Abstract
Background
Once weight loss is achieved, the challenge is to maintain this benefit. This review reports on the effectiveness of programs for weight-loss maintenance, as part of a larger review examining treatments for overweight and obese adults.
Methods
We updated the search of a 2011 review on screening and management of overweight and obese adults. Four databases were searched. For inclusion, participants had to have lost weight in treatment and then been randomly assigned to a weight-maintenance intervention or control conditions. Studies from the 2011 review that met the criteria were included. Data were extracted and pooled (where possible) for outcomes related to weight-loss maintenance.
Results
Eight studies were included. Compared with control participants, intervention participants regained less weight (mean difference [MD] –1.44 kg, 95% confidence interval [CI] –2.42 to –0.47), regardless of whether the intervention was behavioural (MD–1.56 kg, 95% CI –3.10 to –0.02) or pharmacologic plus behavioural (MD −1.39 kg, 95% CI –2.86 to 0.08). Intervention participants also showed better weight maintenance than the control participants in terms of waist circumference (MD –2.30 cm, 95% CI –3.45 to –1.15) and body mass index (MD –0.95 kg/m2, 95% CI –1.67 to –0.23). Participants undergoing pharmacologic plus behavioural interventions were more likely to maintain a loss of 5% or more of initial body weight than those in the control group (risk ratio [RR] 1.33, 95% CI 1.15 to 1.54); no difference was found for maintaining a weight loss of 10% or more (RR 1.76, 95% CI 0.75 to 4.12).
Interpretation
Moderate quality evidence shows that overweight and obese adults can benefit from interventions for weight maintenance following weight loss. However, there is insufficient evidence on the long-term sustainability of these benefits. Registration: PROSPERO no. CRD42012002753
The health-related risks associated with overweight and obesity in adults are well documented.1–3 Some of the health issues that obese adults are at a greater risk for include type 2 diabetes, coronary artery disease, stroke, depression and certain cancers. Unfortunately, the prevalence of overweight and obesity in adults is rising in Canada, tripling from 6.1% in 1985 to 18.3% in 2011.4
Recent reviews of moderate quality evidence have shown that multiple strategies for weight loss in adults, including a combination of reduced dietary intake, increased physical activity and behaviour modification, have led to reductions in body weight.5,6 To that end, the identification of interventions that sustain and prolong the benefits of weight loss is needed, because maintaining weight loss continues to be a conundrum for individuals and health care providers and represents an increasing burden on the health care system.1,4
This work was part of a larger systematic review conducted to provide an updated synthesis of the effectiveness of behavioural and pharmacologic interventions for treating overweight and obesity in adults.7 The goal of this review was to examine the effectiveness of weight-loss maintenance programs in adults using weight-related outcomes. Specifically, we sought to answer the following question: In adults aged 18 years or older who were overweight or obese, but not morbidly obese, and who lost weight in a prior active-phase treatment intervention, what is the effect of behavioural and pharmacological interventions on weight-loss maintenance?
Methods
Search strategy
Our protocol for examining the evidence on treatments for overweight and obesity in adults was designed to update a search conducted for a recent review by the US Preventive Services Task Force on this topic.8 We examined their search and determined that it addressed our key questions; we added a database (Embase) and a filter to restrict studies to those published in English or French because of limited resources for handling papers in other languages. To avoid duplication we planned to bring forward any of their included studies that met our criteria. We searched MEDLINE, Cochrane Central Register of Controlled Trials, PsycINFO and Embase from September 2010 (the date of the last search by the US Preventive Services Task Force) up to and including Apr. 19, 2013. The search strategy is provided in Appendix 1 (available at www.cmajopen.ca/content/3/1/E47/suppl/DC1). Reference lists from the US Preventive Services Task Force review8 and other reviews were searched for relevant studies not captured by our search. All citations were uploaded to a web-based systematic review software program for screening and data extraction.
Population, intervention, comparator, outcome and setting statement
Details regarding the population, intervention, comparator, outcomes and setting for this review are provided in Box 1.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for this review are provided in Box 2.
