Abstract
Background
Statins have a beneficial effect on bone mineral density (BMD) and lean mass in some studies of HIV-uninfected adults, however this has never been investigated in the setting of HIV infection.
Design
HIV-infected subjects on stable antiretroviral therapy with a low-density lipoprotein cholesterol level of ≤ 130 mg/dL and evidence of heightened immune activation or inflammation were randomized to rosuvastatin 10mg daily or placebo for 96 weeks.
Methods
This was a prespecified interim analysis at 48 weeks. Between-group and within group differences were compared; multivariable regression models were constructed.
Results
72 subjects were randomized to statin therapy and 75 to placebo. Modest 48 week relative increases in trochanter BMD (0.9%; 95% CI: -0.9, 0.6%) and total hip BMD (0.6%; 95% CI: 0.0, 1.1%) in the statin arm were significantly greater than placebo (p<0.05). The relationship between statin use and total hip BMD change was robust to adjustment of age, gender, race, and smoking status (p=0.02) and strengthened by inclusion of baseline (p=0.01) and week 48 change in sTNFR-1 (p=0.009). Relative increases in total body, trunk and limb fat were similar between statin and placebo arms (p ≥0.58). Although a significant gain in leg lean mass was seen in the statin arm, this was not significantly different compared to placebo (p=0.36).
Conclusions
The improvements seen in total hip BMD after 48 weeks of rosuvastatin therapy support further potential benefits of statin therapy in HIV, beyond a reduction of cardiovascular risk.
Keywords: statin, bone mineral density, lean body mass, body fat, HIV
Background
Combination antiretroviral therapy (ART) has increased the life expectancy of human immunodeficiency virus type 1 (HIV) infected persons so that nearly one-half of the 1.2 million HIV-infected persons in the United States are now age 50 years or older. Although living longer, even HIV-infected persons on effective ART appear to be at greater risk of age-related complications including cardiovascular disease and osteoporosis, due at least partly to enhanced inflammation and immune activation [1, 2].
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are highly effective at decreasing cardiovascular events, with morbidity and mortality reductions seen well beyond the magnitude of decrease in cholesterol and attributed to the anti-inflammatory benefit [3]. We have recently shown that statins improve several markers of immune activation and inflammation in HIV-infected persons on stable ART [4, 5].
In addition to the cardiovascular benefits, studies in older adults without HIV suggest that statins may exert an additional beneficial effect on bone mineral density (BMD) and on measures of body composition. Although statin use was not associated with a decreased risk of non-traumatic fractures in a large HIV-infected cohort [6], the effect of statins on BMD in HIV is unknown. Among HIV-infected persons, increases in subcutaneous fat [7] and hip circumference [8] were observed with randomized statin initiation, suggesting a possible role of statins in the treatment of lipoatrophy. Furthermore, although myotoxic adverse effects of statins are reported among both HIV-infected and –uninfected older adults, statin use has been associated with favorable changes in lean mass among older, HIV-uninfected adults [9, 10]. Thus, a theoretical benefit of statins could extend to the bone and body composition of persons aging with HIV. In a double-blinded placebo-controlled trial of rosuvastatin, we hypothesized that statin therapy would be associated with an improvement in BMD, gain in limb fat without a gain in central fat, and gain in limb lean mass compared to placebo, and that these changes would be explained through improvements in inflammation and immune activation.
Methods
Study Design and Participants
The SATURN-HIV (Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV) study is a randomized, double-blinded, placebo-controlled trial designed to measure the impact of daily rosuvastatin at 10mg on cardiovascular disease and skeletal health, as previously described [4, 5, 11, 12]. Randomization was 1:1 and stratified by protease inhibitor use at study entry, by the presence or absence of osteopenia (at either hip or spine) at study entry, and by presence or absence of coronary calcifications by CT scan. Randomization lists were generated by the study statistician and provided directly to the investigational pharmacist who administered study drug. This report details the results of a pre-specified, interim analysis of BMD, fat, and lean mass changes from baseline to week 48. Enrollment occurred between March 2011 and August 2012, when target enrollment was obtained. Eligible subjects were HIV-infected adults ≥ 18 years of age with a fasting low-density lipoprotein (LDL) cholesterol of ≤130 mg/dL and either a high sensitivity C-reactive protein (hsCRP) level of ≥2 mg/L and/or ≥19% activated CD8+ T-cells (CD8+CD38+HLA-DR+). Additional eligibility criteria included receipt of stable ART for ≥12 weeks with cumulative ART duration of ≥6 months, HIV-1 RNA ≤1,000 copies/mL, and no history of fragility fractures. Subjects were excluded for an active or chronic inflammatory condition (besides HIV), prior myocardial infarction, pregnancy/lactation, receipt of systemic chemotherapy or steroids, diabetes mellitus or uncontrolled thyroid disease, or use of anabolic agents, growth hormone, >81 mg aspirin daily, or bone therapy (bisphosphonates, teriparatide, calcitonin). The study is registered on clinicaltrials.gov (NCT01218802) and was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH). Written informed consent was provided by all participants.
