Abstract
The brain relies on a constant supply of glucose, its primary fuel, for optimal function. A taste-independent mechanism within the CNS that promotes glucose delivery to the brain has been postulated to maintain glucose homeostasis; however, evidence for such a mechanism is lacking. Here, we determined that glucokinase activity within the hypothalamic arcuate nucleus is involved in regulation of dietary glucose intake. In fasted rats, glucokinase activity was specifically increased in the arcuate nucleus but not other regions of the hypothalamus. Moreover, pharmacologic and genetic activation of glucokinase in the arcuate nucleus of rodent models increased glucose ingestion, while decreased arcuate nucleus glucokinase activity reduced glucose intake. Pharmacologic targeting of potential downstream glucokinase effectors revealed that ATP-sensitive potassium channel and P/Q calcium channel activity are required for glucokinase-mediated glucose intake. Additionally, altered glucokinase activity affected release of the orexigenic neurotransmitter neuropeptide Y in response to glucose. Together, our results suggest that glucokinase activity in the arcuate nucleus specifically regulates glucose intake and that appetite for glucose is an important driver of overall food intake. Arcuate nucleus glucokinase activation may represent a CNS mechanism that underlies the oft-described phenomena of the “sweet tooth” and carbohydrate craving.
Introduction
It has been suggested that glucose, the brain’s primary fuel source, regulates food intake (1). Although glucose injections into the CNS reduce food intake, glucose is preferred to other types of food by rodents and humans (2–4). Therefore, a mechanism to detect glucose in food and promote the intake of glucose-rich foods is likely to exist. The regulation of glucose intake has been studied extensively (5, 6). A system regulating glucose intake, driven by hedonic responses generated in the limbic system, has been identified (7). Dopamine is thought to be important in the hedonic response to glucose (8). Evidence suggests that nonhedonic nontaste systems are important in regulating glucose intake (9) and that metabolism of glucose is an important factor (10). However, a nontaste-dependent homeostatic mechanism within the hypothalamus or elsewhere in the brain has proved elusive.
Glucokinase is a member of the hexokinase family of enzymes, which phosphorylates glucose to form glucose-6 phosphate (11). Glucokinase is expressed in the liver, pancreas, and CNS (11, 12). Glucokinase is expressed in 2 isoforms: a hepatic form expressed in the liver and a neuroendocrine form expressed in the pancreas and CNS (13). The 2 isoforms are produced by the utilization of different promoters. The isoforms have the same kinetic properties but different functions (13). Within the β cells and glucose-sensitive neurons glucokinase is part of a glucose-sensing system (14). The mechanism used for the glucose-sensing system is thought to be similar in both β cells and neurons (14). Glucose entry into the cell is via the GLUT-2 transporter (15). Metabolism of glucose by glucokinase results in closure of the ATP-sensitive potassium (KATP) channels. This results in depolarization of the cell and release of hormone via a calcium-dependent mechanism.
In the pancreas and liver, glucokinase has an important role in regulating glucose homeostasis (11). Its role in the CNS is less clear. In the hypothalamus, glucokinase is expressed in regions, including the arcuate nucleus, ventromedial nucleus (VMN), paraventricular nucleus (PVN), and lateral hypothalamic area (LHA), which regulate energy homeostasis and are part of a CNS glucose-sensing system (14, 16, 17). In the VMN, glucokinase is involved in mediating the hormonal counterregulatory responses to hypoglycemia and does not regulate energy homeostasis (18, 19). While a role for hypothalamic glucokinase in regulating energy homeostasis has been proposed, it has never been confirmed (12, 14). We hypothesized that glucokinase in the hypothalamic arcuate nucleus was involved in the regulation of energy homeostasis. We found that increased arcuate nucleus glucokinase activity increased food intake and weight gain in rats. It also increased glucose intake in preference to other food types. These effects were mimicked by intra-arcuate administration of glibenclamide, which blocks the KATP channel. Converse effects were obtained by reducing glucokinase activity in the arcuate nucleus and with intra-arcuate injection of diazoxide, a KATP channel activator. We identified a likely mechanism involving P/Q voltage-gated calcium channels, NPY release, and action via neuropeptide Y (NPY) Y1 and Y5 receptors. These data support a role for arcuate glucokinase acting through the KATP channel in the regulation of dietary glucose intake.
Results
Glucokinase activity in the arcuate nucleus is regulated by fasting.
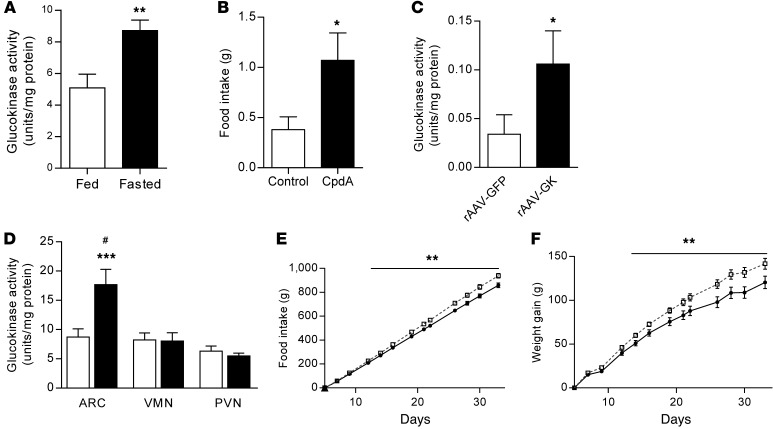
To determine whether glucokinase activity is regulated by nutritional state and in which hypothalamic regions this occurred, we measured glucokinase activity in hypothalamic nuclei of male Wistar rats following a 24-hour fast. Fasting increased glucokinase activity specifically in the arcuate nucleus 1.71-fold compared with controls. Glucokinase activity in the VMN and PVN was not altered (Figure 1A and Supplemental Figure 1, A and B; supplemental material available online with this article; doi:10.1172/JCI77172DS1). The arcuate nucleus plays a pivotal role in the metabolic sensing and control of food intake (20). Therefore, alterations in the activity of the glucokinase may represent a physiological appetite control mechanism that is influenced by nutritional state.
Figure 1. Effect increased arcuate nucleus glucokinase activity on food intake.
(A) Arcuate nucleus glucokinase activity in ad libitum chow–fed and 24-hour–fasted male Wistar rats (n = 8). (B) Normal chow intake 1 hour after intra-arcuate injection of 0.5 nmol CpdA or vehicle (control) in male Wistar rats (n = 10). (C) Glucokinase activity in HEK293 cell lysates after transfection with either rAAV-GFP or rAAV-GK (n = 6). (D) Glucokinase activity in arcuate nucleus (ARC), VMN, and PVN of male Wistar rats following intra-arcuate injection of either rAAV-GFP (iARC-GFP, white bars) or rAAV-GK (iARC-GK, black bars) (n = 8–11). (E) Cumulative food intake and (F) weight changes in iARC-GFP (black squares) and iARC-GK (white squares) rats after recovery from surgery (n = 12 iARC-GFP; n = 15 iARC-GK). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding control values; #P < 0.0001 versus all other groups.
Increased glucokinase activity in the arcuate nucleus increases food intake and body weight gain.
To investigate the role of arcuate nucleus glucokinase activity on food intake, we increased glucokinase activity in the arcuate nucleus. Injection of the glucokinase activator, compound A (CpdA) (17, 21), into the arcuate nucleus of adult male Wistar rats increased food intake during the first hour after administration compared with control injections (control, 0.38 ± 0.13 g; CpdA, 1.07 ± 0.27 g; mean ± SEM) (Figure 1B).
To determine the long-term effect of increased glucokinase in the arcuate nucleus, we used recombinant adeno-associated virus (rAAV) to overexpress the neuroendocrine isoform of glucokinase (rAAV-GK) in this nucleus. The enzymatic activity of the construct was tested in vitro in HEK293 cells. Cells transfected with rAAV-GK had significantly increased glucokinase activity compared with cells transfected with the control virus expressing GFP (rAAV-GFP) (Figure 1C). We stereotactically injected rAAV-GK into the arcuate nucleus of adult male Wistar rats (referred to herein as iARC-GK rats) and rAAV-GFP into the arcuate nucleus of control rats (referred to herein as iARC-GFP rats). In keeping with other studies using similar protocols (22), glucokinase activity was increased specifically in the arcuate nucleus of iARC-GK rats by 2.03 ± 0.30–fold compared with that in controls (Figure 1D). Glucokinase activity was not altered in either the VMN or the PVN (Figure 1D). Consistent with this, increased glucokinase expression was specifically localized to the hypothalamic arcuate nucleus in iARC-GK rats (Supplemental Figure 2, A and B). Expression of GFP, as detected by immunocytochemistry, was also limited to the arcuate nucleus in iARC-GFP animals (Supplemental Figure 2C). These results confirm the validity of iARC-GK rat as a model of increased arcuate nucleus glucokinase activity.