Study selection, quality assessment and data abstraction
Titles and abstracts of articles were reviewed independently by 2 research team members. Those articles marked for inclusion by either team member went on to full-text screening, which was also done independently by 2 researchers. Randomized controlled trials were assessed using the Cochrane Collaboration’s tool for risk-of-bias assessment.9 Overall strength of the evidence (identified as high, moderate, low or very low quality) was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system framework (GRADEpro version 3.2; available at www.ims.cochrane.org/revman/gradepro).10 One team member completed full data abstraction and a second member verified all extracted data, the risk-of-bias ratings and the strength of evidence assessment. All data were checked in a third round of verification. At all levels, interrater disagreements were resolved through discussion.
Data analysis
Longest available follow-up was considered as the data point of interest for weight-loss maintenance across all studies. For the meta-analyses, means and standard deviations (SDs) were used for continuous outcomes (i.e., change in weight in kg, body mass index [BMI] and waist circumference) and number of events were used for binary outcomes (i.e., maintenance of loss of 5% or more and 10% or more of initial body weight). The DerSimonian and Laird random-effects model with inverse variance method was used to generate the summary measures of effect in the form of mean difference (MD) for continuous outcomes and risk ratio (RR) for binary outcomes.11 Cochran’s Q (α = 0.05) and I2 (≥ 75% = high heterogeneity) statistics were used to quantify statistical heterogeneity between studies. For the outcome of change in weight (kg), we did a sensitivity analysis based on focus of intervention (behavioural and pharmacologic plus behavioural). For significant binary outcomes we calculated absolute risk reduction and number needed to treat; the latter were calculated using the absolute numbers computed by the GRADE software. GRADE estimates the absolute number per million using the control group event rate and RR with the 95% confidence interval (CI) obtained from the meta-analysis. Statistical analyses were performed using RevMan version 5.3 and GRADEpro.
Results
Search results
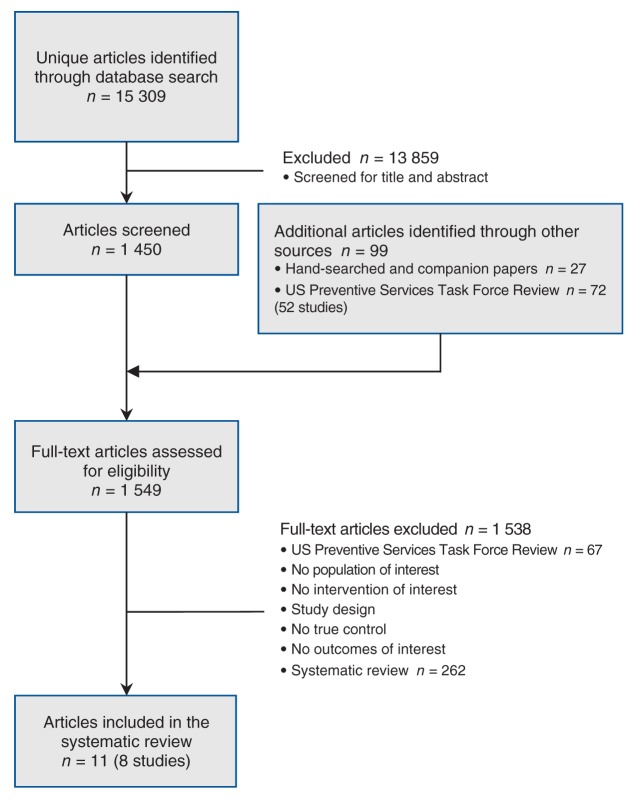
The results of the search and selection are shown in Figure 1. The search found 15 309 citations, which were screened by title and abstract. To the pool of recently published papers retained for full-text screening (n = 1450), we added 27 papers located through hand searching and 52 older studies (72 papers) that appeared in the 2011 US Preventive Services Task Force review8 for consideration. At the end of the process, 8 studies (11 papers) were included that concerned weight maintenance interventions following weight loss: 5 studies (5 papers)12–16 were brought forward from the US Preventive Services Task Force review that met our criteria, and 3 studies (6 papers)17–22 were found through our search of recent literature.
Figure 1:
Selection of studies evaluating the effectiveness of strategies for weight maintenance in adults treated for overweight and obesity.
