Abstract
This meta-analysis examined the effects of process-based cognitive training (49 studies) in the domains of executive function and working memory in older adults (>60 years). The interventions resulted in significant effects on the trained task (pre-to-posttest net gain: MSD = 0.5 compared to active control, MSD = 0.8 compared to passive control; net posttest effect: MSD = 1.2 compared to active control, MSD = 1.1 compared to passive control), significant near transfer (pre-post: MSD = 0.3, 0.3; posttest: MSD = 0.6, 0.4); far-transfer effects were significant in 3 out of 4 comparisons (pre-post: MSD = 0.2, 0.2; net gain at posttest: MSD = 0.3, 0.2, ns). We detected small differences in training-induced improvements between working-memory and executive-functioning training, but none between older adults and the younger-adult samples included in these studies, adaptive and non-adaptive training, or active and passive control conditions. Gains did not vary with total training time.
Keywords: cognitive plasticity, cognitive aging, cognitive control training, transfer of training, meta-analysis
Introduction
Scientific interest in cognitive interventions designed to maintain or improve cognitive functions in the aging brain has been rapidly increasing over the last decade. Numerous studies investigating the effects of such interventions showed that plasticity (i.e., the potential modifiability of a person’s cognitive abilities and brain activity) is considerable up to very old age (Buitenweg, Murre, & Ridderinkhof, 2012; Hertzog, Kramer, Wilson, & Lindenberger, 2008; Karbach & Schubert, 2013; Lustig, Shah, Seidler, & Reuter-Lorenz, 2009; Noack, et al., 2009). Aside from significant performance improvements on the trained tasks, many studies reported near transfer to tasks not explicitly trained, but measuring the same construct as the training task, and far transfer to tasks measuring a different construct. However, these transfer effects are not consistent across studies and have inspired heated recent debates (e.g., Melby-Lervag & Hulme, 2013; Redick et al., 2013; Shipstead, Redick, & Engle, 2012). One reason for the inconsistent pattern of results may be that the large differences in terms of the type, intensity, and duration of the training regimes as well as different methodologies adopted across studies hamper the comparability of their findings. Trained individuals were compared to active control groups in some designs and to passive no-contact groups in others and the training regimes ranged from a few days to months of training (Noack, Lövdén, & Schmiedek, 2014).
Three basic categories of interventions can be distinguished: Strategy-based trainings (e.g., training in the method of loci) typically result in large and often long-lasting improvements on the training task, but induce only limited transfer (Rebok, Carlson, & Langbaum, 2007; Verhaeghen, Marcoen, & Goossens, 1992). Multi-domain training interventions are more complex and engage multiple cognitive processes (e.g., video-game training), yielding broad, but oftentimes small transfer effects (e.g., Basak, Boot, Voss, & Kramer, 2008; Park et al., 2014). Similar findings have been reported after multi-domain process-based interventions (Schmiedek, Lövdén, & Lindenberger, 2010). The main disadvantage of multi-domain trainings is that their complex nature makes it hard to determine which specific features of the training regime induced transfer.
In contrast, process-based training protocols target more general processing capacities, such as speed of processing or executive functions, that usually show a marked age-related decline (e.g., Li et al., 2004). The term executive functions (EF) refers to a set of higher-level control processes supporting the adaptation to changing environments and task demands. They include working memory (WM), inhibition, and cognitive flexibility (e.g., Miyake et al., 2000). Some process-based interventions, mainly focusing on EF, have resulted in promising transfer up to very old age (e.g., Brehmer, Westerberg & Bäckman, 2012; Karbach & Kray, 2009; Zinke et al., 2014), suggesting that process-based training might be more efficient at eliciting transfer than strategy-based interventions. Yet, a systematic comparative analysis across such training studies is still missing. In accordance with the typical terminology in the field, we made a distinction between WM training, aimed at improving scores on tests for WM capacity (e.g., Operation Span) or tests of WM functioning (e.g., N-Back), and EF training, aimed at improving performance on tests of dual-task performance, inhibition and interference control, task switching, and general forms of attention. (We note that N-Back training is often considered WM training (e.g., Shipstead et al., 2012), but since it is also considered an updating task, we also analyzed these two types of tasks separately.)
Another issue that has been debated in the cognitive aging literature concerns age-related differences in cognitive plasticity -- age differences in the magnitude of training and transfer effects. Strategy-based memory-training studies have repeatedly provided evidence for larger training gains in younger adults than in older adults (e.g., Brehmer, et al., 2007; Lindenberger, Kliegel, & Baltes, 1992; Lövdén, Brehmer, Li, & Lindenberger, 2012; Verhaeghen et al., 1992; Verhaeghen & Marcoen, 1996; but see Gross et al., 2012). These magnification effects suggest that younger adults show more benefits because they have more efficient cognitive resources to acquire and implement new strategies. In contrast, process-based EF training studies revealed larger training-related gains in older adults than in younger adults (e.g., Bherer, Kramer, & Peterson, 2008; Cepeda, Kramer, & Gonzalez de Sather, 2001; Karbach & Kray, 2009; Kramer, Larish, & Strayer, 1995; Kray, Eber, & Karbach, 2008). These compensation effects suggest that younger adults are already functioning at a more optimal level with less room for performance improvements. Although these and other findings indicate that process-based training may be more beneficial for older adults than strategy-based approaches, a comprehensive analysis across studies is needed before more general conclusions regarding age differences in the effectiveness of process-based cognitive interventions can be drawn. In the current study, we restricted our analyses of age differences to studies that included both younger and older adults, so that differences between age groups were not confounded with other variables included in the studies. Results of this analysis are of high relevance both for the understanding of the cognitive and neural underpinnings of cognitive plasticity and for the adaptation of training interventions to populations with specific needs, for instance individuals in old-old age or in clinical settings.