Clinical Assessments
At the initial visit, self-reported demographics, medical and HIV treatment history were obtained, with confirmation by medical records. Targeted physical exam included height and weight measurements. Body mass index (BMI) was calculated using the weight (kg) divided by the height (m) squared. Blood samples were collected after a 12-hour fasting period. HIV-1 RNA level and CD4+ lymphocyte count were obtained as part of routine clinical care.
Dual-Energy X-ray Absorptiometry
Dual-energy X-ray absorptiometry (DXA) of whole body, lumbar spine (L1-4) and left hip was performed in anteroposterior view using Lunar Prodigy Advance machine (GE Healthcare). Peripheral fat depot (limb fat) and central fat depots (trunk fat) from whole body DXA were used in the analysis. Total lean mass was defined as fat-free, bone-free mass as measured by DXA; peripheral lean mass (arm and leg) were also used in the analysis. Technicians used the same machine on the same subject throughout the study. All DXA scans were read at Case Medical Center by an experienced radiologist blinded to study information. Subjects were labeled with osteopenia if their T-score was ≤-1 and osteoporosis if T-score was ≤ -2.5 at either total hip or lumbar spine [13].
Markers of inflammation and activation
Plasma markers of inflammation and activation were measured as previously reported [5]. Concentrations of soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble tumor necrosis factor-α receptor (sTNFR)-I, interleukin-6 (IL-6), lipoprotein-associated phospholipase A2 (Lp-PLA2), soluble CD163 (sCD163), and soluble CD14 (sCD14) were determined by ELISAs (R&D Systems, Minneapolis, MN and diaDexus, San Francisco, CA). T lymphocytes and monocytes were phenotyped by flow cytometry as previously described [5].
Statistical Analysis
This was a pre-specified, preplanned interim analysis to assess changes from baseline to 48 weeks in BMD, fat, and lean mass. Continuous measures were described by medians and interquartile ranges and nominal variables with frequencies and percentages. Nominal variables were compared using χ2 analysis or Fisher exact test. For between-group and within group comparisons (at baseline and baseline to 48 week changes), normally distributed variables were compared using t tests or paired t test respectively; non-normally distributed variables were compared using Wilcoxon rank-sum or signed-rank test tests, respectively. Multivariable regression models were constructed to examine the effect of covariates on the association between statin and relative BMD change. Age, sex, race, and smoking were included in all models. Additional covariates were included singly and included clinical characteristics (baseline and week 48 change in body mass index, limb and trunk fat, and total lean mass; family history of hip fracture; week 48 change in LDL), HIV-specific characteristics (baseline and change in HIV-1 RNA, current and nadir CD4+ lymphocytes, current protease inhibitor or tenofovir-containing regimen, duration of protease inhibitor), inflammation (baseline and week 48 change in IL-6, sTNFR-1, sVCAM, Lp-PLA2) and activation (week 48 change in sCD14, sCD163, %CD14+CD16+ monocytes, %CD14dimCD16+ monocytes and %CD38HLA-DR+ CD4+ and CD8+ lymphocytes). Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Study Population
One-hundred and forty-seven HIV-1-infected persons enrolled and were assigned to receive rosuvastatin (72 subjects) or placebo (75 subjects). The groups were well balanced between arms (Table 1). Overall, the median age was 47 years, the median CD4+ lymphocyte count was 613 cells/μL, and 78% had an HIV-1 RNA < 50 copies/mL. The majority of participants were male, African American, smokers and taking tenofovir-containing ART regimens. Thirty-five (24%) subjects met criteria for osteopenia or osteoporosis at the hip and 32 (22%) met criteria for osteopenia or osteoporosis at the lumbar spine.
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | Statin (n=72) | Placebo (n=75) |
|---|---|---|
| Age (years) | 45.6 (41.1-51.4) | 46.9 (39.2-53.6) |
| Male | 58 (81) | 57 (76) |
| African American | 51 (71) | 52 (69) |
| Current smoker | 43 (60) | 54 (72) |
| Body mass index (kg/m2) | 26.6 (23.4-30.0) | 27.2 (23.5-30.5) |
| Family history hip fracture | 6 (8) | 12 (16) |
| Hepatitis C | 5 (7) | 7 (9) |
| CD4+ lymphocyte count (cells/μL) | 608 (440-848) | 627 (398-853) |
| Nadir CD4+ T-cell count (cells/μL) | 173 (84-312) | 190 (89-281) |
| HIV-1 RNA <50 copies/mL | 56 (78) | 58 (77) |
| Antiretroviral therapy duration (months) | 63 (37-119) | 71 (39-116) |
| Current protease inhibitor-containing regimen | 36 (50) | 36 (48) |
| Current tenofovir-containing regimen | 64 (89) | 66 (88) |
| Total hip osteopenia or osteoporosis | 18 (25) | 17 (22) |
| Lumbar spine osteopenia or osteoporosis | 19 (26) | 13 (17) |
Values presented as median (25th, 75th percentile) or number (%).