Characteristics of included studies
Five studies were conducted in the US and 3 originated in Europe. Sample sizes ranged from 55 to 1032 participants. Six studies included both sexes, one included women only and one did not report sex. Participants across all studies ranged in age from 43 to 59 years at baseline. In every instance, participants had completed an active weight-loss phase and were then assigned to a behavioural or pharmacologic and behavioural intervention aimed at maintaining their weight loss or to a control group. The duration of the intervention ranged from 6 to 36 months. Table 1 provides a summary of the characteristics of the 8 included studies; more details for each study are reported in Appendix 2 (available at www.cmajopen.ca/content/3/1/E47/suppl/DC1). Most studies were rated as having an unclear or a high risk of bias. Ratings for risk of bias for the included studies are summarized in Table 2.
Table 1: Characteristics of included studies.
| Study | Sex | Mean age, yr | No. of participants* | Type of intervention for prior weight loss | Intervention type | Control | Intervention length, mo. | Location | Publication date |
|---|---|---|---|---|---|---|---|---|---|
| Champagne et al.17 Svetkey et al.18 |
M + F |
55 |
1032 |
Six-month diet plus group-based support |
Lifestyle (delivery strategies: Internet or personal contact) |
Self-directed |
30 |
US |
2011 |
| Davidson et al.12 |
M + F |
43 |
306 |
120 mg orlistat 3×/d for 1 yr plus controlled-energy diet |
120 mg orlistat 3×/d plus weight-maintenance diet |
Placebo plus same weight-maintenance diet |
12 |
US |
1999 |
| Hauptman et al.13 |
M + F |
42 |
273 |
120 mg orlistat 3×/d for 1 yr plus energy-reduced diet |
120 mg orlistat 3×/d plus weight-maintenance diet |
Placebo plus same weight-maintenance diet |
12 |
US |
2000 |
| Hill et al.14 |
M + F |
46 |
369 |
Six-month hypoenergetic diet plus encouraged to exercise |
120 mg orlistat 3×/d plus dietary and behavioural counselling |
Placebo plus same diet and behavioural components |
12 |
US |
1999 |
| Richelsen et al.15 |
M + F |
47 |
309 |
Eight-week very low-energy diet |
120 mg orlistat 3×/d plus energy-restricted diet and dietary and lifestyle counselling |
Placebo plus same diet and behavioural components |
36 |
Scandinavia |
2007 |
| Rickel et al.19 Perri et al.20 Radcliff et al.21 |
F |
59 |
234 |
Six-month group-based lifestyle program |
Six-month group-based lifestyle program |
Newsletters with tips and recipes |
6 |
US |
2011 |
| Sjöström et al.16 |
M + F |
45 |
261 |
120 mg orlistat 3×/d for 1 yr plus hypocaloric diet |
120 mg orlistat 3×/d plus weight-maintenance diet |
Placebo plus weight-maintenance diet |
12 |
Europe |
1998 |
| Thomas et al.22 | Not reported | 45 | 55 | Not reported (recruited from a weight loss clinic) | Weekly emails from a dietician with dietary, behavioural and exercise advice | No contact | 6 | UK | 2011 |
Note: F = female, M = male. *If a study included multiple treatment arms with different doses of orlistat, only the 120 mg group was included.
Table 2: Risk-of-bias ratings for included studies using Cochrane Collaboration’s tool9.
| Study | Sequence generation | Allocation concealment | Blinding of participants/personnel | Blinding of outcome assessors | Incomplete reporting | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Champagne et al.17 Svetkey et al.18 |
U |
L |
H |
L |
L |
L |
L |
| Davidson et al.12 |
U |
U |
U |
U |
H |
L |
H |
| Hauptman et al.13 |
U |
U |
U |
U |
H |
H |
U |
| Hill et al.14 |
U |
U |
U |
U |
L |
L |
H |
| Richelsen et al.15 |
L |
L |
U |
U |
L |
L |
H |
| Rickel et al.19 Perri et al.20 Radcliff et al.21 |
U |
U |
H |
L |
L |
L |
L |
| Sjöström et al.16 |
L |
U |
U |
U |
L |
L |
H |
| Thomas et al.22 | L | L | H | U | L | L | L |
Note: H = high risk of bias, L = low risk of bias, U = unclear risk of bias.