Therefore, the aim of the present study was to apply meta-analytic techniques to quantitatively investigate the extent to which process-based cognitive training improved cognitive functions in older age. Meta-analyses allow for summarizing the association of two variables across different studies by yielding overall effect sizes (ES) as well as ESs for each study and for testing the influence of moderator variables. Given that EF and WM training seem to be particularly beneficial for older adults and can result in widespread transfer, we focused on training interventions targeting these domains. Our study extends previous meta-analyses (Hindin & Zelinski, 2012; Karr, Areshenkoff, Rast, & Garcia-Barrera, 2014; Melby-Lervag & Hulme, 2013) by including a sizeable number of recently published training studies and by systematically comparing age-differences in the effects of different types of process-based EF and WM training across the adult lifespan.
Methods
We searched Science Direct databases (PsycInfo, PsycArticles) with the following key terms: (1) executive-functions training, (2) cognitive-control training, (3) working-memory training, (4) updating training (5) inhibition training, and (6) switching training, in combination with the terms (7) older adults and/or (8) aging. We also checked the references in each of the collected articles for studies overlooked. Our search was concluded in December 2013. Studies were included if (a) they contained a process-based EF or WM training or practice condition consisting of repeated exposure to the relevant task (this excluded multi-component treatments, such as games, training batteries including other types of tasks, or combinations of cognitive and pharmacological or physical exercise interventions); (b) they examined at least one sample of healthy older adults (mean age >60 years); (c) the data were reported in a format amenable to meta-analysis; and (d) the study was published in the English language in a peer-reviewed journal.
The final sample consisted of 49 articles, containing 61 different experiments or independent subject groups (see supporting online material). Some of these studies included a passive control group, where subjects were retested at approximately the same time interval as the training group without receiving any additional treatment; some included an active control group, where subjects were retested at approximately the same time interval as the training group without receiving additional treatment that qualifies as WM or EF training (i.e., filling out questionnaires, physical training, computer training, attending educational lectures, trivia learning, game playing, visual search, or quizzes). Some of the studies also included one or more samples of younger adults. Selected characteristics of the studies are reported in Table 1. Range of mean ages was 17–31 for younger adults and 63–87 for older adults.
Table 1.
Selected characteristics of studies included in the meta-analysis
| All studies (k = 61) |
Executive control studies (k = 48) |
Working memory studies (k = 13) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Number of sessions | 9.81 | 14.85 | 7.96 | 15.19 | 16.66 | 11.61 |
| Duration per session (hr) | 0.87 | 0.40 | 0.96 | 0.42 | 0.67 | 0.25 |
| Total number of hours spent in training | 8.93 | 16.06 | 8.24 | 17.75 | 10.69 | 11.05 |
| Total time between pre and post (days) | 24.16 | 31.38 | 21.46 | 33.92 | 32.25 | 21.28 |
| Number of subjects (older adults) | 21.34 | 13.98 | 20.69 | 14.18 | 23.73 | 13.48 |
| Average age (older adults) | 69.42 | 3.45 | 69.25 | 2.71 | 70.01 | 5.49 |
| Number of subjects (younger adults) | 21.51 | 16.40 | 21.12 | 16.96 | 25.50 | 9.66 |
| Average age (younger adults) | 22.45 | 2.69 | 22.20 | 2.66 | 24.97 | 1.70 |
| Percentage of studies that | ||||||
| included younger adults | 55 | 65 | 23 | |||
| included near transfer tasks | 56 | 44 | 100 | |||
| included far transfer tasks | 44 | 29 | 100 | |||
| included a passive control condition | 30 | 29 | 31 | |||
| included an active control condition | 39 | 31 | 69 | |||
| included adaptive training | 21 | 6 | 77 | |||
The first analysis concerned gain scores: We calculated treatment gains as the mean standardized difference between post-test and pretest, (Mpost–Mpre)/SDpooled. This statistic tells us how many standard deviations separate the subjects prior to versus after treatment. When mean or SD were not reported, inferential statistics, if available, were used to determine ES. All ESs were corrected for sample size (Hedges & Olkin, 1985). Comparison between treatment gains in training groups with those in passive and active control groups reveals whether or not the training gain is due to the specific intervention rather than to retest or placebo or (re)activation effects.
A second analysis concerned the net treatment effect at posttest, expressed as the mean standardized difference between trained and control subjects, (Mtrained–Mcontrol)/SDpooled, weighted for sample size. All ESs were coded such that positive ESs denote better (i.e., faster or more accurate) performance. Some effects are expressed as difference scores (e.g., dual-task costs, task-switching costs, flanker effects, Stroop effects). In case the difference score was not provided, we calculated it from the relevant conditions; SD of the difference score was calculated from the component conditions using a between-condition correlation of .9 for Stroop, Trail Making, and flanker tests, and .8 for task switching and dual task paradigms tasks; these estimates were based on our own previous data as well as other’s1. For Stroop, we restricted ourselves to RT measures.