Compared with controls, iARC-GK rats had increased cumulative food intake, gained more weight, and had increased body fat when fed a standard chow diet (at 33 days, cumulative food intake: iARC-GFP, 1,094.3 ± 22.39 g; iARC-GK, 1,194.1 ± 24.92 g; cumulative weight gain: iARC-GFP, 151.67 ± 8.86 g; iARC-GK, 175.33 ± 4.87 g; body fat: iARC-GFP, 22.12% ± 1.73%; iARC-GK, 28.0% ± 1.62%) (Figure 1, E and F, and Supplemental Figure 3A). Lean mass, brown adipose tissue (BAT) weight, BAT Ucp1 mRNA expression, and glucose and insulin levels were unaltered (Supplemental Figure 3, B–H). Two-hour food intake following a 48-hour fast was no different between the 2 groups (Supplemental Figure 3I). These data suggest that increased arcuate nucleus glucokinase activity increases food intake and bodyweight and support a role for arcuate nucleus glucokinase in the control of energy homeostasis.
Increased glucokinase activity in the arcuate nucleus increases glucose intake but not fructose intake.
Since glucokinase is part of the glucose-sensing mechanism, we tested whether increased arcuate nucleus glucokinase activity affected the intake of glucose or fructose, a closely related sugar, which is not a substrate for glucokinase (23). Both pharmacologic and genetic activation of arcuate nucleus glucokinase increased intake of 2% and 10% w/v glucose solution (Figure 2, A–C). In contrast, intake of fructose solution was not affected (Figure 2, B and C). These data suggest that, in terms of sugar intake, arcuate nucleus glucokinase activity may specifically regulate glucose intake.
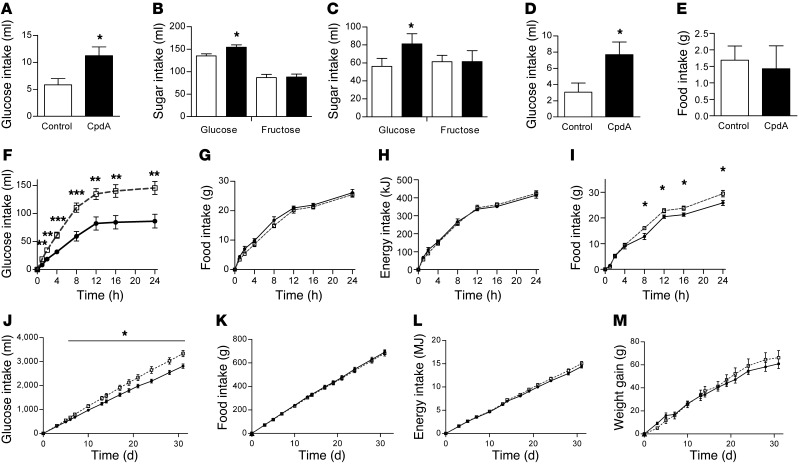
Figure 2. Effect of increased arcuate nucleus glucokinase activity on glucose intake.
(A) 2% w/v glucose solution intake 1 hour after intra-arcuate injection of CpdA or control in rats (n = 7). Twenty-four hour intake of (B) 2% w/v and (C) 10% w/v glucose and fructose solutions in iARC-GFP (white bars) and iARC-GK rats (black bars) (n = 8). (D) 2% w/v glucose solution intake and (E) food intake 1 hour after intra-arcuate injection of CpdA or vehicle in rats with ad libitum access to 2% w/v glucose solution and normal chow (n = 7). (F) 2% w/v glucose solution intake, (G) food intake, and (H) energy intake in iARC-GFP (black circles) and iARC-GK (white squares) rats during a 24-hour feeding study, with ad libitum access to 2% w/v glucose solution and normal chow intake (n = 7). (I) Food intake in iARC-GFP (black circles) and iARC-GK (white squares) rats (n = 7) during a 24-hour feeding study, with ad libitum access to normal chow diet only. (J) 10% w/v glucose, (K) food intake with normal chow, (L) total energy intake, and (M) weight changes in iARC-GFP (black circles) and iARC-GK (white squares) rats with ad libitum access to normal chow diet and 10% w/v glucose given 4 weeks after rAAV microinjection (n = 8). Data are presented as mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.001.
Increased glucokinase activity in the arcuate nucleus increases glucose intake in preference to chow.
We hypothesized that arcuate nucleus glucokinase selectively stimulates intake of glucose-rich foods in preference to other foods. To investigate whether increased glucokinase activity in the arcuate nucleus increased glucose intake in preference to chow, we measured the intake of glucose and chow when ad libitum access to both was available. In this setting, injection of a glucokinase activator into the arcuate nucleus resulted in a 2.52 ± 0.51–fold increase in 2% w/v glucose intake in the first hour after injection (Figure 2D), which is consistent with our previous finding (Figure 2A). In contrast to when chow was presented alone (Figure 1B), CpdA did not increase chow intake when it was presented alongside glucose (vehicle, 1.69 ± 0.4 3g; CpdA, 1.43 ± 0.70 g) (Figure 2E).
Similarly, in iARC-GK rats, 24-hour intake of 2%, 10%, and 20% w/v glucose was significantly increased (Figure 2F and Supplemental Figure 4, A and D). Twenty-four hour chow intake and total energy intake (derived from both chow and glucose) were not different between the 2 groups when glucose was available in addition to chow (Figure 2, G and H, and Supplemental Figure 4, B, C, E, and F). When chow was presented alone, chow intake and therefore energy intake were increased in iARC-GK rats compared with controls (iARC-GFP, 25.86 ± 1.01 g; iARC-GK, 29.46 ± 1.27 g) (Figure 2I). This suggests that the increase in chow intake produced by glucokinase activation was driven by an increased appetite for glucose.
To determine whether this effect was sustained long term, iARC-GK and iARC-GFP rats were given ad libitum access to 10% w/v glucose and standard chow. Increased arcuate nucleus glucokinase activity resulted in increased glucose intake (at 31 days, iARC-GFP, 2,812.9 ± 91.89 ml; iARC-GK, 3,337.1 ± 131.39 ml) (Figure 2J). The previously observed increase in food intake, energy intake, and body weight gain in iARC-GK rats that occurred with chow alone did not occur when a glucose solution was available in addition to the chow diet (at 31 days, food intake: iARC-GFP, 691.94 ± 19.41 g; iARC-GK, 682.14 ± 22.84 g; energy intake: iARC-GFP, 14.41 ± 0.33 MJ; iARC-GK, 15.07 ± 0.42 MJ; weight gain: 61.00 ± 4.17 g; iARC-GK, 66.25 ± 6.12 g) (Figure 2, K–M). These data support a role for arcuate glucokinase in selectively regulating glucose intake and support our hypothesis that the orexigenic effect of increasing arcuate nucleus glucokinase activity is the result of an increased drive to consume glucose.
Decreased glucokinase activity in the arcuate nucleus decreases food intake and glucose intake.
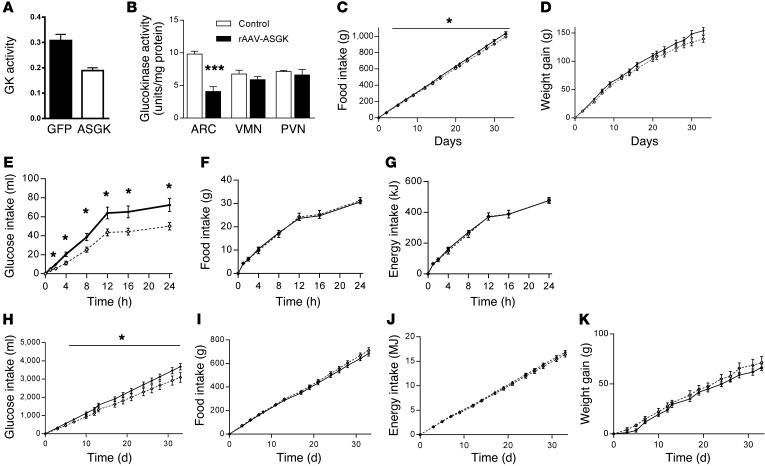
To examine the physiological role of arcuate glucokinase in the regulation of energy homeostasis and glucose consumption, we used rAAV encoding antisense glucokinase (rAAV-ASGK), which has previously shown to be effective at reducing glucokinase activity in vivo (24). The activity of the construct was tested in vitro in Hep g2 cells. Cells transfected with rAAV-ASGK had markedly reduced glucokinase activity compared with that of cells transfected with rAAV-GFP (Figure 3A). We stereotactically injected rAAV-ASGK into the arcuate nucleus of adult male Wistar rats (referred to herein as iARC-ASGK rats); iARC-GFP rats were used as the control. Glucokinase activity was decreased specifically in the arcuate nucleus of iARC-GK rats to approximately 50% of that of control iARC-GFP rats (Figure 3B). Glucokinase activity was not altered in the VMN or the PVN (Figure 3B). iARC-ASGK rats consumed less food (at 33 days, food intake: iARC-GFP, 1,039 ± 17.9 g; iARC-ASGK, 994 ± 14.1 g) (Figure 3C), and they also gained less weight, although this did not reach statistical significance (iARC-GFP, 153.9 ± 6.0 g; iARC-ASGK, 139 ± 6.2 g) (P = 0.06), when fed a standard chow diet compared with controls (Figure 3D). We tested the effect of reduced glucokinase activity in the arcuate nucleus on 24-hour glucose intake in the presence of ad libitum access to normal chow. In this setting, intake of 2% w/v glucose solution was reduced in the iARC-ASGK group compared with that in the iARC-GFP group throughout the 24-hour period (Figure 3E) and intake of chow and total energy intake were not different between the groups (Figure 3, F and G).
