Abstract
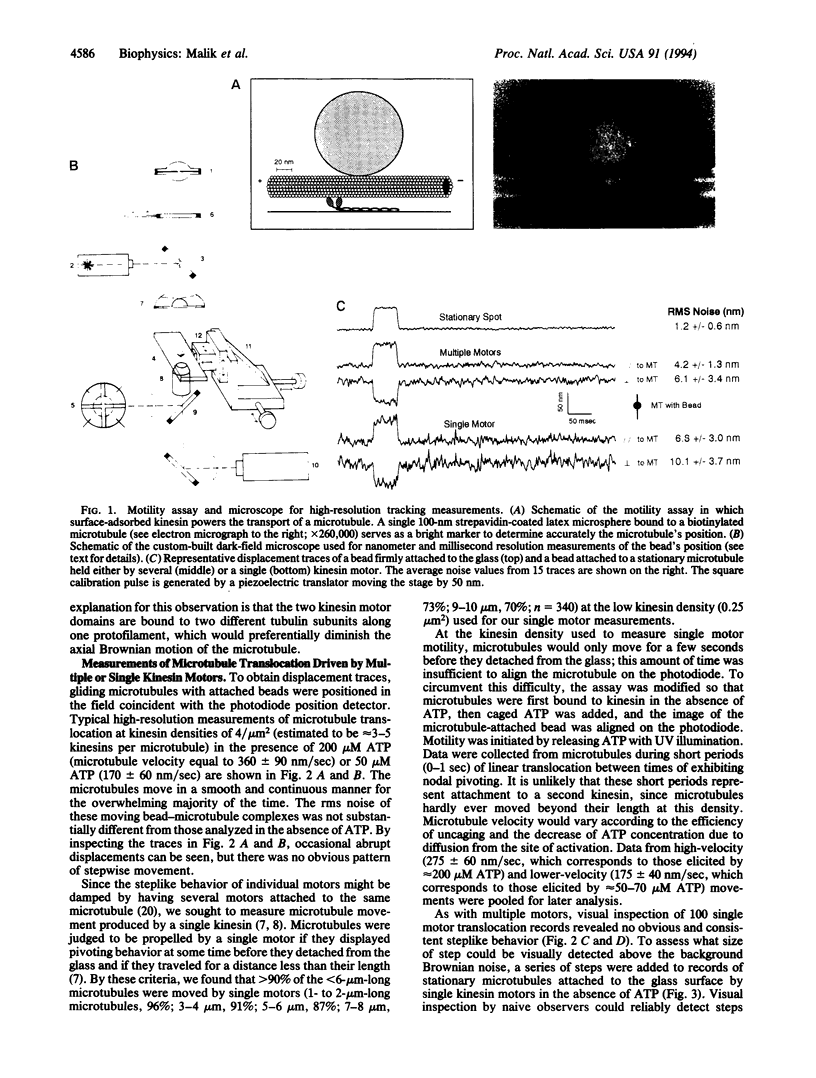
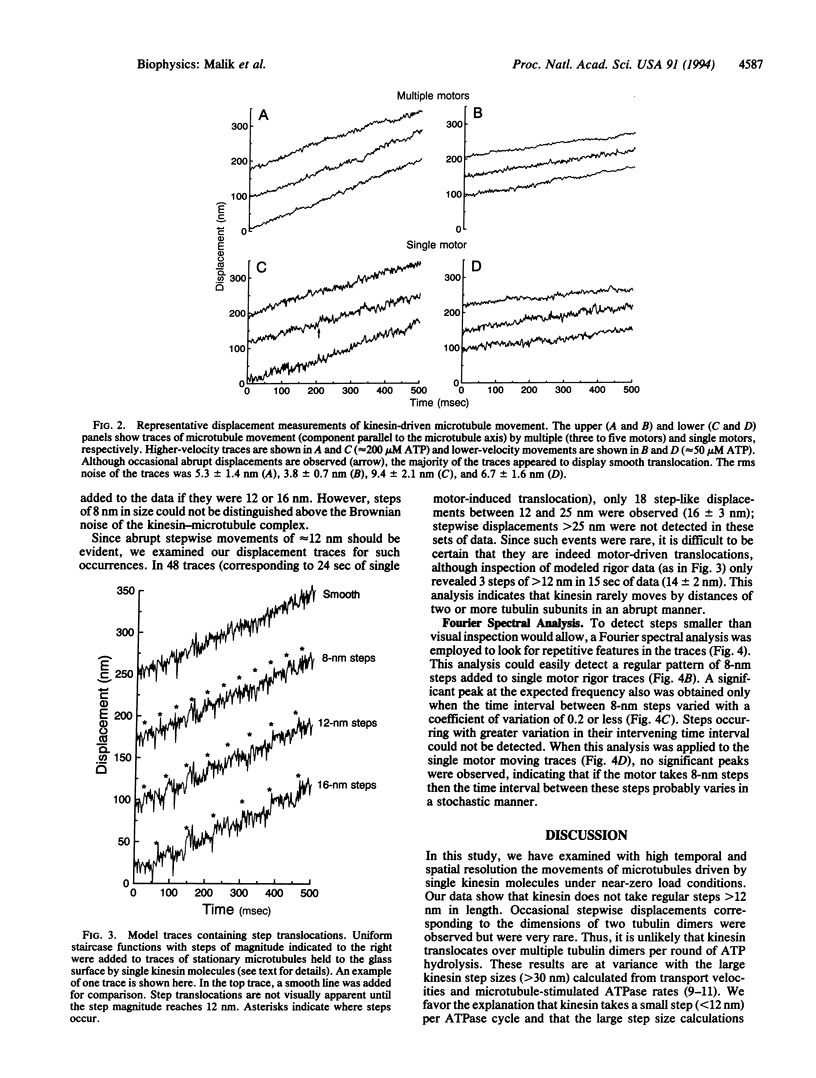
Kinesin is a microtubule-based motor protein that contains two identical force-generating subunits. The kinesin binding sites along the microtubule lie 8 nm apart (the dimension of the tubulin dimer), which implies that kinesin must translocate a minimum distance of 8 nm per hydrolysis cycle. Measurements of kinesin's microtubule-stimulated ATPase activity (approximately 20 ATP per sec) and velocity of transport (approximately 0.6 micron/sec), however, suggest that the net distance moved per ATP (approximately 30 nm) may be greater than one tubulin dimer under zero load conditions. To explore how kinesin translocates during its ATPase cycle, we constructed a microscope capable of tracking movement with 1-nm resolution at a bandwidth of 200 Hz and used this device to examine microtubule movement driven by a single kinesin motor. Regular stepwise movements were not observed in displacement traces of moving microtubules, although Brownian forces acting on elastic elements within the kinesin motor precluded detection of steps that were < 12 nm. Though individual steps of approximately 16 nm were occasionally observed, their infrequent occurrence suggests that kinesin rarely moves abruptly by distances of two or more tubulin subunits during its ATP hydrolysis cycle. Instead it is more likely that kinesin moves forward by the distance of only a single tubulin subunit under zero load conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Gelles J., Schnapp B. J., Sheetz M. P. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988 Feb 4;331(6155):450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- Gittes F., Mickey B., Nettleton J., Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993 Feb;120(4):923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. Kinesin ATPase: rate-limiting ADP release. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6314–6318. doi: 10.1073/pnas.85.17.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B. C., Marchese-Ragona S. P., Gilbert S. P., Cheng N., Steven A. C., Johnson K. A. Decoration of the microtubule surface by one kinesin head per tubulin heterodimer. Nature. 1993 Mar 4;362(6415):73–75. doi: 10.1038/362073a0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J., Vale R. D. Movement of microtubules by single kinesin molecules. Nature. 