Abstract
The delta opioid receptor (DOR) has raised much interest for the development of new therapeutic drugs, particularly to treat patients suffering from mood disorders and chronic pain. Unfortunately, the prototypal DOR agonist SNC80 induces mild epileptic seizures in rodents. Although recently developed agonists do not seem to show convulsant properties, mechanisms and neuronal circuits that support DOR-mediated epileptic seizures remain to be clarified. DORs are expressed throughout the nervous system. In this study we tested the hypothesis that SNC80-evoked seizures stem from DOR activity at the level of forebrain GABAergic transmission, whose inhibition is known to facilitate the development of epileptic seizures. We generated a conditional DOR knockout mouse line, targeting the receptor gene specifically in GABAergic neurons of the forebrain (Dlx-DOR). We measured effects of SNC80 (4.5, 9, 13.5 and 32 mg/kg), ARM390 (10, 30 and 60 mg/kg) or ADL5859 (30, 100 and 300 mg/kg) administration on electroencephalograms (EEGs) recorded in Dlx-DOR mice and their control littermates (Ctrl mice). SNC80 produced dose-dependent seizure events in Ctrl mice, but these effects were not detected in Dlx-DOR mice. As expected, ARM390 and ADL5859 did not trigger any detectable change in mice from both genotypes. These results demonstrate for the first time that SNC80-induced DOR activation induces epileptic seizures via direct inhibition of GABAergic forebrain neurons, and supports the notion of differential activities between first and second-generation DOR agonists.
Keywords: Delta opioid receptor, Conditional Knockout, Epileptic seizures, delta agonist, biased agonism, in vivo
Introduction
Delta opioid receptors (DOR) have emerged during the last decade as a major player for the modulation of chronic pain, the control of emotional processes and regulation of some aspects of addiction including impulsivity. Preclinical studies, using both genetic and pharmacological approaches, have emphasized the beneficial contribution of DOR agonists to reduce chronic pain [1, 2] and anxiety/depressive-like behaviors [3]. DOR agonists have entered clinical trials in order to treat mood disorders [4].
The development of new delta drugs encountered untoward effects of DOR agonists, in particular their convulsive properties. The first non-peptidic agonists BW373U86 and SNC80 were described to mediate brief and non-lethal convulsions in rodents [5, 6]. In addition, pro-convulsive effects of SNC80 were also reported in rhesus monkeys [7]. New agonists were developed with less or no adverse effects on epileptic thresholds, such as ADL5859, ADL5747 [8, 9] or KNT-127 [10] for example. Mechanisms underlying the distinct DOR agonists effects on behavioral responses may involve differential intracellular processes, a concept referred as to biased agonism or functional selectivity, and those involved in DOR agonist-dependent convulsant activity remain to be clarified [2].
Genetic and pharmacological studies have demonstrated that BW373U86 and SNC80-induced seizures are mediated by DORs [6, 11, 12]. At present however, the precise neuroanatomical site, as well as neurotransmitter systems involved in SNC80-induced epileptic seizures are unknown. The contribution of GABAergic systems in the onset and spreading of absence seizures has been long established, and for example, progressive decrease of GABAergic phasic inhibition in the hippocampus was shown in a rat model of spontaneous seizures [13]. DORs are broadly expressed in the nervous system [14]. In the forebrain, a main site for the control of epileptic seizures [15], DORs are expressed in cortex and hippocampus with well-characterized expression in GABAergic neurons for hippocampus [16, 17].
Here we tested the hypothesis that DORs expressed in GABAergic neurons of the forebrain are responsible for SNC80-induced seizures. To this aim, we used a conditional knockout mouse line [18] with a specific DOR gene deletion in these neurons. In these mice, DOR binding is significantly decreased at the level of hippocampus, and intact in the cortex. We tested effects of SNC80 (high proconvulsant activity), as well as ARM-390 and Adolor-5859 (low proconvulsant activity) in Dlx-DOR and control littermates. As expected, ARM-390 and Adolor-5859 had no effect in any mouse line. Remarkably SNC80-induced modifications of electroencephalogram recordings (EEGs) were abolished in Dlx-DOR mice, demonstrating for the first time that SNC80-evoked convulsions arise from direct inhibition of forebrain GABAergic neurons.
