further support the important role of the anterior insula in the neural mechanism of social influence on human behavior (Klucharev et al., 2009; Berns et al., 2010; Campbell-Meiklejohn et al., 2010; Izuma and Adolphs, 2013).
The parietal-temporal cortex was active at all stages of belief updating (by social and private information). Importantly, activity of the parietal-temporal cortex was modulated by interindividual differences in the weighting of private information: stronger overweighting of private information was associated with decreased activity in the parietal-temporal cortex during the final stage of belief updating. Previous human and nonhuman studies consistently associated the parietal cortices with number processing (Dehaene et al., 1998, 2003) and with the resolution of uncertainty in tasks with limited knowledge about the correct action to take (Volz et al., 2003, 2004; Huettel et al., 2005, 2006; Kiani and Shadlen, 2009; Symmonds et al., 2011). Our results suggest that people with stronger numerical processing of private information in the parietal cortices are less biased toward private information and estimate uncertainty closer to the Bayesian optimal solution; however, this makes them more prone to start an informational cascade. Overall, we suggest a 2-fold neural mechanism of overweighting of private information in informational cascades: (i) increased activity of the inferior frontal gyrus/anterior insula and (ii) decreased activity in the parietal-temporal cortex. At a later stage during decision-making, these two neural signals could be integrated via the direct anatomical connection between insula and posterior parietal cortex (Cavada and Goldman-Rakic, 1989). Further experiments are needed to explore this hypothesis.
We found a large overlap of activations evoked by increased uncertainty during belief updating by private information and during decision-making (see supplementary fMRI results). In both time windows, we observed uncertainty-related activity of the DMPFC, anterior insula, parietal cortex and DLPFC. A meta-analysis by Mohr et al. (2010) showed that these brain regions are more strongly activated for decision risk as compared with anticipation risk. In our task, all relevant information was already available after the presentation of private information. Therefore, participants had the opportunity to form a decision (i.e. select a stock) before the response cue. Thus, in our task it is difficult to differentiate the neural effects related to belief updating and decision-making at the last stages of a trial. Interestingly, in contrast to Stern et al. (2010), we did not find activity in the dorsal anterior cingulate cortex during belief updating in general (even when using a very low uncorrected threshold of 0.10). This discrepancy could be caused by the differences in the statistical analysis and/or design of the two studies. In contrast to our study, participants in the evidence accumulation task used by Stern et al. (2010) (i) rated uncertainty after each of the information cues, (ii) received only private information, (iii) received a feedback after every trial and (iv) had the opportunity to decline a decision. Behrens et al. (2008) showed that the anterior cingulate gyrus is involved in social learning. However, to clarify the exact role of the anterior cingulate cortex in belief updating further studies are needed. Additional studies will also help to generalize the observed mechanisms to different social environments. In particular, we used a decision scenario in which participants inferred information from hypothetical behavior of others. Therefore, it appears necessary to examine situations in which social information is inferred from real behavior of other people in the future.
Taken together, we show that private information conflicting with social information activates brain regions associated with risk and uncertainty. Furthermore, activity of the inferior frontal gyrus/anterior insula and the parietal-temporal cortex were modulated by interindividual differences in the overweighting of private information. The behavioral results indicate that such interindividual differences can influence the probability that a cascade starts. By and large, our results suggest a profound role of the uncertainty-related neural activity in the formation of informational cascades.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Markus Klarhoefer, Oliver Schürmann and Stefan Thommen for assistance in the fMRI experiments and Andreas Pedroni and Peter Mohr for helpful comments.
This work was supported by the Swiss National Science Foundation (SNSF grant no. 100014-130352) to Vasily Klucharev and Jörg Rieskamp and by the Switzerland–Russian Scientific & Technological Cooperation Program, the Russian Targeted Federal Program ‘Scientific and scientific-pedagogical personnel of innovative Russia’ (contract 8488, by a grant from the Russian Foundation for Basic Research [RFBR 11-06-00449-a]).