Figure 3. Effect of decreased arcuate nucleus glucokinase activity on food and glucose intake.
(A) Glucokinase (GK) activity in Hep G2 cell lysates after transfection with either rAAV-GFP or rAAV-ASGK (n = 6). (B) Glucokinase activity in arcuate nucleus, VMN, and PVN of male Wistar rats following intra-arcuate injection of either rAAV-GFP (iARC-GFP) or rAAV-ASGK (iARC-ASGK). (C) Cumulative food intake and (D) weight changes in iARC-GFP (black diamonds) and iARC-ASGK (white diamonds) rats after recovery from surgery (n = 11). (E) 2% w/v glucose solution intake, (F) food intake, and (G) energy intake in iARC-GFP (black diamonds) and iARC-ASGK (white diamonds) rats during a 24-hour feeding study, with ad libitum access to 2% w/v glucose solution and normal chow intake (n = 11). (H) 10% w/v glucose, (I) food intake with normal chow, (J) total energy intake, and (K) weight changes in iARC-GFP (black diamonds) and iARC-ASGK (white diamonds) rats, with ad libitum access to normal chow diet and 10% w/v glucose given 4 weeks after rAAV microinjection (n = 11). Data presented as mean ± SEM *P < 0.05, ***P < 0.001 versus corresponding control values.
To determine whether this effect was sustained long term, iARC-ASGK and iARC-GFP rats were given ad libitum access to 10% w/v glucose and standard chow. Decreased arcuate nucleus glucokinase activity resulted in significantly lower glucose intake (at 33 days, iARC-GFP, 3,674 ± 173.8 ml; iARC-ASGK, 3,090 ± 263.8 ml) (Figure 3H). The previously observed decrease in food intake and body weight gain in iARC-ASGK rats that occurred with chow alone did not occur when a glucose solution was available in addition to the chow diet (at 33 days, food intake: iARC-GFP, 684.8 ± 21.2 g; iARC-ASGK 711.0 ± 22.2 g; energy intake: iARC-GFP, 16.82 ± 0.37 MJ; iARC-ASGK, 16.36 ± 0.49 MJ; weight gain: iARC-GFP 66.6 ± 3.3 g; iARC-ASGK, 71.4 ± 6.1 g) (Figure 3, I–K).
Pharmacologic modulation of the KATP channel in the arcuate nucleus mimic the effects of altered glucokinase activity.
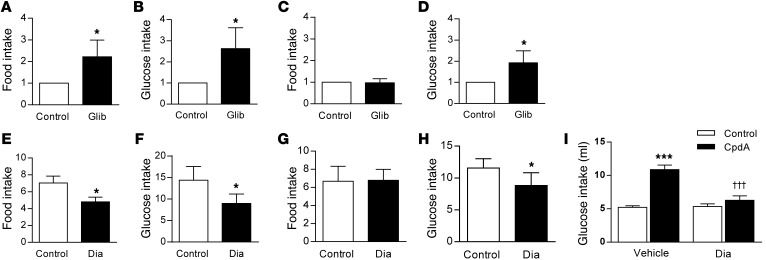
One signaling mechanism downstream of glucokinase is the KATP channel. We therefore hypothesized that inhibition of KATP channels may mediate the effect of arcuate nucleus glucokinase on food intake and glucose appetite. To investigate whether arcuate glucokinase may be acting via inhibiting the KATP channels, either the KATP channel inhibitor, glibenclamide, or the KATP channel activator, diazoxide, was injected into the arcuate nucleus of Wistar rats. Four hours after injection of glibenclamide, food intake was increased compared with that after control injections when chow was available alone (2.22 ± 0.77 relative to control) (Figure 4A). Similarly, 2% w/v glucose intake (2.73 ± 0.99 relative to control) was significantly increased 4 hours after glibenclamide injection compared with that after control injections when glucose was presented alone (Figure 4B). When both chow diet and glucose were present, only glucose intake was increased following glibenclamide injection, compared with that after control injection (1.93 ± 0.57 relative to control), with chow intake unaffected (0.97 ± 0.19 relative to control) (Figure 4, C and D).
Figure 4. Effect of activating and inhibiting arcuate nucleus KATP channels on food intake and glucose appetite.
(A) Chow intake relative to that of control-treated rats 4 hours after intra-arcuate injection of 2 nmol glibenclamide (Glib) or vehicle (control) in rats (n = 12). (B) 2% w/v glucose solution intake relative to that of control-treated rats 4 hours after intra-arcuate injection of 2 nmol glibenclamide or vehicle in rats (n = 11). (C) Chow intake and (D) 2% w/v glucose intake relative to that of control-treated rats 4 hours after intra-arcuate injection of 2 nmol glibenclamide or vehicle in rats with ad libitum access to 2% w/v glucose and normal chow (n = 10). (E) Chow intake relative to that of control-treated rats 1 hour after intra-arcuate injection of 1 nmol diazoxide (Dia) or vehicle in rats (n = 9). (F) 2% w/v glucose intake relative to that of control-treated rats 1 hour after intra-arcuate injection of 1 nmol diazoxide or vehicle in rats (n = 9). (G) Chow intake and (H) glucose intake relative to that of control-treated rats 1 hour after intra-arcuate injection of 1 nmol diazoxide or vehicle in rats with ad libitum access to 2% w/v glucose and normal chow (n = 9). (I) 2% w/v glucose solution intake 1 hour after intra-arcuate injection of vehicle CpdA or 1 nmol diazoxide administered alone (control) or followed by injection of 0.5 nmol CpdA in rats (n = 10). Data are presented as mean ± SEM. *P < 0.05; ***P < 0.001 versus control; †††P < 0.001 versus CpdA vehicle injected.
Conversely, injection of diazoxide into the arcuate nucleus inhibited food intake when chow was presented alone compared with that after control injections (Figure 4E and Supplemental Figure 5A). Glucose intake was also reduced following injection of diazoxide compared with that after control injections (Figure 4F and Supplemental Figure 5B). However, when both glucose and chow were available, only the consumption of glucose was decreased following diazoxide injection, whereas that of chow remain unchanged (Figure 4, G and H, and Supplemental Figure 5, C and D).
The role of KATP channels in mediating the increased glucose intake stimulated by glucokinase activation in the arcuate nucleus was investigated by coadministration of diazoxide and CpdA into the arcuate nucleus in fed animals. As before, injection of CpdA increased intake of glucose, and this increased glucose intake was blocked by coadministration of diazoxide (Figure 4I). Under the conditions used, diazoxide given alone did not affect glucose intake when administered alone (Figure 4I). These data are in accord with KATP channels having a role in transducing the effects of arcuate nucleus glucokinase on glucose appetite.
Effects of arcuate nucleus glucokinase on hypothalamic AMPK, ACC, and FAS.