Materials and Methods
Conditional knockout (Dlx-DOR) and total knockout (CMV-DOR) mice were obtained and genotyped as described in [18]. The DOR pattern of expression in Ctrl and Dlx-DOR mice was described previously [18] and is summarized in Figure 1. Two independent cohorts were assessed and each was composed of 8 mice per genotype. Only male mice were used in all experiments, aged 2–6 months, maintained in standard conditions (12 h dark/light cycle light on at 7 am) with food and water ad libitum, except during the EEG recording sessions. Mice were habituated to their new experimental environment and handled for 1 week before starting the experiments. All experimental procedures were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the local ethical committee (Comité d’éthique pour l’expérimentation animale IGBMC-ICS).
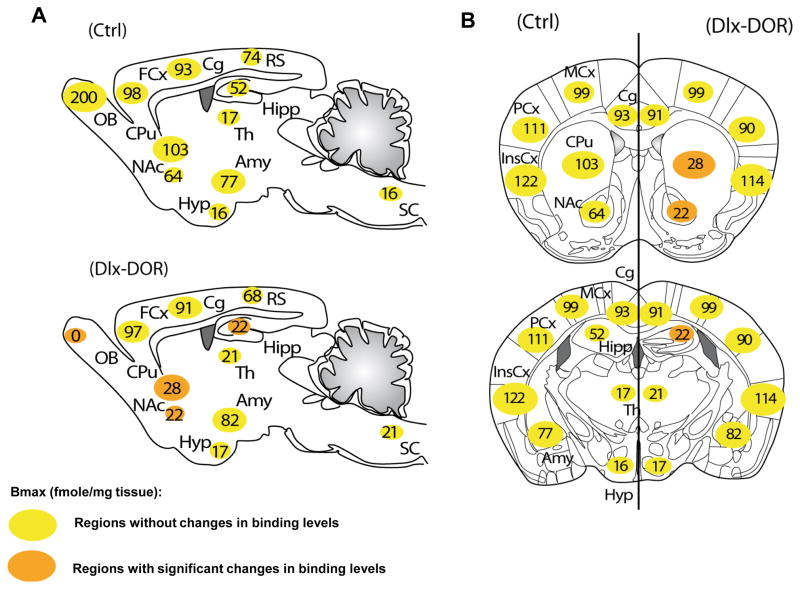
Fig. 1. Summary of the anatomical distribution of delta opioid receptors in Ctrl and Dlx-DOR mice.
The DOR activity in Ctrl and Dlx-DOR mice that was described previously [18]. (A) Sagittal sections in Ctrl mice at the top, in Dlx-DOR mice at the bottom; (B) Coronal sections at 2 different anterio-posterior levels (bregma 0.98 mm; bregma −1.46 mm) in Ctrl on (left side) and Dlx-DOR mice (right side). Quantification of DOR expression levels in fmole/mg of tissue. In Ctrl mice, DORs are particularly abundant in the OB, cortical regions (FCx, Cg, MCx, PCx and InsCx), amygdala and striatum (CPu and NAc). DORs are also expressed at moderate levels in the Hipp, RS, and at much lower level in Hyp, Th and SC. Orange circles represent brain regions showing detectable change of DOR expression in Dlx-DOR as compared to Ctrl mice. DORs are fully removed in the OB; strongly in the CPu and NAc; and partially in the Hipp of Dlx-DOR mice. Abbreviations: Amy, Amygdala; Cg, Cingulate cortex; CPu, Caudate Putamen; FCx, Frontal cortex; Hipp, Hippocampus; Hyp, Hypothalamus; InsCx, Insular cortex; MCx, Motor cortex; NAc, Nucleus Accumbens; OB, Olfactory Bulb; PCx, Parietal cortex; RS, Retrosplenial; SC, Spinal Cord; Th, Thalamus. n=5–6.
One week after their arrival in the animal facility, mice were anesthetized via intraperitoneal injection of a ketamine 1% / xylazine 0.5% solution and went through stereotaxic surgery. Four tungstene electrodes were positioned on the skull, one over the frontal cortex and one over the hippocampus on each side, as previously described [19, 20]. A fifth electrode was positioned at caudal level, over the cerebellum, and served as reference. The animals were allowed to recover for 24 h individually housed, and then one week in their normal environment.
