Abstract
Importance
Daily bathing of critically ill patients with the broad spectrum, topical antimicrobial agent chlorhexidine is widely performed and may reduce healthcare-associated infections.
Objective
To determine if daily bathing of critically ill patients with chlorhexidine decreases the incidence of healthcare-associated infections.
Design, setting, and participants
A pragmatic cluster-randomized, cross-over study of 9,340 patients admitted to five adult intensive care units of a tertiary medical center in Nashville, Tennessee
Intervention
Units performed once-daily bathing of all patients with disposable cloths impregnated with 2% chlorhexidine or non-antimicrobial cloths as a control. Bathing treatments were performed for a 10-week period followed by a two-week washout period during which patients were bathed with non-antimicrobial disposable cloths, before crossover to the alternate bathing treatment for 10 weeks. Each unit crossed over between bathing assignments three times during the study
Main Outcome and Measures
The primary prespecified outcome was a composite of central line-associated blood stream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), ventilator-associated pneumonia (VAP), and Clostridium difficile infections. Secondary outcomes included rates of clinical cultures positive for multi-drug resistant organisms, blood culture contamination, healthcare-associated bloodstream infections, and rates of the primary outcome by ICU.
Results
A total of 55 and 60 infections occurred during chlorhexidine and control bathing periods, respectively (4 and 4 CLABSI, 21 and 32 CAUTI, 17 and 8 VAP, 13 and 16 C. difficile infections, respectively, between chlorhexidine and control bathing periods). The primary outcome rate was 2.86 per 1000 patient-days and 2.90 per 1000 patient-days during chlorhexidine and control bathing periods, respectively (rate difference, −0.04; 95% CI, −1.09 to 1.01; P=0.95). After adjusting for baseline variables, no difference between groups in the rate of the primary outcome was detected. Chlorhexidine bathing did not change rates of infection-related secondary outcomes including hospital-acquired bloodstream infections, blood culture contamination, or clinical cultures yielding multi-drug resistant organisms. In a prespecified subgroup analysis, no difference in the primary outcome was detected in any individual ICU.
Conclusion and Relevance
In this pragmatic trial, daily bathing with chlorhexidine did not reduce the incidence of healthcare-associated infections including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, or C. difficile. These findings do not support daily bathing of critically ill patients with chlorhexidine.
Trial Registration
ClinicalTrials.gov number, NCT02033187
Keywords: [3–10] Chlorhexidine, Bathing, Intensive care unit, Infection control, Ventilator associated event, Catheter associated urinary tract infection, Central line associated blood stream infection, Clostridium difficile, Blood stream infection
INTRODUCTION
Infections acquired during hospitalization (healthcare-associated infections) are associated with increased hospital length of stay, rates of death, and increased costs1–3. Substantial effort is devoted to preventing healthcare-associated infections through practices designed to reduce the transmission of nosocomial pathogens, such as hand hygiene, bundles for insertion and care of devices, and isolation of patients with multi-drug resistant organisms (MDROs)4,5.
The skin of hospitalized patients is a reservoir for pathogens and invasion by skin flora is thought to be a mechanism contributing to healthcare-associated infections6. Chlorhexidine is a broad-spectrum topical antimicrobial agent that, when used to bathe the skin, may decrease the bacterial burden thereby reducing infections. Several observational and quasi-experimental studies have found that daily bathing with chlorhexidine results in decreased skin colonization with MDROs, decreased rates of bloodstream infections, and reduced Clostridium difficile infections (CDI) (reviewed in7). A recent multicenter cluster-randomized trial demonstrated that bathing patients with chlorhexidine reduced MDRO acquisition and hospital-acquired bloodstream infections (HA-BSI)8, and chlorhexidine bathing is incorporated into some expert guidelines9. These results, however, have not been replicated and the effect of chlorhexidine bathing on other infections is unclear. Furthermore, chlorhexidine increases costs and unnecessary exposure may result in the development of chlorhexidine resistance10,11. Therefore, we conducted a cluster-randomized trial to evaluate the effect of chlorhexidine bathing on the rates of multiple healthcare-associated infections among critically ill adults.
