Abstract
Hepatocytes and their in vitro models are essential tools for preclinical screening studies for drugs that affect the liver. Most of the current models primarily focus on hepatocytes alone and lack the contribution of non-parenchymal cells (NPCs), which are significant through both molecular and the response of the NPCs themselves. Models that incorporate NPCs alongside hepatocytes hold the power to enable more realistic recapitulation and elucidation of cell interactions and cumulative drug response. Hepatocytes and liver sinusoidal endothelial cells (LSECs) account for ∼80% of the liver mass where the LSECs line the walls of blood vessels, and act as a barrier between hepatocytes and blood. Culturing LSECs with hepatocytes to generate multicellular physiologically relevant in vitro liver models has been a major hurdle since LSECs lose their phenotype rapidly after isolation. To this end, we describe the application of collagen gel (1) in a sandwich and (2) as an intervening extracellular matrix layer to coculture hepatocytes with LSECs for extended periods. These coculture configurations provide environments wherein hepatocyte and LSECs, through cell–cell contacts and/or secretion factors, lead to enhanced function and stability of the cocultures. Our results show that in these configurations, hepatocytes and LSECs maintained their phenotypes when cultured together as a mixture, and showed stable secretion and metabolic activity for up to 4 weeks. Immunostaining for sinusoidal endothelial 1 (SE-1) antibody demonstrated retention of LSEC phenotype during the culture period. In addition, LSECs cultured alone maintained high viability and SE-1 expression when cultured within a collagen sandwich configuration up to 4 weeks. Albumin production of the cocultures was 10–15 times higher when LSECs were cultured as a bottom layer (with an intervening collagen layer) and as a mixture in a sandwich configuration, and native CYP 1A1/2 activity was at least 20 times higher than monoculture controls. Together, these data suggest that collagen gel-based hepatocyte-LSEC cocultures are highly suitable models for stabilization and long-term culture of both cell types. In summary, these results indicate that collagen gel-based hepatocyte-LSEC coculture models are promising for in vitro toxicity testing, and liver model development studies.
Introduction
Liver is a complex multifunctional organ with a large range of functions, supporting almost every other organ in the body, and is the prime organ for drug metabolism.1–4 Several in vitro models comprising of microsomes, cell lines, primary hepatocytes, and liver slices have been developed to provide predictive information related to drug toxicity.5–10 Primary hepatocytes are relatively easy to isolate and retain most of the important in vivo functions for several days in monolayer static culture,11–13 and up to 2 weeks or longer when cocultured with stromal cell support,14–16 under collagen gel or on micropatterned surfaces,15–17 and continue to be the most studied for drug screening purposes in various formats.9,10,18 A major advance in primary hepatocyte culture is the use of a collagen gel sandwich, wherein primary hepatocytes are cultured between two layers of type I collagen gel for extended time periods.11,13 However, this method still lacks the contribution of non-parenchymal cells (NPCs) and the importance of the drug response of NPCs in addition to hepatocytes is being slowly realized. This necessitates the development of models which incorporate NPCs, while maintaining long-term phenotype and function.
Within the liver, hepatocytes and liver sinusoidal endothelial cells (LSECs) comprise ∼80% of the total cell mass.19–21 In the liver sinusoid, LSECs and hepatocytes are arranged in layers with the intervening space occupied by the extracellular matrix (ECM) of the perisinusoidal space (space of Disse); collagen I is one of the major components in this matrix.22–25 While hepatocytes are primarily responsible for various secretion and metabolic functions of the liver; LSECs line the sinusoids, isolating hepatocytes from the sinusoid flow and are first to be exposed to various toxic and benign factors circulating through the hepatic sinusoids.26–28 There is growing evidence that LSECs also participate in various metabolic activities, and are the primary target for some hepatic toxicants.15,29–34 Although LSECs have a significant role in drug exposure and toxicity, their use in drug screening, alongside hepatocytes, is limited as they lose their phenotype rapidly after isolation and are not viable in monoculture beyond a few days.29–38
Several strategies have previously been proposed to culture LSECs while retaining their phenotype.37–43 LSECs, cultured in media supplemented with lipids and oleic acid, in particular, was shown to maintain their metabolic and endocytotic activity for up to 5 days by influencing Akt/PKB and ERK signaling pathways.39 Another study with culturing LSECs and hepatocytes in a three-dimensional (3D) microreactor shows retention of LSEC phenotype expression for 13 days, primarily through modulation of vascular endothelial growth factor in the culture media.38 Coculturing LSECs as a layer of cells on top of hepatocytes sandwiched within a multilayer polyelectrolyte maintained the phenotype of LSECs for up to 12 days, whereas monocultures lost their phenotype within 3 days.37 Despite these advances in culture of LSECs alone, or with hepatocytes, their long-term culture and viability is still poorly understood.44,45 While several strategies for LSEC culture have been proposed,15,39,46 long-term maintenance of LSECs remains challenging.