Subject Disposition and Safety Data
Nineteen subjects (6 statin; 13 placebo) withdrew prior to the week 48 analysis: 16 were lost-to-follow-up. One subject on placebo was diagnosed with diabetes and dropped out. Two subjects on placebo withdrew due to Grade 2 myalgias with normal creatine phosphokinase (CPK) levels. One subject in the statin group stopped treatment at week 5 due to hospitalization for hydration to treat grade 3 myalgias without rhabdomyolysis or renal compromise, but continued to be followed on study, off study drug. Myalgias resolved soon after study drug was discontinued. Three subjects on placebo changed ART regimens between baseline and 48 weeks: one replaced didanosine with abacavir, one switched from emtricitabine/tenofovir to abacavir/lamivudine, and one switched from lamivudine/zidovudine to emtricitabine/tenofovir and maraviroc. Overall, 14 subjects (7 statin, 7 placebo) had HIV-1 RNA >50 copies/mL at week 48. Neither baseline nor 48-week change in HIV-1 RNA or CD4+ lymphocyte counts differed between the groups.
Changes in BMD
Overall, changes in BMD from baseline to week 48 were modest, with significant relative gains in the statin arm in total hip BMD (0.6% [0.0, 1.1%]) and trochanter BMD (mean 0.9% [95% CI: 0.0, 1.7%]) and losses in the placebo arm in total hip BMD (0.6% [-1.3, 0.2%] and trochanter BMD (-0.7% [-1.8, 0.4%], Figures 1a and 1b. Compared to placebo, statin therapy was associated with a statistically significant increase in total hip and trochanter BMD (p<0.05). Lumbar spine BMD did not change significantly in either study arm, and was not significantly different between the two arms (Figure 1c). The relationship between statin use and change in total hip BMD was robust to adjustment for bone-related covariates of age, gender, race, and smoking status (p=0.02).
Figure 1.
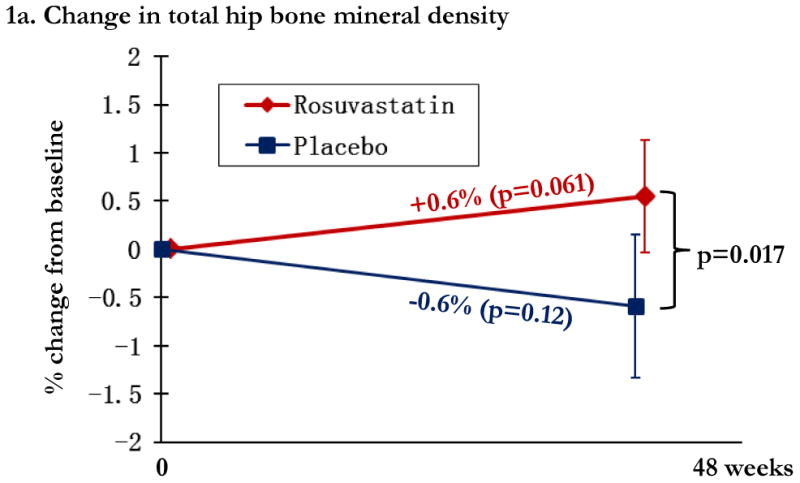
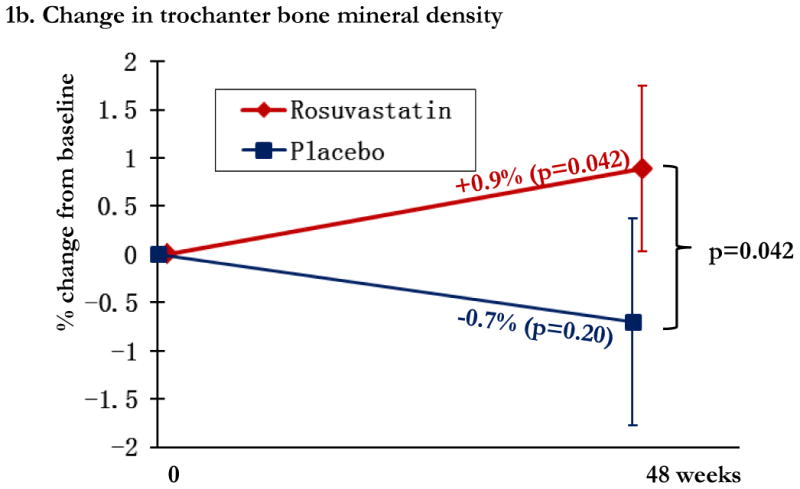
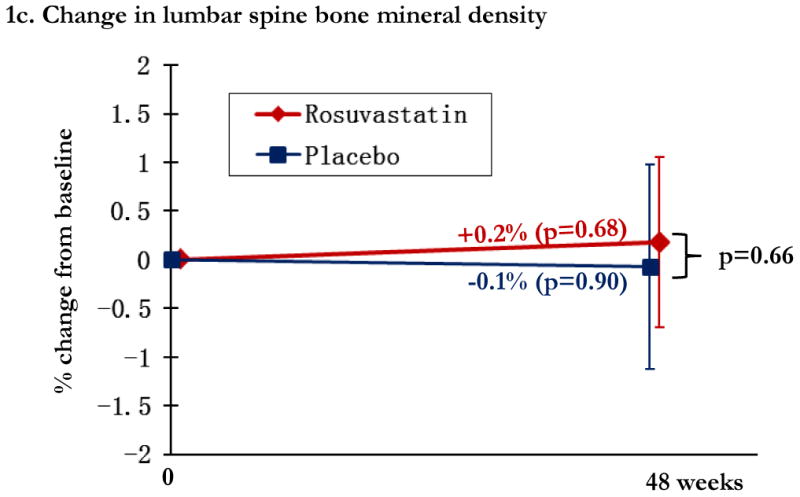
Regional changes in bone mineral density (BMD) by study arm, with total hip BMD (1a), trochanteric BMD (1b), and lumbar spine (1c). Changes in the rosuvastatin arm are shown in red and changes in the placebo arm are shown in blue. Within group relative changes and p values are indicated on the slope line in the respective color. P values for the comparison between study arms is provided in black font and indicated by the bracket.