For each of the included studies we recorded the following variables: age, number of participants, duration per session, number of sessions, pre-post interval, type of intervention (training, passive control, active control), and type of measure. We classified all measures into one of three types: (a) target measures (tasks explicitly practiced in the training groups); (b) near-transfer measures (tasks not explicitly trained, but measuring the same construct as the construct trained; e.g., if N-Back, a WM task, was trained, then Operation Span would be a near-transfer task; if a task-switching training involved two tasks A and B, a test alternating tasks C and D would be a near-transfer task); and (c) far transfer measures (tasks measuring a different construct than the construct trained; e.g., if WM was trained, a task-switching task or a reasoning test would be far-transfer tasks).
Initially, ESs were calculated for each dependent measure in each study; these were collapsed into a single estimate as appropriate (e.g., averaging all target measures within a study to form a single-point estimate for target measure per study), so that only a single estimate per study entered the final comparisons. Pooling of ESs within each grouping of interest was done by calculating a mean ES (d+), weighted for sample size (cf. Hedges & Olkin, 1985).
Results
Gain scores
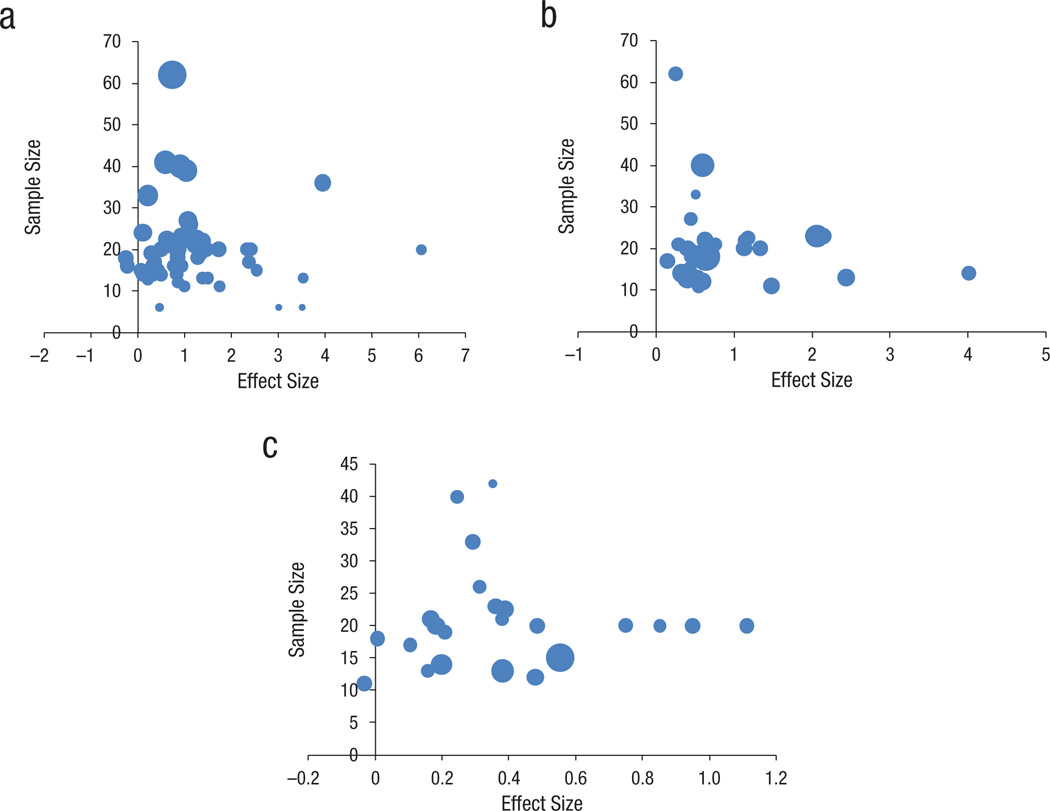
The funnel plots in Figure 1 serve to investigate publication bias in the gain scores. ESs in the trained groups (X-axis) are plotted against sample size (Y-axis); the size of the bubbles is proportionate to the precision of measurement, as indexed by 1/SE (thus, larger bubbles denote more precise measurements). The plot for target measures (panel A) is not significantly asymmetric, suggesting a lack of publication bias; Egger’s (Egger, Smith, Schneider, & Minder, 1997) bias=1.19, p=.67. Only the single largest effect size was an outlier according to disjoint cluster analysis (Hedges & Olkin, 1985). Removing this data point from analysis did not alter the results substantially; therefore, we conducted all analyses on the full data set. The plot for near-transfer measures (panel B), however, was significantly asymmetrical, Egger’s bias=5.76, p=.03. The skew suggests that there are fewer studies with negative results than might be expected. We therefore conducted our analyses both on the near-transfer gain scores as found and on the average weighted ES corrected for publication bias, using the Duval and Tweedle (2000) trim–and-fill correction. Far-transfer data showed no indication of publication bias (Panel C), Egger’s bias=0.54, p=.79.
Figure 1.
Funnel plots for effect sizes (pre to posttest gain) on (A) target measures, (B) near-transfer measures, and (C) far-transfer measures (bubble size denotes 1/SE, as a measure of precision). Only Panel B shows significant asymmetry.