Previously, we have developed a 3D model mimic of an in vivo cellular arrangement by overlaying cardiac endothelial cells on top of collagen-embedded hepatocytes.47,48 This work demonstrated that the collagen gel-based culture configuration induces the early recovery of hepatocytes following cell isolation with albumin and fibrinogen protein secretion and gene expression increasing by twofold by day 4 of culture. Surprisingly, early recovery was not caused by growth factors or cytokines, or by secreted ECM, but rather by the release of the amino acid proline by endothelial cells, which promoted the hepatocellular production of new collagen.48
This work extends the sandwich-collagen gel model to a coculture system consisting of hepatocytes and LSECs. The premise of this approach is providing native cues to the hepatocyte and endothelial cell cocultures, since collagen is one of the major components in the space of Disse.22 LSECs were cultured with hepatocytes (above, below, or together) in a coculture in different arrangements while enabling exposure to secretion factors through the matrix. Standard collagen sandwich culture of hepatocytes was extended to enable layering LSEC above, below, or as a mixture with hepatocytes (Fig. 1). A key feature of this model is the use of subconfluent (∼ 50%) coverage of either cell type. While two configurations separated LSECs and hepatocytes with an intervening layer, a third configuration enabled the formation of hepatocyte-LSEC contacts. Stability of cocultures were established using hepatocyte specific secretory (albumin and urea) and enzymatic (CYP1A) assays as well as immunostaining of endothelial surface markers [sinusoidal endothelial 1 (SE-1)] for up to 4 weeks. LSECs maintained their phenotype when cultured with hepatocytes as the bottom most layer (with an intervening collagen gel) or as a mixture, and hepatocytes in these configurations produce higher albumin when compared with other configurations, and this function is sustained for up to 4 weeks. Methods described in this work for the separation and use of cocultures could enable the development of a drug-screening platform, which incorporates other NPCs in addition to hepatocytes.
FIG. 1.
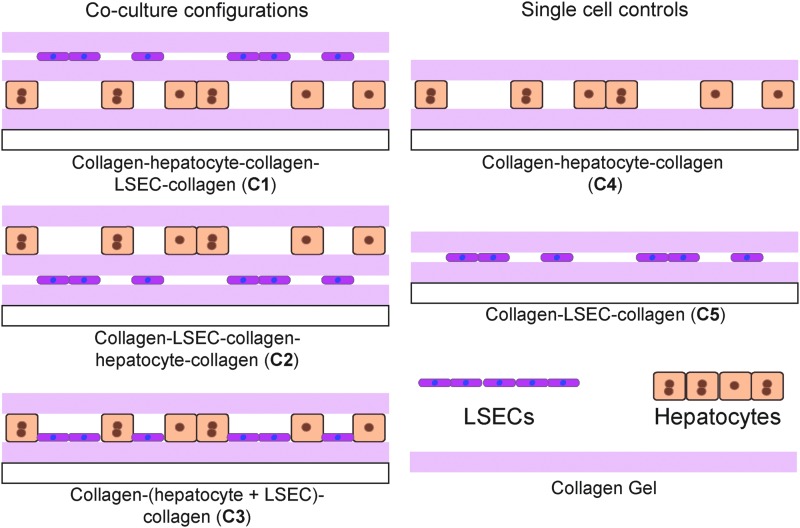
Schematic showing hepatocyte and liver sinusoidal endothelial cell (LSEC) coculture configurations. Hepatocytes and LSECs are cocultured as stacked layers in sandwich collagen gel. Coculture configurations with collagen–hepatocyte–collagen–LSEC–collagen (C1), collagen–LSEC–collagen–hepatocyte–collagen (C2), and collagen–(hepatocyte+LSEC)–collagen (C3) were prepared and single cell controls with collagen–hepatocyte–collagen with 50% hepatocyte coverage (C4) and collagen–LSEC–collagen (C5) were prepared.
Materials and Methods
Materials
Hepatocyte culture media was prepared with high glucose (4.5 g/L) Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 0.02 mg/L epidermal growth factor, 0.01428 mg/L Glucagon, 7.5 mg/L Hydrocortisone, 500 U/L Insulin, 2 mM Glutamine, and 2% Penicillin–Streptomycin. Type I Collagen was prepared by extracting acid-soluble collagen from Lewis rat-tail tendons as previously reported.11 Percoll was purchased from GE Biosciences. Rat albumin (Cat. No. 0855952) and peroxidase-conjugated sheep IgG fraction to rat albumin was purchased from MP Biomedicals (Cat. No. 0855776). The Urea assay kit was purchased from Stanbio laboratory (Cat. No. 0580–250). Earle's balanced salt solution (EBSS; Cat. No. 14155063), Live Cell Imaging Solution (Cat. No. A14291DJ), the Live/Dead Viability kit (Cat. No. L-3224), and the NucBlue Live Ready Probes Reagent (Cat. No. R37605) were purchased from Life Technologies. Hepatic sinusoidal endothelial cell antibody (SE-1; Cat. No. NB110-68095G) was purchased from Novus Biologicals. All other chemical reagents were purchased from Sigma.
Primary rat hepatocyte isolation
Hepatocytes were obtained from female Lewis rat using two-step collagenase protocol. Two to 3 month old female Lewis rats (Charles River Laboratories) weighing 180–200 g were used as a hepatocyte source and were maintained in accordance with the National Research Council guidelines. Experimental protocols were approved by the Subcommittee on Animal Care, Committee on Research at Massachusetts General Hospital. Using a modification on the two-step collagenase perfusion method,49,50 involves purification of cell suspension by means of centrifugation over Percoll. About 200–300 million cells were obtained from one rat isolation with viability between 85% and 95% (evaluated by Trypan Blue exclusion) and used immediately.
Primary rat LSEC separation
LSECs were separated from NPC fraction using density separation in a Percoll gradient.51 The NPC fraction was obtained from the same liver perfusions as the hepatocyte isolations. The supernatant remaining after the primary rat hepatocyte separation contained the LSECs and other liver cell types (NPC fraction). Briefly, the NPC fraction was aliquoted into 50 mL conical tubes and centrifuged at 300 g for 15 min. The supernatant was discarded and the pellet was resuspended in ice cold phosphate buffered saline (PBS). Typically, the NPC fraction from a rat was resuspended in 20 mL PBS. A Percoll gradient was prepared in 50 mL conical tubes with 15 mL of 50% Percoll (isotonic) on the bottom and 20 mL of 25% Percoll (isotonic) layered on top of it. Ten milliliters of the NPC fraction (in PBS) was carefully placed on the 25% Percoll layer and centrifuged at 900 g for 25 min, without brake. At the end of the centrifugation, the layer between 25% and 50% percoll gradient (10–17.5 mL level) was collected and diluted in ice cold PBS and centrifuged at 900 g for 25 min (without brake) to pellet the cells. Any remaining Percoll was aspirated and the cells were suspended in fresh hepatocyte culture media (10 mL). To remove any contaminating cells (Kupffer), the cell fraction was incubated on a 10-cm-diameter tissue culture dish for 1–2 min and the nonadherent cells were collected. From each isolation, 30–40 million LSECs were obtained and used immediately for preparing cocultures.
