Abstract
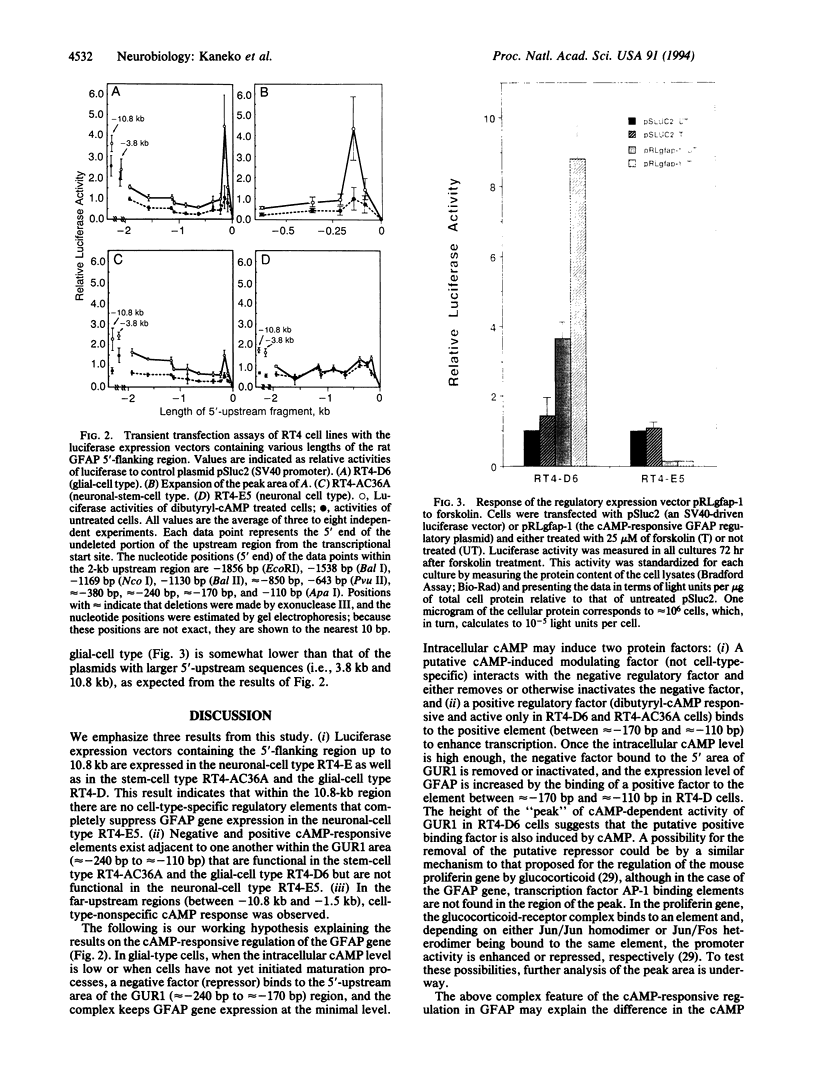
Expression of the rat glial fibrillary acidic protein (GFAP) gene is responsive to the intracellular level of cAMP. We have examined the sequence 5'-upstream of the transcription start site of the rat GFAP-encoding gene to determine the elements responsible for regulating the cAMP response. The RT4 cell lines consist of a neural stem-cell type RT4-AC and its three derivative cell types, one glial-cell type, RT4-D, and two neuronal-cell types, RT4-B and RT4-E. GFAP is expressed in the stem-cell type and the glial-cell type but is not expressed in the neuronal-cell types. Luciferase expression vectors containing various areas of the 10.8-kb region upstream of the transcription start site of the GFAP gene were transiently transfected into these RT4 cells. The effect of cAMP was examined by quantitating the transient expression of luciferase. We found that (i) the 5'-upstream region alone (up to 10.8 kb) allows expression of the GFAP gene in the stem-cell type, the glial-cell type, and a neuronal-cell type; (ii) there are negative and positive cAMP-responsive elements that are juxtaposed within the region between -240 bp and -110 bp upstream and are functional in the stem-cell and glial-cell types but are not functional in the neuronal-cell type RT4-E; (iii) there may be elements that respond to dibutyryl-cAMP in all three RT4 cell types within the region from 2 kb to 10.8 kb upstream of the transcription start site; and (iv) a regulatory luciferase plasmid pRLgfap-1, containing both the upstream and downstream regulatory regions of the GFAP gene, not only expresses luciferase but also responds to forskolin in the stem-cell type and the glial-cell type. This regulatory plasmid, however, does not express in the neuronal-cell type with or without the forskolin treatment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcarek J. M., Cowan N. J. Structure of the mouse glial fibrillary acidic protein gene: implications for the evolution of the intermediate filament multigene family. Nucleic Acids Res. 1985 Aug 12;13(15):5527–5543. doi: 10.1093/nar/13.15.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Intermediate filaments in nervous tissue. Cell Muscle Motil. 1985;6:75–96. doi: 10.1007/978-1-4757-4723-2_4. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Donahue L. M., Schaller K., Sueoka N. Segregation of Na(+)-channel gene expression during neuronal-glial branching of a rat PNS-derived stem cell line, RT4-AC. Dev Biol. 1991 Oct;147(2):415–424. doi: 10.1016/0012-1606(91)90299-i. [DOI] [PubMed] [Google Scholar]
- Droms K., Sueoka N. Cell-type-specific responses of RT4 neural cell lines to dibutyryl-cAMP: branch determination versus maturation. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1309–1313. doi: 10.1073/pnas.84.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985 Jun;8(4-6):203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Freeman M. R., Beckmann S. L., Sueoka N. Regulation of the S100 protein and GFAP genes is mediated by two common mechanisms in RT4 neuro-glial cell lines. Exp Cell Res. 1989 Jun;182(2):370–383. doi: 10.1016/0014-4827(89)90242-5. [DOI] [PubMed] [Google Scholar]