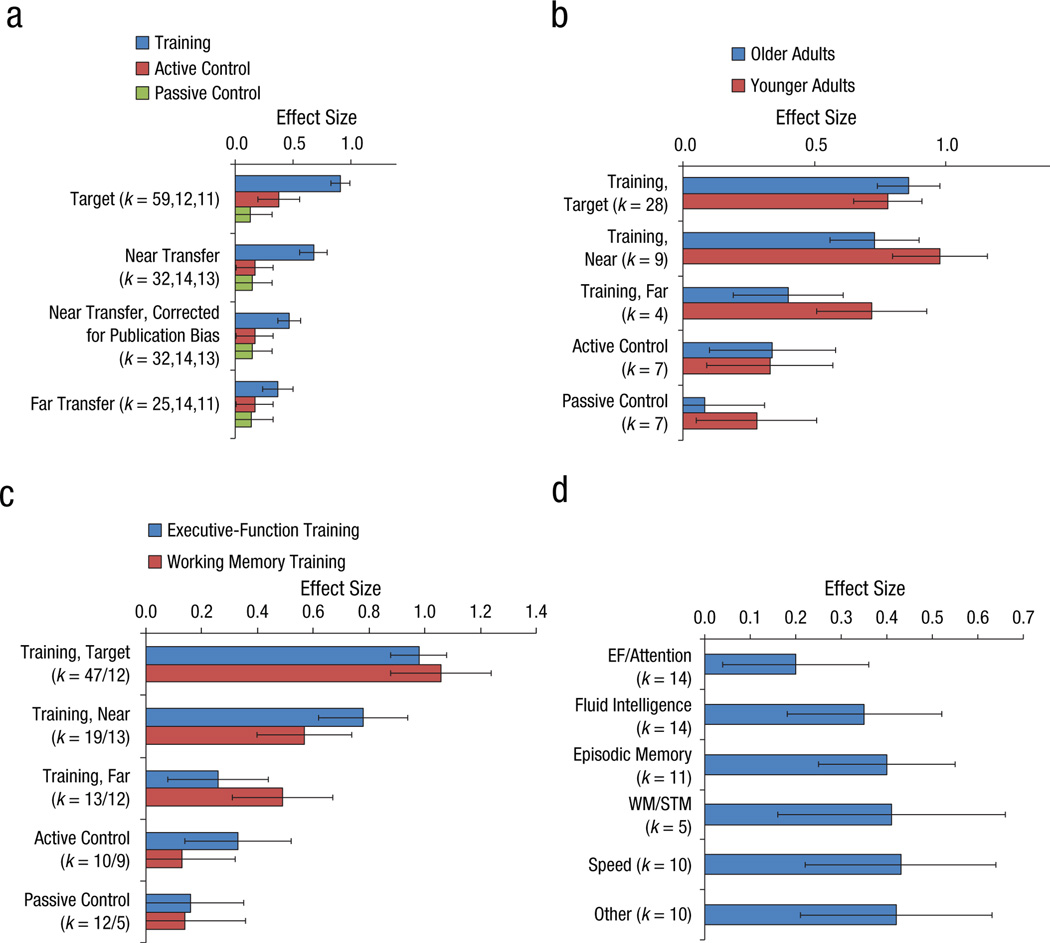
Figure 2, Panel A shows the overall effectiveness of training on target and transfer measures (older adults only). Effect sizes were heterogeneous for target measures (QW=286.53) and near transfer measures (QW=96.03) within the trained groups, suggesting that the ESs within these groupings were highly variable. Five conclusions emerge. First, all effects in the trained group – effects on target measures, and near and far transfer effects – are significantly larger than zero. Second, training leads to significantly larger improvements on target measures than either control treatment does (active control: QB=30.01 or; passive control: QB=51.86, p<.001). The training-related effect on the target measure is 0.91. Active control groups show an ES of 0.38, passive control groups an ES of 0.13. Third, the effects of training on near-transfer and far-transfer measures are reliably smaller (as demonstrated by non-overlap in the 95% confidence intervals) than those on target measures (0.68 uncorrected/0.47 corrected, 0.37, and 0.91, respectively). Fourth, transfer effects in the trained groups are also reliably larger than the effects of either control treatment (one exception is the marginal effect for far transfer in the trained groups compared to active control, QB=3.66, p=0.056 for a two-tailed test; for far transfer in the trained groups compared to passive control, QB=4.17, p<0.05; near transfer vs. active, QB=26.53, p<.001; near transfer vs. passive, QB=26.11, p<.001). Net gain of training is about 0.50 SD (0.30 SD after removing publication bias) for near-transfer tasks and 0.20 SD on far-transfer tasks. Finally, active and passive control treatments yield statistically indistinguishable effects (target measures: QB=3.33, p=0.068; near transfer: QB=0.03, p=.86; far transfer: QB=0.07, p=.79); we note that the effect on target measures is marginally significant, suggesting that active control treatment might lead to larger effects on target measures than passive treatment, maybe because of Hawthorne or other expectancy effects.
Figure 2.
Averaged effect sizes (pre to posttest gain) (A) by treatment and type of measure, (B) by age group (only studies that included both younger and older adults were included), (C) as a function of training type, and (D) as a function of the type of transfer measure. Error bars denote 95% confidence intervals. Note: k denotes the number of studies; the count is reported in order of presentation on the graph; EF=executive function, WM=working memory, STM=short-term memory.
To explain the heterogeneity of effect sizes within the trained subjects, we conducted two random-effects meta-regression analyses, one on target measures, one on near-transfer measures, using the following predictors: Age, total time spent in training, type of training (0=executive control; 1=working memory), and whether or not the training was adaptive. The fit was poor for both analyses (R2=.04 and .11, resp.) and none of the predictors were significant.
Panel B splits the data by age group. We restricted our analyses to studies including both younger and older adults, so that differences between age groups cannot be ascribed to any of the other variables included in the studies. Due to the low number of comparisons within the younger-adults sample, we collapsed over measures within the active and passive control groups. The conclusion is simple: No reliable age differences were detectable within the set of studies gathered here (largest QB=2.45, p=.12). Again, ESs were heterogeneous for target measures (QW=145.34 for older and 177.29 for younger adults) and near transfer measures (QW=30.00 for younger and 88.55 for older adults) within the trained groups.