Coculture sample preparation
Samples were prepared in 12-well plates with 200 μL of collagen gel per layer. Collagen gel mixture was prepared by mixing Type I collagen (1.25 mg/mL) and 10×DMEM in a ratio 9:1 at 4°C and used immediately. Collagen mixture (200 μL) was added to each well and allowed to gel for 1 h at 37°C before addition of media. Cocultures were prepared with 0.25 million hepatocytes and 1 million LSECs, whereas single cell controls contained 0.25 million hepatocytes or 1 million LSECs in sandwich configurations.
Collagen–hepatocyte–collagen–LSEC–collagen (C1) samples were prepared by (1) seeding 0.25 million hepatocytes on a collagen gel and stabilization for 24 h, (2) addition of a collagen gel on top and incubation for 24 h, (3) addition of 1 million LSECs on top, followed by the (4) addition of a collagen gel on top.
Collagen–LSEC–collagen–hepatocyte–collagen (C2) samples were prepared by (1) seeding 1 million LSECs on a collagen gel and stabilization for 24 h, (2) addition of a collagen gel on top and incubation for 24 h, (3) addition 0.25 million hepatocytes on top, followed by the (4) addition of a collagen gel on top.
Collagen–(hepatocyte+LSEC)–Collagen (C3) samples were prepared by (1) seeding a mixture of 1 million LSECs and 0.25 million hepatocytes on a collagen gel and stabilization for 24 h and (2) addition of a collagen gel on top. Single cell controls were prepared by seeding cells on a collagen gel followed by the addition of a second collagen layer on top after 24 h.
Control samples were prepared with (1) 0.25 million hepatocytes (C4), (2) 1 million LSECs (C5) in collagen gel sandwich configurations.
All cultures were maintained in 400 μL hepatocyte culture media at 37°C and 10% CO2 and media was replaced every 24 h. Media samples collected while changing media were stored at 4°C for analysis with Day 1 considered 24 h after the top collagen gel was added on top of the configurations. In the case of C1 and C2, cocultures were prepared from two different isolations over a period of 3 days. The coculture (C3) was prepared from hepatocytes and LSECs from the same isolation.
Albumin enzyme-linked immunosorbent assay
Albumin concentration in the media was measured using an in-house enzyme-linked immunosorbent assay (ELISA) protocol. Briefly, 96-well high-binding ELISA plates were coated with 5 μg/well rat albumin in 100 μL PBS and incubated overnight at 4°C. Coated plates were washed with PBS-Tween (0.05%) at least four times and 50 μL of the media or standards were added to the wells in the plate. Each plate has a set of standards. Peroxidase-conjugated albumin antibody was diluted 1:10,000 in PBS-tween and 50 μL was added to each well and incubated overnight at 4°C or for 2 h at 37°C. At the end of incubation, the plates were washed with PBS-Tween (0.05%) at least four times. A substrate solution of o-Phenylenediamine dihydrochloride (400 μg/mL) and 4 μM hydrogen peroxide was prepared in a citric acid buffer. Fifty microliters of the solution was added to each well and incubated for 5 min. Reaction was stopped by the addition of 50 μL of 8 N sulfuric acid and the absorbance was measured at 490 nm.
Urea assay
Urea concentration in the media was measured using a Stanbio Urea BUN assay kit with the protocol provided by the manufacturer. Briefly, Urea assay reagent was prepared by mixing two parts of the acid reagent and one part of the color reagent (150 μL/well; ∼15 mL per 96 wells). To 10 μL of media or standards, 150 μL of the urea reagent mix was added and incubated at 60°C for 90 min. At the end of incubation, the plate was allowed to cool to room temperature (5–10 min) and absorbance was measured at 520 nm.
CYP 450 (EROD) assay
Cytochrome P450 1A1 activity of the cocultures was evaluated using 7-ethoxyresorufin. Samples were induced with 3-methylcholantherene (3-MC; 2 μM) for 48 h before the assay in 2× daily media (800 μL). Control samples were incubated with vehicle (dimethyl sulfoxide) under similar conditions. Media in both control and induced samples were not changed for 48 h. At the end of the incubation, wells were rinsed with EBSS at least three times to remove any Phenol red from the media and collagen gel. To each of the wells, 500 μL of the substrate mixture (10 μM 7-ethoxyresorufin+80 μM Dicumarol in EBSS) was added and incubated at 37°C. Hundred microliters of the reagent was withdrawn at 5, 10, 15, and 25 min intervals. Fluorescence from the collected sample was measured at λex=525±10 nm and λem=580±10 nm. The rate of resorufin production was calculated by preparing resorufin standards in EBSS.
Endothelial cell staining
Endothelial cells were identified by staining for surface expression of SE-1. Briefly, cells were washed with fresh media and incubated with a solution of 1:1000 dilution of hepatic sinusoidal endothelial cell antibody (SE-1) and NucBlue stain for 1 h at 37°C. Samples were washed in the live cell imaging solution and images were obtained using a fluorescent microscope.
