Abstract
Gamma-aminobutyric acid B receptors (GABABRs) are heterodimeric G-protein coupled receptors, which mediate slow synaptic inhibition in the brain. Emerging evidence suggests astrocytes also express GABABRs, although their physiological significance remains unknown. To begin addressing this issue, we have used imaging and biochemical analysis to examine the role GABABRs play in regulating astrocytic Ca2+ signalling. Using live imaging of cultured cortical astrocytes loaded with calcium indicator Fluo-4/AM, we found that astrocytic GABABRs are able to induce astrocytic calcium transients only if they are pre-activated by P2 purinoceptors (P2YRs). The GABABR-mediated calcium transients were attenuated by the removal of extracellular calcium. Furthermore, P2YRs enhance the phosphorylation of astrocytic GABABR R2 subunits on both serine 783 (S783) and serine 892 (S892), two phosphorylation sites that are well known to regulate the activity and the cell surface stability of GABABRs. Collectively these results suggest that P2YR mediated signaling is an important determinant of GABABR activity and phosphorylation in astrocytes.
1. Introduction
Astrocytes, the most abundant cell type in the central nervous system (CNS), are accepted to play essential roles in brain function by supporting neuronal viability and vascular integrity (Attwell et al., 2010). In addition, astrocytes release glutamate, D-serine and adenosine triphosphate (ATP), a process that has been termed gliotransmission which regulates neuronal excitability and synaptic transmission (Haydon and Carmignoto, 2006). Whilst astrocytes are not electrically active, their properties are subject to regulation via dynamic changes in intracellular Ca2+ signalling, events that are believed to play a critical role in coordination of astrocyte communication and gliotransmission (Haydon and Carmignoto, 2006; Wang et al., 2009). Astrocytes express a plethora of neurotransmitter receptors, including those activated by adenosine, ATP, glutamate and GABA (Haydon and Carmignoto, 2006). Whilst the roles glutamatergic receptors and purinoreceptors play in regulating astrocyte activity have been addressed (Cornell-Bell et al., 1990; Fumagalli et al., 2003; James and Butt, 2002), the role GABA receptors play in these processes are not as well understood.
GABABRs are G-protein coupled receptors that mediate slow and prolonged inhibitory signalling in the brain via the activation of Gi/o type G-proteins leading to inhibition of adenylyl cyclase (AC). Structurally, GABABRs are obligate heterodimers composed from R1 and R2 subunits (Bowery et al., 2002; Couve et al., 2000). The effector coupling and stability of GABABRs are subject to modulation via the phosphorylation of serine residues 783 and 892 within GABABR R2 subunit. (Couve et al., 2002; Kuramoto et al., 2007). Significantly, phosphorylation of S783 is regulated via the activation of N-Methyl-D-aspartate receptors (NMDAR), and this process plays a key role in determining neuronal morphology, in addition to cognitive behaviours (Terunuma et al., 2014; Terunuma et al., 2010b). In addition to neurons, GABABR subunits are expressed in astrocytes and other types of glia (Charles et al., 2003; Lee et al., 2011; Oka et al., 2006). However, the role GABABRs play in regulating astrocyte activity remains largely speculative.
In this study, we examined the mechanisms regulating GABABR signalling in astrocytes. Our experiments reveal that GABABR receptors induce Ca2+ transient in astrocytes but only after pre-activation of P2 purinoceptors. In parallel with this, we demonstrated that purinoceptors enhance the phosphorylation of S783 and S892 in the R2, events that are accepted to increase GABABR activity. Therefore, our results reveal an unexpected role for purinoceptors in facilitating astrocytic GABABR signalling.
2. Material and methods
2.1 Cultured astrocytes
Cerebral cortical astrocytes from P0-1 C57/Bl6 mice were cultured as described previously (Mungenast, 2011; Zhang et al., 2004). Dissected cortex were treated with 0.25 % tripsin, triturated in minimum essential medium (MEM) and transferred into flasks. They were grown to confluence at 37 °C in a humidified 5 % CO2 atmosphere. After 7-10 days, flasks were washed with cold Earle’s balanced salt solution (EBSS), and fed with cold modified MEM before shaking at 260 rpm for 3 days. Remaining adherent cells were dissociated by using 0.1 % tripsin, and plated onto coverslips. Cells were used after 4-6 days in culture (Zhang et al., 2004). All procedures have been approved by Tufts University’s Institutional Animal Care of Use Committee (IACUC).
2.2 Cell surface biotinylation assay
Labelling of surface proteins for steady-state assays were performed as reported previously in cultured cortical neurones (Fairfax et al., 2004).
2.3 Cyclic AMP (cAMP) assay
The measurement of cAMP in cultured astrocytes was performed using ELISA based kit (Cell Biolabs).