During the test, mice were equipped with five single-contact electrodes. Mice were individually tested in a Plexiglas cylinder and EEG traces were continuously recorded during 3 h. EEG recordings were performed on freely moving animals. Basal EEG trace was monitored for 2 h, then the animal received the drug and EEG traces were monitored for 1 h. Cohort 1 received SNC80 at 4.5, 9, 13.5 and 32 mg/kg. SNC80 (Tocris Bioscience, Bristol, UK) was dissolved in saline and injected intraperitoneally. Cohort 2 received ARM390 at 10, 30 and 60 mg/kg followed by ADL5859 at 30, 100 and 300 mg/kg. ARM390 (AstraZeneca, Montreal, Canada) was administered orally by gavage, as described [21]. ADL5859 (Adolor Corporation, Exton, PA) was dissolved in distilled water and administered by gavage orally as described previously [8, 9]. A period of one week between each dose and two weeks between different compounds were applied in order to allow a sufficient washout period. The behavior of animals was observed during the whole recording sessions. The different seizure events were quantified though analysis of EEG recordings. Seizure patterns were the following: (1) myoclonies; (2) isolated or repeated clonic seizures; and (3) tonico-clonic seizures leading to status epilepticus. Bilateral spike-and-wave discharges (SWS) were scored as a reminiscence of absence seizures. A representative trace is shown in Figure 2. Latency for first occurrence of each event, number of events (Figure 3) and percentage of mice that expressed each seizure events were determined (Figure 4).
Fig. 2. EEG recordings from Dlx-DOR (top) and Ctrl mouse (bottom) after SNC80 administration.
A representation EEG recording session is shown. Recording starts 2 m in after SNC80 injection (32 mg/kg, s.c.). On the Ctrl mouse trace, a spike-and-wave discharge (SWS) is observed at the beginning of the session, followed by 18 myoclonic events which in turn lead to clonic seizure. No characteristic events are observed on Dlx-DOR mouse trace.
Fig. 3. Epileptic seizures induced by SNC80.

(A) Latency before the first seizure event and (B) duration of the seizure are represented. Highest doses (9, 13.5 and 32 mg/kg) of SNC80 decreased latency before seizure and increased duration of seizures in Ctrl (black bars) mice, whereas no detectable change occurred in Dlx-DOR and CMV-DOR mice. n= 8 per genotype. All data are presented as means ± S.E.M. Drug pharmacokinetics was analyzed by using repeated-measures ANOVA followed by Student’s t test for individual time points and pharmacological effects by two-way ANOVA for drug and genotype effects followed by Bonferroni post hoc analysis (*p<0.05; **p<0.01;***p<0.001).
Fig. 4. SNC80-induced EEG patterns.
Graphs represent the percentage of Ctrl, Dlx-DOR and CMV-DOR mice that showed (A) SWS, (B) myoclonic and (C) clonic seizures on EEG records. No detectable change occurred on EEG recordings for Dlx-DOR and CMV-DOR mice. (D) The number of myoclonies per period of 20 min was measured. n= 8 per genotype. All data are presented as means ± S.E.M (*p<0.05; **p<0.01; ***p<0.001).
Results
SNC80-induced seizures are abolished in Dlx-DOR mice
The non-peptidic DOR agonist SNC80 is described as a pro-convulsant drug in rats [6, 12]. We examined the effects of SNC80 administration on the latency to first seizure and total duration of seizures in Ctrl, Dlx-DOR and CMV-DOR mice (Figure 3). At the low dose (4.5 mg/kg), SNC80 did not evoke detectable change in any of the three groups. At the 9 mg/kg SNC80 dose, EEG recordings were modified in Ctrl mice, but this effect was not significant (latency before seizure, F(2, 20) = 2.782; p>0.05; duration of seizure, F(2, 20) = 2.52; p>0.05, two-way ANOVA). At higher doses (13.5 and 32 mg/kg), SNC80 produced seizures in Ctrl mice, reflected by dose-dependent decrease of latency before the first seizure (Figure 3A) as well as increase of seizure duration (Figure 3B). Two-way ANOVA indicated a statistically significant genotype effect at 13.5 mg/kg on both latency before first seizure (F(2, 20) = 5.205; p<0.05) and duration of seizure (F(2, 20) = 4.175; p<0.05). Post hoc analysis confirmed that SNC80, administered at 13.5 mg/kg, induced significant decrease of the latency to first seizure (p<0.05, Bonferroni/Dunn test) and enhanced duration of seizures (p<0.05, Bonferroni/Dunn test) in Ctrl mice compared to the two other genotypes. At the highest dose (32 mg/kg), two-way ANOVA revealed a strongly significant effect of SNC80 on both latency before seizure (F(2, 20) = 17.217; p<0.001) and duration of seizure (F(2, 20) = 14.708; p<0.001). Similarly, post hoc analysis revealed that SNC80 administered at 32 mg/kg induced a significant decrease of latency (p<0.001, Bonferroni/Dunn test) and increase in duration (p<0.001, Bonferroni/Dunn test) in the Ctrl mice, as compared to the other genotypes.
