Abstract
This review presents a general model for the understanding of pain, placebo, and chronification of pain in the framework of cognitive neuroscience. The concept of a computational cost-function underlying the functional imaging responses to placebo manipulations is put forward and demonstrated to be compatible with the placebo literature including data that demonstrate that placebo responses as seen on the behavioural level may be elicited on all levels of the neuroaxis. In the same vein, chronification of pain is discussed as a consequence of brain mechanisms for learning and expectation. Further studies are necessary on the reversal of chronic pain given the weak effects of treatment but also due to alarming findings that suggest morphological changes in the brain pain regulatory systems concurrent with the chronification process. The burden of chronic pain is devastating both on the individual level and society level and affects more than one-quarter of the world's population. Women are greatly overrepresented in patients with chronic pain. Hence, both from a general standpoint and from reasons of health equity, it is of essence to advance research and care efforts. Success in these efforts will only be granted with better theoretical concepts of chronic pain mechanisms that maps into the framework of cognitive neuroscience.
Keywords: Neural mechanisms, Chronic pain, Model-driven approach to brain function, Cognitive neuroscience
This communication carries such a perspective on chronic pain and pain regulation. Several scientific leaps forward have been noted in the understanding of learning and memory as well as in the understanding of the brain as a complex dynamic system, but these advancements have only to a limited extent influenced the research and clinical practice of pain medicine. The main argument here is that the neural mechanisms underlying chronic pain should be understood in the context of a model-driven approach to understand brain function; ie, the models of mainstream cognitive neuroscience. A comprehensive explanation should be based on a general model, or else the findings from different studies and scientific niches cannot be reconciled.
The seminal article of Craig with the description of pain as a homoeostatic emotion12 moved the field away from the concept of a “searching for pain center” in the brain to a systems-oriented understanding. The experience of pain was put in a behavioural perspective and the dynamics of the preprogrammed complex emotional reactions to acute pain were explained in terms of a dynamic regulatory system. Just as in all other expressions of emotion, the homoeostasis model for understanding pain provides both a basis for a prolongation of the feeling state but also, at the same time, an effective measure of social communication to alert others of, eg, danger. In addition, such a mechanism also serves to raise empathic responses in the group. The understanding of pain as a homoeostatic emotion has also contributed to the understanding of affective comorbidity in different pain syndromes because the mechanisms of both lowered mood and anxiety are based on similar regulatory mechanisms.53 However, the mentioned mechanisms are mostly represented in the phylogenetically old components of the central nervous system. The role of the cerebral cortical regulation in chronic pain remains a challenge to fully explain.
The main survival value of the brain is to provide timely information on dangers and correct motivation for different behaviour in real-time. All brain processing takes time and hence preprogamming of complex responses is an effective time saver.2 Some of the oldest and most effective responses have developed during evolution and provide the basis for several of the behaviours that have been denoted as human universals.15 One of them is the acute pain reaction, but also the general sickness response should be mentioned in this context. No matter how complex these responses may seem, they illustrate that preprogrammed series of events may be instantiated fully in the nonaware domain. The more set a behaviour is, the less the voluntary control, ie, less cortical executive control.
Although fast reactions provide a general survival value, a repeated stereotype reactive behaviour leads to the opposite. If a predator can fully read the coming behaviour of the prey, the catch would be an easy one. Over the course of evolution, the addition of cortical regulation has added variation and inhibition of automatic responses and thereby lowered the predictability. This could only be achieved by increase of the capacity for information processing in the brain, ie, the development of the brain is all about information processing.2,43
Increasingly, the view on the brain function as a machinery for predictions has gained support. We and others have pointed out that this also is an important principle in pain perception and regulation and especially for the subjective reporting of pain. A recent comprehensive review summarises very well the standing in the placebo research field.48 This communication has the aim to further the description and suggest concepts for the understanding of these phenomena that can be challenged in empirical studies. Predictions entail the ability to internally maintain models of the world and to constantly update those with sparse multisource bits of information, principles that have been well established in sensory-motor learning.52 In short, upon an execution of a movement, an efferent copy is made as to serve as an internal model for the coming sensory feedback. This minimises the need for further computations as only discrepancies between the inner model and the received sensory feedback is ground for corrective behaviour, whereas expected input may be blocked from entering the motor-planning system. This understanding also explains why it is impossible to tickle oneself.6 The nature of the cerebral representations of the internal models is still debated. Kurzweil29 has proposed a theory that has gained traction. He has modelled a cortical unit as the unitary construct for cortical function where pattern recognition is central, and although it represents a rigid construction, it has a very flexible function. He argues that such a system provides the means for a hierarchical representation that allows for constant updates, sparse information processing, and thereby the necessary speed and precision. His notion of a regular structure in the connections in the cerebral cortex as a distinguishing feature has gained support recently when the regular structure of the cerebral cortex has been possible to read by means of high-resolution diffusion magnetic resonance imaging.50 Also, Kurzweil's suggestion for the construction of the cortical architecture satisfies the needs for a multilevel system in which Bayesian principles for model optimisation can be harboured.27 The Bayesian principles posit that all current information is related to prior beliefs (ie, predictions).