Panel C splits the older-adult data from Panel B into WM and EF training. The two types of training do not differ reliably in their effects on cognition (there are marginal effects on near transfer, QB=3.16, p=0.075, and far transfer, QB=3.03, p=0.082, going in opposite directions). ESs were again heterogeneous for target measures (QW=353.97 for EF and 28.06 for WM) and near transfer measures (QW=65.90 for EF and 29.98 for WM) for the trained groups. Further splitting the WM training sample into training on working memory capacity (WMC) and N-Back tasks yielded a significant difference on target measures only (WMC, 8 studies: 0.93; N-Back, 4 studies: 1.44; QB=6.10, p<.05).
Panel D zooms in on the far transfer effects, splitting the data in smaller categories of cognitive measures (note that this also dilutes statistical power, resulting in a wider CI). The CI of all types of measures overlap, suggesting that all benefit in equal amounts. All ESs are significant.
Net treatment effects at posttest
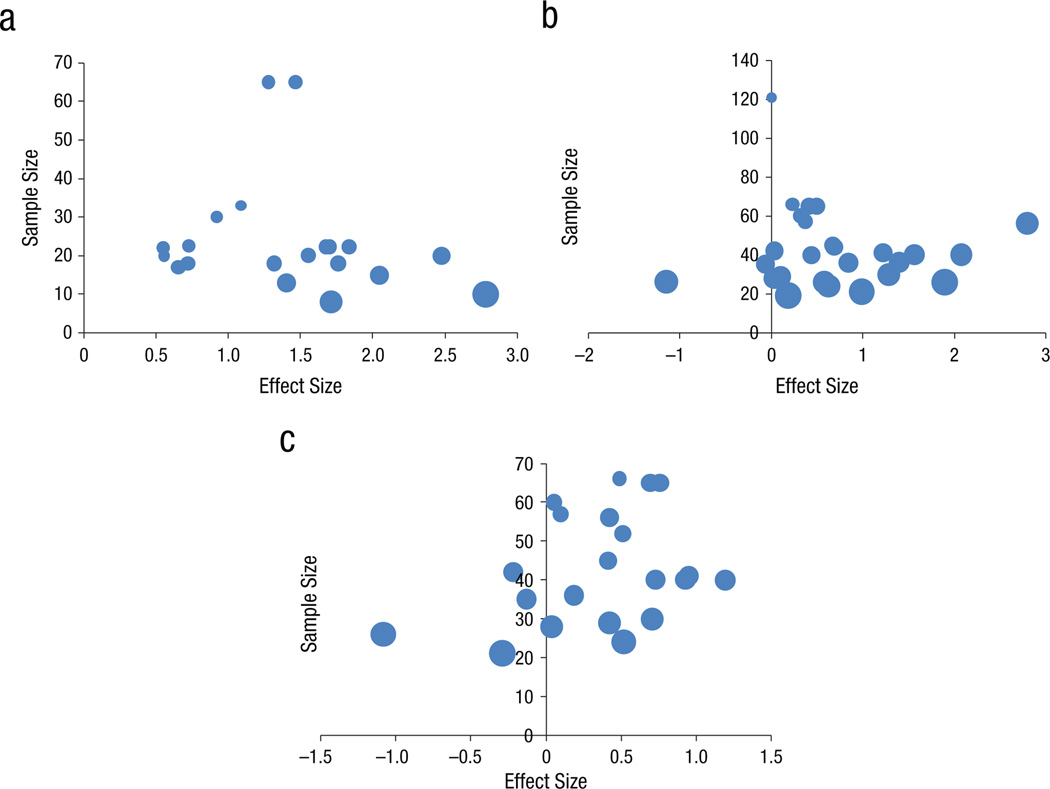
Figure 3 presents the funnel plots used to investigate publication bias in the net treatment effects. The plot for target measures (panel A) was significantly asymmetric; Egger’s bias=14.95, p=.035. Neither the near-transfer plot (Egger’s bias=8.16, p=.11), nor the far-transfer plot (Egger’s bias=2.32, p=.35) showed significant asymmetry. We therefore conducted our analyses on target measures both on the net gains as found and on the average weighted ES corrected for publication bias after trim-and-fill correction.
Figure 3.
Funnel plots for effect sizes (net effect, operationalized as the difference between effect size in experimental conditions minus effect size in control conditions) on (A) target measures, (B) near-transfer measures, and (C) far-transfer measures (bubble size denotes 1/SE, as a measure of precision). Only Panel A shows significant asymmetry.