Multivariate models were created to further explore the relationship between statin use and relative change in total hip BMD. The estimated difference in hip BMD with assignment to statin was 0.92% (0.15, 1.68%; p=0.019) in a model including age, race, gender, and smoking status. For additional models, we included age, race, gender, and smoking status, and then introduced a single additional variable. The estimated difference in hip BMD with statin use did not change significantly when including either family history of hip fracture; use of tenofovir; duration of protease inhibitor; HIV-1 RNA; current or nadir CD4+ lymphocyte count; baseline or 48 week relative change in trunk or limb fat, or total lean mass. The estimated difference in relative hip BMD change with statin use was weakened by inclusion of the 48 week change in BMI (estimate 0.87% [0.12, 1.6%]; p=0.02).
Because of the potential effect of inflammation on BMD changes, we then explored the addition of baseline or the week 48 change in inflammatory and activation markers on the relationship between statin use and hip BMD. The week 48 changes in these markers in SATURN-HIV have recently been reported [14]. Briefly, significantly larger reductions in lymphocyte activation, sCD14, and Lp-PLA2 were observed with statin use. In multivariate models including age, race, gender, and smoking status, we introduced separately each marker of interest to determine the impact on change in hip BMD. The estimated change in BMD with statin use was strengthened only by inclusion of baseline (estimate 0.96% [0.22, 1.7]; p=0.01) and week 48 change in sTNFR-1 (estimate 1.0% [0.25, 1.79%]; p=0.009).
Changes in Fat Mass
Modest and not statistically significant relative increases in total body fat (5.5% [-0.9, 11.9]; p=0.093), trunk fat (6.1% [−1.3, 13.6&; p=0.11), and limb fat (5.3%; [-0.4, 11.0]; p=0.070) were seen in the statin arm from week 0 to 48; these changes were not significantly different than placebo (p ≥ 0.58).
Changes in Lean Mass
In the statin arm, total lean mass increased 0.8% (p=0.10), leg lean mass 1.5% (p=0.013), and arm lean mass was unchanged (p=1.0). Differences between statin and placebo arms in relative lean mass change were not significant at any location (all p>0.23), Figure 2a-c. CPK increased by 17.2% ([2.5, 31.9%]; p=0.02) in the statin arm, compared to 16.2% ([1.5, 30.9%]; p=0.03) in the placebo arm; between group differences were not significant (p=0.93).
Figure 2.