METHODS
Study Design
We performed a pragmatic cluster-randomized, crossover, controlled study involving patients admitted to five adult intensive care units at a tertiary care medical center between July 2012 and July 2013. The neurological, surgical, and trauma units contain 34, 34, and 31 ICU and step down beds, respectively, and the cardiovascular and medical units contain 27 and 34 ICU beds. Each unit is staffed by critical care nurses and nurse practitioners with 24-hour physician coverage. Units performed once-daily bathing of all patients with cloths impregnated with 2% chlorhexidine (2% Chlorhexidine Gluconate Cloths, Sage Products, Cary, IL) or with disposable non-antimicrobial cloths (Comfort Bath, Sage Products, Cary, IL) as a control. Due to differences in the scent and appearance of the cloths, blinding of patients, treating physicians, nurses, and unit staff was not possible. Infection control personnel responsible for adjudicating infection outcomes according to standardized definitions were blinded to the treatment assignments. Each unit was randomized to a bathing sequence by generating five numbers from one-two at random using software available at www.randomizer.org. Each number in the sequence corresponded to one of the five ICUs. Those assigned a one began with chlorhexidine bathing and those assigned a two began with control bathing. Bathing assignment alternated thereafter. Bathing treatments were performed for a 10-week period followed by a two-week washout period during which patients were bathed with non-antimicrobial disposable cloths, before crossover to the alternate bathing treatment for 10 weeks. Each unit crossed over between bathing assignments three times during the study (Figure 1).
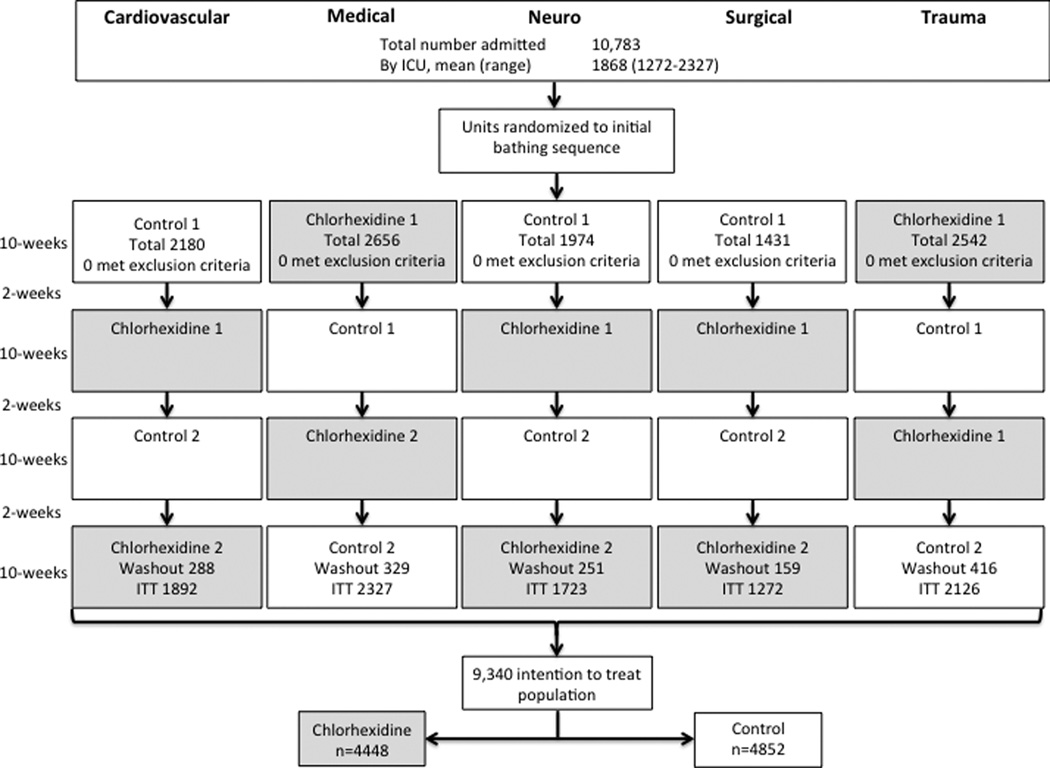
Figure 1. Recruitment, Randomization, and Patient Flow.