The theories on the mirror systems in the brain44 points in the same direction and suggests that information from others are corepresented with the representation of the self in the cortex, albeit that the self also entails self-agency as an separate line of information processing.36
These general principles provide a basis not only for the understanding both of the chronification process of pain but also for the basis of different treatment paradigms in different pain syndromes. Our ability to verify the existence of the above-mentioned mechanisms has been advanced by studies of the placebo phenomenon. In such studies, it is possible to explicitly manipulate the internal model. We have left the era when the debate focused on whether a placebo effect is real or not.46 When placebo is understood in the context of dynamic complexity, that debate becomes redundant. Any notion that placebo would be constantly present and thus remains constant over time is incorrect because placebo rests on the discrepancy between the inner representational model and the current information inflow. If such a discrepancy remains over time, the inner model will update, and with the diminishing computational difference, and the placebo effect will disappear with time.
The important first evidence that placebo mechanisms could be a target for systematic studies, and thereby become biomedically anchored, was provided by the important study by Levine et al.32 more than 2 decades before the advent of functional neuroimaging. Levine demonstrated, in an elegant model-driven design, that placebo analgesia could rationally be tied to the function of the endorphin system. Since then, many behavioural studies have validated the original findings by Levine et al., and some have added new information about the neurobiology of placebos, also beyond the endorphin systems and extending the mechanisms also to other transmitter-specific systems.1,10 However, the original study by Levine et al. provided the essential conceptual framework that has since then formed a foundation for current theories of placebo mechanisms. There was a significant advance when some early accounts of a functional cerebral anatomy of placebo responses were published.14,34,41 All of these demonstrated that incongruence between the inner representation and the current input lead to a cost-function in terms of increased activity in cerebral regions attributed to evaluative and emotional components.
The brain is a complex system, ie, it is composed of interconnected parts that as a whole exhibit one or more properties (behaviour, memory with different time properties) not obvious from the properties of the individual parts. At the same time, local modules can hold several functions, and this economises with wiring and thereby with energy and time.7 The function in discrete units may also change dramatically over time through mechanisms of learning.29 Several theories now point in the direction that any internal representation models of the external world is done directly in the systems for perception, in line with the less explicit models of mirror theories.27 Grounded theories on cognition are gaining popularity with its ability to house the need for internal representation models of the environment directly bound to functional units. Grounded theories on cognition allow for computational sparseness and a decreased need for synchrony in time. Grounded theories on cognition assume that there is no central module for cognition.42 According to this view, all cognitive phenomena, including amodal cognition such as reasoning, numeric, and language processing, emerge from a variety of bodily, affective, perceptual, and motor processes. Grounded theories on cognition posit dynamic brain–body–environment interactions and perception–action links as the common bases of simple behaviours and complex cognitive and social skills, without representational separations between these domains.4
The core concept of the perception–action loop entails arrows 1-3-4-5 in Figure 1. This loop also serves as the major mechanism for nondeclarative learning (unconscious, implicit skills). The action perception loop concept can be generalised by means of the model of pain as a homoeostatic mechanism12 to also include the dynamics of pain regulation. According to Craig's model, homoeostatic regulation of pain builds its activity on a mix of information based on nociception and expectations of nociceptive input; largely working in a top-down manner (arrow 2). This model work and the understanding of the placebo concept and pain perception have given us the opportunity to test several hypotheses derived from Figure 1 in empirical designs.
Figure 1.