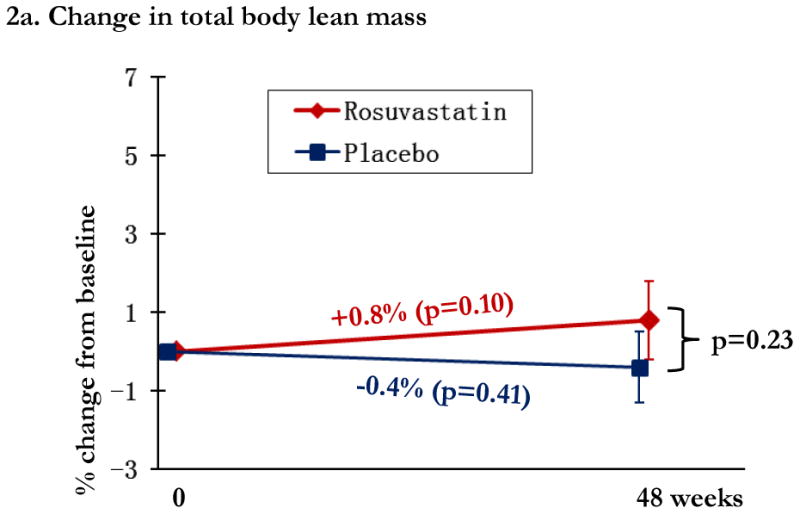
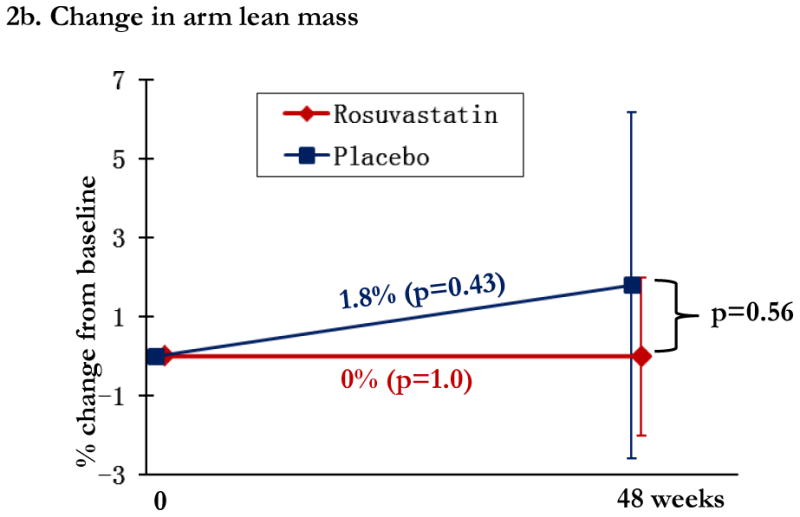
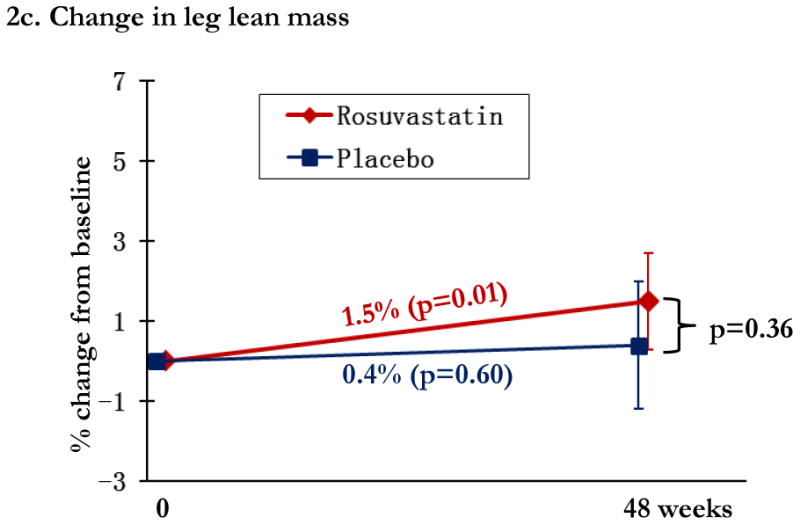
Changes in lean mass by study arm. Changes in the rosuvastatin arm are shown in red and changes in the placebo arm are shown in blue. Within group relative changes and p values are indicated on the slope line in the respective color. P values for the comparison between study arms is provided in black font and indicated by the bracket.
Discussion
Statins are potent cholesterol lowering drugs recognized for the additional anti-inflammatory mechanisms. Here, we present the first randomized intervention of statin therapy to assess the impact on changes in BMD, fat, and lean mass among effectively treated, HIV-infected adults with normal LDL cholesterol and increased levels of inflammation or immune activation. In comparison to the 0.6% increase in hip BMD observed in the statin arm of our study, a previously completed randomized trial of HIV-infected participants on stable ART randomized to alendronate or placebo (both arms receiving calcium carbonate 500 mg and vitamin D 200 IU tablets twice daily) demonstrated total hip BMD increases of 3.95% and 1.31%, respectively [15]. Although the small but significant improvement in total hip and trochanter BMD after 48 weeks of statin therapy does not provide support the use of statins as a primary treatment for low BMD, our findings do support a beneficial effect of statins on BMD when used for reduction of cardiovascular events. The improvement in hip BMD was independently associated with baseline and 48 week change in sTNFR-1, suggesting possible mechanistic pathways for the role of statins on BMD in HIV infection.