- Freeman M. R., Sueoka N. Induction and segregation of glial intermediate filament expression in the RT4 family of peripheral nervous system cell lines. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5808–5812. doi: 10.1073/pnas.84.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. L. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976 Oct;56(4):652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- Gandelman K. Y., Pfeiffer S. E., Carson J. H. Cyclic AMP regulation of P0 glycoprotein and myelin basic protein gene expression in semi-differentiated peripheral neurinoma cell line D6P2T. Development. 1989 Jun;106(2):389–398. doi: 10.1242/dev.106.2.389. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Imada S., Sueoka N. Cell-type specific segregation of transcriptional expression of glial genes in the rat peripheral neurotumor RT4 cell lines. J Neurosci Res. 1993 Dec 15;36(6):646–656. doi: 10.1002/jnr.490360605. [DOI] [PubMed] [Google Scholar]
- Imada M., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. I. Isolation and characterization of cell lines and cell type conversion. Dev Biol. 1978 Sep;66(1):97–108. doi: 10.1016/0012-1606(78)90276-2. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Schwann cell precursors and their development. Glia. 1991;4(2):185–194. doi: 10.1002/glia.440040210. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Morgan L., Stewart H. J., Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990 May;109(1):91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Kaneko R., Sueoka N. Tissue-specific versus cell type-specific expression of the glial fibrillary acidic protein. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4698–4702. doi: 10.1073/pnas.90.10.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prince G., Fages C., Rolland B., Nunez J., Tardy M. DBcAMP effect on the expression of GFAP and of its encoding mRNA in astroglial primary cultures. Glia. 1991;4(3):322–326. doi: 10.1002/glia.440040310. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messens J., Slegers H. Synthesis of glial fibrillary acidic protein in rat C6 glioma in chemically defined medium: cyclic AMP-dependent transcriptional and translational regulation. J Neurochem. 1992 Jun;58(6):2071–2080. doi: 10.1111/j.1471-4159.1992.tb10948.x. [DOI] [PubMed] [Google Scholar]
- Miura M., Tamura T., Mikoshiba K. Cell-specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis- and trans-acting promoter elements for astrocyte-specific expression. J Neurochem. 1990 Oct;55(4):1180–1188. doi: 10.1111/j.1471-4159.1990.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sarid J. Identification of a cis-acting positive regulatory element of the glial fibrillary acidic protein gene. J Neurosci Res. 1991 Feb;28(2):217–228. doi: 10.1002/jnr.490280209. [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Kume-Iwaki A., Goldman J. E. Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia. 1988;1(5):346–354. doi: 10.1002/glia.440010507. [DOI] [PubMed] [Google Scholar]
- Tardy M., Fages C., Le Prince G., Rolland B., Nunez J. Regulation of the glial fibrillary acidic protein (GFAP) and of its encoding mRNA in the developing brain and in cultured astrocytes. Adv Exp Med Biol. 1990;265:41–52. doi: 10.1007/978-1-4757-5876-4_4. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y., Sueoka N. In vitro segregation of different cell lines with neuronal and glial properties from a stem cell line of rat neurotumor RT4. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6305–6309. doi: 10.1073/pnas.75.12.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomozawa Y., Sueoka N., Miyake M. Clonal sublines of rat neurotumor RT4 and cell differentiation. V. Comparison of Na+ influx, Rb+ efflux, and action potential among stem-cell, neuronal, and glial cell types. Dev Biol. 1985 Apr;108(2):503–512. doi: 10.1016/0012-1606(85)90053-3. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]