No sign of seizure could be detected in any of the two mutant lines (Figure 3). Thus, CMV-DOR were insensitive to the DOR agonist, as previously described [6], confirming that SNC80-induced seizures are specifically mediated by DORs. Remarkably, Dlx-DOR mice were equally insensitive to SNC80, demonstrating that DORs expressed in forebrain GABAergic neurons are essential for this effect.
We further examined types of seizure events produced by SNC80, including spike-and-wave discharges (SWS), clonies and myoclonies (Figure 4) and determined the proportion of mice exhibiting the different seizure events in the three genotypes (Figure 4A–D). In Ctrl mice, SNC80 at 13.5 mg/kg induced a significant increase in percentage of mice that showed clonic seizures (Figure 4C) (genotype effect, F(2, 101) = 5.217; p<0.05; Bonferonni Post-hoc analysis, Ctrl vs. Dlx-DOR mice p<0.05; Ctrl vs. CMV-DOR mice p<0.05) and 32 mg/kg (genotype effect, F(2, 101) = 17.391; p<0.001; Bonferonni Post-hoc analysis, Ctrl vs. Dlx-DOR mice p<0.001; Ctrl vs. CMV-DOR mice p<0.001). Further, SNC80 also induced myoclonic seizures in all mice from the Ctrl group, and at three doses (9, 13.5 and 32 mg/kg) (Figure 4C–D). Two-way ANOVA revealed that the percentage of mice expressing myoclonic seizures was affected by the genotype (F (2, 101) = 50.04; p<0.001). Post-hoc analysis confirmed that a significant proportion of Ctrl mice showed SNC80-induced myoclonies at 9 mg/kg (p<0.001, Bonferroni/Dunn test), 13.5 mg/kg (p<0.001, Bonferroni/Dunn test) and 32 mg/kg (p<0.001, Bonferroni/Dunn test), as compared to Dlx-DOR and CMV-DOR mice. Finally, no tonico-clonic seizures were detected in Ctrl mice showed (data not shown), in line with the notion that epileptogenic effects of SNC80 are mild [12].
In CMV-DOR mice, SNC80 produced no changes on spike-and-wave discharges, myoclonic, clonic and tonico-clonic seizures. This again is consistent with previous studies showing lack of convulsant effects of SNC80 upon behavioral observation of DOR knockout mice [6]. Dlx-DOR mice injected with SNC80 showed few SWS discharges at the highest doses (Figure 4A), and no sign of seizure was detected, including clonic, myoclonic and tonic-clonic seizures (Figure 4B–D). The scoring of seizure events, therefore, further confirms that DORs expressed in forebrain GABAergic neurons are necessary for convulsing SNC80 effects
ARM-390 and Adolor-5859 show no convulsant properties
Several studies have reported that second-generation delta drugs do not show convulsant properties [8, 21, 22]. To verify this, we also tested effects of DOR agonists of this category (ARM-390 and ADL-5859) in our experimental system. As expected, we found that neither ARM-390 (0, 30 and 60 mg/kg) nor ADL5859 (30, 100 and 300 mg/kg) modified EEG traces in any the three groups of mice (data not shown), even at the highest dose.
Discussion
In the present study, we confirm pro-convulsive effects of the non-peptidic delta agonist SNC80 in normal mice. This pharmacological activity of SNC80 was previously demonstrated upon behavioral observation [11] and, to our knowledge, this is the first report of EEG modifications in mice after SNC80 administration. Classical pentylenetetrazole treatment produces strong and long lasting crises, and may eventually lead to the animal death [23], however SNC80 seizures are reported to be mild [12]. In accordance, pro-convulsing effects of SNC80 were brief and mild since no strong tonico-clonic seizures could be observed along the study.
The GABAergic system is known as a critical neurotransmitter system involved in epileptic seizures [15]. Here, conditional knockout mice characterized by a genetic deletion of DOR in forebrain GABAergic neurons, especially in hippocampus, striatum and olfactory bulb [18], did not respond to SNC80 under conditions were EEG recordings are strongly modified in Ctrl mice. This clear-cut observation demonstrates that the subset of receptors expressed in forebrain GABAergic neurons indeed mediate convulsing effects SNC80. Furthermore, DORs are strongly expressed on GABAergic neurons [16] and their inhibitory activity on these neurons normally leads to increase local network excitability. The pro-convulsant effect of SNC80, therefore, likely results from enhanced excitation of forebrain networks. Notably we observed that pentylenetetrazole has similar convulsant effects in Dlx-DOR and control mice (not shown), suggesting that forebrain DOR-expressing GABA neurons, which mediate the mild pro-convulsant effect of SNC80, may not be major players in the general epileptogenic activity of PTZ.