A total of 10,783 patients were admitted to the participating ICUs during the study period. Each ICU was randomized to an initial bathing treatment for a 10-week period followed by a two week washout prior to crossover into the alternate bathing treatment. Each unit crossed between treatments three times during the study period. Therefore, each unit received two non-sequential 10-week periods of chlorhexidine bathing alternating with two non-sequential 10-week periods of control bathing. The 1443 patients admitted during washout periods were excluded from the analysis per protocol. The number of patients admitted during each bathing period is shown.
Bathing was performed once daily according to the manufacturer’s instructions with sequential cloths used to rinse all body surfaces. Patients that became soiled after the initial daily bath were allowed to be bathed a second time in that day with bathing cloths maintaining the randomization. The face was not bathed to avoid exposure of the mucous membranes to chlorhexidine. The cardiovascular ICU used chlorhexidine cloths for a single, preoperative bathing of patients undergoing cardiac surgery regardless of the unit treatment assignment at the time. However, routine daily bathing of patients was performed according to the study bathing assignment. All other units were supplied only with the assigned cloths and the alternate cloths were not available during each bathing period. Prior to the study, two units were using daily chlorhexidine bathing in routine care and three were not. Before the study began, nurses on each unit were instructed to use only the available cloths and were reminded of proper bathing technique. All other infection control and cleaning procedures, including the use of contact precautions for patients colonized or infected with multi-drug resistant organisms, were performed according to the usual practice of each unit throughout the study period. Active surveillance for multi-drug resistant organism colonization was not done.
All patients admitted to the cardiovascular, medical, neurological, surgical, and trauma ICUs during the study period were included. Patients were excluded if they were known to have an allergy to chlorhexidine, were admitted with burns or toxic epidermal necrolysis/Stevens-Johnson syndrome, or if the treating physician felt bathing would be unsafe. Patients admitted during a washout period were excluded from the primary analysis.
The chlorhexidine impregnated and non-antimicrobial cloths were purchased from Sage Products (Cary, IL) who had no input into study design, implementation, or data analysis. The study was approved by the Vanderbilt University Institutional Review Board with waiver of consent.
This study was conceived as an institutional quality improvement project, and underwent IRB review as is our practice with approval of the study design, endpoints, and analysis plan on May 7, 2012. As is characteristic of some quality improvement efforts, this trial was not registered with clinicaltrials.gov at that time. After patient enrollment was completed but before any data analyses were conducted we realized the novel design and size of this study might be of interest to others and registered the study at clinicaltrials.gov on January 8, 2014. The study endpoints are concordant between the IRB-approved protocol, a detailed statistical analysis plan dated November 26, 2013, those specified in the trial registration, and the results reported in this manuscript. Healthcare-associated bloodstream infections were added as a secondary endpoint because they became available electronically during the course of the study. The complete data set was available to investigators for analysis on February 4, 2014. No data analyses were conducted during the study or prior to trial registration.
Study Outcomes and Definitions
Since individual healthcare-associated infections are rare events, the analysis plan specified a composite primary outcome including central line-associated blood stream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), possible or probable ventilator-associated pneumonia (VAP), or Clostridium difficile infection. Infections were determined using Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) definitions by trained infection control personnel, who were blinded to the bathing assignment12. Secondary outcomes included the rates of each individual infection included in the primary outcome, in-hospital mortality, hospital and ICU length of stay, rates of clinical cultures positive for multi-drug resistant organisms (number of positive cultures per 1000 patient-days), blood culture contamination (number of contaminants per 1000 patient-days), healthcare-associated bloodstream infections (HA-BSI), and rates of the primary outcome by ICU. Additional definitions of infection-related outcomes are available in the online supplement.
