Abstract
Importance
Autism Spectrum Disorders (ASD) aggregates in families, but the individual risk and to what extent this is caused by genetic factors, or shared or non-shared environment remains unresolved.
Objective
To provide estimates of familial aggregation of ASD.
Design, Setting and Participants
A population based cohort of all Swedish children born 1982–2007. We identified all twins, full siblings, maternal and paternal half siblings and cousin pairs and all diagnosis of ASD to 31-December-2009.
Main Outcome Measure(s)
The relative recurrence risk (RR) measure familial aggregation of disease. The RR is the relative risk of autism in an participant given a sibling or cousin has the diagnosis, compared with the risk in a participant with no diseased family member. We calculated RR for both ASD and Autistic Disorder (AD). We estimated how much of the probability of developing ASD can be related to genetic (additive and dominance) and environmental (shared and non-shared) factors.
Results
In the sample of 2,049,899 children, 14,516 obtained an ASD diagnosis of which 5,689 were AD. The ASD RR was estimated to 153.0 (95%CI 56.7–412.8; 27 vs 6,273 per 100,000 person-years) for monozygotic twins, 8.2 (95%CI 3.7–18.1; 55 vs 805 per 100,000 person-years) for dizygotic twins, 10.3 (95%CI 9.4–11.2; 49 vs 829 per 100,000 person-years) for full-siblings, 3.3 (95%CI 2.6–4.2; 94 vs 492 per 100,000 person-years) for maternal half siblings, 2.9 (95%CI: 2.2–3.7; 85 vs 371 per 100,000 person-years) for paternal half siblings, and 2.0 (95%CI: 1.8–2.2; 49 vs 155 per 100,000 person-years) for cousins. The RR pattern was similar for AD but of slightly higher magnitude. We found support for a disease etiology including only additive genetic and non-shared environmental effects. The ASD heritability was estimated to 0.50 (95%CI 0.44–0.55) and the AD heritability was estimated to 0.54 (95%CI 0.44–0.64).
Conclusion and Relevance
Among children born in Sweden, heritability of ASD and AD were estimated to be approximately 50%. For an individual, the risk of autism is increased 10 fold if a full sibling has the diagnosis and about 2 fold if a cousin has the diagnosis. These findings may inform counseling families with affected children.
INTRODUCTION
Autism Spectrum Disorders (ASD) is affecting almost 1% of the population, and defined by impairments in social interaction and communication and the presence of restricted interests and repetitive behaviors. Autistic disorder (AD) is most profound form of ASD1.
Family studies found that ASD aggregates in families and early twin studies estimated the proportion of the phenotype variance due to genetic factors (the heritability), to be about 90%2–6, making it the most heritable of all developmental disorders. As a consequence, etiological research in ASD, focus predominantly on genetic factors7. While recent twin studies support high heritability5,6 a large twin study7 indicated substantial role for shared environmental influences. Results of family studies also raise questions about the relative influence of genetic factors8 and contribute to uncertainty regarding the etiology of ASD.
Furthermore, previous studies have limitations. Twin studies often having only small samples limiting the reliability when studying rare diseases such as ASD. None of the previous studies represent a prospective population based random sample which raises concerns for potential biases introduced by population selection. Restricted follow up time, and possible differences in etiology for different ASD subtypes may also limit reliability.
Also while heritability estimates provide a valuable metric for the effects of genetic factors in the population, they do not provide any information on individual risk. Detailed etiological models will require accounting for risk on a population level, as well as providing quantitative information in a given individual, thus allowing for individualized disease prevention and treatment9. Recurrence risk express the risk of yet another affected family member in an already affected family. The relative recurrence risk measure this recurrence in relation to disease in families not yet affected but can be interpreted and compared between groups which may differ in disease prevalence. Consequently, there is a need for reliable estimates of heritability for ASD, as well as combine these population-based estimates with individual-level risk estimates providing a more precise and complete picture of the etiology of ASD.
To that goal we conducted a longitudinal cohort study of all births in Sweden between 1982 and 2007. Using all pairs of monozygote (MZ) and dizygote (DZ) twins, full siblings, half siblings and cousin pairs in the population we determined the family clustering of ASD by estimating relative recurrence risk (RR) within families, and assessed the importance of genetic vs. environmental factors associated with ASD.
METHODS
Study Population
A birth-cohort of all children born alive in Sweden January 1, 1982 to December 31, 2006 was established using data from Swedish national registers including the Medical Birth Register10, Multi Generation Register11, Patient Register12–14, Twin register15 and Statistics Sweden registers for vital statistics. Single-child families were excluded from the cohort. Twin zygosity was obtained from the Twin Registry, and was determined by DNA analysis in 86% of same-sex twins. For the remainder, an algorithm based on five parent-reported items assessing twin similarity, was used. The Swedish Multi Generation register contain identifiers for the parents of all children born 1932 and onwards. This allowed us to determine family relations; full- and maternal and paternal half-siblings and cousins using the unique identifiers of the parents and grand fathers of all Swedish children born 1982 through 2006. Cousins were derived between full siblings only. Further details in online appendix A. The study was approved by the ethics committee at the Karolinska Institutet, Stockholm, Sweden. Informed consent was waived by the ethics committee. Data are collected routinely by Swedish government agencies and were merged and anonymized by an independent government agency (Statistics Sweden), and the code linking the personal identification numbers to the new case numbers was destroyed immediately after merging. Therefore, informed consent was not required.