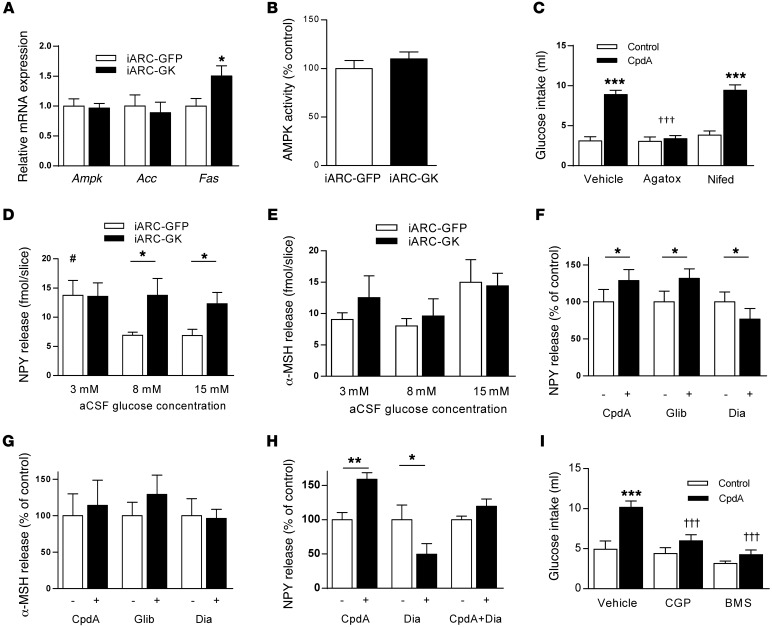
We investigated possible mechanisms by which increased arcuate nucleus glucokinase activity may be exerting its effects on food and glucose intake. Increasing arcuate nucleus glucokinase activity did not affect either expression or activity of hypothalamic adenosine monophosphate–activated protein kinase (AMPK), a key cellular energy sensor that is influenced by cellular ATP levels (ref. 25 and Figure 5, A and B). In addition, there was no difference in expression of the metabolic sensing enzyme acetyl CoA carboxylase in iARC-GK animals compared with that in controls (ref. 26 and Figure 5A). There was a small increase in fatty acid synthase expression in iARC-GK animals compared with that in controls (Figure 5A). This enzyme catalyzes the synthesis of long-chain fatty acids and modulates food intake (27).
Figure 5. Effect of increased arcuate nucleus glucokinase on NPY and α-MSH and effect of NPY antagonist and calcium channel blockade on glucokinase activator–stimulated glucose intake.
(A) Hypothalamic Ampk, Acc, and Fas expression in iARC-GFP and iARC-GK rats (n = 9). (B) Hypothalamic AMPK activity in hypothalami from iARC-GFP and iARC-GK rats (n = 10). (C) 2% w/v glucose solution intake 1 hour after intra-arcuate injection of vehicle ω-agatoxin-IVA (Agatox) or nifedipine (Nifed) and subsequent injection of CpdA or control in rats (n = 15). (D) Glucose-induced NPY release from hypothalamic slices of iARC-GFP or iARC-GK rats (n = 7 iARC-GFP; n = 8 iARC-GK). (E) Glucose-induced α-MSH release from hypothalamic slices of iARC-GFP or iARC-GK rats (n = 7 iARC-GFP; n = 8 iARC-GK). (F) NPY release from rat hypothalamic slices, following treatment with CpdA, glibenclamide, or diazoxide (n = 10–12). (G) α-MSH release from rat hypothalamic slices, following treatment with CpdA, glibenclamide, or diazoxide (n = 6–9). (H) NPY release from rat hypothalamic slices, following treatment with CpdA or diazoxide alone and in combination (CpdA+Dia) (n = 10–12). (I) 2% w/v glucose solution intake 1 hour after intra-peritoneal injection of vehicle CGP-71683 (CGP) or BMS-193885 (BMS) and subsequent intra-arcuate injection of CpdA or control in rats (n = 14). Data are presented as mean ± SEM. *P < 0.05 versus corresponding control values; #P < 0.05 versus control values from 8 mM and 15 mM glucose; **P < 0.00001 versus corresponding CpdA-injected group; †††P < 0.0001 versus vehicle CpdA-injected group; ***P < 0.0001.
The effects of glucokinase on glucose intake are dependent upon calcium channel activity.
To investigate whether the effects of arcuate nucleus glucokinase activity were dependent upon calcium channel activity specific calcium channel blockers were used. Either nifedipine, specific for L-type calcium channels, or ω-agatoxin IVA, specific for P/Q-type channels, was injected into the arcuate nucleus, followed by injection of CpdA and measurement of intake of 2% w/v glucose. Injection of nifedipine alone did not alter glucose intake at 1 hour (Figure 5C) or at subsequent time points (Supplemental Figure 6A), and it did not have any effect on glucose intake stimulated by CpdA at either 1 hour (Figure 5C) or subsequent time points (Supplemental Figure 6A). Injection of ω-agatoxin IVA alone did not affect glucose intake either at 1 hour (Figure 5C) or subsequent time points (Supplemental Figure 6A). In contrast, injection of ω-agatoxin IVA completely blocked the stimulatory effects of CpdA on glucose intake at 1 hour (Figure 5C) and up to 8 hours after injection (Supplemental Figure 6A). These data suggest that the effects of glucokinase on glucose intake are dependent upon P/Q-type calcium channels.
Effects of altered glucokinase activity on NPY release.
Release of NPY from ex vivo hypothalamic slices in response to increasing glucose concentrations was altered in iARC-GK animals compared with that in iARC-GFP controls. Treatment of hypothalamic slices from iARC-GFP rats with increasing concentrations of glucose resulted in a decrease in NPY release (Figure 5D). In contrast, treatment of hypothalamic slices from iARC-GK rats with increasing concentrations of glucose did not affect NPY release. Release of α-melanocyte–stimulating hormone (α-MSH) from ex vivo hypothalamic slices from either iARC-GFP or iARC-GK rats was not affected by changes in glucose concentration (Figure 5E).
A similar response was seen when hypothalamic slices were treated with either CpdA, a glucokinase activator, or glibenclamide, which blocks KATP channels. Treatment with either of these 2 pharmacologic agents increased NPY release from ex vivo hypothalamic slices (Figure 5F). In contrast, treatment of ex vivo hypothalamic slices with diazoxide, which activates KATP channels, reduced NPY release (Figure 5F). Release of α-MSH from hypothalamic slices was not altered by any treatment (Figure 5G). The increased release of NPY from the hypothalamic slices stimulated by glucokinase activation appears to be dependent on closure of the KATP channel, since treatment of ex vivo slices with diazoxide blocked the effect of CpdA (Figure 5H). These data indicate that the effects of arcuate nucleus glucokinase on energy homeostasis may be mediated by the orexigenic neurotransmitter NPY.
The effects of glucokinase activation on glucose intake are blocked by NPY receptor antagonists.
To investigate whether NPY was physiologically important in the effects of arcuate nucleus glucokinase activity on glucose intake, NPY receptor antagonists were used. Either BMS-193885, a NPY Y1–specific antagonist, or CGP-71683, a NPY Y5–specific antagonist, was injected intraperitoneally, followed by injection of CpdA into the arcuate nucleus. Injection of BMS-193885 alone did not affect intake of glucose at 1 hour (Figure 5I) or at any subsequent time point. Injection of BMS-193885 did attenuate the CpdA-stimulated intake of glucose at 1 hour (Figure 5I) and all time points up to 8 hours (Supplemental Figure 6B). Similarly injection of CGP-71683 alone did not affect glucose intake at 1 hour (Figure 5I) or at any subsequent time point (Supplemental Figure 6B). However, injection of CGP-71683 did attenuate the CpdA-stimulated intake of glucose at 1 hour (Figure 5I) and all time points up to 8 hours (Supplemental Figure 6B). These data support a physiological role for NPY in the effects of arcuate glucokinase in the regulation of glucose consumption via both the Y1 and the Y5 receptor.
Discussion
It has been suggested that glucose sensors, such as glucokinase, have a role in the hypothalamic regulation of food intake (1, 14). Increased glucokinase activity has been postulated but not demonstrated to reduce food intake as part of a glucostatic control system. We found that glucokinase activity increased in the arcuate nucleus following a fast. Expression of glucokinase is regulated by fasting in a tissue-specific manner. Expression of hepatic glucokinase is reduced by fasting (28), while expression of the neuroendocrine form of glucokinase in the pancreas is unaltered by fasting (28). In contrast, expression of the neuroendocrine form of glucokinase in the hypothalamus is increased by fasting (29). The mechanism underlying the differences in the regulation of expression is unclear; however, it is likely to be the result of differences in the promoter sequence used and the combination of transcription factors expressed in each tissue. In addition to this, regulation of glucokinase occurs at a transcriptional level. It has been demonstrated recently that glucokinase activity in the hypothalamus is regulated by glucokinase regulatory protein (GKRP) (30). GKRP regulates glucokinase activity by binding to it and inactivating it by sequestering it within the nucleus (31). In the liver, the binding of GKRP to glucokinase is increased by hypoglycemia and fasting, resulting in reduced glucokinase activity, which is reduced in response to these stimuli. However, in the hypothalamus, binding of GKRP to glucokinase is inhibited by fasting and increased by hyperglycemia (30). Therefore, glucokinase activity in the hypothalamus would be increased with fasting, in line with our findings.