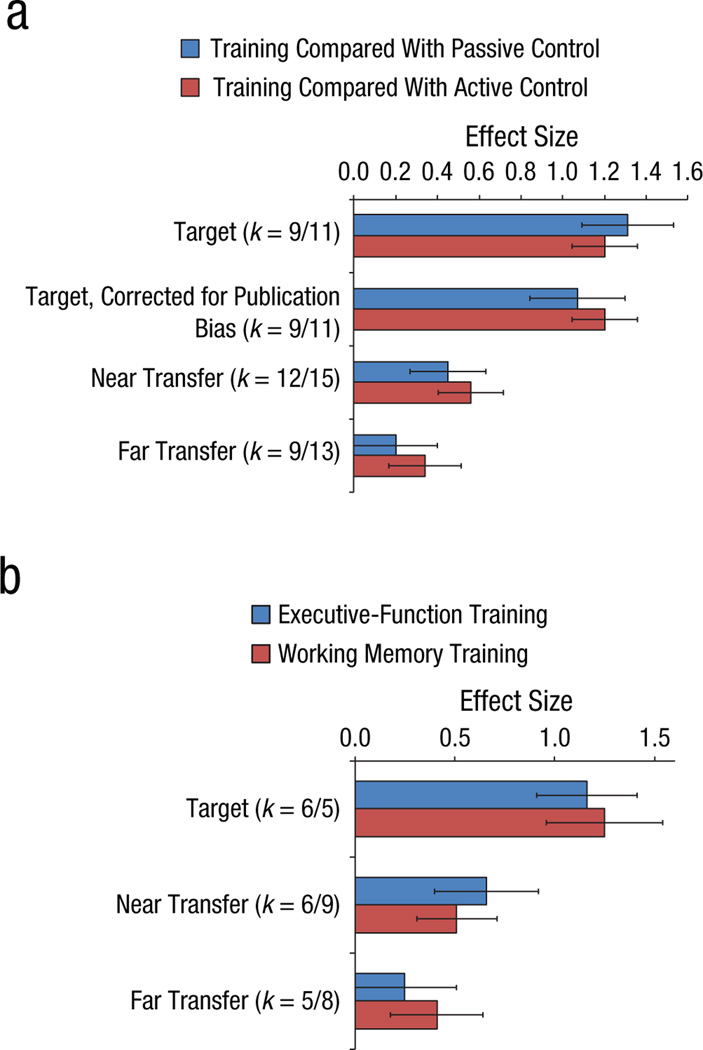
Figure 4 shows the average ESs for the net effect at posttest. (Sample size is smaller than in the previous analyses simply because not all studies provided both (or even one) type of control condition(s). The main result from Panel A is clear: All effects are significantly larger than zero. This indicates that the effects of WM or EF training are reliably larger than those of either passive or active control treatment, not only for target measures, but for measures of near and far transfer as well. A second result is that the net effect of treatment does not vary reliably with the type of control training. Panel B breaks down the effects by type of training. This analysis was performed on the treatment-active control contrast only because the treatment-passive control contrast yielded extremely small samples.
Figure 4.
Average effect sizes (A) for the net effect at posttest for the treatment tasks (all effect sizes significantly larger than zero) and (B) as a function of training type for studies with active control conditions (all effect sizes significantly larger than zero, except for far-transfer measures in executive function training). Error bars denote 95% confidence intervals. Note: k denotes the number of studies; the count is reported in order of presentation on the graph.
With the exception of the EF far-transfer effect, which is marginally significant (lower limit of the 95% CI is −0.013, two-tailed p=.063), all effects are significant, and the two types of training do not differ in their net effects.
Discussion
The main goals of this meta-analysis were: (a) Testing the extent of cognitive benefits after process-based cognitive training in younger and older adults, including improvements in tasks of near and far transfer, and (b) investigating age-related differences in training and transfer effects.
The results regarding training improvements are clear: First, WM and EF training lead to significant and large improvements in the trained tasks. The raw gain is about 0.9 SD; net gain after subtracting the effects of active control treatment is about 0.5 SD; net gain after subtracting the effects of passive control treatment is about 0.8 SD; net treatment effect at posttest after correction for publication bias is about 1.1 SD. Second, WM and EF training result in clear and quite large transfer effects to near-transfer tasks measuring the same construct as the task trained. (One could consider these the effects of training at the level of the latent variable.) The gain is about 0.7 SD (or 0.5 SD after statistically removing publication bias); net gain, after subtracting the effects of control treatment, is about 0.5 SD (or 0.3 SD after statistically removing publication bias); net treatment effect at posttest is about 0.5 SD. Third, WM and EF training result in clear but smaller transfer effects to far-transfer tasks measuring a different cognitive construct than the task trained. The gain is about 0.4 SD; net gain, after subtracting the effects of control treatment, is about 0.2 SD; net treatment effect at posttest is about 0.25 SD. (Note that the evidence is not completely univocal under two-tailed testing assumptions. The net effect on gain scores was significant in three out of the four relevant comparisons; p-value for the one non-significant effect was .056 in a two-tailed comparison. Likewise, the net effect post-treatment was significant in three out of four comparisons; only the difference between EF training and the active control group was marginal, two-tailed p = .063. Observe that if one accepts a one-tailed logic – which seems defensible here – all effects involving far transfer are significant.) Of particular interest is the finding that gain on measures of fluid intelligence was not negligible (0.35 SD) – suggesting that process-based training generalizes to tasks that are potentially extremely relevant for daily functioning. (Hindin & Zelinksi, 2012, and Karr et al., 2014, reported similar effects in their meta-analysis; they, however, did not make an explicit distinction between near and far transfer, included multi-domain training groups or samples with cognitive impairments in their analyses.)