2.4 Confocal calcium imaging in cultured astrocytes
For calcium imaging in cultured astrocytes, cells were plated on glass cover slips. The measurement of intracellular [Ca2+] was performed using the acetoxymethyl-ester form of the fluorescent dye Fluo-4 (Fluo-4/AM; Invitrogen) as described previously (D’Ascenzo et al., 2007; Xie et al., 2010). The dye was dissolved in dimethyl sulfoxide (DMSO) (5 mg/ml), and this stock solution was stored at −20 °C. Before the experiments, the stock solution was diluted in Normal Hippocampal Saline (NHS; in mM: 140 NaCl, 5 KCl, 10 D-glucose, 2 CaCl2, 2 MgSO4, 10 HEPES, 6 Sucrose, pH 7.35), containing 0.15% pluronic F-127 (Sigma-Aldrich) as described previously (D’Ascenzo et al., 2007). The working concentration of dye was 2 μg/ml. The cultured cells were incubated for 20 min in the dye-containing NHS, washed two times with NHS, and then incubated for another 30 min in a dye-free solution allowing time for hydrolysis of the ester dye. The imaging procedure took place at room temperature. Fluorescence was excited at 488 nm. ATP (100 μM, Sigma-Aldrich), baclofen (100 μM, Tocris) and other drugs for stimulation were applied through a perfusion system equipped with a pinch valve to control the duration of application (Xie et al., 2010). Fluorescence intensity was measured from individual astrocytes as the average intensity of fluorescence in a region of interest corresponding to the cell soma using Metamorph software package. The fluorescent signal at a given time point was expressed as ΔF/F0 = (F1 - F0)/F0, where F0 and F1 are the value of the fluorescence in astrocytes at rest and at the given time point, respectively (D’Ascenzo et al., 2007).
2.5 Western blot
Cultured cortical astrocytes were first lysed in 1 % SDS, 50 mM NaF, 1 mM EDTA, then diluted with 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM NaF, 2 mM Na3VO4, 10 mM Na4P2O7, 10 μg/mL leupeptin, 1 μg/mL aprotinin, 10 μg/mL antipain and 250 μg/mL 4-(2-Aminoethl) benzenesulfonyl fluoride hydrochloride, to reduce concentration of SDS to 0.1%. Soluble material was then subjected to immunoblotting with antibodies against GABABRs, phospho-S783/phospho-S892 or pan-R1 and R2 antibodies that recognize C-terminal epitopes in the respective proteins have been described previously (Terunuma et al., 2014). The phosphorylation and expression of AMPK was examined as described in (Terunuma et al., 2010b). Membrane were then probed with HRP-conjugated secondary antibodies and detected by SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific). The luminescence images were captured by Luminescent image analyser (LAS3000, Fujifilm) and the intensity of bands were measured by Image J. Data were analysed using GraphPad Prism and statistical significance were determined using on-way ANOVA or paired t-test.
3. Results
3.1 Functional GABABRs are express on the surface of cultured cortical astrocytes
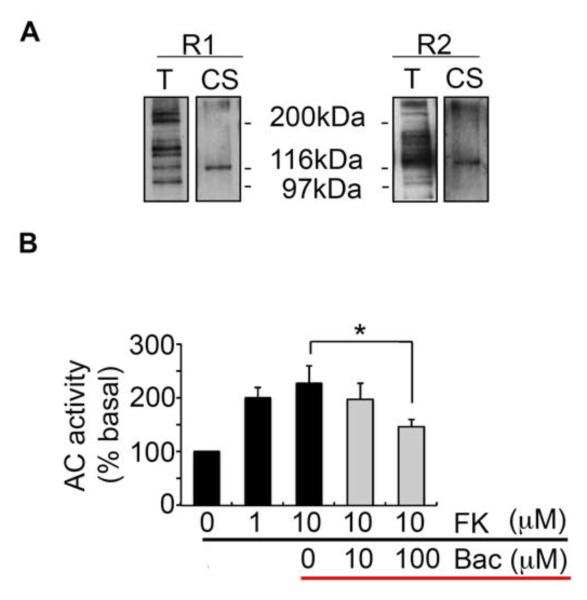
To initiate our studies, we examined GABABR expression in cultured astrocytes prepared from P0-1 mouse cerebral cortex using immunoblotting coupled with biotinylation. Western blotting of total lysates suggested that astroctyes express multiple isoforms of the R1 and R2 subunits (Fig. 1A), and biotinylation confirmed that astroctyes express both R1 and R2 subunits on their plasma membranes (Fig. 1A). In order to identify the functionality of astrocytic GABABRs, we assessed the effects of GABABR agonist baclofen on adenosine-3′-5′-cyclic monophosphate (cAMP) accumlutaion. Exposure of astrocytes to forskolin, an AC activator, significantly increased cAMP levels (10 μM: 227 ± 31.78, p = 0.0008), an effect that was reduced by baclofen (100 μM: 146 ± 13.78, p = 0.0413, compared to 10 μM forskolin) (Fig. 1B). Collectively, these results suggest that astrocytes express functional GABABRs.