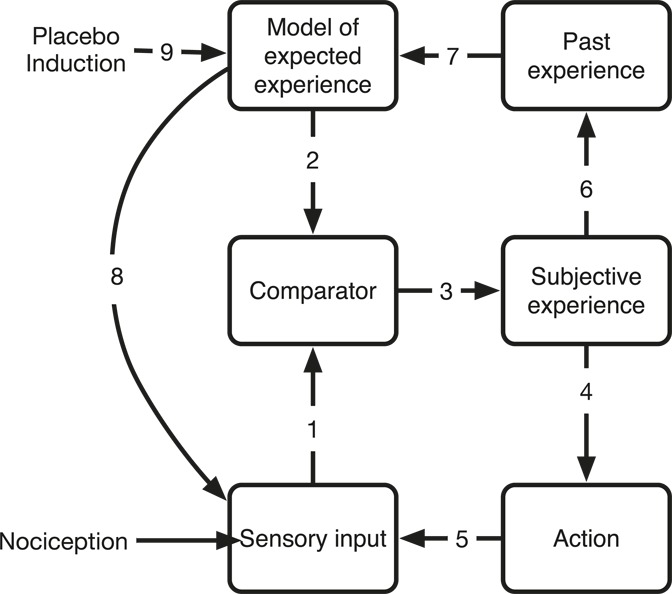
The sensory input is fed directly to the model comparator that is distributed along the sensory axis (1) that compares the input with the model of the expected information (2). The resulting subjective experience (3) that feeds motivation by contributions to action (4), its feedback (5), and memory (6) that in turn influences the expectancy model (7). The sensory axis may be influenced by top-down regulation of the lower-order systems. (8). The expectation model may also be directly influenced directly (9) of, eg, context, instructions, or other perturbations such as a placebo induction.
In the suggested model, all sensory input is compared with the expectancy of that particular input, directly in the primary input region, and any deviance will incur a cost-function that is measurable as an activation (arrows 1 and 2). Hence, the computationally intensive comparison function will be activated both in situations where the input is stronger than expected but also importantly in situations with lesser input than expected.9 The ultimate difference in such a comparison system is of course if the expectation is no input but instead a sensory input is evoked as a surprise. In such situations with low predictability, the subjective experience of nociception seems to amplify.45 Predictability of a nociceptive input entail all dimensions, and lack of predictability in time and intensity may be modelled separately.8 The concept of adaptability is connected to the concept of pattern recognition in space and time. A prediction from the general model as presented in Figure 1 is that withholding information will incur a computational cost as this also invokes a mismatch between the input and the expectancy. We tested this in a set of volunteers and demonstrated how a time-locked visual and sensory stimulus quickly allowed for associative learning, and the generation of a robust expectancy model. When the time lock was broken, the model for predictability was rendered useless the cost was noted in all the systems of relevance, ie, both in sensory regions and in regions associated with timing and pattern recognition.8
Conversely, model preparedness for a change that ultimately does not take place will also evoke a computational cost. We tested this in a paradigm where a sensory stimulus (tickling) was administered in a random manner. Each stimulus was preceded by a visual cue. Hence, after the induction of the expectation (visual cue), it was possible to vary the experiment so that the visual cue was not always followed by the sensory stimulus. The findings illustrated the power of a predictive model in the ability to set a whole system in preparedness given strong enough prior.9 In an earlier study, we also demonstrated how inhibition of motor function could be demonstrated as a cost-function using cerebral blood flow (CBF) measurements with positron emission tomography. We induced itch with a small dose of intradermal histamine, with all its motivational drive to scratch, but gave strict verbal instruction not to scratch.20 The motor programme for scratching is presumably well encoded, and hence a number of brain regions connected to the actual behaviour of scratching were all activated. This report has later been confirmed and extended in the Irene Tracey's Laboratory.31
A placebo manipulation explicitly reframes the model of expectation, and hence can move in either direction as a placebo or nocebo response.11 Such a reframing of expectancy is, as noted above, an unstable state, specifically if the subject is exposed several times to the same discrepancy. A waning of the placebo response with time is a natural result of the presented model.11
Sensory illusions also have the capacity to incur changes in brain activation as a response to inconsistencies between sensory input and sensory expectation. The thermal grill illusion was first used in brain imaging by Craig et al.13 The application of the thermal grill illusion results in an awkward painful experience paired with thalamic hypoactivity.13,33 The illusion is a result of an unexpected thermal sensation using a grill where every other grid element is warm and the others are cool. It is likely that the unpleasantness alarm is set off because of the inadequacy of our inner model of what to expect and demonstrate that model-based sensory interpretation is present along the sensory axis.