Injection of the glucokinase activator, CpdA, into the arcuate nucleus increased food intake. This is opposite from the effect proposed by the glucostatic hypothesis of food intake, although it is consistent with the increase in glucokinase activity detected following a fast. Many orexigenic agents demonstrate increased activity or expression in response to fasting, whereas anorexic agents demonstrate the opposite response (20). To investigate whether this effect was sustained, rAAV-GK was stereotactically injected into the arcuate nucleus of adult male iARC-GK rats. On a chow diet, iARC-GK animals consumed more food than iARC-GFP animals, in accord with the findings following pharmacologic activation. To investigate the physiological importance of this, we reduced arcuate nucleus glucokinase activity by injecting rAAV-ASGK into the arcuate nucleus of iARC-ASGK rats. On a chow diet, iARC-ASGK animals consumed less food than iARC-GFP animals. Together, these data suggest that increased arcuate nucleus glucokinase activity may constitute an orexigenic signal. This is consistent with previous work revealing increased hypothalamic glucokinase mRNA expression in diet-induced obese and obese-prone rats (32). Previous studies have failed to demonstrate an orexigenic effect of glucokinase (19). However, they did not specifically target the arcuate nucleus. In addition, they used methods that do not alter glucokinase activity specifically and that are known to alter food intake via other mechanisms (33). These effects on food intake are unlikely to be secondary to changes in circulating glucose or insulin levels, since neither fasting nor fed glucose and insulin levels were changed in the iARC-GK animals compared with those in the iARC-GFP animals.
Since glucokinase is an integral part of the glucose-sensing mechanism in the pancreas and specialized neurons (14, 17), we hypothesized that arcuate nucleus glucokinase activity may specifically regulate the consumption of glucose rather than just energy. This was tested by allowing the rats access to a source of pure glucose, either alone or in the presence of chow. Following injection of CpdA, intake of glucose was increased in iARC-GK rats compared with controls, while intake of fructose was unaffected. This suggests that the increase in food intake depends on the presence of glucose in the food. When glucose and chow were presented together, glucose intake was enhanced, while chow intake was unaffected by injection of CpdA. We found a similar effect in long-term studies comparing iARC-GK animals with iARC-GFP animals. In this situation, in contrast to our previous results when only chow was available, there was no difference in either chow intake or body weight between the 2 groups, while glucose intake was increased in the iARC-GK group compared with that in the controls. This supports the hypothesis that the increase in food intake in animals with increased glucokinase activity is in part driven by an increased physiological drive for glucose consumption. This hypothesis is further supported by the finding that, in iARC-GKAS animals with reduced glucokinase activity in the arcuate nucleus, glucose intake was reduced but chow intake was unaffected when both were freely available.
Intracerebroventricular injection of glucose has been established to decrease food intake as part of the system of metabolic regulation of food intake (14, 19, 34). Our data would seem contrary to this effect. However, there are several glucose-sensing regions in the brain, both within and outside the hypothalamus. It is likely that activation of these regions following injection of glucose into the brain is substantially different to that resulting from glucose ingestion.
The metabolic regulation of food intake by glucose is well established (14, 19, 34, 35), while homeostatic taste-independent mechanisms promoting glucose intake have been postulated but not demonstrated in mammals. Such a mechanism would increase intake of foods rich in glucose. Throughout evolutionary history, such foods have been difficult to obtain, and glucose is a vital nutrient for some tissues, including the brain. Therefore, a mechanism to maximize intake of glucose-rich foods when available would be advantageous. A similar mechanism has been found recently in invertebrates (36). In rodents and humans, glucose intake leads to a coordinated response between homeostatic and hedonic pathways regulating sensing, reward, and feeding behavior (7, 37). This study suggests that, within the arcuate nucleus, glucokinase is an integral part of the homeostatic CNS glucose-sensing mechanism that regulates glucose appetite. Our data does not exclude the possibility that other nuclei that express glucokinase within the hypothalamus or brain stem have a role in regulating glucose intake or homeostasis, as the effect of altered glucokinase activity in these regions was not tested. Data from invertebrate, rodent, and human studies suggest that food intake is regulated around a fixed macronutrient ratio (38, 39). Our results suggest that arcuate nucleus glucokinase regulates the set point for glucose and that altering arcuate nucleus glucokinase activity alters the set point for glucose consumption (40). Such a set point would explain the normalization in body weight gain in the iARC-GK and iARC-GKAS animals given access to glucose. The iARC-GK animals were able to sate their glucose appetite without intake of other nutrients. The glucose solution has a lower energy content per unit of glucose and thus the overall energy intake of these rats was reduced compared with those that only had access to chow. This same effect was observed with the controls in the rAAV-GKAS study. This mechanism would be specific for glucose, since other monosaccharides, for example, fructose, are not substrates for glucokinase and thus would not activate this pathway. Therefore, the maintenance of a glucose source in the diet of obese individuals may be important to satiate glucose appetite, given that hypothalamic glucokinase expression is elevated in animal models of obesity (32). This homeostatic system may explain the effects of foods with different glycemic index values on weight loss (41). Foods that have a high glycemic index value, which release large amounts of glucose quickly, would activate the arcuate glucokinase pathway. This would encourage further food intake. Foods with a low glycemic index value would not activate this system, and so further intake would not be stimulated. It is possible that the recent rise in fructose consumption in Western-type diets may not satiate the glucose appetite, resulting in consumption of more calories (42).
Possible downstream signaling mechanisms were examined. The expression and activity of AMPK in the hypothalamus was unaffected by increased glucokinase activity in the arcuate nucleus. This suggests that AMPK is not an important mediator of the observed changes in food and glucose intake and increasing arcuate nucleus glucokinase activity does not change cellular energy availability (25). This is not surprising, since, as in β cells, energy production via glycolysis is dependent upon hexokinase I and not glucokinase (hexokinase IV). A slight increase in hypothalamic fatty acid synthase expression occurred in iARC-GKS rats. This may be a part of the mechanism underlying the increased food and glucose intake observed in the iARC-GK rats (27). The increase in FAS expression could be the result of increased ChREBP activation (43), driven by increased glucose-6-phosphate concentrations (44). Although activation of ChREBP would also be expected to increase ACC expression (43), this was not observed.
The subcellular localization and close proximity of glucokinase and membrane-bound KATP channels may allow glucokinase and KATP channels to function as a glucose-sensing complex (14). This would allow glucokinase to alter local ATP levels and change KATP channel activity, without affecting cellular ATP. Such compartmentalization of glycolytic enzymes, glycolysis, and ATP production has been demonstrated in a variety of cells, including neuronal cells (45, 46). Injection of the KATP channel inhibitor glibenclamide resulted in the same response as increased glucokinase activity in the arcuate nucleus. Injection of diazoxide, a KATP channel activator, produced the opposite effect. In addition coadministration of diazoxide blocked the increase in glucose intake stimulated by CpdA. These results are supportive of a role for KATP channels in the actions of glucokinase in the arcuate nucleus. KATP channels are expressed in a small number of neurons, which do not express glucokinase (16). It is possible that the effects of glibenclamide and diazoxide on food and glucose intake are being mediated by these neurons rather than glucokinase-expressing neurons. However, given the effects that closely parallel those of increased glucokinase activity in the arcuate nucleus and the well-characterized coupling between glucokinase and KATP channels (16, 17, 32, 47, 48), KATP channels appear a likely mechanism for transducing the effects of glucokinase on glucose appetite via a mechanism analogous to glucose-stimulated insulin release in the pancreas (14). These results are in keeping with activation of hypothalamic KATP channels by leptin (49, 50) and the observation that mice with targeted deletion of Kir6.2 have increased food intake (51). These findings also have important implications for the potential use of glucokinase activators and sulfonylureas in the treatment of type 2 diabetes. The increased food intake in response to arcuate nucleus glibenclamide administration could explain the increase in body weight seen in patients treated with sulfonylurea class agents (52).
The mechanism linking glucokinase to increased glucose intake is also likely to require activity of P/Q calcium channels. Injection of the specific P/Q channel blocker ω-agatoxin IVA completely blocked the increase in glucose intake stimulated by CpdA. Injection of the L-channel blocker nifedipine was without effect on CpdA-stimulated glucose intake. P/Q channels are expressed presynaptically throughout the CNS and are important in neurotransmitter release (53). Activity of these channels has been shown previously to mediate the release of NPY from hypothalamic slices (54).
A possible cellular mechanism underlying the changes in food and glucose intake is increased activity of arcuate NPY neurons, since there were changes in release of NPY in response to glucose from ex vivo hypothalamic slices from iARC-GK rats. As has been reported previously (55), increasing glucose concentrations in the artificial CSF (aCSF) resulted in reduced NPY release from control hypothalami. In contrast, there was no decrease in NPY release from iARC-GK hypothalami with increasing glucose concentrations. These results were mimicked by the treatment of ex vivo hypothalamic slices with either CpdA, a glucokinase activator, or glibenclamide, which blocks KATP channels. Both of these treatments resulted in increased NPY release from hypothalamic explants. Conversely, treatment of hypothalamic slices with diazoxide, which opens KATP channels, reduced NPY release and blocked the release of NPY stimulated by CpdA. A role for NPY in mediating this effect is supported by the finding that the increase in glucose intake stimulated by injection of CpdA into the arcuate nucleus could be attenuated by the administration of either a specific NPY Y1 receptor or NPY Y5 receptor antagonist. This suggests that NPY acting on both the Y1 and Y5 receptors is involved in the regulation of glucose intake (16, 48). NPY has been shown to preferentially increase carbohydrate intake compared with other dietary constituents (56). These findings also fit with the increased NPY expression, but unaltered POMC expression, in mice with targeted deletion of Kir6.2, a component of KATP channel, suggesting that the KATP channel may play a role in the regulation of NPY signaling in the arcuate nucleus (51). The finding of Parton et al. that transgenic mice expressing KATP channels unresponsive to ATP in POMC neurons had normal body weight (57) further supports this potential mechanism.