Statistical Analysis
The study was conducted over one year. The approximately 10,000 patients expected to be admitted to the participating ICUs based on the previous year’s admissions would provide at least 95% power to detect a change in the primary outcome of 0.1 infections per 1000 patient-days. Using an intention-to-treat-design, each patient was analyzed according to the bathing assignment of the unit at the time of admission regardless of length of stay or the number of days they were bathed. Patients whose hospital stay bridged a crossover event, and therefore changed bathing treatment, were analyzed according to their initial bathing assignment. The primary analysis was a comparison of the infection rate (number of infections per 1000 patient-days) between groups using a Poisson regression model. All events meeting an outcome definition were included. Therefore repeated infections from an individual patient were included as events in the analysis. Five, 24, 23, and 34 patients contributed multiple events to the primary outcome, clinical cultures positive for multi-drug resistant organisms, healthcare-associated bloodstream infections, and blood culture contamination, respectively.
Pre-specified secondary analyses included tests for a chlorhexidine effect for each individual infection comprising the primary outcome, differences in hospital and ICU length of stay as well as rates of healthcare-associated bloodstream infections, blood culture contamination, and cultures positive for multi-drug resistant organisms using a Mann-Whitney U test or Poisson model where appropriate. Adjusted estimates of chlorhexidine effect were obtained using a logistic and Poisson model. Covariates included age, sex, race (white, non-white, or unknown), admission ICU, study time, University HealthSystem Consortium expected mortality (UHC, Chicago IL)13, comorbid conditions, and admission WBC, along with bathing assignment. Race was collected from an administrative database based on patient self-reporting. Effectiveness of chlorhexidine was also assessed by comparing the primary outcome occurrence rate within each ICU using Poisson regression. Sensitivity analyses were performed including an analysis where patients receiving both bathing treatments were excluded, an as-treated analysis to account for a study protocol violation, and a group-level analysis performed on the unit clusters as opposed to analyses of individual patients. A logistic regression model with the same covariates and primary predictors of treatment assignment described above including an interaction term for treatment assignment and infection status was used to estimate the effect of chlorhexidine on the outcome of in-hospital mortality as well as its interaction with our primary outcome. All tests were two-tailed with a significance threshold of P < 0.05. The statistical analysis was performed with R (version 2.10.1, www.r-project.org, the R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics (version 22, Armonk, NY).
RESULTS
Enrollment and Patient Characteristics
A total of 10,783 patients were admitted to the five participating ICUs during the study period (Figure 1). None met exclusion criteria. The 1,443 patients admitted during washout periods were excluded from the analysis per protocol. Therefore, 9,340 patients were included in the primary analysis with 4,488 patients in the chlorhexidine bathing periods and 4,852 patients in the control bathing periods. Baseline patient characteristics were balanced between the control and intervention periods with regard to age, gender, race, comorbid conditions, and baseline laboratory data (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics.