Fig. 1.
Surface expression of astrocytic GABABRs and dose-dependent inhibition of cAMP levels by baclofen. A. Surface biotinylation assay confirmed expression of GABABRs on the plasma membrane of cultured cortical astrocytes. B. Baclofen treatment significantly reduced the production of cAMP (*p<0.05, paired-t test, n=4).
3.2 Induction of astrocytic Ca2+ transients by GABABRs
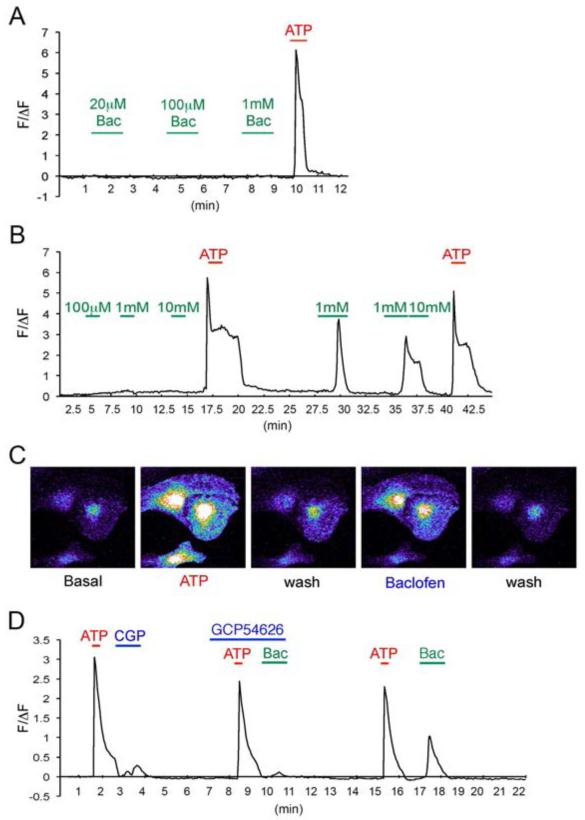
To determine whether astrocytic GABABRs modulate Ca2+ signalling, cultures were loaded with Fluo-4/AM and dynamic changes in cytosolic Ca2+ levels were analysed using time-lapse confocal microscopy (Parri and Crunelli, 2003; Simard et al., 2003). Exposure of astrocytes to increasing concentrations of baclofen did not lead to any significant changes in intracellular Ca2+ levels (Fig. 2A). In contrast, subsequent exposure of astrocytes to the P2 purinoceptor agonist ATP rapidly increased intracellular Ca2+ levels, consistent with published studies (Fig. 2A and 2B) (Fischer et al., 2009; King et al., 1996; Li et al., 2003). Importantly, after exposure to ATP, evidence of small baclofen induced Ca2+ transients was observed and did not occlude subsequent responses to ATP (Fig. 2A and 2B). To assess whether GABABR signalling is facilitated by pre-exposure to ATP, we used the GABABR antagonist CGP54626. Pre-treatment of astrocytes with 1μM CGP54626 abolished baclofen-evoked Ca2+ transients (Fig. 2C). However, CGP54626 alone did not have any effect on ATP-dependent increases in Ca2+(Fig. 2C).
Fig. 2.
Calcium imaging in cultured astrocytes. A. Time course of Ca2+ fluorescence changes (ΔF/F0) by baclofen before and after ATP (100 μM) treatments. Data points are averages of 7 cells in the imaging field in one experiment. The horizontal bar represents the application of stimuli. Green: baclofen, Red: 100 μM ATP. B. An example of images of cultured cortical astrocytes loaded with the Fluo-4/AM. Cells were stimulated with 100 μM ATP for 15 sec, washed until the calcium levels are reduced to baseline, then stimulated again with 100 μM baclofen for 1 min. C. Representative Ca2+ fluorescence changes (ΔF/F0) of baclofen-induced calcium increase with/without GABABR antagonist CGP54626 (1 μM). Data points are averages of 6 cells in the imaging field in one experiment. The horizontal bar represents the application of stimuli. Green: 100 μM baclofen (Bac), Red: 100 μM ATP, Blue: 1 μM CGP54626.