Ascertainment of autism and psychiatric diagnosis
In Sweden all infants and preschool children regularly undergo routine medical and developmental examinations. At age 4 a mandatory developmental assessment (motor, language, cognitive and social development) is conducted. Children with suspected developmental disorders are referred for further assessment by a specialized team in a child psychiatry unit or habilitation service. Diagnostic information is reported to the Patient Register. The register has nearly complete national coverage12 of psychiatric diagnoses since 1973. With a rare disease the sensitivity is a smaller problem than the specificity of the diagnostic codes. For this we rely on previous validation studies of psychiatric codes generally12,14 and for autism specifically16. With prospective follow-up until 31 December 2009. Autistic disorder (AD) was defined by codes from the “International Classification of Diseases”, version 9 (ICD-9) 299. A/B/X and version 10 (ICD-10) F84.0 while ASD also included ICD-10 F84.1 (Atypical autism), F84.5 (Asperger’s syndrome), F84.8 (Other pervasive developmental disorders) and F84.9 (Pervasive developmental disorder, unspecified).
Covariates
We considered several factors that might confound or modify the familial associations. Parental psychiatric history has been associated with autism in the offspring. Parental psychiatric history was classified as present/not-present for each parent separately using any psychiatric diagnosis at any time before the birth of the oldest child in a siblings or cousins pair using ICD 7th–10th revisions (eTable 4). We also obtained information on paternal and maternal age at birth of the child, birth year and sex.
Statistical methods
Relative recurrence risk
The RR for siblings is the risk of autism diagnosis in a sibling of an autistic child compared with a sibling to a non-autistic child. We calculated RR in families of different genetic relatedness; full-siblings, maternal and paternal half-siblings and cousins. Cousin-pairs were defined as cohort members having the same grandparents, but no parents in common. To allow a direct comparison between cousin RR and sibling RR we did not consider cousins between single-child-families.
We estimated the RR for ASD by Cox proportional hazards regression using the sibling attained age as underlying time scale17. Each individual in a sibling or cousin pair was entered into the cohort and followed for a diagnosis of autism starting from the age of one or from 1 January 1987, which ever came last. Each sibling/cousin was then followed to his first autism diagnosis, death, emigration or death or emigration of his non-autistic sibling or 31 December 2009, whichever came first. The exposure (autistic or non-autistic sibling) was treated as a time-varying covariate in the models. Each sibling in a family typically contribute to the calculations in two ways: as an exposed sibling and as a proband per pair. A sibling may also occur in more than one pair. Consequently we used robust standard errors to account for the dependence between (pairs of) individuals in a family18. Further details of the RR calculations is given in online appendix A.
For descriptive purposes we calculated the cumulative probability of ASD up to the age of 20 years (i.e. the prevalence) using the Cox regression. For the calculation of RR the Cox regression makes an implicit assumption of hazards ratios constant across time (age of the sibling). We verified the validity of this assumption by plotting the Schoenfeld residuals19.
A change in RR for later birth cohorts may be due to truncation of follow-up time or due to changes in incidence. The children born 1982 are followed for 28 years while the children born 2006 are only followed for three years. In the Cox model this could show up as a violation of the proportional hazards assumption which we tested for. To address this further we calculated the RR by birth cohorts using all available follow-up time.
The RR was calculated separately for monozygotic and dizygotic twins, full siblings, maternal and paternal half siblings as well as for cousins. We excluded twins from the sibling analyzes. We considered several factors that might confound the RR including parental psychiatric history, parental age, birth year and sex of the exposing sibling. As parental psychiatric history and parental age may be on a causal path between familial risk and adverse developmental outcome we fitted models adjusting for confounding with and without these covariates. We treated the covariates categorically as sex of the exposed sibling and of the proband, birth cohort (1982–86, 1987–91, 1992–96, 1997–2001, 2002–06), maternal age (≤35 years, >35 years) and paternal age (≤40 years, >40 years) of the exposed sibling, and paternal and maternal psychiatric history (yes/no) at birth of the oldest sibling.
Heritability
Autism diagnosis is a dichotomy (yes/no). By assuming a continuous normally distributed trait is underlying the observed autism diagnosis the correlation of autism diagnosis between family members can be estimated. These are called tetrachoric correlations and are frequently calculated in family and twin studies to obtain approximate estimate of the genetic and non-genetic influences. Next we fitted liability-threshold models20(pp43–77) pp43–77. using MZ- and DZ twins, full siblings and paternal and maternal half siblings and cousins to decompose the variance in liability into a factor for additive genetic effect reflecting inherited additive effects of different alleles, non-additive genetic factors reflecting interaction effects between alleles at the same gene locus, shared environmental factors reflecting non-genetic influences that contribute to similarity within pairs of siblings and non-shared environmental factors reflecting experiences that make sibling pairs dissimilar. From each family one sibling pair was randomly included in the calculations.
Using likelihood ratio tests we compared the full model versus different smaller sub-models obtained by dropping both or only one of the four genetic and environment parameters in order to explain the observed data and pattern of variance using as few parameters as possible. The proportion of the ASD liability contributed by genetic factors, the heritability, was then calculated as the variance associated with the genetic term(s) divided with the total variance. Details of the models are presented in online appendix X.
All calculations were done for ASD and AD separately. All tests of statistical hypothesis were done on the two-sided 5% level of significance. We used SAS software version 9.3 and the R software version 2.15.2 (Linux 64-bit package ‘survival’ for Cox regression; package ‘OpenMx’ version 1.3.1–217922 for heritability).