Live/Dead assay
The Live/Dead assay was performed on the samples using a commercial kit from Life Technologies as per the manufacturer's protocol. Briefly, to 10 mL of media, 5 μL of Calcein AM reagent, 20 μL Ethidium Homodimer, and 10 drops of NucBlue stain were added. Five hundred microliters of this reagent was added to the sample and incubated at 37°C, 10% CO2 for 15 min. Media in the sample were replaced with Live Cell Imaging Solution and imaged on a microscope.
Results
Creation of hepatocyte–LSEC sandwich cocultures
The coculture configurations in this work require hepatocytes and LSECs from two different isolations and cultured within collagen gel layers (see “Materials and Methods” section). Cell isolation protocols were established for repeated isolation of hepatocytes and LSECs with high purity and viability. Hepatocytes from subsequent isolations were obtained with high viability (>85%) and had similar secretion functions (Supplementary Figs S1 and S2; Supplementary Data are available online at www.liebertpub.com/tec) and >90% of the LSECs expressed SE-1 (Supplementary Fig. S3). In a standard 12-well plate, a 50% surface area coverage was achieved by seeding 0.25 million hepatocytes and 1 million LSECs and this ratio was maintained in all the culture configurations. The surface coverage of these cells is chosen based on the cell size, wherein LSECs have much smaller size when compared with hepatocytes.
Morphology and viability of hepatocytes and LSECs in various configurations
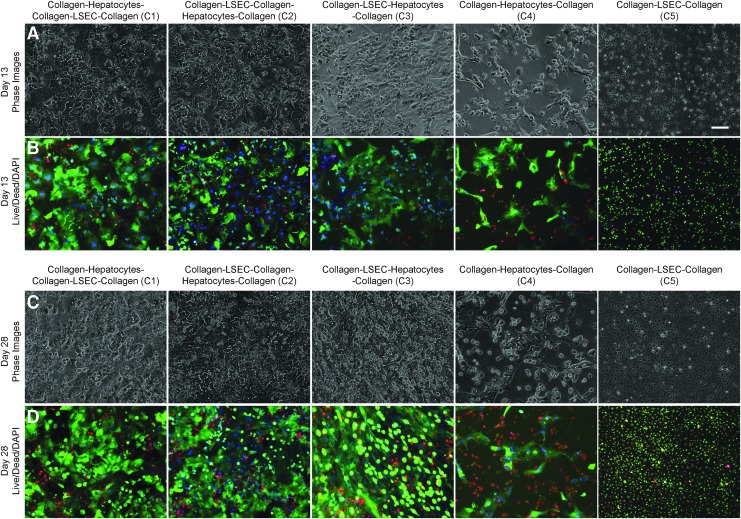
Phase contrast microscopy was used to identify the morphological differences of cells in various coculture configurations within sandwich cultures up to 4 weeks. Staining using Calcein AM (fluorescent green), Ethidium Homodimer-1 (fluorescent red) and 4′,6-diamidino-2-phenylindole (fluorescent blue) was used to assess cell viability. Fluorescence images of the cells in various configurations at Days 13, and 28 are shown in Figure 2, and Days 7 and 20 are shown in Supplementary Figure S4. In case of C3, C4, and C5, all the cells were within the same plane (in a sandwich), whereas in the case of C1 and C2, hepatocytes and LSECs were in two different layers, in close proximity. In configuration C3, hepatocytes show typical cuboidal morphology with LSECs occupying the remaining space; with high viability at 13 and 28 days of culture. In the other configurations C1 and C2, where the cells were originally separated with a collagen gel layer in between, cells eventually appeared to reorganize within the layer to form a contiguous layer of cells. Similar to C3, hepatocytes in the configurations C1 and C2 maintained their morphology as seen in phase contrast images of the cocultures. However, in the control monoculture (C4), hepatocytes seem to show deteriorated morphology and viability by 4 weeks. This could be due to subconfluent seeding of hepatocytes in the control monoculture, and the hepatocyte seeding was kept the same in all the culture configurations (C1–C4). In addition, LSECs cultured alone within the collagen sandwich (C5) retained morphology and show high viability over the 4 week period (Fig. 2 and Supplementary Figs S4 and S6).
FIG. 2.
Phase contrast and Live/Dead microscopy images of coculture configurations on Days 13 (A, B) and 28 (C, D) of culture. C1, C2, and C3 show stable hepatocyte morphology with high viability up to 4 weeks. C4 shows loss of hepatocyte morphology and viability when compared with coculture configurations. C5 (LSEC) show good cell morphology and viability up to 4 weeks. Scale bar is 100 μm.
Expression of SE-1 marker in various configurations
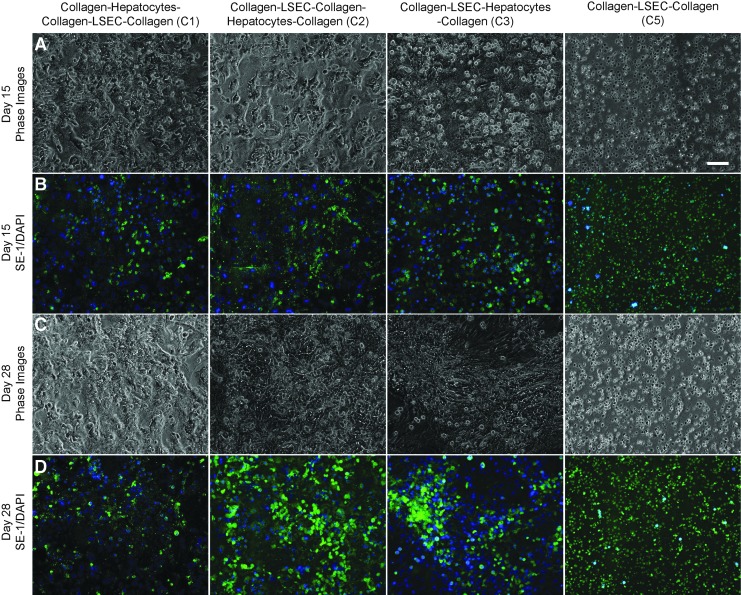
A major premise of this study is the preservation of LSECs phenotype in coculture with hepatocytes. To evaluate the maintenance of LSEC phenotype, the configurations C1, C2, and C3, and the LSEC only control (C5) were stained with SE-1 at regular intervals for up to 4 weeks. LSECs cultured as the bottom most layer (C2) and as a mixture with hepatocytes (C3) maintained expression of SE-1 up to 4 weeks, whereas configuration C1 showed a decrease (Fig. 3 and Supplementary Figs S5, S7–S9). These results indicate that the LSECs maintain their phenotype in the case of C2 and C3 configurations, whereas the phenotypical expression in C1 is lost rapidly (Fig. 3 and Supplementary Figs S5 and S7). Interestingly, SE-1 expression is retained by LSECs cultured alone in the collagen sandwich (C5), with high viability (Fig. 2 and Supplementary Fig. S5) over 4 weeks.