To achieve an agreement between expected value and the input, either one can be adjusted. The general behavioural pain response to avoid further nociceptive input is part of such an adjustment. Removing the limb away from a heat-source is part of such a scheme. Adaptational changes may also be incurred in the sensory pathways. Thus, upon repeated sensory stimuli, the primary sensory response could wane quickly as a result of adaptation in all components of the sensory system.49
We manipulated the instructions in a pain experiment to elucidate contextual information as a basis for a manipulation of a pain experience. We told the research subjects that the painful stimulus (cold pressure test, [one hand in a bucket of circulating ice water]) would either last 60 or 120 seconds. Without the subjects' knowledge, all measurements of the brain activity were made during the first minute. Thus, the nociceptive input was the same, but the context was different because of the information that some sessions would incur a more prolonged pain stimulus. The main finding was a suppressed activity bilaterally in the amygdala that was interpreted as mirroring the adaptive response and need to suppress the emotional response as to endure the experimental situation.38
A specific trait of all different forms of adaptation (learning) is the variation of the time span for different forms of learning. Although social learning often rides on evolutionary facilitated mechanisms for rapid learning, other forms of learning such as skill learning may need robust learning efforts with multiple repetitions to reach the desired level. The representation of change in different models of expectancy probably varies in the same way. The understanding of placebo as a modification of expectations necessitates the possibility to quickly update and refresh one's expectations. A number of scientific articles have demonstrated such an update of expectancies, and one of the first studies of sensory expectation16 elegantly demonstrated this.
Placebo model designs for brain imaging should ideally be anchored in cognitive theory or they run the risk of becoming phenomenological in their interpretations with poor explanatory power of the neurophysiology of placebo and pain experience. Given the general concept that the model in Figure 1 presents, a placebo response should be possible to invoke in any homoeostatic system, eg, in all emotional domains. Our first generalisation outside the domain of pain and pain regulation was an experiment where we manipulated the expected emotional reaction to images with aversive content (International Affective Picture System images30). We made an induction of expectations by suggesting that we could lower the level of anxiety evoked by the image by injection of a benzodiazepine (oxazepam) and removing the blocking of this effect by a benzodiazepine antagonist. Indeed, during the induction of the placebo, we injected both the benzodiazepine and the blocker (lanexat) and the subjects rated among other things the invoked unpleasantness of the pictures. As expected, the rating decreased during benzodiazepine and increased after the block. On day 2, the subjects returned and were studied with functional magnetic resonance imaging (fMRI). The same scripted instructions were given as in day 1 but the injections were all exchanged for saline. The event-related fMRI demonstrated that the same modulatory network, including the rostral anterior cingulate cortex and the lateral orbitofrontal cortex, was involved in both emotional placebo and placebo analgesia. However, the top-down target of manipulation was situated, as the theory would predict, in the extrastriatal visual cortices and the amygdala.39 A confirmation of the relevance of this finding was recently published from a paradigm where we manipulated the instruction overtly to study top-down regulation of the incurred by aversive pictures in a variation of previous experiment.37 We simply gave the instruction to reappraise the emotional content to its fullest emotional expression or to reappraise with suppression. The latter induced a top-down reappraisal based on cortical control demonstrated as an activation of the dorsolateral prefrontal cortex for both unpleasant and neutral content, whereas the lateral orbitofrontal cortex only changed its activity to reappraisal of pictures with negative emotional content subtracted from reappraisal of neutral pictures.19 Notably, because this paradigm included no perturbation of the expectancy model, no activation was incurred in the rostral anterior cingulate cortex.