Finally we also performed a few sensitivity analyses. We calculated ASD RR adjusting for 1-year birth cohorts using natural splines. To challenge that the ASD RR were dependent on family size, due to stoppage or fertility related, we calculated the full siblings RR in sub-groups of family size (eTable 5).
RESULTS
The cohort included a total of 2,049,973 unique siblings/cousins; 2,642,064 full sibling pairs, 432,281 maternal half sibling pairs, 445,531 paternal half sibling pairs and 37,570 twins and 5,799,875 cousin pairs. We found 14,516 cases of ASD of which 5,689 (39%) had a diagnosis of AD (Table 1). The male/female sex ratio was 2.7 for ASD cases and 2.4 for AD cases.
Table 1.
Confounder and baseline characteristics across sibling relations; the study participant and his exposing proband. Count, percent, median and percentiles of the participants.
| Variable | Full Siblings | Maternal Half Siblings | Paternal Half Siblings | Cousins | DZ Twins | MZ Twins |
|---|---|---|---|---|---|---|
| Participants | 1,788,009 | 288,671 | 286,705 | 1,241,166 | 29,032 | 8,338 |
| Participant Pairs (discordant) | 2,641,822 (34,465) | 432,114 (8,896) | 445,335 (8,179) | 5,798,842 (73,615) | 29,424 (411) | 8,354 (56) |
| Boys (%) | 51.5% | 51.2% | 51.1% | 51.5% | 51.0% | 47.2% |
| ASD cases (%) | 12,033 (0.67%) | 2,955 (1.02%) | 2,538 (0.89%) | 8,073 (0.65%) | 215 (0.74%) | 41 (0.49%) |
| AD cases (%) | 4,762 (0.27%) | 1,000 (0.35%) | 877 (0.31%) | 2,996 (0.24%) | 97 (0.33%) | 21 (0.25%) |
| Maternal Psych History (%)# | 39,233 (2.2%) | 18,419 (6.4%) | 14,475 (5.0%) | 25,180 (2.0%) | 908 (3.1%) | 196 (2.4%) |
| Paternal Psych. History (%)# | 38,427 (2.1%) | 15,666 (5.4%) | 16,137 (5.6%) | 23,778 (1.9%) | 792 (2.7%) | 200 (2.4%) |
| Maternal age ≥35 (%) | 209,941 (11.7%) | 41,019 (14.2%) | 37,624 (13.1%) | 132,881 (10.7%) | 1,216 (14.6%) | 6,590 (22.7%) |
| Paternal age ≥35 (% | 149,650 (8.3%) | 26,685 (9.2%) | 40,900 (14.3%) | 79,668 (6.4%) | 776 (9.3%) | 4,020 (13.8%) |
| Birth Year, Median (p5-p95) | 1993 (1984–2005) | 1993 (1983–2005) | 1993 (1983–2005) | 1993 (1984–2004) | 1996 (1983–2005) | 1994 (1982–2002) |
| Age at ASD diagnosis, Median (p5-p95) | 13 (4–22) | 13 (4–22) | 13 (4–23) | 13 (4–22) | 11 (4–21) | 10 ( 4–25) |
| Person Years, Median (p5- p95) | 14 (4–23) | 10 (3–20) | 10 (3–20) | 15 (4–24) | 13 (4–25) | 14 (7–26) |
ASD: Autism Spectrum Disorder; AD: Autistic Disorder (infantile autism); p5: 5th percentile, p95: 95th percentile.
Note: Besides the “Sibling pairs”, the statistics (cound, percent, median and percentiles) are calculated across unique participants in each group.
Pairs are the pairs of siblings (cousins) who enter the statistical analysis;
At birth of the proband, that is, at the birth of the oldest sibling in the family
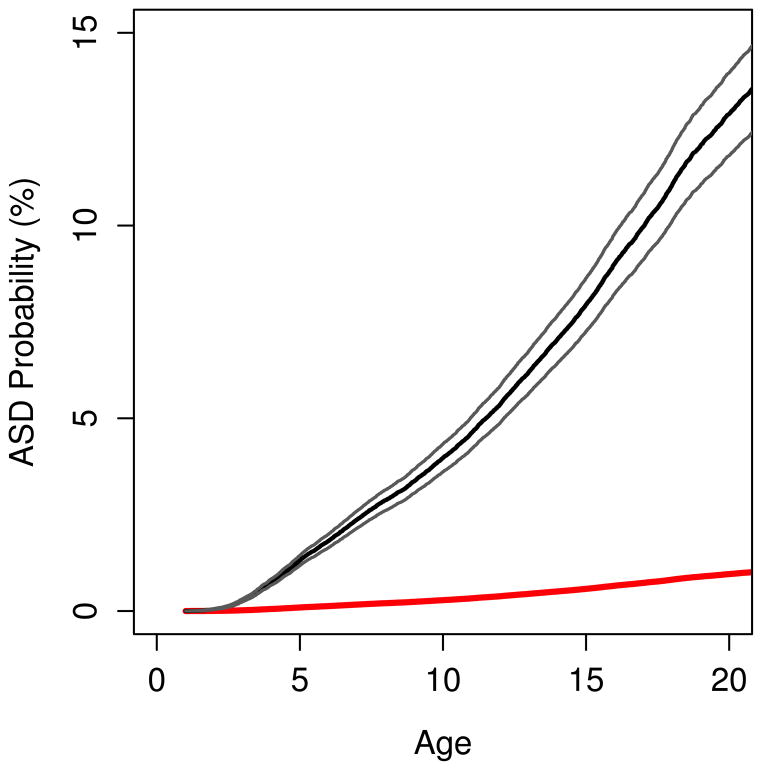
For individuals with a sibling with ASD the cumulative probability of an ASD diagnosis at age 20 was estimated to 13% compared with 1.2% for individuals without an ASD sibling (figure 1).