Maintaining weight loss
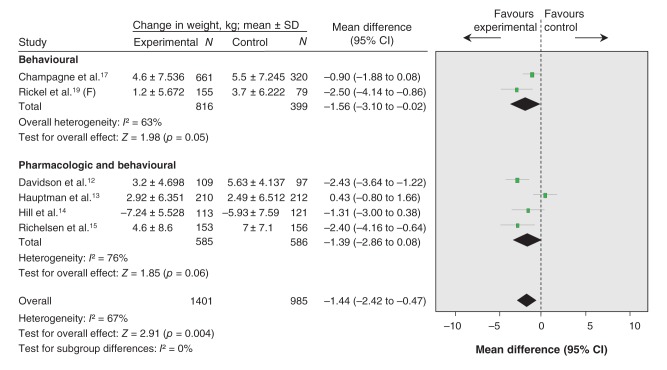
Six trials of moderate quality (downgraded for risk of bias) were included in the meta-analysis assessing weight maintenance as measured in kg (Figure 2, Table 3).12–15, 17,19 Pooled analysis showed that intervention participants (n = 1401) regained less weight than control participants (n = 985) (MD –1.44 kg, 95% CI –2.42 to –0.47, I2 = 67%). In all studies except one,14 there was less weight regain in the intervention group than in the control group. In the study by Hauptman and colleagues,13 the intervention group regained more weight than the control group, although there was no statistically significant difference. Pooled analysis for 2 behavioural (lifestyle) interventions showed that intervention participants regained less weight than the control groups (MD –1.56 kg, 95% CI –3.10 to –0.02, I2 = 63%).17,19 One additional 6-month behavioural intervention study, which could not be pooled, found that the intervention group maintained a greater median weight loss (9.6 kg, interquartile range [IQR] 10.9) than the control group (7.8 kg, IQR 5.9).22 No significant difference in effect for weight regain was seen for the 4 pharmacological plus behavioural interventions compared with control (MD –1.39 kg, 95% CI –2.86 to 0.08, I2 = 76%).12–15 The test for subgroup differences found no evidence that the effect of treatment differed based on type of intervention: behavioural (lifestyle) versus pharmacological (orlistat) plus behavioural (χ21 = 0.02, p = 0.88, I2 = 0%).
Figure 2:
Effect of weight-maintenance interventions on mean weight loss or gain (kg), overall and by type of intervention (behavioural [lifestyle] or pharmacologic [orlistat] plus behavioural). Values less than 0 indicate a change in weight in favour of the intervention (i.e., less weight regain). CI = confidence interval, F = female, N = total number of participants.
Table 3: Effect of weight-maintenance interventions on continuous weight outcomes.
| Outcome; intervention type | Meta-analysis, MD (95% CI) |
Statistical heterogeneity (within-group), p value (I2 value, %) |
Test for between-group differences, p value (I2 value, %) |
No. of participants | No. of studies | Quality of evidence rating* |
|---|---|---|---|---|---|---|
|
Maintenance of weight, kg | ||||||
| Overall |
–1.44 (–2.42 to –0.47) |
0.010 (67) |
NA |
2386 |
6 |
Moderate |
| Behavioural |
–1.56 (–3.10 to –0.02) |
0.10 (63) |
0.88 (0) |
1215 |
2 |
Moderate |
| Pharmacological + behavioural |
–1.39 (–2.86 to 0.08) |
0.006 (76) |
1171 |
4 |
Low |
|
|
Maintenance of BMI, kg/m2 | ||||||
| Behavioural |
–0.95 (–1.67 to –0.23) |
NA |
NA |
234 |
1 |
Moderate |
|
Maintenance of waist circumference, cm | ||||||
| Pharmacological + behavioural | –2.30 (–3.45 to –1.15) | NA | Na | 309 | 1 | Moderate |
Note: BMI = body mass index, MD = mean difference, NA = not applicable. *Grading of Recommendations Assessment, Development and Evaluation (GRADE) quality of evidence rating reflects confidence in the estimate of effect assessed through 5 domains of the evidence (risk of bias, indirectness, imprecision, inconsistency and reporting bias): moderate = downgraded for risk of bias, low = downgraded for risk of bias and imprecision.
Maintaining body mass index
A single study of moderate quality (downgraded for risk of bias) that examined a 6-month intervention using counselling approaches to support older women in rural areas in making lifestyle changes provided data for the outcome of maintaining BMI (Table 3).19 After completing the program, intervention participants (n = 155) showed a smaller change in BMI compared with the control group (n = 79) (MD –0.95 kg/m2, 95% CI –1.67 to –0.23).