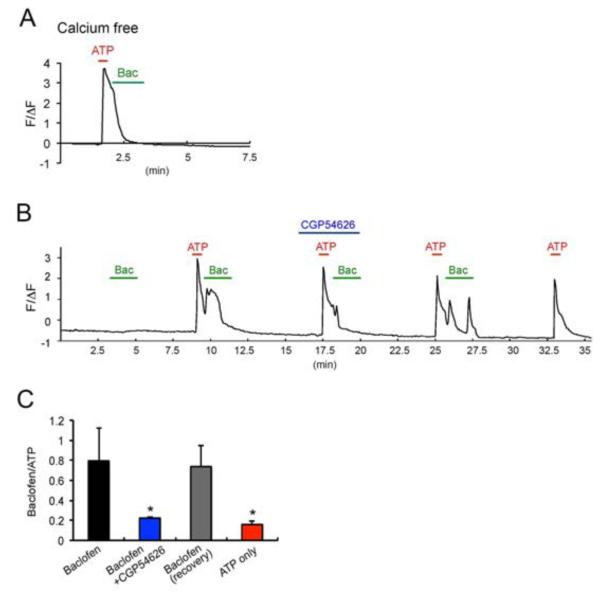
To further assess the role of ATP in promoting GABABR activity in astrocytes, we compared the ratios of baclofen-induced Ca2+ transients under varying conditions (Baclofen/ATP) (Fig. 3A and 3B). These experiments confirmed that baclofen-induced Ca2+ transients were only seen after exposure to ATP, an effect blocked by CGP54626 (36.08 ± 9.87 % compared to ATP-baclofen, p = 0.0015). However these effects were seen in the majority of cultured astrocytes (>80%). The effects of CGP54626 on baclofen-dependent modulation were reversible after extensive washing with extracellular solution (109.89 ± 39.226 compared to first ATP-baclofen stimulation, p = 0.407) (Fig. 3B; recovery). To examine the origins of baclofen-induced Ca2+ transients, we used Ca2+ free extracellular solutions. In multiple experiments performed on distinct cultures, the removal of extracellular Ca2+ prevented baclofen induced Ca2+ transients, but not on those induced by ATP (Fig. 3C).
Fig. 3.
Baclofen-induced Ca2+ waves are ATP and extracellular calcium dependent. A. Representative trace of long-term imaging of baclofen-induced Ca2+ fluorescence changes (ΔF/F0). Data points are averages of 13 cells in the imaging field in one experiment. The horizontal bar represents the application of stimuli. Green: 100 μM baclofen, Red: 100 μM ATP, Blue: 1 μM CGP54626. B. Summary histograms showing that baclofen induces Ca2+ transients and it is reduced by pre-treatment with CGP54626 (blue). The second baclofen treatment (recovery, grey) after wash induced Ca2+ transient. **p<0.01, ***p<0.001, compare to first ATP-baclofen stimulation (black) n=. C. Representative trace of baclofen-induced Ca2+ fluorescence changes (ΔF/F0) after removal of extracellular calcium. Data points are averages of 8 cells in the imaging field in one experiment. The horizontal bar represents the application of stimuli. Green: 100 μM baclofen, Red: 100 μM ATP.
Collectively, these studies strongly suggest the ability of GABABRs to modulate astrocytic Ca2+ signalling is facilitated by ATP.
3.3 ATP Stimulation Increases Phosphorylation of Astrocytic GABABRs
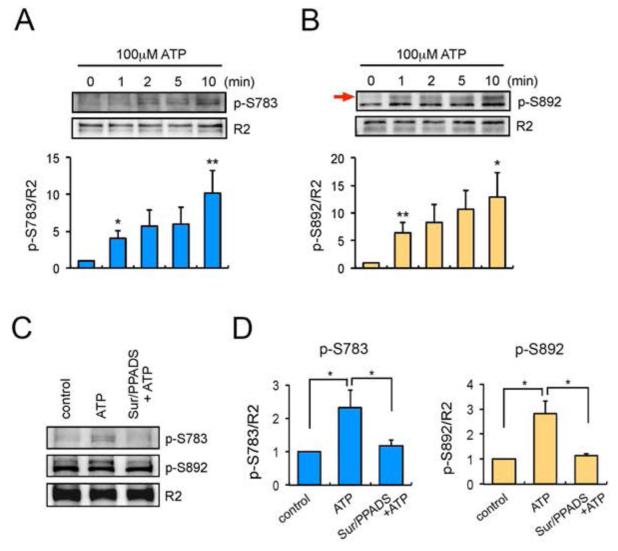
To analyse the mechanisms by which ATP regulates GABABR activity, we examined its effects on the phosphorylation of S783 and S892 within the receptor R2 subunit, accepted substrates of 5′-AMP (AMPK) and cAMP-(PKA) dependent protein kinases, respectively (Couve et al., 2002; Terunuma et al., 2010b). To do so, we used phospho-specific antibodies against these residues, and the ratio of p-S783/R2 and p-S892/R2 was then compared between treatments (Couve et al., 2002; Kuramoto et al., 2007). Exposure of astrocytes to ATP stimulation significantly increased S783 and S892 phosphorylation in a time-dependent manner (Fig. 4A and 4B). ATP-induced phosphorylation of both residues was prevented by pre-application of the P2 purinoceptor antagonists, 300 μM Suramin and 100 μM pyridoxal phosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS) (Fig. 4C and 4D) (p-S783: p = 0.037; p-S892: p = 0.018 compared to ATP alone). Therefore, these results demonstrate that in astrocytes, P2 purinoceptors regulate the phosphorylation of S783 and S892 within the GABAB R2 subunit.