FIG. 3.
Phase contrast and sinusoidal endothelial 1 (SE-1) expression of LSECs within cocultures on Days 15 (A, B) and 28 (C, D) of culture. C1 showed decrease in SE-1 expression whereas C2 and C3 show high SE-1 expression up to 4 weeks. C5 (LSECs only) shows similar levels of SE-1 expression. Scale bar is 100 μm.
Hepatocyte function (albumin and urea) comparison in various coculture configurations
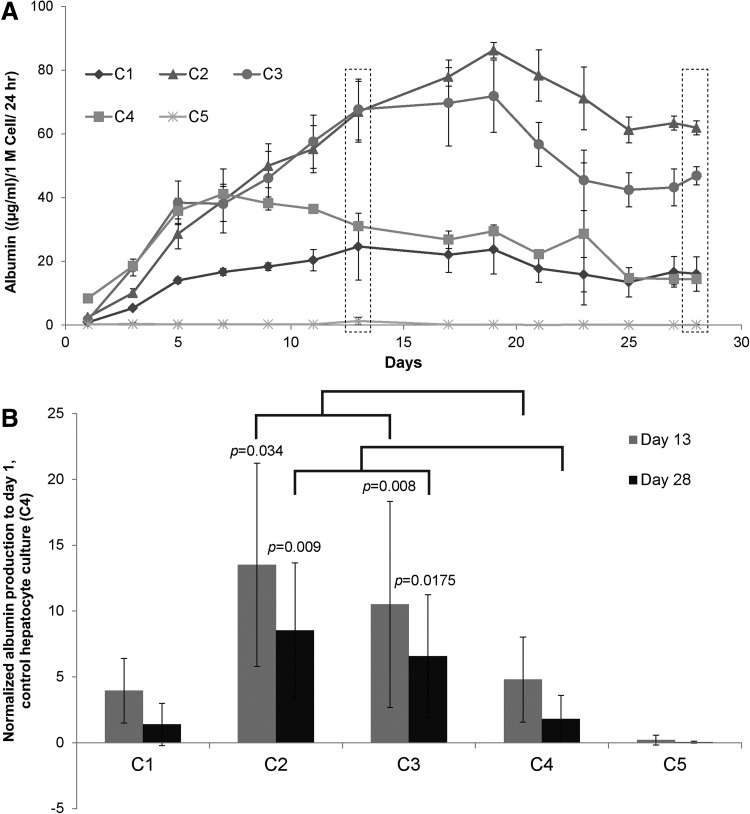
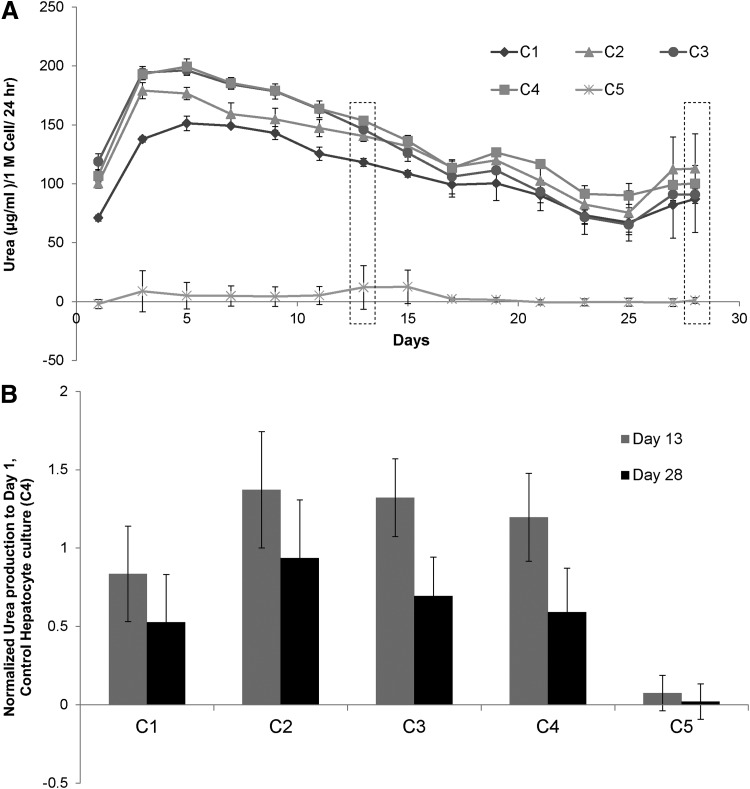
Urea and albumin production were monitored to determine whether LSECs stabilized the hepatocytes and influence their function in various configurations. Normalized albumin and urea secretions of primary hepatocytes in various culture configurations, over a period of 4 weeks, are shown in Figures 4 and 5, respectively. Our results indicate that hepatocytes have higher albumin secretion in C2 and C3 configurations whereas C1 has similar albumin secretion when compared with the control hepatocyte culture (C4). Albumin secretion by hepatocytes in C2 and C3 increased up to Day 9 and remained stable for up to 4 weeks. To compare results from different experiments, data in each experiment were normalized to secretion of albumin from Day 1 of control hepatocyte culture (C4). Figure 4B shows the fold increase in the production of albumin in various coculture configurations when compared with control hepatocytes from n=3 experiments, which includes hepatocytes and LSECs from six isolations. Results are expressed as mean±standard deviation and statistical significance was analyzed by Student's t-test. Differences were considered significant at p<0.05 and are shown in the figure accordingly. Figure 4A is a representative time course of albumin production, whereas Figure 4B is the averaged data from multiple experiments normalized to control hepatocyte culture. While hepatocytes in control (C4) and configuration C1 show similar albumin secretion (4–5 times), hepatocytes in C2 and C3 configurations show a higher albumin secretion (10–13 times) after 2 weeks. In contrast to albumin, the urea secretion levels were similar across different culture configurations (Fig. 5A, B).
