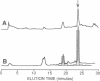
Abstract
Dityrosine is a sporulation-specific component of the yeast ascospore wall that is essential for the resistance of the spores to adverse environmental conditions. Dityrosine in vivo exists in both the LL and DL configurations and is part of an insoluble macromolecule of unknown structure. Here we present data indicating that dityrosine of the yeast spore wall is biosynthesized by a different mechanism than dityrosine in other biological systems--e.g., the hard fertilization membrane of the sea urchin egg. We identified two soluble, low molecular weight LL-dityrosine-containing spore wall precursors in extracts of sporulating cells and one precursor containing L-tyrosine. By expression of the previously described sporulation-specific genes DIT1 and DIT2 in vegetative cells, it was shown that DIT1 catalyzes the reaction leading from L-tyrosine to the tyrosine-containing precursor. DIT2, which is a member of the cytochrome P450 superfamily, is responsible for the dimerization reaction leading to the dityrosine-containing precursors. Epimerization of LL- to DL-dityrosine is one of the latest steps in spore wall formation and takes place after the dityrosine-containing precursors are incorporated into the spore wall. On the basis of these findings we suggest a biosynthetic pathway for the top layer of the yeast spore wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. O. Characterization of a new type of cross-linkage in resilin, a rubber-like protein. Biochim Biophys Acta. 1963 Feb 5;69:249–262. doi: 10.1016/0006-3002(63)91258-7. [DOI] [PubMed] [Google Scholar]
- Amadò R., Aeschbach R., Neukom H. Dityrosine: in vitro production and characterization. Methods Enzymol. 1984;107:377–388. doi: 10.1016/0076-6879(84)07026-9. [DOI] [PubMed] [Google Scholar]
- Briza P., Breitenbach M., Ellinger A., Segall J. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 1990 Oct;4(10):1775–1789. doi: 10.1101/gad.4.10.1775. [DOI] [PubMed] [Google Scholar]
- Briza P., Ellinger A., Winkler G., Breitenbach M. Characterization of a DL-dityrosine-containing macromolecule from yeast ascospore walls. J Biol Chem. 1990 Sep 5;265(25):15118–15123. [PubMed] [Google Scholar]
- Briza P., Ellinger A., Winkler G., Breitenbach M. Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. J Biol Chem. 1988 Aug 15;263(23):11569–11574. [PubMed] [Google Scholar]
- Briza P., Winkler G., Kalchhauser H., Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986 Mar 25;261(9):4288–4294. [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger-Van Rij N. J. Electron microscopy of germinating ascospores of Saccharomyces cerevisiae. Arch Microbiol. 1978 Apr 27;117(1):73–77. doi: 10.1007/BF00689354. [DOI] [PubMed] [Google Scholar]
- LaBella F., Keeley F., Vivian S., Thornhill D. Evidence for dityrosine in elastin. Biochem Biophys Res Commun. 1967 Mar 21;26(6):748–753. doi: 10.1016/s0006-291x(67)80137-2. [DOI] [PubMed] [Google Scholar]
- Law D. T., Segall J. The SPS100 gene of Saccharomyces cerevisiae is activated late in the sporulation process and contributes to spore wall maturation. Mol Cell Biol. 1988 Feb;8(2):912–922. doi: 10.1128/mcb.8.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn R. R., Magee P. T. Development of the spore wall during ascospore formation in Saccharomyces cerevisiae. J Cell Biol. 1970 Mar;44(3):688–692. doi: 10.1083/jcb.44.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B. Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1971 Apr;17(4):507–510. doi: 10.1139/m71-084. [DOI] [PubMed] [Google Scholar]
- Muthukumar G., Suhng S. H., Magee P. T., Jewell R. D., Primerano D. A. The Saccharomyces cerevisiae SPR1 gene encodes a sporulation-specific exo-1,3-beta-glucanase which contributes to ascospore thermoresistance. J Bacteriol. 1993 Jan;175(2):386–394. doi: 10.1128/jb.175.2.386-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammer M., Briza P., Ellinger A., Schuster T., Stucka R., Feldmann H., Breitenbach M. DIT101 (CSD2, CAL1), a cell cycle-regulated yeast gene required for synthesis of chitin in cell walls and chitosan in spore walls. Yeast. 1992 Dec;8(12):1089–1099. doi: 10.1002/yea.320081211. [DOI] [PubMed] [Google Scholar]
- Schneider J. C., Guarente L. Vectors for expression of cloned genes in yeast: regulation, overproduction, and underproduction. Methods Enzymol. 1991;194:373–388. doi: 10.1016/0076-6879(91)94028-b. [DOI] [PubMed] [Google Scholar]
- Sutter T. R., Loper J. C. Disruption of the Saccharomyces cerevisiae gene for NADPH-cytochrome P450 reductase causes increased sensitivity to ketoconazole. Biochem Biophys Res Commun. 1989 May 15;160(3):1257–1266. doi: 10.1016/s0006-291x(89)80139-1. [DOI] [PubMed] [Google Scholar]
- Zaitsu K., Eto S., Ohkura Y. High-performance liquid chromatographic determination of dityrosine in biological samples. J Chromatogr. 1981 Feb 27;206(3):621–624. doi: 10.1016/s0021-9673(00)88936-0. [DOI] [PubMed] [Google Scholar]