Fig. 4.
ATP stimulation increases phosphorylation of GABABRs. A and B. Cortical astrocytes were treated with 100 μM ATP for 0-10 min. Total lysates were subjected to SDS-PAGE and visualised by immunoblotting with anti-p-S783 antibodies (A), anti-p-S892 antibodies (B) or GABABR2 antibodies. Integrated intensities were calculated by densitometry measurements of immunoblots in Image J and were normalised to averages of R2 subunits. Bar graphs are shown as a change relative to control (0 min) and represent mean value ± SEM of 3 independent experiments. *p<0.05, **p<0.01, one-way ANOVA. The black arrow on panel B is pointing the band representing p-S892. C. Cortical astrocytes were treated with P2 purinoceptor antagonists Suramin (300 μM) and PPADS (100 μM) for 15 min prior to ATP (100 μM) stimulation. ATP was then applied for 10 min and the phosphorylation of S783 (p-S783) and S892 (p-S892) were visualised by immunoblotting. D. Normalised quantification of p-S783 (left) and p-S893 (right). Data are shown as a change relative to control and represent mean ± SEM of 5 independent experiments for p-S783 and 3 independent experiments for p-S892. *p<0.05, one-way ANOVA.
3.4 ATP-mediated S783 phosphorylation in GABABR2 is regulated by CaMKK activated AMPK
Studies in neurons suggested that S783 phosphorylation is mediated by AMPK (Kuramoto et al., 2007). The activity of AMPK is critically dependent upon phosphorylation of Threonine 172 (T172), which is facilitated by Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) upon NMDAR activation (Mairet-Coello et al., 2013; Terunuma et al., 2010b). Therefore, we examined whether CaMKK-AMPK signalling pathways also mediate S783 phosphorylation in astrocytes.
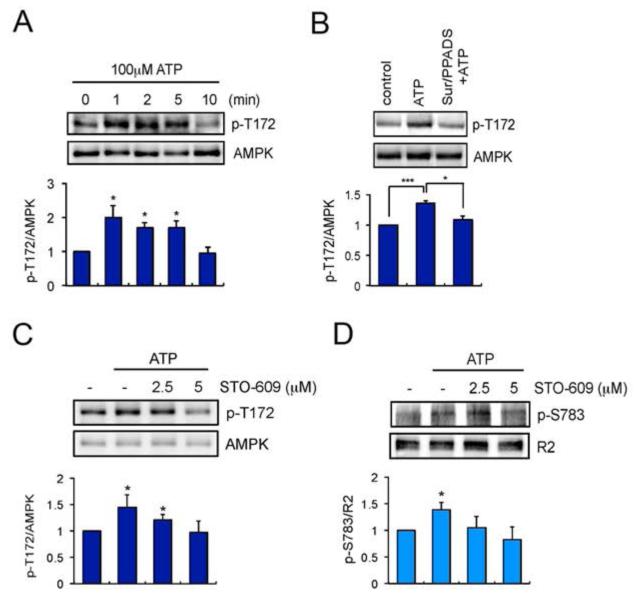
To test this, we examined the effects of ATP on phosphorylation of T172 in AMPK using phospho-specific antibodies. ATP induced a rapid increase in T172 phosphorylation (1 min: 2.0 ± 0.34, p = 0.0214 compared to 0 min), which returned to baseline after 10 min (10 min: 0.9494 ± 0.1760, p = 0.3940 compared to 0 min) (Fig. 5A). To determine if ATP-induced T172 phosphorylation is mediated by P2 purinoceptors, cultures were pre-treated with Suramin and PPADS. ATP induced phosphorylation of T172 was abolished by P2 purinoceptor antagonists (1.08 ± 0.0676, p = 0.014 compared to ATP alone, p = 0.15 compared to control). In order to assess the role CaMKK plays in ATP-induced phosphorylation of AMPK as an upstream kinase, we used the CaMKK inhibitor STO-609 (Fig. 5C). ATP dependent T172 phosphorylation was abolished by STO-609 (p = 0.023 compared to ATP alone) (Fig. 5C).