FIG. 4.
Albumin secretion by hepatocytes cultured in various coculture configurations. (A) Time course albumin secretion of hepatocytes cultured in cocultures. C2 and C3 show higher albumin secretion, whereas C1 shows similar amount of albumin secretion as control hepatocyte cultures (C4). (B) Albumin secretion of various coculture configurations normalized to albumin secreted by control (C4) at day 1 from n=3 experiments. Statistical analysis is done with respective control values (C4) on days 13 and 28.
FIG. 5.
Urea synthesis by hepatocytes cultured in various coculture configurations. (A) Time course urea synthesis of hepatocytes cultured in cocultures in different configurations. All the cultures with hepatocytes show similar urea secretion. (B) Urea secretion of various coculture configurations normalized to urea secreted by control (C4) at day 1 from n=3 experiments. Urea secretions by hepatocytes were similar in various coculture configurations.
Cytochrome P 450 1A activity comparison in various configurations
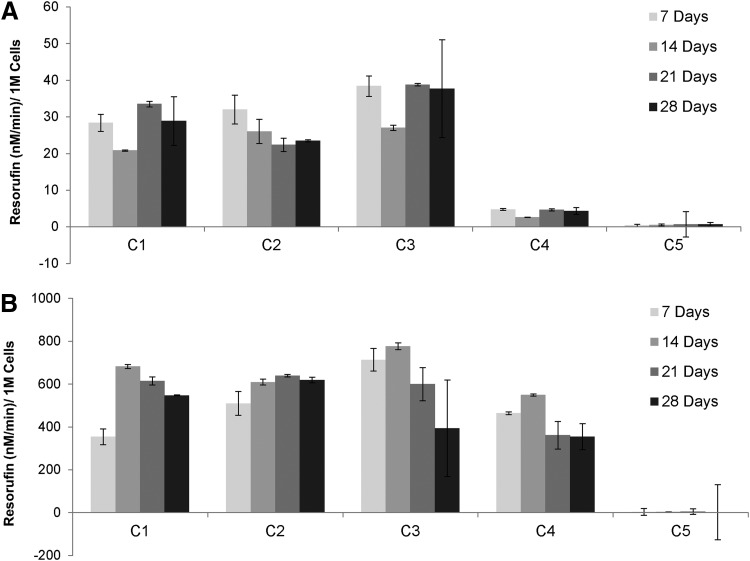
CYP450 activity is a critical function of hepatocytes and is an essential indicator of metabolism of toxins, drugs, and pharmaceuticals.2,52,53 CYP450 activity of the culture configurations was monitored at weekly intervals up to 4 weeks (Fig. 6). CYP1A activity of the hepatocytes in these configurations was measured under native and induced 3-MC (2 μM) conditions. Hepatocytes in configurations C1, C2, and C3 showed 7–10 times higher CYP1A activity when compared with control hepatocyte cultures under native (noninduced) conditions while LSECs have negligible CYP activity (Fig. 6A). The higher activity of the coculture configurations was sustained over a period of 4 weeks, when compared with hepatocyte only monocultures. In contrast to the native activity, under induced conditions (incubation with 2 μM 3-MC), hepatocytes in the coculture configurations C1, C2, and C3 showed similar activity when compared with the hepatocyte only control (C4).
FIG. 6.
CYP 1A1 (EROD) activity of hepatocytes in coculture configurations. (A) Coculture configurations C1, C2, and C3 show high native CYP activity when compared with hepatocyte only (C4) control cultures. (B) Induced cocultures C1, C2, and C3 show similar levels of activity when compared with control hepatocyte cultures (C4).
Discussion
To identify the culture configuration that can enable long-term stable coculture of hepatocytes and LSECs, LSECs were introduced into a collagen gel sandwich configuration along with hepatocytes. Since collagen gel sandwich is already known to promote long-term maintenance of hepatocytes, it could be an ideal culture configuration for incorporating LSECs along with hepatocytes.11,50 The cocultures of hepatocytes and LSECs within a collagen sandwich layer are prepared by culturing LSECs above, below, and with hepatocytes in a collagen gel sandwich. Specifically, LSECs were combined with hepatocytes in multilayer configurations, collagen–hepatocyte–collagen–LSEC–collagen (C1), collagen–LSEC–collagen–hepatocyte–collagen (C2), and collagen–(hepatocyte+LSEC)–collagen (C3). As single cell controls, collagen–hepatocyte–collagen (C4) and collagen–LSEC–collagen (C5) were used (Fig. 1; “Materials and Methods” section). While the coculture configurations (C1 and C2) enable cell–cell communication by secreted factors (there is an intervening collagen gel layer to prevent cell–cell contact), C3 allowed for direct cell–cell contact of hepatocytes and LSECs within the same layer. These configurations enable coculture in an environment which allows for (1) cell–cell contact (C3) and (2) cell–cell interaction through secreted factors (C1, C2, and C3) leading to enhanced stability and function.