1989 Nov 9;342(6246):154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Hyman A., Drechsel D., Kellogg D., Salser S., Sawin K., Steffen P., Wordeman L., Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Kamimura S., Mandelkow E. Tubulin protofilaments and kinesin-dependent motility. J Cell Biol. 1992 Aug;118(4):865–875. doi: 10.1083/jcb.118.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E., Cooke R. Simulation of stochastic processes in motile crossbridge systems. J Muscle Res Cell Motil. 1991 Aug;12(4):376–393. doi: 10.1007/BF01738593. [DOI] [PubMed] [Google Scholar]
- Ray S., Meyhöfer E., Milligan R. A., Howard J. Kinesin follows the microtubule's protofilament axis. J Cell Biol. 1993 Jun;121(5):1083–1093. doi: 10.1083/jcb.121.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg L., Vale R. D. Chemomechanical cycle of kinesin differs from that of myosin. Nature. 1993 Jan 14;361(6408):168–170. doi: 10.1038/361168a0. [DOI] [PubMed] [Google Scholar]
- Sadhu A., Taylor E. W. A kinetic study of the kinesin ATPase. J Biol Chem. 1992 Jun 5;267(16):11352–11359. [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Furusawa K., Ohashi S., Toyoshima Y. Y., Okuno M., Malik F., Vale R. D. Nucleotide specificity of the enzymatic and motile activities of dynein, kinesin, and heavy meromyosin. J Cell Biol. 1991 Mar;112(6):1189–1197. doi: 10.1083/jcb.112.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D. A., Scholey J. M. Cytoplasmic microtubule-based motor proteins. Curr Opin Cell Biol. 1993 Feb;5(1):95–104. doi: 10.1016/s0955-0674(05)80014-6. [DOI] [PubMed] [Google Scholar]
- Song Y. H., Mandelkow E. Recombinant kinesin motor domain binds to beta-tubulin and decorates microtubules with a B surface lattice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1671–1675. doi: 10.1073/pnas.90.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A. Optical trapping: Motor molecules in motion. Nature. 1990 Nov 22;348(6299):284–285. doi: 10.1038/348284a0. [DOI] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Schnapp B. J., Block S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature. 1993 Oct 21;365(6448):721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Shpetner H. S. Motor proteins of cytoplasmic microtubules. Annu Rev Biochem. 1990;59:909–932. doi: 10.1146/annurev.bi.59.070190.004401. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]