Maintaining waist circumference
A single trial of moderate quality (downgraded for risk of bias) comparing a pharmacologic (120 mg orlistat 3 times daily) plus behavioural (standard energy-restricted diet and dietary and lifestyle counselling) strategy with a placebo plus the same behavioural approach provided data for the outcome of maintaining waist circumference (Table 3).15 After 36 months, intervention participants (n = 153) had a smaller increase in waist circumference compared with the control group (n = 156) (MD –2.3 cm, 95% CI –3.45 to –1.15).
Maintenance of loss of 5% or more of initial body weight
Three trials of moderate quality (downgraded for risk of bias) were included in the meta-analysis assessing maintenance of loss of 5% or more of initial body weight (Figure 3, Table 4).13,15,16 All 3 studies compared the effects of a pharmacologic intervention (120 mg orlistat 3 times daily) plus a weight-maintenance diet with the effects of a placebo plus the same weight-maintenance diet. At the postintervention assessment point, intervention participants (n = 496) were 33% more likely to have maintained the loss of 5% or more of their initial body weight compared with the control participants (n = 491) (RR 1.33, 95% CI 1.15 to 1.54, I2 = 14%; absolute risk reduction 12.37%, number needed to treat 8, 95% CI 5 to 18).
Figure 3:
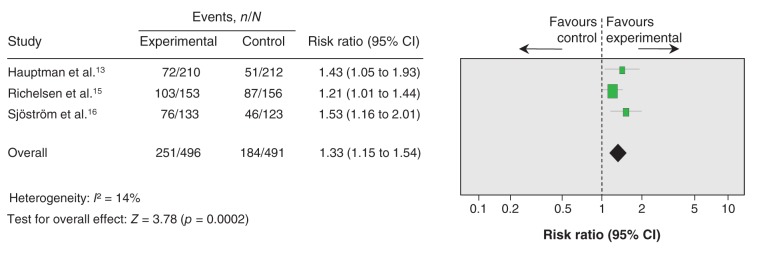
Effect of weight-maintenance interventions (orlistat plus behavioural) on maintaining a weight loss of 5% or more of initial body weight. Values greater than 1 indicate an effect of weight-loss maintenance in favour of the intervention. CI = confidence interval, n = number of participants who maintained a weight loss of 5% or more of initial body weight, N = total number of participants.
Table 4: Effect of weight-maintenance interventions on dichotomous weight outcomes.
| Outcome; intervention type | Effect |
Statistical heterogeneity (within-group), p value (I2 value, %) |
No. of participants | No. of studies | Quality of evidence rating* | ||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | Absolute risk reduction, % | Number needed to treat (95% CI) |
|||||
|
Maintenance of loss of ≥ 5% baseline body weight | |||||||
| Pharmacological + behavioural |
1.33 (1.15–1.54) |
12.37 |
8 (5–18) |
0.31 (14) |
987 |
3 |
Moderate |
|
Maintenance of loss of ≥ 10% baseline body weight | |||||||
| Pharmacological + behavioural | 1.76 (0.75–4.12) | — | — | 0.01 (85) | 731 | 2 | Low |
Note: RR = risk ratio. *Grading of Recommendations Assessment, Development and Evaluation (GRADE) quality of evidence rating reflects confidence in the estimate of effect assessed through 5 domains of the evidence (risk of bias, indirectness, imprecision, inconsistency and reporting bias): moderate = downgraded for risk of bias, low = downgraded for risk of bias and imprecision.
Maintenance of loss of 10% or more of initial body weight
Two trials of low quality (downgraded for risk of bias and imprecision) were included in the meta-analysis assessing maintenance of loss of 10% or more of initial body weight (Table 4).13,15 Both studies compared the effects of a pharmacologic intervention (120 mg orlistat 3 times daily) plus a weight-maintenance diet (n = 363) with the effects of a placebo plus the same weight-maintenance diet (n = 368). After completion of the intervention, there was no difference between groups in terms of maintaining the loss of 10% or more of their initial body weight (RR 1.76, 95% CI 0.75 to 4.12, I2 = 85%).