Fig. 5.
ATP stimulation increases the phosphorylation of AMPK. A. Cortical astrocytes were treated with 100 μM ATP for 0-10 min. Total lysates were subjected to SDS-PAGE and visualised by immunoblotting with anti-p-T172 antibodies or AMPK antibodies. Bar graphs are shown as a change relative to control (0 min) and represent mean value ± SEM of 3 independent experiments. *p<0.05, one-way ANOVA. B. Cortical astrocytes were treated with P2 purinoceptor antagonists Suramin (300 μM) and PPADS (100 μM) for 15 min prior to ATP (100 μM) stimulation. ATP was then applied for 2 min and the phosphorylation of T172 (p-T172) were visualised by immunoblotting. Data are shown as a change relative to control and represent mean ± SEM of 3 independent experiments. *p<0.05, **p<0.01, one-way ANOVA. C and D. Increased concentration of STO-609 (0-5 μM) inhibited ATP-induced phosphorylation of T172 in the AMPK and S783 in the GABABR2 subunits. Data are shown as a change relative to control (-) and represent mean ± SEM of 3 independent experiments. *p<0.05, **p<0.01, one-way ANOVA and paired t-test.
Whilst the direct effects AMPK plays in regulation of substrates is difficult to determine due to a lack of specific inhibitors, we found that in addition to preventing T172 phosphorylation, STO-609 also prevented ATP-dependent modulation of S783 phosphorylation (p = 0.383 compared to ATP alone) (Fig. 5D). Collectively, these results suggest that exposure of astrocytes to ATP leads to CaMKK dependent activation of AMPK and subsequent phosphorylation of S783.
3.5 ATP-mediated S783 phosphorylation in GABABR2 is through P2YRs
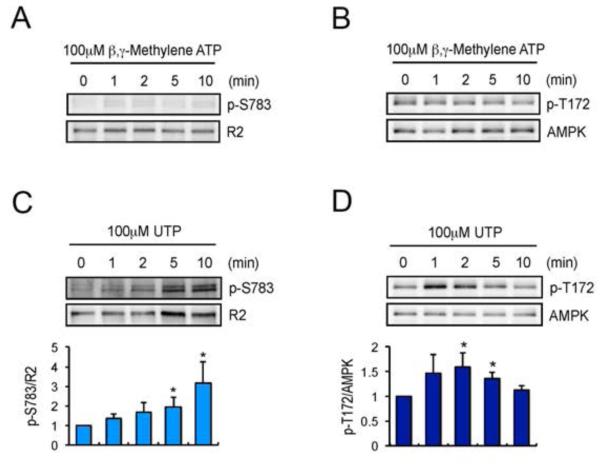
Astrocytes express both ionotropic P2XRs and metabotropic P2YRs (Fam et al., 2003; Fischer et al., 2009; James and Butt, 2002). To establish which P2 purinoceptor(s) mediate AMPK-dependent phosphorylation of S783, we used the P2XR agonist β,γ-methylene ATP (β,γ-meATP) (Coddou et al., 2011). βγ-meATP had no marked effects on the phosphorylation of T172 or S783, suggesting a minimal role of P2XR receptors in regulating AMPK and GABABR phosphorylation (Fig. 6A). Next, we tested the effects of 2-Methylthio ATP (2-meSATP) and UTP which activate P2Y1Rs/P2XRs and P2Y2/P2Y4Rs, respectively, on AMPK/GABABR phosphorylation (Centemeri et al., 1997) (Fig. 6B-D). Both 2-meSATP and UTP significantly increased phosphorylation of S783 and T172 in AMPK (data not shown for 2-meSATP). Therefore, metabotropic P2YRs are likely to be the principle mediators of S783 phosphorylation and AMPK activation in astrocytes.
Fig. 6.
P2YR agonists increase the phosphorylation of GABABR2 and AMPK. A. Cortical astrocytes were treated with P2XR agonist β,γ-meATP for 0-10 min. Total lysates were subjected to SDS-PAGE and visualised by immunoblotting with anti-p-S783, anti-GABABR2, anti-p-T172 or anti-AMPK antibodies. B and C. Astrocytes were treated with P2YR agonist UTP for 0-10 min. Total lysates were subjected to SDS-PAGE and visualised by immunoblotting with anti-GABABR2 antibodies (B), or AMPK antibodies (C). Integrated intensities of phosphorylation were calculated by densitometry measurements of immunoblots in Image J and were normalised to averages of total proteins. Bar graphs are shown as a change relative to control (0 min) and represent mean value ± SEM of 4 independent experiments. *p<0.05, one-way ANOVA. D and E. Phosphorylation of S783 (D) and S892 (E) in R2 subunits were determined by P2YR agonist 2-meSATP. Bar graphs are shown as a change relative to control (0 min) and represent mean value ± SEM of 3 independent experiments. *p<0.05, one-way ANOVA. F. PTX inhibits ATP-mediated S892 phosphorylation in R2 subunit. PTX was applied for 14 hr before 10 min ATP (100 μM) stimulation. Bar graphs are shown as a change relative to control (-) and represent mean value ± SEM of 3 independent experiments. *p<0.05, ***p<0.001, one-way ANOVA.