The results from the coculture experiments suggest that interactions between hepatocytes and LSECs led to (1) stabilization of hepatocytes and retention of LSEC phenotype, (2) LSECs stabilization of hepatocytes, leading to a higher albumin production, (3) higher CYP activity of hepatocytes in coculture with LSECs, and (4) maintenance of stable function for up to 4 weeks. Whereas the configuration C1 shows similar albumin production as the control hepatocyte culture (C4), and a decreased SE-1 expression of LSECs. LSECs within the coculture C1 have either lost their SE-1 expression (Fig. 3 and Supplementary Figs S5 and S7) or did not survive leading to a configuration similar to C4, and with an additional layer of collagen gel on top. In case of C2 and C3, hepatocytes are viable (Fig. 2 and Supplementary Fig. S4) and there is retention of SE-1 expression of LSECs (Fig. 3 and Supplementary Figs S5, S8, and S9), which indicates that secretion factors from both LSECs and hepatocytes are interacting in a mutually beneficial way. An interesting observation is the viable culture of LSECs and retention of SE-1 expression as single cell cultures (C5) (Fig. 3 and Supplementary Fig. S5). Our results indicate that in the coculture configurations C2 and C3, both hepatocytes and LSECs remain viable and maintain their phenotype–which indicates a stable configuration enabled by heterotypic cell interactions. Despite LSECs maintaining their phenotype in the collagen double gel monocultures (C5), in the culture configuration C1, they show loss in expression of SE-1 (Fig. 3 and Supplementary Fig. S7). Further, the entrapment of LSECs within the collagen gel leads to the stabilization of LSECs, and maintain their phenotype with high viability (Figs. 2 and 3). In the case of configurations C2 and C3, LSECs remain viable and maintain their phenotype (Fig. 3 and Supplementary Figs S8 and S9) and thus are able to secrete factors which enhance hepatocyte function. This is consistent with previous reports where other cell types are known to enhance albumin function of hepatocytes by a number of mechanisms, including direct cell–cell contact and/or soluble factors.15,48 The configuration C1 shows lower albumin secretion when compared with C3 and C4. This could be due to the loss of LSECs in C1 and perhaps due to the presence of two layers of collagen gel on top of hepatocytes, adversely affecting the albumin function. Despite these differences in albumin secretion of coculture C1 from C2 and C3, the native CYP 1A1 activity was observed to be similar over the 4 week period. Further, the native CYP 1A1 activity of hepatocytes in C1 was higher than the control monoculture (C4).
In this study, all four configurations result in long-term culture of hepatocytes. The urea function is similar in all four configurations suggesting that cues provided by LSECs do not influence urea secretion. In the case of CYP function, the induced CYP activity is similar for all four configurations suggesting that 3-MC mediated induction of CYP activity is the dominant mechanism, which most likely masks any potential effect of LSECs. By contrast, LSECs enhanced native CYP activity of hepatocytes in all the coculture configurations (C1–C3). The albumin function across different culture configuration is more complicated. LSECs do enhance albumin secretion (C2 and C3) of hepatocytes. Although in the case of C1 configuration, the presence of two layers of collagen on top of hepatocytes most likely detrimentally affected albumin function. Elucidation of the exact mechanism through which C1 configuration reduces albumin function and not urea and CYP activity will require further study. It is plausible that the albumin function of hepatocytes is more sensitive to the ECM environment (single vs. two layers of collagen on top) as compared to other functions. In fact in a prior study,48 proline mediated ECM remodeling greatly influenced albumin function of hepatocytes whereas urea and CYP function were not affected.
Conclusions
In summary, our results indicate that coculture of hepatocytes with primary LSECs in a collagen gel sandwich with a 50% surface coverage retains the LSEC phenotype while enhancing hepatocyte function. Hepatocytes cultured with LSECs in the same plane or with hepatocytes cultured on top of LSECs show higher albumin function when compared with hepatocyte alone for up to 4 weeks. Further, hepatocyte coculture with LSECs showed an increased native CYP activity when compared with pure hepatocyte cultures suggesting enhanced cell–cell interactions within the cocultures. Hepatocyte activities within the matrix and coculture are controlled by the organized network, secretion factors, and cell–cell interactions with LSECs in the in vitro model. This model provides a simple method to recreate a matrix and geometry and an in vivo microenvironment of the liver. We envision that the described methods to coculture hepatocytes with LSECs provides a convenient platform for engineering hepatocyte cocultures with NPCs for drug screening applications and generation of in vitro liver models.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health NIH-5UH2TR000503, NIH-K99DK095984 for A.B., NIH-F32DK098905 for W.J.M., and the Shriners Hospital Postdoctoral Fellowship number 84202 for B.U.
Disclosure Statement
No competing financial interests exist.