Figure 1.
Age-cumulative probabilities for ASD diagnosis in sibling with and without a sibling with an earlier ASD diagnosis. 95% two-sided point wise confidence bands for exposed siblings.
Dashed line: Cumulative probability of an autism diagnosis up to this age for siblings with a sibling proband with an autism diagnosis. Solid line: Cumulative probability of an autism diagnosis up to this age for siblings with a sibling proband free from an autism diagnosis.
Relative recurrence risk
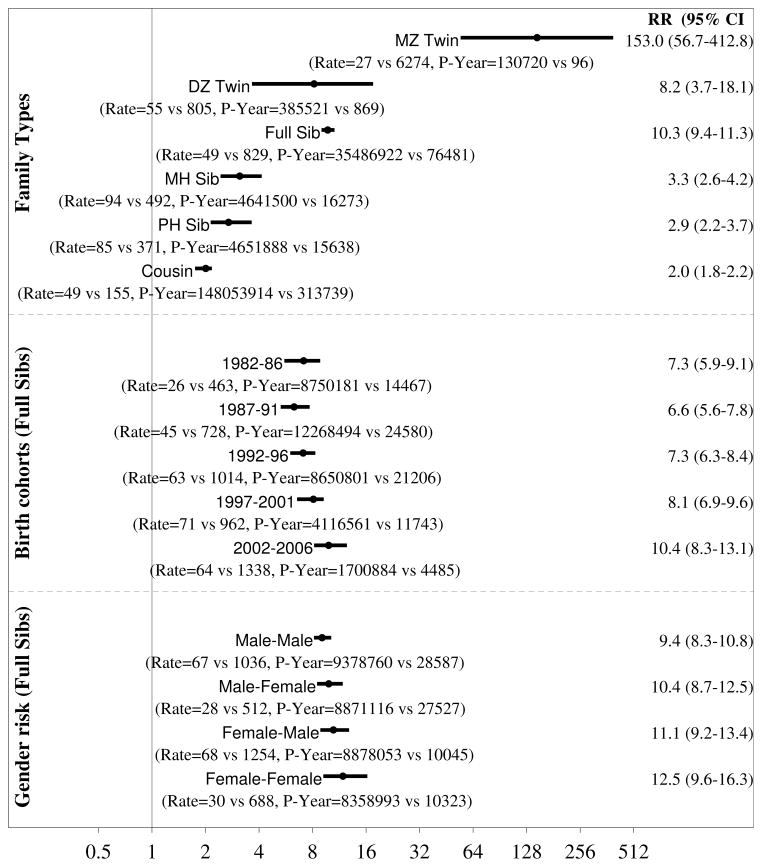
Figure 2 presents adjusted RR for ASD and associated two-sided 95% confidence intervals for the different degrees of genetic distance between family relatives. The RR remained stable after adjustment for sex, parental psychiatric history and parental age. There was some support for confounding attributable to birth cohorts (figure 2, bottom panel). When adjusting for 5-year birth cohorts, sex, parental age and parental psychiatric history the RR was 153.0 (95%CI 56.7–412.8; 27 vs 6,273 per 100,000 person-years) for monozygotic twins, 8.2 (95%CI 3.7–18.1; 55 vs 805 per 100,000 person-years) for dizygotic twins, 10.3 (95%CI 9.4–11.2; 49 vs 829 per 100,000 person-years) for full siblings, 3.3 (95%CI 2.6–4.2; 94 vs 492 per 100,000 person-years) for maternal half siblings, 2.9 (95%CI 2.2–3.7; 85 vs 371 per 100,000 person-years) for paternal half siblings and 2.0 (95%CI 1.8–2.2; 49 vs 155 per 100,000 person-years) for cousins. For crude RR see eTable 3.
Figure 2.
ASD adjusted relative recurrence risks for full and maternal (MH) and paternal (PH) half siblings, cousins and DZ twins. Point estimates and two-sided 95% confidence intervals. MZ twins not shown.
Male-Female indicate risk in female exposed to a male relative. The star for MZ twin indicate a truncated right confidence too wide to fit the figure. Adjusted: Models adjusting for birth cohort and sibling and proband sex and paternal and maternal psychiatric history at birth of the child and older maternal age (≤35, > 35) and older paternal age (≤40, > 40); MH: Maternal half siblings, PH: Paternal half siblings; Old Pa: Paternal age > 40; Yng Pa: Paternal age ≤40; Old Ma: Maternal age > 35; Yng Ma: Maternal age ≤35; Fa Psych; With a paternal psychiatric history; Fa Psych: With a paternal psychiatric history; With a maternal psychiatric history; Ma Psych: With a maternal psychiatric history; Parental psychiatric history was measured at birth of the first sibling in the family.