The massively parallel structure of the brain still has a hierarchical organisation along the neuroaxis. We hypothesise that a placebo response may be instantiated at any level as all levels carry the same principle and an internal comparator with a prior. Thus, any level of an adaptive system could potentially be a target for a placebo manipulation. Generalising the mechanism of placebo to an induced mismatch between the expected value and the input, it should be possible to get a placebo also by induction through the unaware domain. In a study,22 we demonstrated the ability to evoke this learnt association through stimuli unaware to the subject as to evoke a placebo response. The method was visual presentation of backward masking, ie, inducing associations to the pain stimulus by 13-millisecond exposures of a pictured face directly followed by a scrambled image. The subject remains unaware of the face by this procedure. Interestingly, standard pain stimuli varied in subjective experience according to the preceding contextual conditioning that had been made. That behavioural study has now been followed up with an imaging study.21 Because conditioning was used with different cues toward placebo and nocebo, it was possible to study the functional response to the induction of each. The major finding was that the expected cost-function was expressed in areas pertaining to positive emotion (eg, orbitofrontal cortex), whereas the non-conscious nocebo elicited activations of the thalamus, amygdala, and hippocampus. Our results show that conditioned pain response modifications can be elicited independently of conscious awareness. We note that although these findings are in harmony with the presented general model and clearly support the concept of hierarchically organisation of the brain, they may seem to be at variance with some influential writings on the subject. Notably, the Benedetti group, several years ago, demonstrated that verbal instruction could fully annihilate a conditioned placebo response by means of a top-down mechanism.5
Thus, our model posits that if a cognitive instruction is the basis for a placebo response, this should have an instantiation in the frontal cortex. In a reanalysis40 of our previous articles,41,39 we were able to demonstrate a separation between the cortical activation based on nonspecific placebo vs that of specific drug treatment. Although the effects in the rostral anterior cingulate cortex (ACC) were activated in both treatment conditions, the orbitofrontal activation was only seen in the placebo condition. In a direct challenge of the difference between placebo and drug analgesia, the finding of separable frontal activations related to expectancies was replicated.3 This points to the immense importance of furthering the studies where drug treatment and psychological treatment are combined in the treatment of chronic pain. A hypothesis, that needs to be further studied, is that the ineffectiveness of drug regimes in chronic pain may be coupled with the inability to activate the proper learning mechanisms to readjust the homoeostatic pain mechanisms that have been altered in chronic pain.
Placing placebo mechanisms into general brain function brings on the hypothesis that a functional correlate to a placebo response should be possible to detect along the effector axis of the appropriate system (arrow 8). Already in our first article, we could demonstrate that the placebo response incurred systems appropriate top-down regulation at the level of the brainstem including periaqueductal gray and medulla.41 Several other studies have replicated our finding17 and correspondingly direct imaging of the opiate systems adds confirmation to this conclusion.54,55 An important extension was made when the Hamburg group developed a proper and reproducible method to study fMRI in the spinal fMRI.17 They have directly demonstrated the top-down effects of a placebo manipulation in the spinal cord,17 and that this response is modulated the same way as other responses by attention.47
Since placebo is instantiated in the same system as the underlying function, it is expected that the dynamics of placebo is reflected in the dynamics of the specific response, ie, the better response there is to a drug, the better the placebo response. This has been reported many times before by us41,39,22 and others and is therefore of no real surprise but rather a confirmation that the experiment has been well designed. Importantly, variations in the intensity of the induction of the placebo has been reported to also influence the activity in the primary region for coding of the mismatch between expectancy and sensory input, namely the rostral anterior cingulate cortex.18
Moving this framework to the understanding of chronic pain could be of benefit for the understanding of the adaptive processes including learning that ultimately leads to a centralised pain syndrome not dependent on nociceptive input for its self-perpetuating nature. The centralisation process is fully compatible with the above model and central components of the learning model.
We tested our framework in a series of studies of fibromyalgia. Earlier data have demonstrated that the descending pain inhibitory system has a diminished function in this disorder.35 Induction of pain activates both the ascending components of the nociceptive system and also the descending system emanating in the rostral forebrain (insula and rostral ACC). In a cross-sectional study between subjects with fibromyalgia and healthy control subjects, we demonstrated a diminished response in the mentioned regions, most notably in the rostral ACC.23 The regulation of the descending system is partly dependent on opioid mechanisms and genetic variations in this regulation of system alter the capacity for descending.28 It is still not clear whether such detected variations are part of the pathophysiology of fibromyalgia. In further analysis of fibromyalgia, the functional connectivity in the forebrain was studied. As hypothesised, the functional networks in the forebrain were changed in the patients with fibromyalgia.25 These functional changes were also parallelled with changes in the structure of the brain in line with the notion that central plasticity is critical for the transition from acute to chronic pain. Patients with fibromyalgia displayed a distinct overlap between decreased cortical thickness, decreased brain volumes, and decreased functional regional coherence in the rostral anterior cingulate cortex.26 In line with the theory of continuous changes due to plasticity, the morphometric changes were more pronounced with longer exposure to fibromyalgia pain. Detailed analyses remain if early structural and functional brain alterations in response to long-standing pain exist also in other forms of chronic pain disorders.