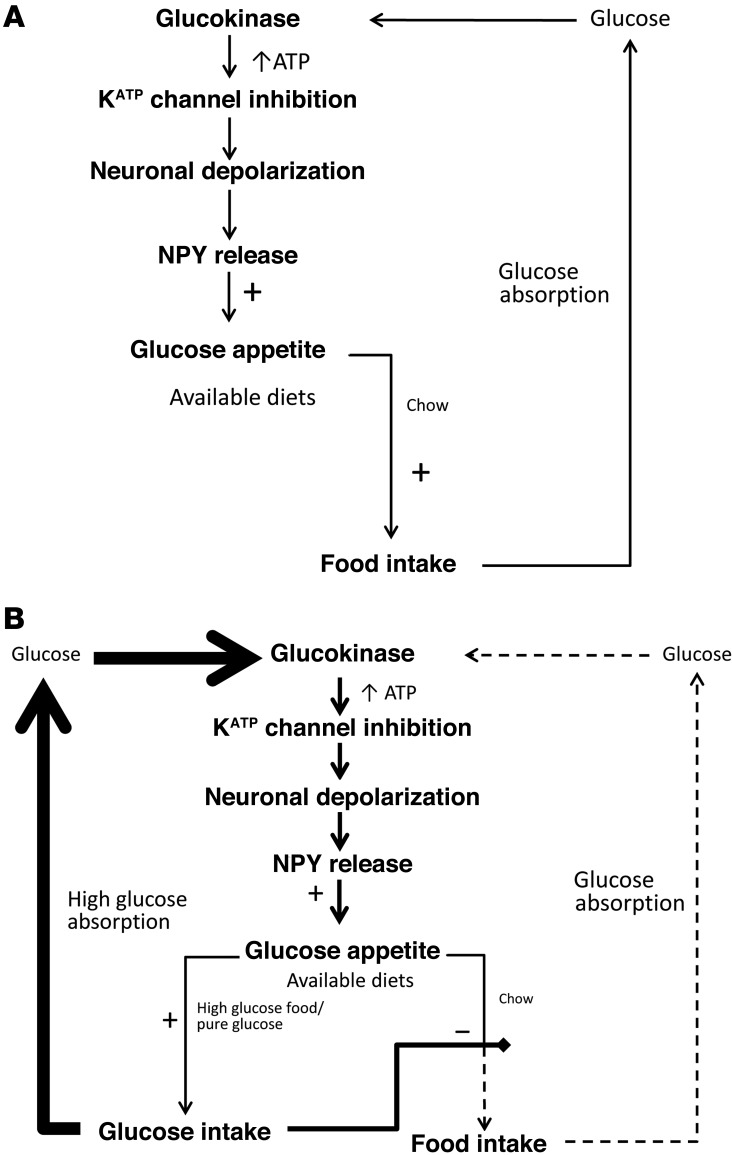
It is therefore plausible that food with high levels of glucose causes increased activation of arcuate nucleus NPY neurons, via glucokinase, KATP channels, and P/Q voltage-gated calcium channels, promoting further intake of that food in a positive feedback loop (Figure 6). This system would promote the intake of glucose-rich foods above that of those containing lower levels of glucose.
Figure 6. Schematic representation of the proposed mechanism by which arcuate nucleus glucokinase regulates glucose intake.
(A) Representation when only one diet is available. When mixed food is ingested, glucose is released and enters the arcuate nucleus. Metabolism of the glucose by glucokinase results in closure of the KATP channel and increased NPY release. The increase in NPY stimulates further food intake. The normal satiety process would terminate the feeding response. (B) Representation when a rich source of glucose is available in addition to normal diet. If pure glucose or a food high in glucose is available, the positive feedback loop will be more active. This will result in a preferential intake of the food with a high glucose concentration and reduced intake of the mixed diet.
Overall, these results suggest that a requirement for glucose, rather than calories alone, partly drives food intake and that arcuate nucleus glucokinase can regulate this specific intake of glucose. In the absence of a source of pure glucose, the increased physiological drive for glucose, resulting from greater glucokinase activity in the arcuate nucleus, drives the intake of mixed nutrient foods. This hypothalamic system may form part of the postingestive reward pathway of glucose (7, 58) or form a separate homeostatic regulatory system regulating glucose appetite, analogous to that described recently in invertebrates (36). A role in taste-driven intake is less likely, as this is thought to be more responsive to fructose and sweeteners (59), which do not appear to activate this glucokinase-based glucose-sensing system, maximizing intake of what was until recently a rare resource. Evidence supports the existence of this pathway in humans. Patients with mutations that decrease glucokinase activity have reduced body weight (60). Conversely, patients who have mutations associated with increased activity of the neuroendocrine form of glucokinase have increased body weight (61).
Our results suggest that arcuate nucleus glucokinase is acting as part of a glucose-sensing system, analogous to that in the β cell, as part of a central macronutrient regulatory system. This glucose-sensing system regulates glucose appetite and energy homeostasis. Glucose from food enters arcuate nucleus neurons by GLUT-2 and is phosphorylated by glucokinase to glucose-6-phosphate, which is linked to closure of KATP channels; this induces neuronal depolarization and entry of calcium via P/Q calcium channels, leading to release of NPY. Increased arcuate nucleus glucokinase activity could lead to a positive energy balance and increased preference for glucose. Diet-induced obese and diet-induced obese–prone rats have increased arcuate nucleus glucokinase mRNA expression, suggesting that this system may play a role in the development of obesity (32). This mechanism may explain the observation that diets high in carbohydrate are associated with weight gain in mice (62) and why low glycemic index diets produce weight loss (41). It also provides a possible CNS mechanism to explain the often-described phenomena of the “sweet tooth” and carbohydrate craving, particularly for high glycemic index foods.
Methods
Animal housing conditions.
Adult male Wistar rats (230–280 g, Charles River) were housed in single cages and maintained under a controlled environment (temperature 21°C–23°C, 12-hour light/12-hour dark cycle, lights on at 07:00 hours), with ad libitum access to chow (RM1; SDS Diets) and water, except where stated.
Glucokinase plasmid construction.
Full-length glucokinase cDNA was isolated and amplified by PCR from pCMV4.GKB1 encoding full-length glucokinase cDNA (gift from Mark Magnuson, Vanderbilt University Medical Center, Nashville, Tennessee, USA) and cloned into pTR-CGW (gift from Joost Verhaagen, Nederlands Herseninstituut, Amsterdam, The Netherlands) (63).
rAAV production.
rAAV was produced, isolated, and purified as described previously (63). The titer for rAAV-EGFP was 5.04 × 1012 genome particles per ml, that for rAAV-GK was 2.96 × 1012 genome particles per ml, and that for rAAV-ASGK was 3.42 × 1012 genome particles per ml.
rAAV microinjections.
Animal surgical procedures and handling were carried out as previously described (63). 1 μl of either rAAV-GK or rAAV-GFP was stereotaxic injected according to coordinates of Paxinos and Watson (64) (3.4 mm caudal, ± 0.5 mm lateral to the bregma, and 9.5 mm below the surface of the skull).
Starting body weights for studies with normal chow were as follows: iARC-GFP, 270.08 ± 3.04 g, and iARC-GK, 273.60 ± 2.75 g. Starting body weights for long-term studies with normal chow and 10% w/v glucose were as follows: iARC-GFP, 428.63 ± 11.58 g, and iARC-GK, 449.25 ± 5.60 g. Acute 24-hour normal chow and sugar intake feeding studies commenced at lights out (19:00 hours). Rats were killed during the early light phase by decapitation. Hypothalami, whole brains, and weighed interscapular BAT were collected and stored at –70°C. Correct targeting of rAAV to the arcuate nucleus was confirmed experimentally in several ways. Brains from one group of animals (n = 15) injected with rAAV-GK were subject to in situ hybridization to detect glucokinase and assessed for localization to the arcuate nucleus. In all animals, expression of glucokinase was found to be localized to the arcuate nucleus. Brains from one group of rats injected with rAAV-GFP were subject to either in situ hybridizations for expression of WPRE (n = 12), a component of the rAAV construct, or immunocytochemistry for GFP (n = 2). In all of these animals, expression was found to be limited to the arcuate nucleus. This process was repeated in a second group of animals injected with rAAV-GKAS or rAAV-GFP. The brains of all of these animals were subject to in situ hybridization for WPRE expression. In all of these animals, expression was found to be limited to the arcuate nucleus.