Interpretation
Main findings
The principle finding from this body of evidence is that there are benefits to interventions for weight-loss maintenance in adults who successfully complete treatment for overweight or obesity. The pooled-effect estimates for all but one of the weight outcomes were significant in favour of the interventions. Compared with the control-group participants, intervention participants regained less weight (kg), experienced smaller increases in BMI and waist-circumference measurements, and were more likely to maintain their loss of 5% or more of their initial body weight. It is estimated that, for every 8 patients treated for weight maintenance, 1 patient will maintain their loss of 5% or more of initial body weight. There was no significant effect for the outcome of maintaining the loss of 10% or more of initial body weight. Where we were able to compare the effectiveness of behavioural interventions versus pharmacologic plus behavioural, there was no significant difference between the two.
Comparison with other studies
The findings from this review parallel the emerging literature on the effectiveness of weight-maintenance programs.5,6 A recent systematic review confirmed that a multifaceted approach using dietary, physical and behavioural interventions was effective for weight maintenance.5 However, the contributing studies in the review included various prospective designs and narrative summaries of the findings from individual studies.5 Another meta-analysis of 11 studies compared multiple weight-maintenance programs and calculated an average weight loss of 3.2 kg over a period of 17.6 months of extended care programming (Hedge’s g 0.385, 95% CI 0.281 to 0.489, p < 0.0001).23 The mean quality score of the studies included in the review was 6 (moderate) out of a possible 11 according to the Physiotherapy Evidence Database scale.23
Limitations
There was a small number of studies (n = 8) available to address the effectiveness of weight-maintenance interventions and great variability in the interventions studied. Specifically, the interventions included diet, exercise, lifestyle and pharmacotherapy approaches; moreover, some interventions were deployed through technology, which increased the heterogeneity across studies. Some control groups received dietary counselling or information above and beyond usual care, which may not reflect usual practice. There were too few studies (n < 10) to assess reporting bias.24 Additional limitations in methodology (i.e., unclear risk of bias and imprecision) reduced the strength of evidence, resulting in moderate-quality and sometimes low-quality ratings, which in turn reduce confidence in the pooled estimates of observed effects. A filter applied in the original search to include papers published only in English and French meant papers about relevant interventions that were available only in other languages were not captured.
Clinical implications, future research and conclusion
Recognizing the issue of the increasing overweight and obesity rates in Canada, long-term strategies are needed to address the sustainability of weight loss and to define effective weight-loss maintenance (i.e., amount of weight loss over what duration). Although this review did not examine the potential risks or harms associated with these interventions, future studies may want to examine adverse effects, such as cost (financial and time), the risk for potential injury with increased or sustained physical activity and the adverse effects of sustained pharmacotherapy.
The main clinical implication of our findings is that modest weight loss (5% reduction in initial body weight) achieved through prior treatment (behavioural, pharmacologic or both) is more likely to be maintained with some form of continued intervention. The challenge facing health care professionals is to convey the importance of maintaining lifestyle changes to help patients sustain weight loss and avoid weight regain. Future research could benefit from longer term follow-up to observe the duration of weight loss and to study the health consequences of repeated weight cycling.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/3/1/E47/suppl/DC1
Box 1: Description of population, intervention, comparator, outcomes and setting.
Population
• Adults (aged 18 years or older) who were overweight (body mass index [BMI] 25–29.9 kg/m2) or obese (BMI 30–39.9 kg/m2), but not morbidly obese (BMI ≥ 40 kg/m2), and who lost weight in a prior active-phase treatment intervention
Intervention
• Interventions for weight maintenance that were either behavioural (diet, exercise, diet plus exercise or lifestyle), pharmacologic (orlistat or metformin) or some combination of both
Comparator
• No intervention, usual care, placebo or minimal intervention (e.g., informational newsletter)
Outcomes
• Maintenance of weight loss (kg), BMI (kg/m2), waist circumference (cm) and loss of 5% or more or 10% or more of initial body weight
Settings
• Feasible for use in or referral from primary care
Box 2: Inclusion and exclusion criteria.