Thus chronic pain may be a result of continuous centralisation based on learning and recoding where plasticity carries changes both in functional connectivity and morphology. This line of explanation for the pathophysiology of chronic pain would be supported if reversal of the cerebral physiological changes were possible to demonstrate upon successful treatment. We tested whether the cognitive behavioural therapy would be an effective treatment in chronic pain and whether some of the above-mentioned changes were revisable. Cognitive behavioural therapy is based on learning theory and can potentially target different aspects of the pain response such as mood, suffering, and actual pain experience.51 A dominant theory for the effects of such treatment is the cortical control theory. In an fMRI study,24 patients in the treatment group reported larger improvement of fibromyalgia on the Patient Global Impression of Change measure, and also improved depression and anxiety symptoms, compared with the waiting-list controls. Surprisingly, there were no effects on clinical pain or pain sensitivity measures. An analysis of fMRI scans revealed that CBT led to increased activations in the ventrolateral prefrontal/lateral orbitofrontal cortex. These are all regions associated with executive cognitive control. These finding were in line with the behavioural measures and showed that the treatment did not reverse the changes in the descending pain pathways but rather pointed toward an increased access to executive regions for reappraisal of pain.
The model-based design of studies based on learning theories has given several clues on the cerebral functional and structural basis of the chronic pain experience. Hard work remains before these initial findings may be generalised, and above all, to clarify to what extent the changes are reversible. There are some indications of a limited reversibility given data both from clinical practice but also worrying data on changes in brain morphology,26 but this awaits full characterisation and above all on the reversibility. The quest for effective treatments in chronic pain syndromes rests on a deepened mechanistic understanding of the pathophysiology. The combination of experimental and clinical neuroimaging has promises to provide part of the much needed further understanding.
Conflict of interest statement
The author has no conflicts of interest to declare.
This work is supported by The Swedish Research Council 2012-1999, 2009-3191, the Osher Center for Integrative Medicine, Karolinska Institutet, Stockholm County Council, and the Knut and Alice Wallenberg Foundation.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Reference
- [1].Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arbib MA. The handbook of brain theory and neural networks. MIT press, 2002. [Google Scholar]
- [3].Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci 2012;32:8053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barsalou LW. Grounded cognition. Annu Rev Psychol 2008;59:617–45. [DOI] [PubMed] [Google Scholar]
- [5].Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 2003;23:4315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1998;1:635–40. [DOI] [PubMed] [Google Scholar]
- [7].Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012;13:336–49. [DOI] [PubMed] [Google Scholar]
- [8].Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage 2006;32:1804–14. [DOI] [PubMed] [Google Scholar]
- [9].Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. Tickling expectations: neural processing in anticipation of a sensory stimulus. J Cogn Neurosci 2000;12:691–703. [DOI] [PubMed] [Google Scholar]
- [10].Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci 2005;6:545–52. [DOI] [PubMed] [Google Scholar]
- [11].Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. PAIN 2010;151:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci 2003;26:303–7. [DOI] [PubMed] [Google Scholar]
- [13].Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science 1994;265:252–5. [DOI] [PubMed] [Google Scholar]
- [14].de la Fuente-Fernandez R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet Neurol 2002;1:85–91. [DOI] [PubMed] [Google Scholar]
- [15].Diener E, Kanazawa S, Suh EM, Oishi S. Why people are in a generally good mood. Pers Soc Psychol Rev 2014. pii: 1088868314544467. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [16].Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Jr, Raichle ME. Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 1995;373:249–52. [DOI] [PubMed] [Google Scholar]
- [17].Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009;326:404. [DOI] [PubMed] [Google Scholar]
- [18].Geuter S, Eippert F, Hindi Attar C, Buchel C. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 2013;67:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, Schalling M, Ingvar M, Ohman A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One 2012;7:e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neurosci Lett 1999;262:61–4. [DOI] [PubMed] [Google Scholar]