Cannulation.
Intra-arcuate cannulation was performed as previously described (22). Cannulas (Plastics One) were stereotaxically implanted unilaterally into the arcuate nucleus. At the end of the injection studies, cannula placement was confirmed histologically in all animals. One microliter of Indian ink was injected via the cannula. The brains were removed and fixed in 4% formaldehyde, dehydrated in 40% sucrose, and frozen in isopentane cooled in liquid nitrogen. Brains were sliced into 15-μm coronal sections on a cryostat (Bright), and correct arcuate nucleus placement was determined by microscopy according to the position of the Indian ink. All cannula were correctly targeted to the arcuate nucleus.
Injection of pharmacologic agents.
Rats received intra-arcuate injections at between 09:30 and 10:30 hours with either drug or vehicle control on 2 separate days, 72 hours apart. Rats were fasted overnight for studies when diazoxide was administered alone; all other studies were in ad libitum–fed animals. All compounds were administered in a 1 μl volume over a period of 1 minute. Doses were based on previous experiments (18, 65, 66). Data are presented as food intake and glucose intake relative to those in corresponding rats treated with control during the crossover study.
Calcium channel studies.
Rats received intra-arcuate injections at between 09:30 and 10:30 hours, with study days being at least 72 hours apart. First, rats received an intra-arcuate injection of 5 nmol nifedipine, 1 μg ω-agatoxin IVA, or saline in random order. Fifteen minutes later, they received an intra-arcuate injection of either 0.5 nmol CpdA or vehicle in random order, and glucose intake was subsequently measured. All compounds were administered in a 1-μl volume over a period of 1 minute. Doses were based on previous experiments (67–69).
NPY antagonist studies.
Rats received injections at between 09:30 and 10:30 hours, with study days being at least 72 hours apart. First, rats received an intra-peritoneal injection of 10 mg/kg BMS-193885, 10 mg/kg CGP-71683, or vehicle in a volume of 100 μl in random order; the antagonists were chosen as they are able to cross the blood-brain barrier. Fifteen minutes later, they received an intra-arcuate injection of 5 nmol CpdA or vehicle in random order, and glucose intake was subsequently measured. Intra-arcuate injections were administered in a 1-μl volume over a period of 1 minute. Doses were based on previous experiments (70, 71).
Glucokinase activity assays.
Sections 880-μm thick were cut from frozen brains using a freezing Shandon sled microtome and mounted on slides. Slides were kept on dry ice. Arcuate nucleus, VMN, and PVN were removed using a 22-gauge neuro punch (Fine Science Tools) (64). Nuclei were homogenized in 200 μl ice-cold buffer containing 0.0107 M MgCl2, 5 mM sodium EDTA, 0.15 M KCl, and 0.07% w/v 2-mercaptoethanol. The homogenate was centrifuged at 16,000 g for 40 minutes at 4°C. Glucokinase activity was determined spectrophotometrically using an NADP+-coupled assay with glucose-6-phosphate dehydrogenase. Twenty microliters of homogenate was added to 1 ml reaction reagent containing 100 mM GlyGly, 45 μM 5-thio-d-glucose-6-phosphate, 1 M MgCl2, 0.5 mM 3-O-methyl-N-acetylglucosamine, 200 mM ATP, 12.5 mM NADP, 2 M glucose, and 0.4 units glucose-6-phosphate dehydrogenase (type IX, from Baker’s yeast). Each reaction was undertaken in triplicate. The reaction was incubated at 37°C for 1 hour. Absorbance at 340 nm was measured and compared with a standard curve for glucokinase. Sample protein concentration was determined by BCA protein assay (Pierce).
AMPK activity assay.
AMPK assays on hypothalami were undertaken as described previously (25).
In situ hybridization.
In situ hybridization using antisense glucokinase cDNA riboprobe corresponding to nucleotides 15–116 of rat glucokinase was performed on 15-μm frozen brain sections.
Hormone and glucose assays.
Plasma insulin and leptin concentrations were determined by enzyme-linked immunosorbent assay (Crystal Chem), and plasma glucose was determined by a glucose oxidase assay kit (Randox) using the manufacturer’s protocol.
Body composition.
Body composition was calculated as previously described (72).
Quantitative PCR.
Quantitative PCR was performed on hypothalamic cDNA using TaqMan Gene Expression Assays (primer assay ID: glucokinase Rn00561265_m1, Ampk Rn00576935, Acc Rn01456589_m1, Fas Rn01463550_m1, 18S 4310893E). mRNA expression relative to housekeeping gene (18S) was calculated according to the ΔΔCT method.
Northern blot.
Expression of Ucp1 mRNA in interscapular BAT was measured by Northern blot with normalization to oligo dT.
Hypothalamic explant static incubation system.
The static incubation system used was a modification of the method previously described to measure neurotransmitter release from hypothalamic explants in response to glucose or pharmacologic agents (57, 73). Following a 2-hour equilibration period in aCSF, hypothalamic explants were incubated for 45-minute periods in 600 μl aCSF containing 3, 8 (baseline), and 15 mM glucose in randomized order for experiments assessing glucose-stimulated neurotransmitter release in hypothalami from iARC-GFP rats and iARC-GKS rats. For experiments assessing hypothalamic neurotransmitter release following treatment with drugs, hypothalamic explants were incubated for 45 minutes in 600 μl aCSF (baseline control), followed by 45 minutes in 600 μl aCSF containing 30 μM CpdA (Merck), 100 μM Glibenclamide (Sigma-Aldrich), or 200 μM Diazoxide (Sigma-Aldrich). Viability of the tissue was verified by 45-minute incubation in aCSF containing 56 mM KCl. Only hypothalami that showed secretion over baseline in response to 56 mM KCl were used in the analysis. At the end of each incubation period, supernatants were removed and tested for NPY or α-MSH release by radioimmunoassay, as described previously (74, 75).
Statistics.
Results are shown as mean and SEM. A generalized estimating equation was used to compare cumulative data between control and treatment groups. Data from glucokinase activity assays and calcium channel blocker and NPY antagonist studies were analyzed using 1-way analysis of variance, followed by post-hoc Holm-Sidak test. A paired Student’s t test was used to compare food intake and glucose intake data from crossover studies. An unpaired 2-tailed Student’s t test was used to compare all other data. Significance was set at P < 0.05 for all analyses.
Study approval.
All animal experiments were approved by the home office under the United Kingdom Animals (Scientific Procedures) Act 1986 (70/7229).
Supplementary Material
Acknowledgments
We thank K. Murphy for reading the manuscript and Mark Magnuson, Vanderbilt University Medical Center, Nashville, and Joost Verhaagen, Nederlands Herseninstituut, Amsterdam, for providing the pCMV4.GKB1 plasmid encoding full-length pancreatic glucokinase cDNA and pTR-CGW plasmid, respectively. This work was funded by Biotechnology and Biological Sciences Research Council (BBSRC) project grant number BB/I00842X. The Section of Investigative Medicine is funded by grants from the Medical Research Council, BBSRC, and National Institute for Health Research (NIHR) as well as the NIHR Imperial Biomedical Research Centre Funding Scheme, an Integrative Mammalian Biology Capacity Building Award, and an FP7-HEALTH-2009-241592 EurOCHIP grant. S.S. Hussain was funded by a Wellcome Trust Clinical Research Fellowship grant number 090792/Z/09/A. G. Bewick and W.S. Dhillo are funded by NIHR career development fellowships. E. Richardson was funded by a BBSRC capacity-building award in integrative mammalian biology studentship (grant number BB/E52708X).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(1):337–349. doi:10.1172/JCI77172.
Gavin Bewick’s present address is: Division of Diabetes and Endocrinology, King’s College London, Guy’s Campus, London, United Kingdom.