In Dlx-DOR mice, receptors are deleted in GABAergic neurons from olfactory bulb (100% deletion), striatum (65–81% deletion) and hippocampus (57% deletion). Although receptors responsible for epileptogenic effects of SNC80 remain to be precisely determined, it is unlikely that DORs mediate seizure events via olfactory bulb networks. In contrast, both striatal and hippocampal circuitry have been involved in epileptic events [15], and our data therefore suggest that SNC80 convulsant activity operate at the level of DORs in striato-hippocampal networks.
DORs are also expressed in other neuronal populations than GABAergic neurons, and may be present in some glutamatergic neurons, where their activity (anti-convulsant) would counteract DOR activity at GABAergic cells (pro-convulsant). Opposing activities of distinct DOR populations in epileptogenic circuits may also explain the mild convulsant effects of SNC80, as compared to those of pentylenetetrazole, which directly and specifically block GABA receptors [24].
Our analysis shows EEG modifications following administration of SNC80, but not ARM390 or ADL5849 compounds. These findings are in line with previous findings. ARM390 and ADL-5849 (Adolor) were developed for clinical purposes and produce no convulsions or EEG disturbances in the rat [8, 9]. To our knowledge, our results indicate for the first time the absence of detectable effect of ARM-390 compound on epileptic seizures. An interesting correlate is the lack of trafficking effects of both compounds that, in contrast to SNC80, do not trigger receptor endocytosis in vivo [21, 22]. It is likely that active forms of DOR bound to ARM390 or ADL5849 differ from SNC80-bound receptors, engaging distinct intracellular signaling pathways within epilepsy-associated circuits that do not trigger seizure events. This is another example of biased agonism at DOR in vivo [25], and the identification of differentially recruited effector pathways require further investigation.
Highlights.
SNC80 induces epileptic seizures at the level of forebrain GABAergic neurons.
ARM-390 and ADL5859, other DOR agonists, do not produce epileptic seizures.
Differential DOR agonist activities operate in corticomesolimbic circuitry.
Acknowledgments
We thank the Mouse clinical Institute, the animal core facility and the imaging platform at IGBMC for technical support (Illkirch, France). We are grateful to Elise Le Marchand, Thomas Favier, Gilles Duval and Dzemailj Memedov for the animal care. This work was supported by the CNRS, INSERM, and Université de Strasbourg. We would also like to thank the Fondation pour la Recherche Médicale (FRM FDT20120925269), the US National Institutes of Health (NIDA, grant #05010 and NIAAA, grant #16658) for financial support.
Abbreviations
- DOR
delta opioid receptor
- EEG
electroencephalogram
Footnotes
Disclosure/conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–14. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–90. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu Sin Chung P, Kieffer BL. Delta opioid receptors in brain function and diseases. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, et al. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- 5.Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, et al. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–95. [PubMed] [Google Scholar]
- 6.Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002;303:723–9. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- 7.Danielsson I, Gasior M, Stevenson GW, Folk JE, Rice KC, Negus SS. Electroencephalographic and convulsant effects of the delta opioid agonist SNC80 in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:428–34. doi: 10.1016/j.pbb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, et al. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–6. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- 9.Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, et al. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747) J Med Chem. 2009;52:5685–702. doi: 10.1021/jm900773n. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, et al. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–9. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 11.Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology (Berl) 2005;182:588–96. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006;317:1337–48. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- 13.Crunelli V, Cope DW, Terry JR. Transition to absence seizures and the role of GABA(A) receptors. Epilepsy Res. 2011;97:283–9. doi: 10.1016/j.eplepsyres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalonde R, Strazielle C. Brain regions and genes affecting myoclonus in animals. Neurosci Res. 2012 doi: 10.1016/j.neures.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Rezai X, Faget L, Bednarek E, Schwab Y, Kieffer BL, Massotte D. Mouse delta opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell Mol Neurobiol. 2012;32:509–16. doi: 10.1007/s10571-011-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, et al. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience. 2012;221:203–13. doi: 10.1016/j.neuroscience.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu Sin Chung P, Keyworth HL, Martin-Garcia E, Charbogne P, Darcq E, Bailey A, et al. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Biological Psychiatry. 2014 In Press. [Google Scholar]
- 19.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, et al. delta-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loscher W, Honack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8:171–89. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- 24.Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–95. [PubMed] [Google Scholar]
- 25.Pradhan AA, Smith ML, Kieffer BL, Evans CJ. Ligand-directed signalling within the opioid receptor family. Br J Pharmacol. 2012;167:960–9. doi: 10.1111/j.1476-5381.2012.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]