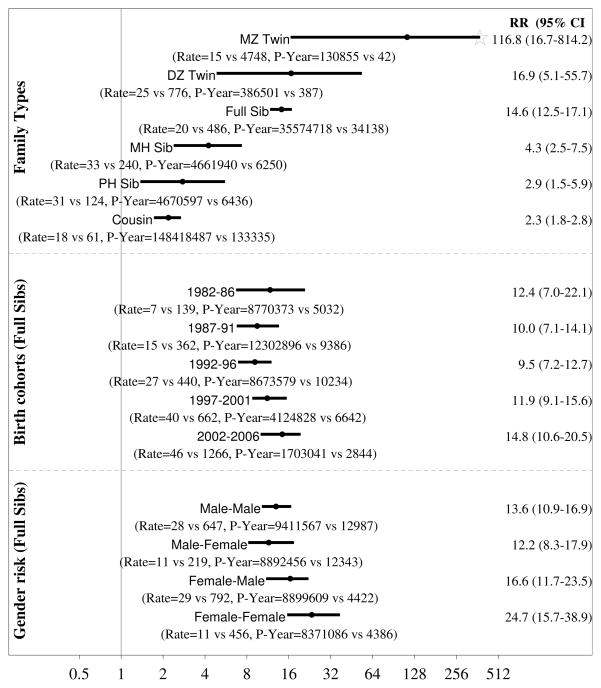
RR for AD are presented in figure 3. Adjusting for 5-year birth cohorts, sex, parental age and parental psychiatric history the RR was 116.8 (95%CI 16.7–814.2; 14 vs 4,748 per 100,000 person-years) for monozygotic twins, 16.9 (95%CI 5.1–55.7; 25 vs 776 per 100,000 person-years) for dizygotic twins, 14.6 (95%CI 12.5–17.1; 124 vs 486 per 100,000 person-years) for full sibling 4.3 (95%CI 2.5–7.5; 33 vs 240 per 100,000 person-years) for maternal half siblings, 2.9 (95%CI 1.5–5.9; 31 vs 124 per 100,000 person-years) for paternal half siblings and 2.3 (95%CI 1.8–2.8; 18 vs 61 per 100,000 person-years) for cousins.
Figure 3.
AD adjusted relative recurrence risks for full and maternal (MH) and paternal (PH) half siblings, cousins and DZ twins. Point estimates and two-sided 95% confidence intervals. MZ twins not shown.
Male-Female indicate risk in female exposed to a male relative. The star for MZ twin indicate a truncated right confidence too wide to fit the figure. Adjusted: Models adjusting for birth cohort and sibling and proband sex and paternal and maternal psychiatric history at birth of the child and older maternal age (≤35, > 35) and older paternal age (≤40, > 40); MH: Maternal half siblings, PH: Paternal half siblings; Old Pa: Paternal age > 40; Yng Pa: Paternal age ≤40; Old Ma: Maternal age > 35; Yng Ma: Maternal age ≤35; Fa Psych; With a paternal psychiatric history; Fa Psych: With a paternal psychiatric history; With a maternal psychiatric history; Ma Psych: With a maternal psychiatric history; Parental psychiatric history was measured at birth of the first sibling in the family.
There was no statistically significant difference in RR between boy or girl offspring or in RR from male or female proband (figure 2, figure 3). The model goodness-of-fit supported the assumption of hazards being proportional over the time of follow-up. For the sensitivity analyzes of ASD RR for full siblings; adjusting for 1-year birth cohorts did not change the results (RR=9.9 (95%CI 9.0–10.8) ) and the ASD RR did not change in sub-groups of family size (online eTable 5).
Heritability
The unadjusted ASD tetrachoric correlation was estimated to 0.54 (SD=0.20) for MZ twins; 0.25 (SD=0.13) for DZ twins; 0.25 (SD=0.02) for full siblings; 0.11 (SD=0.04) for maternal half siblings and to 0.07 (SD=0.05) for paternal half siblings; (eTable1). The correlations for AD are presented in eTable2. The tetrachoric correlations adjusted for sex and birth cohort were almost identical (eTable1, eTable 2).
The model including additive genetic, shared and non-shared environment parameters was chosen as the full model under which nested sub-models were tested. The best fitting model was the model only including additive genetic and non-shared environment parameters (Table 2). Using this model the ASD heritability was estimated to 0.50 (95%CI 0.46–0.56) and the non-shared environmental influence was 0.50 (95%CI 0.44–0.55).
Table 2.
ASD and AD Heritability. Model goodness of fit and variance component estimates.