Prior data from in vitro or in vivo animal models provide evidence that statins impact bone through several distinct pathways. First, statins promote osteogenesis through the depletion of cellular cholesterol, which stimulates the Ras pathway and increases expression of bone morphogenetic protein (BMP)-2, an essential component of osteoblast differentiation [16-18]. Thus, in the current study, although decrease in LDL was not associated with change in BMD, change in other unmeasured cholesterol products may have further stimulated bone formation. Statins also have a direct effect on bone, suppress osteoclastogenesis through inhibition of osteoclasts [19, 20], and increase osteoprotegerin (OPG) mRNA and decrease receptor activator of nuclear factor kappa-B ligand (RANKL) mRNA expression [21]. The RANKL/OPG pathways are tightly interwoven with inflammatory markers including sTNFR-1 [22, 23].
In HIV-uninfected populations, the data supporting a clinically meaningful effect of statins on BMD are discordant and, similar to our findings, of a modest magnitude. A meta-analysis including five case-control, six cohort, and four randomized controlled trials (nearly 35,000 subjects) found small but significant increases in weighted mean changes of total hip, femoral neck, and lumbar spine BMD in statin users compared to placebo or control [24]. Another meta-analysis of eight observational studies found a protective effect of statins on risk of fracture [25]. Similar to our findings of a differential effect on total hip and trochanter BMD, these analyses in HIV-uninfected populations demonstrated a greater improvement in BMD at the hip and a greater protective effect for hip fracture in comparison to other sites, suggesting the possibility that statins have site-specific effects on fracture risk [24, 25]. In contrast, statin use was not associated with significant differences in BMD or fractures among 93,716 participants (7,846 statin-users) in the Women's Health Initiative Study [26]. Statins did not reduce fractures among HIV-infected statin-users compared to those not taking statins in a large observational cohort with 15,135 person-years of follow-up [6]. The present study is the first to study statin effect on BMD in a randomized trial in an HIV-infected population. Because of the heightened inflammatory state associated with HIV, it is conceivable that statins may have an accentuated beneficial effect in this population. Thus, larger and longer studies are needed to assess the effect of statin on fracture risk in the aging HIV population.
The role of statins on body fat in HIV was initially reported from a placebo controlled study of pravastatin where a significant increase in total, limb and subcutaneous fat was seen in subjects randomized to pravastatin versus placebo [7]. A subsequent randomized study failed to find changes in limb, trunk, subcutaneous or visceral fat with pravastatin [27]. In a large HIV-infected cohort, a greater increase in hip circumference was seen among statin-users compared to non-statin users, with a greater increase in thigh circumference in statin-users also taking thymidine analogues [8]. The differing results in our study may be explained by the indication for statin therapy (hyperlipidemia versus heightened inflammation), extensive prior thymidine analogue use in prior studies, differences in lipoatrophy, or differential statin effects. For example, pravastatin but not rosuvastatin led to a significant increase in the anti-atherogenic cytokine, adiponectin, in HIV-uninfected adults with coronary artery disease [28]. Similarly, switching from chronic simvastatin to pravastatin in HIV-uninfected adults led to a significant increase in adiponectin and decrease in CRP without a change in cholesterol [29]. Lastly, we cannot exclude differences in fat content within bone. Age-related bone loss is associated with increased marrow fat, and in vitro data demonstrates that the addition of pravastatin to protease inhibitor-treated mesenchymal stem cells improves cell proliferation and restores osteoblastic potential [30].
As with any therapy, adverse events do occur. Statin use is infrequently associated with myopathy, characterized by elevated CPK. Statin therapy has also been associated with subjective myalgias and evidence of myopathy on biopsy, without CPK elevation [31]. In the current study, myalgias were uncommon in both study arms, with similar CPK changes between study arms. Interestingly, we also detected a significant gain in leg, but not arm or total lean mass within the statin arm, although the change was not significantly different from that in the placebo arm. The effects of statins on lean mass in older adults without HIV vary considerably by study. In observational cohort studies, declines in strength [32] and lean mass [32, 33] are seen among statin-users. A randomized, placebo-controlled trial of high-dose atorvastatin in older adults found a higher rate of muscle complaints but no objective change in strength or performance [34, 35]. In other studies, statin use improved lower-extremity blood flow [36], was associated with better lower-extremity muscle function [37] and slower decline in lower-extremity function [38, 39]. Furthermore, among older adults started on resistance exercise, statin-users had a greater gain in total lean mass than non-statin-users [9].
The primary strength of this study is the double-blinded, randomized assignment to statin therapy, eliminating the prescribing bias found in observational studies of statins. Furthermore, the study is of adequate duration to observe changes in BMD and is relatively large, allowing for adjustment of underlying confounders and consideration of possible mediators.