- [21].Jensen K, Kaptchuk T, Chen X, Kirsch I, Ingvar M, Gollub R, Kong J. A mechanism for nonconscious activation of conditioned placebo and nocebo responses. Cereb Cortex 2014. doi: 10.1093/cercor/bhu275 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A 2012;109:15959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in fibromyalgia reflected in rACC during provoked pain. PAIN 2009;144:95–100. [DOI] [PubMed] [Google Scholar]
- [24].Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. PAIN 2012;153:1495–503. [DOI] [PubMed] [Google Scholar]
- [25].Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 2012;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 2013;65:3293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kilner JM, Friston KJ, Frith CD. The mirror-neuron system: a Bayesian perspective. Neuroreport 2007;18:619–23. [DOI] [PubMed] [Google Scholar]
- [28].Kosek E, Jensen KB, Lonsdorf TB, Schalling M, Ingvar M. Genetic variation in the serotonin transporter gene (5-HTTLPR, rs25531) influences the analgesic response to the short acting opioid remifentanil in humans. Mol Pain 2009;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kurzweil R. How to create a mind. London: Viking Penguin, 2013. [Google Scholar]
- [30].Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 1993;30:261–73. [DOI] [PubMed] [Google Scholar]
- [31].Leknes SG, Bantick S, Willis CM, Wilkinson JD, Wise RG, Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol 2007;97:415–22. [DOI] [PubMed] [Google Scholar]
- [32].Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2:654–7. [DOI] [PubMed] [Google Scholar]
- [33].Lindstedt F, Johansson B, Martinsen S, Kosek E, Fransson P, Ingvar M. Evidence for thalamic involvement in the thermal grill illusion: an FMRI study. PLoS One 2011;6:e27075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am J Psychiatry 2002;159:728–37. [DOI] [PubMed] [Google Scholar]
- [35].Mense S. Descending antinociception and fibromyalgia. Z Rheumatol 1998;57(suppl 2):23–6. [DOI] [PubMed] [Google Scholar]
- [36].Moutoussis M, Fearon P, El-Deredy W, Dolan RJ, Friston KJ. Bayesian inferences about the self (and others): a review. Conscious Cogn 2014;25:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 2002;14:1215–29. [DOI] [PubMed] [Google Scholar]
- [38].Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci 2004;16:1289–301. [DOI] [PubMed] [Google Scholar]
- [39].Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing - induced expectations of anxiety relief activate a generalized modulatory network. Neuron 2005;46:957–69. [DOI] [PubMed] [Google Scholar]
- [40].Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. PAIN 2010;150:59–65. [DOI] [PubMed] [Google Scholar]
- [41].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imagine a shared neuronal network. Science 2002;295:1737–40. [DOI] [PubMed] [Google Scholar]
- [42].Pezzulo G, Barsalou LW, Cangelosi A, Fischer MH, McRae K, Spivey MJ. Computational Grounded Cognition: a new alliance between grounded cognition and computational modeling. Front Psychol 2012;3:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pinker S. How the mind works. Ann N Y Acad Sci 1999;882:119–127; discussion 128–34. [DOI] [PubMed] [Google Scholar]
- [44].Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci 2004;27:169–92. [DOI] [PubMed] [Google Scholar]
- [45].Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci 2004;24:7199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shetty N, Friedman JH, Kieburtz K, Marshall FJ, Oakes D. The placebo response in Parkinson's disease. Parkinson study group. Clin Neuropharmacol 1999;22:207–12. [PubMed] [Google Scholar]
- [47].Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol 2012;22:1019–22. [DOI] [PubMed] [Google Scholar]
- [48].Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med 2010;16:1277–83. [DOI] [PubMed] [Google Scholar]
- [49].Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol 2007;17:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wedeen VJ, Rosene DL, Wang R, Dai G, Mortazavi F, Hagmann P, Kaas JH, Tseng WY. The geometric structure of the brain fiber pathways. Science 2012;335:1628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, Ingvar M, Olsson GL. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur J Pain 2013;17:599–611. [DOI] [PubMed] [Google Scholar]
- [52].Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci 2011;12:739–51. [DOI] [PubMed] [Google Scholar]
- [53].Yalcin I, Barrot M. The anxiodepressive comorbidity in chronic pain. Curr Opin Anaesthesiol 2014;27:520–7. [DOI] [PubMed] [Google Scholar]
- [54].Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005;25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 2009;1156:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]