REFERENCES
- Anderson LR, Holt CA. Information cascades in the laboratory. American Economic Review. 1997;87:847–62. Available: http://www.jstor.org/stable/2951328. [Google Scholar]
- Bach DR, Hulme O, Penny WD, Dolan RJ. The known unknowns: neural representation of second-order uncertainty, and ambiguity. Journal of Neuroscience. 2011;31:4811–20. doi: 10.1523/JNEUROSCI.1452-10.2011. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3166851&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AV. A simple model of herd behavior. Quarterly Journal of Economics. 1992;107:797–817. Available at: http://www.jstor.org/stable/2118364. [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456:245–9. doi: 10.1038/nature07538. Available: http://www.nature.com/nature/journal/v456/n7219/suppinfo/nature07538_S1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo AE, Welch I. On the evolution of overconfidence and entrepreneurs. Journal of Economics and Management Strategy. 2001;10:301–30. Available: 10.1162/105864001316907964. [Google Scholar]
- Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage. 2010;49:2687–96. doi: 10.1016/j.neuroimage.2009.10.070. Available: http://www.sciencedirect.com/science/article/pii/S1053811909011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikhchandani S, Hirshleifer D, Welch I. A theory of fads, fashion, custom, and cultural change as informational cascades. Journal of Political Economy. 1992;100:992–1026. Available: http://www.jstor.org/stable/2138632. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] presented at the 8th International Conference on Functional Mapping of the Human Brain. Neuroimage. 2002;16(2) abstract 497. [Google Scholar]
- Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Current Biology. 2010;20:1165–70. doi: 10.1016/j.cub.2010.04.055. Available: http://linkinghub.elsevier.com/retrieve/pii/S0960982210005956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology. 1989;287:422–45. doi: 10.1002/cne.902870403. Available: http://dx.doi.org/10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chari VV, Kehoe PJ. Financial crises as herds: overturning the critiques. Journal of Economic Theory. 2004;119:128–50. Available: http://www.sciencedirect.com/science/article/pii/S0022053103002254. [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience. 2009;29:12574–83. doi: 10.1523/JNEUROSCI.2614-09.2009. Available: http://www.jneurosci.org/cgi/content/abstract/29/40/12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini RB, Goldstein NJ. Social influence: compliance and conformity. Annual Review of Psychology. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. Available: http://www.annualreviews.org/doi/abs/10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. Available: http://www.nature.com/nrn/journal/v10/n1/full/nrn2555.html. [DOI] [PubMed] [Google Scholar]
- d’Acremont M, Schultz W, Bossaerts P. The human brain encodes event frequencies while forming subjective beliefs. Journal of Neuroscience. 2013;33:10887–197. doi: 10.1523/JNEUROSCI.5829-12.2013. Available: http://www.jneurosci.org/content/33/26/10887.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. Abstract representations of numbers in the animal and human brain. Trends in Neurosciences. 1998;21:355–61. doi: 10.1016/s0166-2236(98)01263-6. Available: http://www.sciencedirect.com/science/article/pii/S0166223698012636. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. Available: http://www.tandfonline.com/doi/abs/10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Dieckmann A, Rieskamp J. The influence of information redundancy on probabilistic inferences. Memory and Cognition. 2007;35:1801–13. doi: 10.3758/bf03193511. Available: http://dx.doi.org/10.3758/BF03193511. [DOI] [PubMed] [Google Scholar]
- Ellis CJ, Fender J. Information cascades and revolutionary regime transitions. The Economic Journal. 2011;121:763–92. Available: http://dx.doi.org/10.1111/j.1468-0297.2010.02401.x. [Google Scholar]
- Goeree JK, Palfrey TR, Rogers BW, McKelvery RD. Self-correcting information cascades. Review of Economic Studies. 2007;74:733–62. Available: http://search.ebscohost.com/login.aspx?direct=true&db=buh&AN=25378406&site=ehost-live. [Google Scholar]
- Huettel SA. Behavioral, but not reward, risk modulates activation of prefrontal, parietal, and insular cortices. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:141–51. doi: 10.3758/cabn.6.2.141. Available: http://link.springer.com/article/10.3758%2FCABN.6.2.141. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25:3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. Available: http://www.jneurosci.org/content/25/13/3304.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–75. doi: 10.1016/j.neuron.2006.01.024. Available: http://www.sciencedirect.com/science/article/pii/S089662730600078X. [DOI] [PubMed] [Google Scholar]