The study is not without limitations. The majority of subjects were less than 50 years of age, male, African American, and with normal LDL cholesterol. Thus, the results are not necessarily generalizable to other populations. Furthermore, the study is specific for effects of rosuvastatin and results may not be generalizable to other statins. Our subjects had relatively normal BMD and were on stable ART. Statins may have had a greater impact on persons with osteoporosis or a greater attenuating effect on the BMD decline seen with ART initiation. Although 48 weeks is adequate for detecting significant changes in BMD, statins are often continued for several years, thus the long-term benefits or consequences should be confirmed in longitudinal studies. Lastly, we did not include measures of muscle strength or performance. These changes are often detected before changes in lean mass, thus we may have been able to detect differences between study arms in muscle function. Of note, both study arms reported a marked increase in self-reported physical activity over 48 weeks (statin arm: 83.7% [-2.7, 170.2% ]; p=0.06; placebo arm: 83.3% [31.5, 135.0%]; p=0.002), without significant differences between arms (p=0.19). These findings suggest that clinically significant differences in function between study arms were unlikely.
In conclusion, randomized assignment to rosuvastatin resulted in small but significant improvements in total hip BMD compared to placebo, which was independently associated with changes in sTNFR-1, but not other inflammation or activation markers, and independent of traditional bone risk factors. Although these modest improvements would not merit treatment of low BMD with statins, our findings provide evidence that statins may have additional benefits in HIV-infected persons beyond the reduction in cardiovascular risk. Longer term follow-up is planned for an additional 48 weeks to verify the safety and efficacy of rosuvastatin in measures of BMD, fat, and lean mass.
Acknowledgments
GAM developed and led the main study protocol and enrolled all study participants. YJ and SMD conducted the study analysis. KME and GAM assisted with the data plan analysis and KME prepared the initial draft of the manuscript. All authors reviewed and edited the manuscript. Results of this study were presented in part at the Conference on Retroviruses and Opportunistic Infections 2014, Boston, MA.
Funding: This project was supported by the National Institutes of Health [NR012642 to GAM and R03AG040594 and K23AG050260 to KME] and the Hartford Foundation Centers for Excellence in Geriatric Medicine (KME). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study drug and matching placebo were donated by AstraZeneca.
Footnotes
Potential Conflicts of Interest: GAM has served as a scientific advisor or speaker for Bristol-Myers Squibb, Tibotec, Gilead, and Merck, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study. SD serves on the DSMB for a Johnson & Johnson-sponsored study.
References
- 1.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Topics HIV medicine. 2009;17:118–123. [PubMed] [Google Scholar]
- 2.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97:33a–41a. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 Weeks of Statin Therapy on Systemic and Vascular Inflammation in HIV-Infected Subjects Receiving Antiretroviral Therapy. J Infect Dis. 2014;209:1156–1164. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overton ET, Kitch D, Benson CA, Hunt PW, Stein JH, Smurzynski M, et al. Effect of statin therapy in reducing the risk of serious non-AIDS-defining events and nonaccidental death. Clin Infect Dis. 2013;56:1471–1479. doi: 10.1093/cid/cit053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallon PW, Miller J, Kovacic JC, Kent-Hughes J, Norris R, Samaras K, et al. Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men--a randomized, placebo-controlled study. AIDS. 2006;20:1003–1010. doi: 10.1097/01.aids.0000222072.37749.5a. [DOI] [PubMed] [Google Scholar]
- 8.Brown TT, Smurzynski M, Wu K, Bosch RJ, McComsey GA. Statin therapy and changes in hip circumference among HIV-infected participants in the ALLRT Cohort. Antivir Ther. 2009;14:853–858. doi: 10.3851/1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riechman SE, Andrews RD, Maclean DA, Sheather S. Statins and dietary and serum cholesterol are associated with increased lean mass following resistance training. J Gerontol A Biol Sci Med Sci. 2007;62:1164–1171. doi: 10.1093/gerona/62.10.1164. [DOI] [PubMed] [Google Scholar]
- 10.Agostini JV, Tinetti ME, Han L, McAvay G, Foody JM, Concato J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55:420–425. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 11.Erlandson KM, O'Riordan M, Labbato D, McComsey GA. Relationships between inflammation, immune activation, and bone health among HIV-infected adults on stable antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:290–298. doi: 10.1097/QAI.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 14.Funderburg NT, Clagett B, Jiang Y, Debanne S, Storer N, Labbato D, et al. Rosuvastatin reduces immune activation and inflammation in treated HIV infection. Presented at the Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. Poster 335. [Google Scholar]