We note that these results are seemingly at odds with other, qualitative literature reviews on transfer effects in younger adults (e.g., Shipstead et al., 2012). Importantly, such qualitative reviews rely, implicitly or explicitly, on vote-counting procedures, that is, they keep track of the proportion of studies that yield a statistically significant effect. The net transfer gain observed in our analysis is about 0.20 SD. Power to detect this small effect with a typical sample size of about 20 subjects is only 16%; conversely, an effect of that size needs a sample of 310 subjects to be detectable with a power of .80. Most studies in the field are thus seriously underpowered and vote-counting methods for data pooling will underestimate the effect greatly. Our results are also inconsistent with recent meta-analyses suggesting that WM training does not yield significant transfer (Melby-Lervag & Hulme, 2013) and that training and transfer effects are largest in very young age groups (i.e., infants, Wass et al., 2012). It should be noted, however, that the findings of these studies do not easily compare to ours, because they included (a) very wide age ranges, from preschoolers to old adults, (b) normally developing and clinical samples (e.g., ADHD, brain injury, schizophrenia), and (c) many different training regimes, such as strategy-based, process-based and multi-domain trainings. Thus, benefits of process-based WM and EF trainings in older adults found in our meta-analysis may have been masked in these previous studies.
We would argue that the process-based interventions summarized here fare very well compared to other known treatments aimed at improving cognition in older adults. First, meta-analyses of the literature have shown that two promising types of training (mnemonic strategies; Verhaeghen et al., 1992, and cognitive speed; Verhaeghen, 2014) do not generalize to untrained measures – EF and WM training, however, clearly do. Second, the one meta-analysis on the effects of fluid ability training (e.g., figural reasoning; Verhaeghen, 2000) showed that this type of training did not yield effects that were reliably larger than those of retest control treatment – EF and WM training effects, in contrast, reliably exceed those of control treatments. A third type of training targeted at cognitive change is aerobic training. In their meta-analysis, Colcombe and Kramer (2003) observed a gain ES on cognition after aerobic exercise training of 0.48 SD, compared with a gain of 0.16 SD after control treatment, thus yielding a net gain of 0.32 SD. The fairest comparison with our own data would be either to near-transfer effects (net gain of .52 SD) or far-transfer effects (net gain of .21 SD). The net gain in cognition (0.36 SD, averaged over near and far transfer) after (on average) 9 hours of EF and WM training is thus comparable in size to the effect observed after (on average) about five months of 45-minute sessions of (presumably daily) aerobic training.
Our second question pertained to age effects in treatment gain. Put succinctly, none were found. This finding goes against the magnification effect often found in strategy training (for an early meta-analysis, see Verhaeghen & Marcoen, 1996), where effects are generally smaller for older than for younger participants, possibly because the correct implementation of complex strategies depends on intact cognitive resources. Even though the present finding is based on a relatively small subset of studies, it suggests that prolonged practice with a task results in comparable gains for younger and older adults, a conclusion in line with a recent meta-analysis on practice effects in other elementary tasks, namely choice reaction time, serial reaction time, memory scanning and visual search (Verhaeghen, 2014).
One additional finding was the absence of a dose-response relationship on target or near-transfer measures (cf. Karr et al., 2014). One possible explanation is that researchers are very good at goldilocking their treatments: They provide, by skill or sheer luck, just the right amount of practice. Another, perhaps more likely explanation is that other factors – such as the specific type of treatment, or the population trained - overshadow the effects of length of treatment.
Finally, there are a few limits that to our knowledge cannot be addressed by the present meta-analysis – points we would like to offer as suggestions for further study. First, little is known about the durability of training effects. Even though the longevity of training-induced gains is considered a key measure for the value of an intervention, follow-up assessments are not consistently reported across studies and vary from a few weeks up to several years (e.g., Willis et al., 2006). Second, although process-based training reliably and positively impacts fluid intelligence, which is presumably correlated with real-life cognition, we have no actual data on the generalizability of the effects of WM or EF training to daily life (for the long-term effects of fluid ability training on everyday functioning, see Rebok et al., 2014). Third, a deeper study into individual differences in effectiveness, and especially in the likelihood of eliciting transfer effects, would be desirable (see Jaeggi, Buschkuehl, Shah, & Jonides, 2013; Titz & Karbach, 2014; Zinke et al., 2014). Finally, more studies into plasticity-related changes in the substrate, that is, the effects of process-based training on brain structure and/or function, would be desirable as well. The few existing neuroimaging studies assessing plasticity in the aging brain yielded heterogeneous findings, providing evidence for training-induced structural changes, but also both increases and decreases in cortical activity. These activation changes are thought to reflect shifts in strategy or processing and increased neural efficiency, respectively (Lustig et al., 2009).
Summarized, we found that process-based training of EF and WM in old age is highly effective, and leads to reliable small to medium-sized transfer effects to both the latent construct trained and the wider cognitive system. No age differences were noted in this form of plasticity. These results suggest that EF and WM training might be a useful tool for cognitive intervention in at least normal old age.
Supplementary Material
Acknowledgments
This study was supported by NIH grant AG-16201.
Footnotes
The authors developed the concept of the study together. J. Karbach performed the search of the literature and P. Verhaeghen analyzed the data. Both authors wrote parts of the manuscript and approved the final version.
We thank Daniel Spieler, Thomas Hutcheon, and Tilo Strobach for providing us with estimates.
Contributor Information
Julia Karbach, Saarland University.
Paul Verhaeghen, Georgia Institute of Technology.