- Hung AA, Plott CR. Information cascades: replication and an extension to majority rule and conformity-rewarding institutions. American Economic Review. 2001;91:1508–20. Available: http://www.jstor.org/stable/2677936. [Google Scholar]
- Izuma K, Adolphs R. Social manipulation of preference in the human brain. Neuron. 2013;78:563–73. doi: 10.1016/j.neuron.2013.03.023. Available: http://www.sciencedirect.com/science/article/pii/S0896627313002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324:759–64. doi: 10.1126/science.1169405. Available: http://www.sciencemag.org/content/324/5928/759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–51. doi: 10.1016/j.neuron.2008.11.027. Available: http://www.sciencedirect.com/science/article/pii/S0896627308010209. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. Available: http://www.sciencedirect.com/science/article/pii/S0896627305006574. [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR. Neural processing of risk. Journal of Neuroscience. 2010;30:6613–19. doi: 10.1523/JNEUROSCI.0003-10.2010. Available: http://www.jneurosci.org/content/30/19/6613.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöth M, Weber M. Information aggregation with random ordering: cascades and overconfidence. The Economic Journal. 2003;113:166–89. Available: http://onlinelibrary.wiley.com/doi/10.1111/1468-0297.00091/abstract. [Google Scholar]
- Oberholzer-Gee F. Nonemployment stigma as rational herding: a field experiment. Journal of Economic Behavior and Organization. 2008;65:30–40. Available: http://www.sciencedirect.com/science/article/pii/S0167268106001119. [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–48. doi: 10.1016/s1053-8119(03)00251-9. Available: http://www.sciencedirect.com/science/article/pii/S1053811903002519. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–90. doi: 10.1016/j.neuron.2006.06.024. Available: http://www.sciencedirect.com/science/article/pii/S0896627306005046. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human Insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. Available: http://www.jneurosci.org/content/28/11/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat RM, Chater N, Frith C. Herding in humans. Trends in Cognitive Sciences. 2009;13:420–8. doi: 10.1016/j.tics.2009.08.002. Available: http://www.sciencedirect.com/science/article/pii/S1364661309001703. [DOI] [PubMed] [Google Scholar]
- Selten R. Axiomatic characterization of the quadratic scoring rule. Experimental Economics. 1998;1:43–62. Available: http://dx.doi.org/10.1007/BF01426214. [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neuroscience Letters. 2008;430:92–7. doi: 10.1016/j.neulet.2007.10.030. Available: http://www.sciencedirect.com/science/article/pii/S0304394007011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Gonzalez R, Welsh RC, Taylor SF. Updating beliefs for a decision: neural correlates of uncertainty and underconfidence. Journal of Neuroscience. 2010;30:8032–41. doi: 10.1523/JNEUROSCI.4729-09.2010. Available: http://www.jneurosci.org/content/30/23/8032.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmonds M, Wright ND, Bach DR, Dolan RJ. Deconstructing risk: separable encoding of variance and skewness in the brain. Neuroimage. 2011;58:1139–49. doi: 10.1016/j.neuroimage.2011.06.087. Available: http://www.sciencedirect.com/science/article/pii/S105381191100752X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. Journal of Neurophysiology. 2007;97:1621–32. doi: 10.1152/jn.00745.2006. Available: http://jn.physiology.org/content/97/2/1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62:593–602. doi: 10.1016/j.neuron.2009.04.007. Available: http://www.sciencedirect.com/science/article/pii/S0896627309002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cerebral Cortex. 2009;19:916–25. doi: 10.1093/cercor/bhn140. Available: http://cercor.oxfordjournals.org/cgi/content/abstract/19/4/916. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19:271–80. doi: 10.1016/s1053-8119(03)00122-8. Available: http://www.sciencedirect.com/science/article/pii/S1053811903001228. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage. 2004;21:848–57. doi: 10.1016/j.neuroimage.2003.10.028. Available: http://www.sciencedirect.com/science/article/pii/S1053811903006797. [DOI] [PubMed] [Google Scholar]
- Weizsäcker G. Do We follow others when we should? A simple test of rational expectations. American Economic Review. 2010;100:2340–60. Available: http://www.aeaweb.org/articles.php?doi=10.1257/aer.100.5.2340. [Google Scholar]
- Wright ND, Symmonds M, Hodgson K, Fitzgerald THB, Crawford B, Dolan RJ. Approach–avoidance processes contribute to dissociable impacts of risk and loss on choice. Journal of Neuroscience. 2012;32:7009–20. doi: 10.1523/JNEUROSCI.0049-12.2012. Available: http://www.jneurosci.org/content/32/20/7009.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.