- 15.McComsey GA, Kendall MA, Tebas P, Swindells S, Hogg E, Alston-Smith B, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007;21:2473–2482. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
- 16.Ruan F, Zheng Q, Wang J. Mechanisms of bone anabolism regulated by statins. Biosci Rep. 2012;32:511–519. doi: 10.1042/BSR20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 18.Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, et al. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 19.Han G, Chen Y, Hou J, Liu C, Chen C, Zhuang J, et al. Effects of simvastatin on relapse and remodeling of periodontal tissues after tooth movement in rats. Am J Orthod Dentofacial Orthop. 2010;138:550 e551–557. doi: 10.1016/j.ajodo.2010.04.026. discussion 550-551. [DOI] [PubMed] [Google Scholar]
- 20.Ayukawa Y, Yasukawa E, Moriyama Y, Ogino Y, Wada H, Atsuta I, et al. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:336–342. doi: 10.1016/j.tripleo.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Ahn KS, Sethi G, Chaturvedi MM, Aggarwal BB. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. Int J Cancer. 2008;123:1733–1740. doi: 10.1002/ijc.23745. [DOI] [PubMed] [Google Scholar]
- 22.Ochi H, Hara Y, Tagawa M, Shinomiya K, Asou Y. The roles of TNFR1 in lipopolysaccharide-induced bone loss: dual effects of TNFR1 on bone metabolism via osteoclastogenesis and osteoblast survival. J Orthop Res. 2010;28:657–663. doi: 10.1002/jor.21028. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Amer Y, Abbas S, Hirayama T. TNF receptor type 1 regulates RANK ligand expression by stromal cells and modulates osteoclastogenesis. J Cell Biochem. 2004;93:980–989. doi: 10.1002/jcb.20197. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Zhu LP, Yang XL, Huang HL, Ye DQ. HMG-CoA reductase inhibitors (statins) and bone mineral density: a meta-analysis. Bone. 2013;54:151–156. doi: 10.1016/j.bone.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, et al. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 26.LaCroix AZ, Cauley JA, Pettinger M, Hsia J, Bauer DC, McGowan J, et al. Statin use, clinical fracture, and bone density in postmenopausal women: results from the Women's Health Initiative Observational Study. Ann Intern Med. 2003;139:97–104. doi: 10.7326/0003-4819-139-2-200307150-00009. [DOI] [PubMed] [Google Scholar]
- 27.Calmy A, Bloch M, Wand H, Delhumeau C, Finlayson R, Rafferty M, et al. No significant effect of uridine or pravastatin treatment for HIV lipoatrophy in men who have ceased thymidine analogue nucleoside reverse transcriptase inhibitor therapy: a randomized trial. HIV Med. 2010;11:493–501. doi: 10.1111/j.1468-1293.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama H, Saito S, Daitoku K, Fukuda I, Higuma T, Hanada H, et al. Effects of pravastatin and rosuvastatin on the generation of adiponectin in the visceral adipose tissue in patients with coronary artery disease. Fundam Clin Pharmacol. 2011;25:378–387. doi: 10.1111/j.1472-8206.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 29.Kai T, Arima S, Taniyama Y, Nakabou M, Kanamasa K. Comparison of the effect of lipophilic and hydrophilic statins on serum adiponectin levels in patients with mild hypertension and dyslipidemia: Kinki Adiponectin Interventional (KAI) Study. Clin Exp Hypertens. 2008;30:530–540. doi: 10.1080/10641960802251925. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Vallejo SJ, Beaupere C, Larghero J, Capeau J, Lagathu C. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: beneficial effect of pravastatin. Aging Cell. 2013;12:955–965. doi: 10.1111/acel.12119. [DOI] [PubMed] [Google Scholar]
- 31.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 32.Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM. 2009;102:625–633. doi: 10.1093/qjmed/hcp093. [DOI] [PubMed] [Google Scholar]
- 33.Dzien A, Winner H, Theurl E, Dzien-Bischinger C, Lechleitner M. Fat-free mass and fasting glucose values in patients with and without statin therapy assigned to age groups between <60 and >75 years. Obes Facts. 2013;6:9–16. doi: 10.1159/000348573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballard KD, Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, et al. Increases in creatine kinase with atorvastatin treatment are not associated with decreases in muscular performance. Atherosclerosis. 2013;230:121–124. doi: 10.1016/j.atherosclerosis.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker BA, Capizzi JA, Augeri AL, Grimaldi AS, Michael White C, Thompson PD. Atorvastatin increases exercise leg blood flow in healthy adults. Atherosclerosis. 2011;219:768–773. doi: 10.1016/j.atherosclerosis.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation. 2003;107:757–761. doi: 10.1161/01.cir.0000050380.64025.07. [DOI] [PubMed] [Google Scholar]
- 38.Gray SL, Aragaki AK, LaMonte MJ, Cochrane BB, Kooperberg C, Robinson JG, et al. Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc. 2012;60:2206–2214. doi: 10.1111/jgs.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giri J, McDermott MM, Greenland P, Guralnik JM, Criqui MH, Liu K, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47:998–1004. doi: 10.1016/j.jacc.2005.10.052. [DOI] [PubMed] [Google Scholar]