| Model comparison measures | Estimated variances (two-sided 95% confidence intervals) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Models, terms included | #p | -2 LL | Diff -2 LL | p-value | Additive Genetic (“Heritability, narrow sense”) | Dominant Genetic | Shared environment | Non-shared environment | Total Genetic (“Broad sense heritability”) |
| ASD - Autism Spectrum Disorder | |||||||||
| Full model | 14 | 143,910 | x | x | 0.33 (0.00–0.55) | 0.16 (0.00–0.59) | 0.05 (0.00–0.17) | 0.46 (0.24–0.65) | 0.49 (0.21–0.75) |
| Excluding the dominant genetic term | 13 | 143,910 | 0.7 | 0.41 | 0.42 (0.19–0.55) | x | 0.04 (0.00–0.15) | 0.54 (0.45–0.66) | 0.42 (0.19–0.55) |
| Excluding the shared environment term | 13 | 143,911 | 0.8 | 0.38 | 0.44 (0.24–0.55) | 0.13 (0.00–0.51) | x | 0.43 (0.23–0.55) | 0.57 (0.45–0.77) |
| Excluding the additive genetic term | 13 | 143,913 | 3.0 | 0.08 | x | 0.45 (0.18–0.71) | 0.14 (0.07–0.20) | 0.41 (0.21–0.62) | 0.45 (0.18–0.71) |
| Additive genetic + Non- shared enviroment | 12 | 143,911 | 1.2 | 0.55 | 0.50 (0.45–0.56) | x | x | 0.50 (0.44–0.55) | 0.50 (0.45–0.56) |
| Dominant genetic + Non- shared environment | 12 | 143,934 | 23.8 | <0.001 | x | 1.00 (1.00–1.00) | x | 0.00 (0.00–0.00) | 1.00 (1.00–1.00) |
| Shared + non-shared environment term | 12 | 143,923 | 13.3 | 0.001 | x | x | 0.24 (0.21–0.26) | 0.76 (0.73–0.79) | x |
| Non-shared enviroment term only | 11 | 144,178 | 268.8 | <0.001 | x | x | x | 1.00 (1.00–1.00) | x |
| AD - Autistic Disorder | |||||||||
| Full model | 14 | 64,586 | x | x | 0.49 (0.00–0.64) | 0.00 (0.00–0.61) | 0.02 (0.00–0.24) | 0.48 (0.18–0.72) | 0.49 (0.04–0.82) |
| Excluding the dominant genetic term | 13 | 64,586 | 0.0 | 0.99 | 0.49 (0.04–0.64) | x | 0.03 (0.00–0.24) | 0.48 (0.36–0.72) | 0.49 (0.04–0.64) |
| Excluding the shared environment term | 13 | 64,586 | 0.1 | 0.81 | 0.54 (0.25–0.64) | 0.00 (0.00–0.54) | x | 0.46 (0.17–0.56) | 0.54 (0.44–0.83) |
| Excluding the additive genetic term | 13 | 64,591 | 4.5 | 0.03 | x | 0.65 (0.00–0.84) | 0.11 (0.04–0.30) | 0.23 (0.10–0.79) | 0.65 (0.00–0.84) |
| Additive genetic + Non- shared enviroment | 12 | 64,586 | 0.1 | 0.97 | 0.54 (0.44–0.64) | x | x | 0.46 (0.36–0.55) | 0.54 (0.44–0.64) |
| Dominant genetic + Non- shared environment | 12 | 64,646 | 59.4 | <0.001 | x | 1.00 (1.00–1.00) | x | 0.00 (0.00–0.00) | 1.00 (1.00–1.00) |
| Shared + non-shared environment term | 12 | 64,591 | 4.7 | 0.096 | x | x | 0.26 (0.21–0.31) | 0.74 (0.69–0.79) | x |
| Non-shared enviroment term only | 11 | 64,683 | 96.8 | <0.001 | x | x | x | 1.00 (1.00–1.00) | x |
#p: Number of parameters in the model; -2 LL: -2*log-likelihood; Diff df: Number of degrees of freedom for the -2 LL (difference in number of parameters between the model and the ADCE model); Diff -2 LL: 2 * difference in log-likelihood between the model and the ADCE model; p-value: p-value for the testing the hypothesis the parameters not in the model but in the ADCE model are all equal to zero; The “Broad-sense heritability” including both the additive and the dominant genetic while the “narrow-sense heritability” only include the additive genetic component. x: Not applicable. Note: All models adjusted for gender and birth cohort. CI: 95% two-sided confidence interval
In the full model, also including the shared environment, the variance associated with the shared environment was estimated to 0.04 (95%CI 0.00–0.15), non-shared environment to 0.54 (95%CI 0.44–0.66) and heritability to 0.42 (95%CI 0.19–0.55). Using twins only the heritability was estimated to 0.52.
For AD the model only including additive genetic and non-shared environment parameters was the best fitting model as well (Table 2) and the AD heritability was estimated to 0.54 (95%CI 0.44–0.64).
DISCUSSION
Including more than 1.6 million families, to the best of our knowledge, this is the largest population based longitudinal study evaluating familial risk of ASD. The RR of ASD increased with increasing genetic relatedness. Genetic and non-genetic influences on the liability for ASD and AD were similarly important. The RR of ASD is 10.3 (95%CI 9.4–11.3; 49 vs 829 per 100,000 person-years), 3.3 (95%CI 2.6–4.2; 94 vs 492 per 100,000 person-years), 2.9 (95%CI 2.2–3.7; 85 vs 371 per 100,000 person-years) and 2.0 (95%CI 1.8–2.2; 18 vs 61 per 100,000 person-years) for full, maternal and paternal half-siblings and cousins respectively. Heritability of ASD was estimated to 50% (95%CI 46–56), suggesting that genetic factors explain half of the liability for autism. This is considerably lower than the 90% in earlier twin studies2–4 and closer to the 38% (14–67) reported in a recent California twin study7 but estimated with substantially higher precision In a Swedish twin cohort23 of 12,000 children heritability of between 49% and 72% was reported for autistic-like traits (social impairment, communication impairment and restricted and repetitive behavior and interests).
Earlier twin studies showed only minimal non-shared environmental contribution to liability to ASD. The California twin study, in contrast, suggested substantial shared environmental influences. The large family data in our study indicated that such influences have only a negligible effect on ASD etiology. Despite differences in shared maternal prenatal environment, dizygotic twins and full siblings and maternal half siblings and paternal half siblings had comparable risks for ASD. In the presence of a familial confounding, factors effecting all members of a family, the RR is expected to be lower for the dizygotic twin compared with full siblings and for the maternal half-siblings compared with the paternal half-siblings. The interpretation of the RR of autism can be done in a wider context by comparing with the RR of schizophrenia, another neurodevelopmental disease that affect individuals later in life than does autism, with earlier overlap in diagnosis and with shared clinical an etiological features24. In a sample overlapping with the parents and grandparents of our study the RR was estimated to 8.5 for full siblings, 2.5 for half siblings and 2.3 for cousins25.