3.6 ATP-mediated S892 phosphorylation in GABABR2 is via pertussis toxin-sensitive P2YRs
We examined whether P2YRs also regulate phosphorylation of S892 in the R2 subunit, a substrate of PKA (Terunuma et al., 2010a). In contrast to S783, 2-meSATP did not significantly modify S892 phosphorylation. In epithelial cells, ATP-dependent activation of PKA is mediated by pertussis toxin (PTX)-sensitive P2YRs (Liu et al., 1998). Therefore, we incubated cultured astrocytes with 100 ng/ml PTX for 14 hrs and examined ATP-induced S892 phosphorylation. PTX pre-treatment abolished phosphorylation of this residue (Fig. 6F). Consequently, our results suggest S892 phosphorylation is regulated via PTX-sensitive P2YRs, such as P2Y12 and P2Y13Rs.
4. Discussion
Communication between astrocytes and their environment is largely mediated by transient elevations of intracellular Ca2+ in response to a variety of neurotransmitter/gliotransmitters, including ATP, glutamate, acetylcholine and norepinephrine (Haydon and Carmignoto, 2006; Wang et al., 2009). Many of these neurotransmitter events in astrocytes are mediated via GPCRs, which couple to phospholipase C to drive the production of inositol trisphosphate and the subsequent release of Ca2+ from the endoplasmic reticulum, in addition to the activation of AC (Petravicz et al., 2008; Volterra and Meldolesi, 2005). Astrocytes express GABABRs (Charles et al., 2003; Oka et al., 2006), which have been suggested to play a role in regulating synaptic transmission (Ding et al., 2009; Kang et al., 1998). However, the signalling mechanisms GABABRs use to influence astrocyte activity remain to be defined but preliminary studies suggest that they are c
In this current study, we have examined whether astrocytic GABABRs induce Ca2+ transients in astrocytes. Exposure of astrocytes to the GABABR agonist baclofen itself did not increase Ca2+ transients, whilst under the same conditions, ATP induced robust elevations in intracellular Ca2+ levels.
However, after brief exposure to ATP, baclofen-induced Ca2+ transients were evident in astrocytes and could be abolished by the GABABR antagonist CGP54626. In contrast to ATP induced Ca2+ transients, those resulting from the activation of GABABRs were dependent upon extracellular Ca2+. These outcomes support previous studies analysing astrocytic signalling after photothrombosis, a model of cerebral ischaemia. This injury leads to rapid release of ATP and prolonged elevations in astrocytic Ca2+ levels which are partly, mediated by GABABRs (Ding et al., 2009; Rossi et al., 2007). In addition our results are consistent with studies that have shown the effects of GABA on astrocytic Ca2+ signalling can be partially reduced the low affinity GABABR antagonist phaclofen (Nilsson et al., 1993).
GABABR activity in neurons is tightly regulated by receptor phosphorylation (Terunuma et al., 2010a). We have previously identified two phosphorylation sites for serine/threonine protein kinases that regulate GABABR activity. S783 in the R2 subunit is phosphorylated by AMPK, an event that prevents receptor internalisation and enhanced GABABR signalling (Kuramoto et al., 2007; Terunuma et al., 2010b). GABABR effector coupling is subject to similar positive modulation via PKA mediated phosphorylation of the adjacent residue S892, within the R2 subunit (Couve et al., 2002). Here, we found both S783 and S892 are phosphorylated by brief ATP exposure in cultured astrocytes through the activation of P2 purinoceptors. ATP-dependent phosphorylation of S783 in R2 subunit was mediated via P2YR-CaMKK-AMPK signalling. Notably, this mechanism is similar to that employed by neurons to regulate S783 phosphorylation downstream of NMDARs (Terunuma et al., 2010b). Astrocytes express many P2YRs, which increase intracellular Ca2+ levels (Fischer et al., 2009). In our study using specific agonists, we found P2Y1R, P2Y2R and P2Y4R are likely to mediate AMPK activation and subsequent S783 phosphorylation. ATP has also been found to activate PKA through the production of arachidonic acid, leading to activation of AC-linked GPCRs, or the activation of Gs-and Gq/11-coupled P2Y11Rs (Liu et al., 1998; van der Weyden et al., 2000). In astrocytes, we found that S892 phosphorylation is mediated by PTX-sensitive P2YRs. The mechanism(s) underlying the functional modulation and phosphorylation of GABABRs by P2YRs are unknown, however it may reflect synergy between G-protein signalling pathways. Significantly such cross talk has recently been demonstrated between GABABRs and metabotropic glutamate receptors in neurons (Rives et al., 2009).