Studies were included if they met the following criteria:
• Randomized controlled trial of a strategy for weight-loss maintenance of any duration
• Interventions for weight maintenance that were either behavioural (diet, exercise, diet plus exercise or lifestyle), pharmacologic (orlistat or metformin) or some combination of both and aimed at adults 18 years or older who were previously overweight or obese and wo lost weight in a prior active-phase treatment intervention
• Reported data for at least one specified weight outcome (i.e., change in weight [kg], waist circumference or BMI, or loss of 5% or more of initial body weight or 10% or more of initial body weight)
• Results were published in English or French
Studies were excluded if:
• Treatment involved a surgical intervention or a drug other than orlistat or metformin
• Intervention focused on morbidly obese adults (BMI ≥ 40 kg/m2)
• Intervention was conducted in an inpatient hospital, institutional or occupational setting or involved a school-based or faith-based program
• The only available results were published in a language other than English or French
Supplementary Material
Acknowledgements
Rachel Warren and Meghan Kenny contributed to the relevance and quality assessment, and data-extraction phases. We are grateful to Maureen Rice for the search and to Sharon Peck-Reid for database management and formatting of the report. Sarah Connor Gorber and Amanda Shane (Scientific Officers, Public Health Agency of Canada) contributed to the original protocol development and review of drafts of the technical report. Similarly, Paula Brauer, Maria Bacchus, Neil Bell, Elizabeth Shaw, and Harminder Singh (members of the Adult Obesity Working Group of the Canadian Task Force on Preventive Health Care) provided comments on the protocol and initial analyses.
References
- 1.Eckersley RM. Losing the battle of the bulge: causes and consequences of increasing obesity. Med J Aust 2001;174:590-2. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76-9. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002;162:1867-72. [DOI] [PubMed] [Google Scholar]
- 4.Twells LK, Gregory DM, Reddigan J, et al. Current and predicted prevalence of obesity in Canada: a trend analysis. CMAJ Open 2014;2:E18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage S, Farmer A, Apps Eccles K, et al. Healthy strategies for successful weight loss and weight maintenance: a systematic review. Appl Physiol Nutr Metab 2014;39:1-20. [DOI] [PubMed] [Google Scholar]
- 6.Collins, CE, Neve, M, Morgan, P, et al. Effectiveness of interventions with a dietary component on weight loss maintenance: a systematic review. JBI Database Syst Rev Implement Rep 2013;11:317-414. Available: www.joannabriggslibrary.org/jbilibrary/index.php/jbisrir/article/view/620. [DOI] [PubMed]
- 7.Peirson L, Douketis J, Ciliska D, et al. Treatment for overweight and obesity in adult populations: a systematic review and meta-analysis. CMAJ Open 2014;2:E306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc, ES, O’Connor, E, Whitlock, EP, et al. Screening for and management of obesity and overweight in adults. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 9.Cochrane handbook for systematic reviews of interventions. Version 5.1.0. New York: John Wiley & Sons; 2011. [Google Scholar]
- 10.Grading of Recommendations Assessment. Development and Evaluation (GRADE) WorkingGroup; 2000. Introduction. Available: www.gradeworkinggroup.org/.
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 12.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235-42. [DOI] [PubMed] [Google Scholar]
- 13.Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160-7. [DOI] [PubMed] [Google Scholar]
- 14.Hill JO, Hauptman J, Anderson JW, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am J Clin Nutr 1999;69:1108-16. [DOI] [PubMed] [Google Scholar]
- 15.Richelsen B, Tonstad S, Rossner S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 2007;30:27-32. [DOI] [PubMed] [Google Scholar]
- 16.Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998;352:167-72. [DOI] [PubMed] [Google Scholar]
- 17.Champagne CM, Broyles ST, Moran LD, et al. Dietary intakes associated with successful weight loss and maintenance during the Weight Loss Maintenance trial. J Am Diet Assoc 2011;111:1826-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139-48. [DOI] [PubMed] [Google Scholar]
- 19.Rickel KA, Milsom VA, Ross KM, et al. Differential response of African American and Caucasian women to extended-care programs for obesity management. Ethn Dis 2011;21:170-5. [PMC free article] [PubMed] [Google Scholar]
- 20.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med 2008;168:2347-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radcliff TA, Bobroff LB, Lutes LD, et al. Comparing costs of telephone vs face-to-face extended-care programs for the management of obesity in rural settings. J Acad Nutr Diet. 2012;112:1363-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas D, Vydelingum V, Lawrence J. E-mail contact as an effective strategy in the maintenance of weight loss in adults. J Hum Nutr Diet 2011;24:32-8. [DOI] [PubMed] [Google Scholar]
- 23.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129-33. [DOI] [PubMed] [Google Scholar]
- 24.Sterne, JAC, Egger, M, Moher, D. Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Oxford: Cochrane Collaboration; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.