The differences visa-vi earlier research may be attributed to sampling, case ascertainment and analytic approach. Our study used a population based sample continuously following participants from birth. Previous twin studies relied on considerably less robust methodologies for case ascertainment, including self-referral, service registers, and parental reports on diagnosis. Even when detailed diagnostic assessment was done the participation rates were low and it could not be ruled out that participation was associated with presence of an autistic child in the family2, limiting generalizability. We adjusted for birth cohorts, addressing biases due to differences in length of follow-up with study participants in different birth years26. It is unclear how this was addressed and effected previous studies. We believe the effect of such a bias could inflate the shared environment component. Our low precision in RR for MZ and DZ twins illustrate well the problem in earlier small sized twin studies.
Factors effecting the variance for non-shared environment includes a misclassification of cases. This could possibly be due to differences in etiology across the different forms of ASD symptoms. Our data do not support this though as our results for the liability of ASD and AD were essentially the same.
The RR between different pairs of family members reflects the genetic influences on the trait and offers a quantitative measure of familial risk. Thus, the RR has an important interpretation which distinguishes it from the more theoretical measures of heritability. For example while genetic factors account for 50% of individual differences in liability to ASD, a sibling of a proband with ASD who shares 50% of the genes has a 10-fold increase in risk. This can potentially be applied at an individual level for family counseling.
Only few earlier studies have had the possibility to calculate the RR8,27,28. Two studies are presenting self-selected samples8,28 and with limited family data. A recent Danish study provide reliable estimates using an excellent epidemiological sample similar to ours. They show lower RR, RR=7.5 for full siblings but with similar relative relation between full siblings and maternal and paternal half-siblings. Our sample include twice as many cases of ASD and more detailed family data including monozygotic and dizygotic twins and cousins. Our bigger sample also allowed us to investigate sex of offspring in some more detail. Several earlier studies have reported absolute sibling recurrence risk28–34 but absolute risk is a cumulative measure which depends on the length of follow up (higher at age 15 years than at age 5 year) and will differ between populations. As elsewhere in epidemiology, where the relative risk is a preferred measure of disease risk, the RR circumvent these limitations.
This study has multiple strengths including the large, full-nation population-based sample with prospective follow-up and a health system with equal access. In addition to sibling pairs we were also able to include cousins and twins including zygosity information and to adjust for parental psychiatric history. To estimate the RR we used time-to-event methods to avoid introduction of bias due to differences in follow-up time for different participants. Analyzing risk between siblings and not requiring the risk to act from an older to a younger sibling as frequently done will also adjust for potential bias due to changes in prevalence of autism in later years where later born siblings may be expected to have a higher risk of being diagnosed.
Our cohort approach with prospective follow-up, following all participants from birth using clinical registers, avoid selection-biases due to disease status or factors such as parental education. It also avoid problems associated with self-reports and retrospective collection of data.
Limitations include lack of information on parental education or socioeconomic status. In Sweden there is free and equal access to health services minimizing the risk of selection biases. There is a well documented gender bias in autism35, and it has been suggested that females may require greater familial etiologic load to manifest the autistic phenotype36. We did not find support for any sex specific differences in the RR.
CONCLUSION
Among children born in Sweden, heritability of ASD and AD were estimated to be approximately 50%. For an individual, the risk of autism is increased 10 fold if a full sibling has the diagnosis and about 2 fold if a cousin has the diagnosis. These findings may inform counseling families with affected children.
Supplementary Material
eTable 1. ASD. Tetrachoric correlations (SD). Correlations of ASD diagnosis (yes/no) between sibling pairs in the different family relations.
eTable 2. AD. Tetrachoric correlations (SD). Correlations of AD diagnosis (yes/no) between sibling pairs in the different family relations.
eTable 3. Crude (no adjustment for confounding) recurrence risk (RR) and two-sided 95% confidence intervals
eTable 4. Codes by the International used for the psychiatric history
eTable 5. Sensitivity analysis. Relative recurrence risk (RR) for ASD and 2-sided 95% confidence intervals (CI) by family size. Full siblings only.
eTable 6. Person years and rate (cases per 100,000 person years) for ASD among MZ and DZ twins, full siblings and maternal- and paternal- half siblings and cousins and in sub-groups of birth year (1982–86, 87–91, 92–96, 97–2001–86, 87–91, 92–96, 97–2002–06) and gender among full siblings.
eTable 7. Person years and rate (cases per 100,000 person years) for Autistic Disorder (AD) among MZ and DZ twins, full siblings and maternal- and paternal- half siblings and cousins and in sub-groups of birth year (1982–86, 87–91, 92–96, 97–2001–86, 87–91, 92–96, 97–2002–06) and gender among full siblings.
Acknowledgments
This study was supported, in part, by grants from the National Institutes of Health (NIH) (HICHD/NIEHS/NINDS-HD073978 and NIMH-MH097849 ) and by the Beatrice and Samuel A. Seaver Foundation. The funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
There is no conflict of interest for any of the authors.