References
- 1.Guyton A.C., Hall J.E.Textbook of Medical Physiology. Philadelphia, PA: Elsevier, 2006. [Google Scholar]
- 2.Reed S.Essential Physiological Biochemistry: An Organ-Based Approach. Oxford: John Wiley & Sons, Ltd., 2009. [Google Scholar]
- 3.Boyer T.D., et al. . Zakim and Boyer's Hepatology: A Textbook of Liver Disease. 5th Ed. Philadelphia, PA [Edinburgh]: Elsevier Saunders, 2006, 2 v (xv, pp. 1516) [Google Scholar]
- 4.Guillouzo A.Liver cell models in in vitro toxicology. Environ Health Perspect 106Suppl 2,511, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zguris J.C., et al. . Microreactor microfluidic systems with human microsomes and hepatocytes for use in metabolite studies. Biomed Microdevices 7,117, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt R., et al. . New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev 35,213, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hewitt N.J., et al. . Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev 39,159, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bjornsson E.Drug-induced liver injury: Hy's rule revisited. Clin Pharmacol Ther 79,521, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bale S.S., et al. . In vitro platforms for evaluating liver toxicity. Exp Biol Med 239,1180, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soldatow V.Y., et al. . In vitro models for liver toxicity testing. Toxicol Res 2,23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn J.C., Tompkins R.G., and Yarmush M.L.Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Progr 7,237, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan P., et al. . Polyelectrolyte nano-scaffolds for the design of layered cellular architectures. Tissue Eng 12,1553, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Dunn J.C., Tompkins R.G., and Yarmush M.L.Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol 116,1043, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia S.N., Yarmush M.L., and Toner M.Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J Biomed Mater Res 34,189, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Bhatia S.N., et al. . Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 13,1883, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Khetani S.R., and Bhatia S.N.Microscale culture of human liver cells for drug development. Nat Biotechnol 26,120, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Khetani S.R., et al. . Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci 132,107, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Guillouzo A., et al. . Use of hepatocyte cultures for the study of hepatotoxic compounds. J Hepatol 26Suppl 2,73, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Nahmias Y., Berthiaume F., and Yarmush M.L.Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol 103,309, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Goulet F., Normand C., and Morin O.Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology 8,1010, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Morin O., Goulet F., and Normand C.Liver sinusoidal endothelial cells: isolation, purification, characterization and interaction with hepatocytes. Revis Biol Celular 15,1, 1988 [PubMed] [Google Scholar]
- 22.Martinez-Hernandez A., and Amenta P.S.The extracellular matrix in hepatic regeneration. FASEB J: official publication of the Federation of American Societies for Experimental Biology 9,1401, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Laskin D.L.Sinusoidal lining cells and hepatotoxicity. Toxicol Pathol 24,112, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Laskin D.L.Nonparenchymal cells and hepatotoxicity. Semin Liver Dis 10,293, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Arias I.M.The Liver, Arias I.M., ed. West Sussex: John Wiley & Sons Ltd., 2009. [Google Scholar]
- 26.McCuskey R.S.The hepatic microvascular system in health and its response to toxicants. Anat Rec (Hoboken, NJ: 2007) 291,661, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hinson J.A., Roberts D.W., and James L.P.Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196,369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeschke H., et al. . Mechanisms of hepatotoxicity. Toxicol Sci 65,166, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Arias I., et al. The Liver: Biology and Pathology. Philadelphia, PA: Lippincott Williams and Wilkins, 2001 [Google Scholar]
- 30.Fraser R., Dobbs B.R., and Rogers G.W.Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology 21,863, 1995 [PubMed] [Google Scholar]
- 31.McCuskey R.S.Sinusoidal endothelial cells as an early target for hepatic toxicants. Clin Hemorheol Microcirc 34,5, 2006 [PubMed] [Google Scholar]
- 32.Smedsrod B.Clearance function of scavenger endothelial cells. Comp Hepatol 3Suppl 1,S22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smedsrod B., et al. . Cell biology of liver endothelial and Kupffer cells. Gut 35,1509, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieder H., Meyer zum Buschenfelde K.H., and Ramadori G.Functional spectrum of sinusoidal endothelial liver cells: filtration, endocytosis, synthetic capacities and intercellular communication. J Hepatol 15,237, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Badmann A., et al. . TRAIL enhances paracetamol-induced liver sinusoidal endothelial cell death in a Bim- and Bid-dependent manner. Cell Death Dis 3,e447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt M.P., Yin H., and Ju C.Exacerbation of acetaminophen-induced disturbances of liver sinusoidal endothelial cells in the absence of Kupffer cells in mice. Toxicol Lett 194,34, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Kim Y., and Rajagopalan P.3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PLoS One 5,e15456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwa A.J., et al. . Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J: official publication of the Federation of American Societies for Experimental Biology 21,2564, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Hang T.C., et al. . Lipids promote survival, proliferation, and maintenance of differentiation of rat liver sinusoidal endothelial cells in vitro. Am J Physiol Gastrointest Liver Physiol 302,G375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bader A., et al. . 3-D coculture of hepatic sinusoidal cells with primary hepatocytes-design of an organotypical model. Exp Cell Res 226,223, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Kostadinova R., et al. . A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol 268,1, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Domansky K., et al. . Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10,51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLeve L.D., et al. . Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol 287,G757, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Elvevold K., Smedsrod B., and Martinez I.The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol 294,G391, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Friedman S.L.Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88,125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatia S.N., et al. . Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog 14,378, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Jindal R., Patel S.J., and Yarmush M.L.Tissue-engineered model for real-time monitoring of liver inflammation. Tissue Eng Part C Methods 17,113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jindal R., et al. . Amino acid-mediated heterotypic interaction governs performance of a hepatic tissue model. FASEB J 23,2288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seglen P.O.Preparation of isolated rat liver cells. Methods Cell Biol 13,29, 1976 [DOI] [PubMed] [Google Scholar]
- 50.Dunn J., et al. . Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J 3,174, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Smedsrod B., et al. . Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res 241,639, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Roberts R.A., et al. . Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci 96,2, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Rodes J., et al. eds. Textbook of Hepatology. Malden, MA: Blackwell Publishing, 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.