In conclusion, our study suggests GABABR signalling in astrocytes is critically dependent upon the activation of multiple P2 purinoceptor subtypes leading to the phosphorylation of key regulatory residues in the GABABR2. This synergistic interaction between purinoceptors and GABABRs may act as a co-incidence detector to allow the fine-tuning of astrocytic Ca2+ signalling.
Acknowledgement
We thank to the members of the Moss laboratory for discussions during the course of this study. MT is recipient of a National Scientist Development Grant (09SDG2260557) from the American Heart Association. SJM is supported by NIH-NINDS grants, NS051195, NS056359, NS081735, NIH-NIMH grant, MH097446, CURE and Simons Foundation. SJM serves as a consultant for SAGE therapeutics and AstraZeneca, relationships that are regulated by Tufts University and do not impact on this study.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002 doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles KJ, Deuchars J, Davies CH, Pangalos MN. GABAB receptor subunit expression in glia. Molecular and Cellular Neuroscience. 2003;24:214–223. doi: 10.1016/s1044-7431(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB Receptors: A New Paradigm in G Protein Signaling. Molecular and Cellular Neuroscience. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Couve A, Thomas P, Calver AR, Hirst WD, Pangalos MN, Walsh FS, Smart TG, Moss SJ. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat Neurosci. 2002;5:415–424. doi: 10.1038/nn833. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia. 2009;57:767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax BP, Pitcher JA, Scott MG, Calver AR, Pangalos MN, Moss SJ, Couve A. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J Biol Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Kalia LV, Salter MW. Differential frequency dependence of P2Y1- and P2Y2-mediated Ca 2+ signaling in astrocytes. J Neurosci. 2003;23:4437–4444. doi: 10.1523/JNEUROSCI.23-11-04437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Appelt K, Grohmann M, Franke H, Norenberg W, Illes P. Increase of intracellular Ca2+ by P2X and P2Y receptor-subtypes in cultured cortical astroglia of the rat. Neuroscience. 2009;160:767–783. doi: 10.1016/j.neuroscience.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43:218–203. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- Li N, Sul JY, Haydon PG. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J Neurosci. 2003;23:10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lalor D, Bowser SS, Hayden JH, Wen M, Hayashi J. Regulation of arachidonic acid release and prostaglandin E2 production in thymic epithelial cells by ATPgammaS and transforming growth factor-alpha. Cell Immunol. 1998;188:81–88. doi: 10.1006/cimm.1998.1343. [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungenast AE. Diacylglycerol signaling underlies astrocytic ATP release. Neural Plast. 2011;2011:537659. doi: 10.1155/2011/537659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Rönnbäck L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54(3):605–14. doi: 10.1016/0306-4522(93)90232-5. 1993. [DOI] [PubMed] [Google Scholar]
- Oka M, Wada M, Wu Q, Yamamoto A, Fujita T. Functional expression of metabotropic GABAB receptors in primary cultures of astrocytes from rat cerebral cortex. Biochem Biophys Res Commun. 2006;341:874–881. doi: 10.1016/j.bbrc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prézeau L. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Pangalos MN, Moss SJ. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv Pharmacol. 2010a;58:113–122. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Revilla-Sanchez R, Quadros IM, Deng Q, Deeb TZ, Lumb M, Sicinski P, Haydon PG, Pangalos MN, Moss SJ. Postsynaptic GABAB receptor activity regulates excitatory neuronal architecture and spatial memory. J Neurosci. 2014;34:804–816. doi: 10.1523/JNEUROSCI.3320-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Vargas KJ, Wilkins ME, Ramirez OA, Jaureguiberry-Bravo M, Pangalos MN, Smart TG, Moss SJ, Couve A. Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc Natl Acad Sci U S A. 2010b;107:13918–13923. doi: 10.1073/pnas.1000853107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, Conigrave AD, Morris MB. Signal transduction and white cell maturation via extracellular ATP and the P2Y11 receptor. Immunol Cell Biol. 2000;78:369–374. doi: 10.1046/j.1440-1711.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Takano T, Nedergaard M. Astrocytic calcium signaling: mechanism and implications for functional brain imaging. Methods Mol Biol. 2009;489:93–109. doi: 10.1007/978-1-59745-543-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience. 2010;170:992–1003. doi: 10.1016/j.neuroscience.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]