Sven Sandin, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Hollander E, Kolevzon A, Coyle JT. Textbook of Autism Spectrum Disorders. American Psychiatric Pub; 2010. [Google Scholar]
- 2.Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 3.Folstein S, Rutter M. Genetic influences and infantile autism. Nature. 1977;265(5596):726–728. doi: 10.1038/265726a0. [DOI] [PubMed] [Google Scholar]
- 4.Steffenburg S, Gillberg C, Hellgren L, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30(3):405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 6.Ronald A, Happé F, Bolton P, et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 7.Hallmayer J, Cleveland S, Torres A, et al. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantino JN, Todorov A, Hilton C, et al. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Mol Psychiatry. 2013;18(2):137–138. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- 9.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsson O. The Swedish medical birth register. Acta Obstet Gynecol Scand. 2003;82(6):491–492. doi: 10.1034/j.1600-0412.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 11.Ekbom A. The Swedish Multi-generation Register. Methods Mol Biol Clifton NJ. 2011;675:215–220. doi: 10.1007/978-1-59745-423-0_10. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124(6):447–453. doi: 10.1111/j.1600-0447.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 14.Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457–464. doi: 10.1080/08039480500360906. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein P, Sullivan PF, Cnattingius S, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet Off J Int Soc Twin Stud. 2006;9(6):875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 16.Kristjansson E, Allebeck P, Wistedt B. Validity of the diagnosis schizophrenia in a psychiatric inpatient register: A retrospective application of DSM-III criteria on ICD-8 diagnoses in Stockholm county. Nord J Psychiatry. 1987;41(3):229–334. [Google Scholar]
- 17.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 18.Liang K-Y, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 19.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 20.Neale M, Cardon LR. Methodology for Genetic Studies of Twins and Families. Springer; 1992. [Google Scholar]
- 21.Moeller E. Fakta om den svenska familjen. Stockholm, Sweden: Statistics Sweden, SCB; 1994. [Google Scholar]
- 22.Boker S, Neale M, Maes H, et al. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronald A, Larsson H, Anckarsater H, Lichtenstein P. A twin study of autism symptoms in Sweden. Mol Psychiatry. 2011;16(10):1039–1047. doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- 24.Stone WS, Iguchi L. Do Apparent Overlaps between Schizophrenia and Autistic Spectrum Disorders Reflect Superficial Similarities or Etiological Commonalities? North Am J Med Sci. 2011;4(3):124–133. doi: 10.7156/v4i3p124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenstein P, Björk C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36(10):1417–1425. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- 26.Lindström L, Pawitan Y, Reilly M, Hemminki K, Lichtenstein P, Czene K. Estimation of genetic and environmental factors for melanoma onset using population-based family data. Stat Med. 2006;25(18):3110–3123. doi: 10.1002/sim.2266. [DOI] [PubMed] [Google Scholar]
- 27.Grønborg TK, Schendel DE, Parner ET. Recurrence of Autism Spectrum Disorders in Full- and Half-Siblings and Trends Over Time: A Population-Based Cohort Study. JAMA Pediatr. 2013 doi: 10.1001/jamapediatrics.2013.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritvo ER, Jorde LB, Mason-Brothers A, et al. The UCLA-University of Utah epidemiologic survey of autism: recurrence risk estimates and genetic counseling. Am J Psychiatry. 1989;146(8):1032–1036. doi: 10.1176/ajp.146.8.1032. [DOI] [PubMed] [Google Scholar]
- 29.Szatmari P, Jones MB, Zwaigenbaum L, MacLean JE. Genetics of autism: overview and new directions. J Autism Dev Disord. 1998;28(5):351–368. doi: 10.1023/a:1026096203946. [DOI] [PubMed] [Google Scholar]
- 30.Bolton P, Macdonald H, Pickles A, et al. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 31.Chudley AE, Gutierrez E, Jocelyn LJ, Chodirker BN. Outcomes of genetic evaluation in children with pervasive developmental disorder. J Dev Behav Pediatr JDBP. 1998;19(5):321–325. doi: 10.1097/00004703-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Sumi S, Taniai H, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. J Hum Genet. 2006;51(6):518–522. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- 33.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(Suppl 10):3–8. [PubMed] [Google Scholar]
- 36.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ASD. Tetrachoric correlations (SD). Correlations of ASD diagnosis (yes/no) between sibling pairs in the different family relations.
eTable 2. AD. Tetrachoric correlations (SD). Correlations of AD diagnosis (yes/no) between sibling pairs in the different family relations.
eTable 3. Crude (no adjustment for confounding) recurrence risk (RR) and two-sided 95% confidence intervals
eTable 4. Codes by the International used for the psychiatric history
eTable 5. Sensitivity analysis. Relative recurrence risk (RR) for ASD and 2-sided 95% confidence intervals (CI) by family size. Full siblings only.
eTable 6. Person years and rate (cases per 100,000 person years) for ASD among MZ and DZ twins, full siblings and maternal- and paternal- half siblings and cousins and in sub-groups of birth year (1982–86, 87–91, 92–96, 97–2001–86, 87–91, 92–96, 97–2002–06) and gender among full siblings.
eTable 7. Person years and rate (cases per 100,000 person years) for Autistic Disorder (AD) among MZ and DZ twins, full siblings and maternal- and paternal- half siblings and cousins and in sub-groups of birth year (1982–86, 87–91, 92–96, 97–2001–86, 87–91, 92–96, 97–2002–06) and gender among full siblings.