References
- Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology and aging. 2008;23(4):765. doi: 10.1037/a0013494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Transfer Effects in Task-set Cost and Dual-task Cost after Dual-task Training in Older and Younger Adults: Further Evidence for Cognitive Plasticity in Attentional Control in late Adulthood. Experimental Aging Research. 2008;34:188–219. doi: 10.1080/03610730802070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Li SC, Mueller V, von Oertzen TV, Lindenberger U. Memory plasticity across the life span: Uncovering children's latent potential. Developmental Psychology. 2007;43(2):465–477. doi: 10.1037/0012-1649.43.2.465. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bäckman L. Working-memory training in younger and older adults: Training gains, transfer, and maintenance. Frontiers in Human Neuroscience. 2012;6:63. doi: 10.3389/fnhum.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenweg JI, Murre JM, Ridderinkhof KR. Brain training in progress: a review of trainability in healthy seniors. Frontiers in human neuroscience. 2012:6. doi: 10.3389/fnhum.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, De Sather JCMG. Changes in executive function across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–729. [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults A meta-analytic study. Psychological science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedle R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:456–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Parisi JM, Spira AP, Kueider AM, Ko JY, Saczynski JS, Rebok GW. Memory training interventions for older adults: A meta-analysis. Aging & mental health. 2012;16(6):722–734. doi: 10.1080/13607863.2012.667783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta- analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment Effects on Adult Cognitive Development Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychological Science in the Public Interest. 2008;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hindin SB, Zelinski EM. Extended Practice and Aerobic Exercise Interventions Benefit Untrained Cognitive Outcomes in Older Adults: A Meta-Analysis. Journal of the American Geriatrics Society. 2012;60(1):136–141. doi: 10.1111/j.1532-5415.2011.03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, Jonides J. The role of individual differences in cognitive training and transfer. Memory & cognition. 2013:1–17. doi: 10.3758/s13421-013-0364-z. [DOI] [PubMed] [Google Scholar]
- Karbach J, Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science. 2009;12:978–990. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Karbach J, Mang S, Kray J. Transfer of task-switching training in older age: The role of verbal processes. Psychology and aging. 2010;25(3):677. doi: 10.1037/a0019845. [DOI] [PubMed] [Google Scholar]
- Karbach J, Schubert T. Training-induced cognitive and neural plasticity. Frontiers in Human Neuroscience. 2013;7:48. doi: 10.3389/fnhum.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, Garcia-Barrera MA. An Empirical Comparison of the Therapeutic Benefits of Physical Exercise and Cognitive Training on the Executive Functions of Older Adults: A Meta-Analysis of Controlled Trials. Neuropsychology. 2014 doi: 10.1037/neu0000101. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Larish JF, Strayer DL. Training for Attentional Control in Dual Task Settings: A Comparison of Young and Old Adults. Journal of Experimental Psychology: Applied. 1995;1:50–76. [Google Scholar]
- Kray J, Eber J, Karbach J. Verbal self-instructions in task switching: a compensatory tool for action-control deficits in childhood and old age? Developmental Science. 2008;11:223–236. doi: 10.1111/j.1467-7687.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- Li KZH, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley P. Benefits of cognitive dual-task training on balance performance in healthy older adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65:1344–1352. doi: 10.1093/gerona/glq151. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Kliegl R, Baltes PB. Professional expertise does not eliminate age differences in imagery-based performance during adulthood. Psychology & Aging. 1992;7:585–593. doi: 10.1037//0882-7974.7.4.585. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Brehmer Y, Li SC, Lindenberger U. Training-induced compensation versus magnification of individual differences in memory performance. Frontiers in human neuroscience. 2012:6. doi: 10.3389/fnhum.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: A review and future directions. Neuropsychology Review. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Developmental Psychology. 2013;49:270. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex"frontal lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Noack H, Lövdén M, Schmiedek F. On the validity and generality of transfer effects in cognitive training research. Psychological research. 2014 doi: 10.1007/s00426-014-0564-6. [DOI] [PubMed] [Google Scholar]
- Noack H, Lövdén M, Schmiedek F, Lindenberger U. Cognitive plasticity in adulthood and old age: Gauging the generality of cognitive intervention effects. Restorative Neurology & Neuroscience. 2009;27:435–453. doi: 10.3233/RNN-2009-0496. [DOI] [PubMed] [Google Scholar]
- Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, Aamodt W. The impact of sustained engagement on cognitive function in older adults: The Synapse project. Psychological Science. 2014;25:103–112. doi: 10.1177/0956797613499592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Carlson MC, Langbaum JB. Training and maintaining memory abilities in healthy older adults: traditional and novel approaches. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- Rebok GW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society. 2104;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, Engle RW. No evidence of intelligence improvement after working memory training: A randomized, placebo-controlled study. Journal Of Experimental Psychology: General. 2013;142:359–379. doi: 10.1037/a0029082. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: Findings from the COGITO study. Frontiers in Aging Neuroscience. 2010;2:1–10. doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychological bulletin. 2012;138:628. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Titz C, Karbach J. Working memory and executive functions: effects of training on academic achievement. Psychological research. 2014 doi: 10.1007/s00426-013-0537-1. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. The interplay of growth and decline: Theoretical and empirical aspects of plasticity of intellectual and memory performance in normal old age. In: Hill RD, Bäckman L, Stigsdotter Neely A, editors. Cognitive rehabilitation in old age. New York: Oxford University Press; 2000. pp. 3–22. [Google Scholar]
- Verhaeghen P. The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. New York: Oxford University Press; 2014. [Google Scholar]
- Verhaeghen P, Marcoen A. On the mechanisms of plasticity in young and older adults after instruction in the method of loci: evidence for an amplification model. Psychology and aging. 1996;11:164. doi: 10.1037//0882-7974.11.1.164. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: a meta-analytic study. Psychology & Aging. 1992;7:242. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- Wass SV, Scerif G, Johnson MH. Training attentional control and working memory–Is younger, better? Developmental Review. 2012;32(4):360–387. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA: the journal of the American Medical Association. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.