References
- 1.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249(1):13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 2.Thompson DA, Campbell RG. Hunger in humans induced by 2-deoxy-D-glucose: glucoprivic control of taste preference and food intake. Science. 1977;198(4321):1065–1068. doi: 10.1126/science.929188. [DOI] [PubMed] [Google Scholar]
- 3.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs HL. Some physical, metabolic, and sensory components in the appetite for glucose. Am J Physiol. 1962;203:1043–1054. doi: 10.1152/ajplegacy.1962.203.6.1043. [DOI] [PubMed] [Google Scholar]
- 5.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sclafani A. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev. 1987;11(2):131–153. doi: 10.1016/S0149-7634(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 7.Domingos AI, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27(6):1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- 9.Dela Cruz JA, Coke T, Icaza-Cukali D, Khalifa N, Bodnar RJ. Roles of NMDA and dopamine D1 and D2 receptors in the acquisition and expression of flavor preferences conditioned by oral glucose in rats. Neurobiol Learn Mem. 2014;114:223–230. doi: 10.1016/j.nlm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol Behav. 2014;133:92–98. doi: 10.1016/j.physbeh.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matschinsky FM, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55(1):1–12. doi: 10.2337/diabetes.55.01.06.db05-0926. [DOI] [PubMed] [Google Scholar]
- 12.Jetton TL, et al. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem. 1994;269(5):3641–3654. [PubMed] [Google Scholar]
- 13.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66(1):27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53(10):2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 15.Thorens B. GLUT2 in pancreatic and extra-pancreatic gluco-detection (review). Mol Membr Biol. 2001;18(4):265–273. doi: 10.1080/09687680110100995. [DOI] [PubMed] [Google Scholar]
- 16.Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE. Localization of glucokinase gene expression in the rat brain. Diabetes. 2000;49(5):693–700. doi: 10.2337/diabetes.49.5.693. [DOI] [PubMed] [Google Scholar]
- 17.Kang L, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55(2):412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Becker TC, Eiki J, Zhang BB, Dunn-Meynell AA. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes. 2008;57(5):1371–1379. doi: 10.2337/db07-1755. [DOI] [PubMed] [Google Scholar]
- 19.Dunn-Meynell AA, et al. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29(21):7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz MW, Woods SC, Porte D, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 21.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004;12(3):429–438. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Patterson M, et al. Hypothalamic injection of oxyntomodulin suppresses circulating ghrelin-like immunoreactivity. Endocrinology. 2009;150(8):3513–3520. doi: 10.1210/en.2008-0796. [DOI] [PubMed] [Google Scholar]
- 23.Printz RL, Magnuson MA, Granner DK. Mammalian glucokinase. Annu Rev Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara H, et al. Inhibition of pancreatic beta-cell glucokinase by antisense RNA expression in transgenic mice: mouse strain-dependent alteration of glucose tolerance. FEBS Lett. 1995;371(3):329–332. doi: 10.1016/0014-5793(95)00932-Y. [DOI] [PubMed] [Google Scholar]
- 25.Andersson U, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 26.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8(5):579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 27.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288(5475):2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 28.Iynedjian PB, et al. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci U S A. 1989;86(20):7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, et al. Short-term food restriction and refeeding alter expression of genes likely involved in brain glucosensing. Exp Biol Med (Maywood). 2003;228(8):943–950. doi: 10.1177/153537020322800810. [DOI] [PubMed] [Google Scholar]
- 30.Salgado M, et al. Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes. PLoS One. 2014;9(4):e94035. doi: 10.1371/journal.pone.0094035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown KS, Kalinowski SS, Megill JR, Durham SK, Mookhtiar KA. Glucokinase regulatory protein may interact with glucokinase in the hepatocyte nucleus. Diabetes. 1997;46(2):179–186. doi: 10.2337/diab.46.2.179. [DOI] [PubMed] [Google Scholar]
- 32.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51(7):2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan JM, et al. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8(1):45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152(7):2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt MC, Bruning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol Metab. 2013;24(2):76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108(28):11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page KA, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309(1):63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28(3):201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- 39. Abraham S, Lowenstein FW, O’Connell DE. Preliminary findings of the first Health and Nutrition Examination Survey, 1971–72: anthropometric and clinical findings. Rockville, Maryland, USA: US Department of Health, Education, and Welfare, Public Health Service, Health Resources Administration, National Center for Health Statistics; 1975. [Google Scholar]
- 40.Egecioglu E, et al. Hedonic and incentive signals for body weight control. Rev Endocr Metab Disord. 2011;12(3):141–151. doi: 10.1007/s11154-011-9166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst. 2007;(3):CD005105. doi: 10.1002/14651858.CD005105.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bray GA. Fructose: should we worry? Int J Obes (Lond). 2008;32(suppl 7):S127–S131. doi: 10.1038/ijo.2008.248. [DOI] [PubMed] [Google Scholar]
- 43.Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab. 2013;24(5):257–268. doi: 10.1016/j.tem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Li MV, et al. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP). Biochem Biophys Res Commun. 2010;395(3):395–400. doi: 10.1016/j.bbrc.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol Life Sci. 2007;64(23):3069–3083. doi: 10.1007/s00018-007-7332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen BJ, Marmarou A. Functional compartmentalization of energy production in neural tissue. Brain Res. 1992;585(1):190–195. doi: 10.1016/0006-8993(92)91206-t. [DOI] [PubMed] [Google Scholar]
- 47.Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- 48.van den Top M, Lyons DJ, Lee K, Coderre E, Renaud LP, Spanswick D. Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neuroscience. 2007;144(3):815–824. doi: 10.1016/j.neuroscience.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 49.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390(6659):521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 50.Mirshamsi S, et al. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YB, et al. ATP-sensitive potassium channel-deficient mice show hyperphagia but are resistant to obesity. Diabetes Metab J. 2011;35(3):219–225. doi: 10.4093/dmj.2011.35.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 53.Turner TJ, Adams ME, Dunlap K. Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science. 1992;258(5080):310–313. doi: 10.1126/science.1357749. [DOI] [PubMed] [Google Scholar]
- 54.King PJ, Widdowson PS, Doods HN, Williams G. Regulation of neuropeptide Y release by neuropeptide Y receptor ligands and calcium channel antagonists in hypothalamic slices. J Neurochem. 1999;73(2):641–646. doi: 10.1046/j.1471-4159.1999.0730641.x. [DOI] [PubMed] [Google Scholar]
- 55.Murphy BA, et al. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol. 2009;296(4):C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6(6):1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 57.Parton LE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 58.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30(23):8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiol Behav. 1994;56(2):399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 60.Froguel P, et al. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med. 1993;328(10):697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- 61.Christesen HB, et al. The second activating glucokinase mutation (A456V): implications for glucose homeostasis diabetes therapy. Diabetes. 2002;51(4):1240–1246. doi: 10.2337/diabetes.51.4.1240. [DOI] [PubMed] [Google Scholar]
- 62.Solon-Biet SM, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem Biophys Res Commun. 2005;327(4):1088–1093. doi: 10.1016/j.bbrc.2004.12.113. [DOI] [PubMed] [Google Scholar]
- 64. Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. San Diego, California, USA: Academic Press; 1989. [Google Scholar]
- 65.Zhang Y, Zhou J, Corll C, Porter JR, Martin RJ, Roane DS. Evidence for hypothalamic K+(ATP) channels in the modulation of glucose homeostasis. Eur J Pharmacol. 2004;492(1):71–79. doi: 10.1016/j.ejphar.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 66.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS. ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes. 2007;56(4):1120–1126. doi: 10.2337/db06-1102. [DOI] [PubMed] [Google Scholar]
- 67.Shapira S, Adeyemo OM, Feuerstein G. Integrated autonomic and behavioral responses to L/N Ca2(+)-channel blocker omega-conotoxin in conscious rats. Am J Physiol. 1990;259(3):R427–R438. doi: 10.1152/ajpregu.1990.259.3.R427. [DOI] [PubMed] [Google Scholar]
- 68.Jackson HC, Scheideler MA. Behavioural and anticonvulsant effects of Ca2+ channel toxins in DBA/2 mice. Psychopharmacology (Berl). 1996;126(1):85–90. doi: 10.1007/BF02246415. [DOI] [PubMed] [Google Scholar]
- 69.Asakura K, Matsuo Y, Kanemasa T, Ninomiya M. P/Q-type Ca2+ channel blocker omega-agatoxin IVA protects against brain injury after focal ischemia in rats. Brain Res. 1997;776(1):140–145. doi: 10.1016/s0006-8993(97)00975-x. [DOI] [PubMed] [Google Scholar]
- 70.Criscione L, et al. Food intake in free-feeding and energy-deprived lean rats is mediated by the neuropeptide Y5 receptor. J Clin Invest. 1998;102(12):2136–2145. doi: 10.1172/JCI4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antal-Zimanyi I, et al. Pharmacological characterization and appetite suppressive properties of BMS-193885, a novel selective neuropeptide Y(1) receptor antagonist. Eur J Pharmacol. 2008;590(1):224–232. doi: 10.1016/j.ejphar.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 72.Bewick GA, et al. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58(4):840–846. doi: 10.2337/db08-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dhillo WS, et al. Hypothalamic interactions between neuropeptide Y, agouti-related protein, cocaine- and amphetamine-regulated transcript and alpha-melanocyte-stimulating hormone in vitro in male rats. J Neuroendocrinol. 2002;14(9):725–730. doi: 10.1046/j.1365-2826.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 74.Allen JM, Yeats JC, Adrian TE, Bloom SR. Radioimmunoassay of neuropeptide Y. Regul Pept. 1984;8(1):61–70. doi: 10.1016/0167-0115(84)90029-6. [DOI] [PubMed] [Google Scholar]
- 75.Kim MS, et al. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105(7):1005–1011. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.