| Control (n=4852) |
Chlorhexidine (n=4488) |
P value | |
|---|---|---|---|
| Age in years, median (25th–75th)a | 57.0 (42–68) | 56.0 (42–68) | 0.82 |
| Male sex, no. (%)b | 2805 (57.8) | 2586 (57.6) | 0.85 |
| Race, no. (%)b | 0.16 | ||
| White | 4045 (83.4) | 3668 (81.7) | |
| Black | 592 (12.2) | 593 (13.2) | |
| Other | 62 (1.3) | 58 (1.3) | |
| Unknown | 153 (3.2) | 169 (3.8) | |
| Admission ICU, no. (%)b | 0.37 | ||
| Medical | 1215 (25.0) | 1112 (22.9) | |
| Trauma | 1072 (22.1) | 1054 (21.7) | |
| Cardiovascular | 986 (20.3) | 906 (18.7) | |
| Neurological | 925 (19.1) | 798 (16.5) | |
| Surgical | 654 (13.5) | 618 (12.7) | |
| Baseline laboratory data | |||
| Creatinine mg/dL, median (25th–75th)a | 0.98 (0.78–1.34) | 0.98 (0.78–1.32) | 0.96 |
| Hemoglobin gm/dL, mean (SD)a | 12.09 (2.45) | 12.08 (2.45) | 0.92 |
| WBC ×1000/ml, median (25th–75th)a | 10.8 (7.80–15.30) | 10.8 (7.70–15.00) | 0.18 |
| Serum lactate mmol/L, median (25th–75th)a | 1.10 (0.80–1.90) | 1.10 (0.70–1.90) | 0.53 |
| Expected mortality (%),median (25th–75th)a | 1.39 (0.40–6.42) | 1.39 (0.38–6.14) | 0.049 |
| Comorbidities, no. (%) | |||
| Respiratory diseaseb | 3633 (74.9) | 3447 (76.8) | 0.030 |
| Cardiovascular diseaseb | 3669 (75.6) | 3328 (74.2) | 0.10 |
| Renal diseaseb | 1338 (27.6) | 1242 (27.7) | 0.92 |
| Diabetes mellitusb | 1273 (26.3) | 1176 (26.2) | 0.97 |
| Malignancyb | 1005 (20.7) | 950 (21.2) | 0.59 |
SD, standard deviation; mg, milligrams; dL, deciliter; gm, gram; mmol, millimoles; WBC, white blood cell count; expected mortality, University HealthSystem Consortium expected mortality (Chicago, IL);
p-value derived using Mann-Whitney U test;
p-value derived using uncorrected Pearson’s chi-square test; missing data, UHC expected mortality (n=156), lactate (n=5669), hemoglobin (n=151), creatinine (n=108)
Primary Outcome
A total of 55 and 60 infections occurred during chlorhexidine and control bathing periods, respectively (4 and 4 CLABSI, 32 and 21 CAUTI, 8 and 17 VAP, 16 and 13 C. difficile infections, respectively, between control and chlorhexidine bathing periods). The rate of the primary outcome was 2.86 per 1000 patient-days during chlorhexidine bathing and 2.90 per 1000 patient-days during control bathing (rate difference, −0.04; 95% CI, −1.10 to 1.01; P=0.95). After adjusting for age, sex, race, unit of admission, time, comorbid conditions, and admission WBC, no significant difference between groups in the rate of the primary outcome was detected (adjusted risk ratio in treatment group, 0.94; 95% CI, 0.65 to 1.37; P=0.83) (Table 2, Figure 2). Five patients developed more than one infection included in the primary outcome during the study (three during chlorhexidine and two during control bathing).
Table 2.
Primary and Secondary Outcomes.
| Intention to treat | Control | Chlorhexidine |
Difference (CI) |
P value |
||
| No. | 4852 | 4488 | ||||
| Patient-days | 20720.5 | 19201.5 | ||||
| Infections per 1000 patient-days |
Rate (CI) | No. events / no. patients |
Rate (CI) | No. events / no. patients |
||
| Composite primary outcomea | 2.90 (2.16–3.63) | 60/58 | 2.86 (2.11–3.62) | 55/52 | −0.04 (−1.10–1.01) | 0.95 |
| CLABSIa | 0.19 (0.004–0.38) | 4/4 | 0.21 (0.004–0.41) | 4/4 | 0.02 (−0.26–0.30) | 0.91 |
| CAUTIa | 1.54 (1.01–2.08) | 32/31 | 1.09 (0.63–1.56) | 20/21 | −0.45 (−1.16–0.26) | 0.22 |
| C. difficilea | 0.77 (0.39–1.15) | 16/16 | 0.68 (0.31–1.05) | 13/13 | −0.09 (−0.62–0.44) | 0.72 |
| VAPa | 0.39 (0.12–0.65) | 8/8 | 0.89 (0.46–1.31) | 17/17 | 0.5 (0.0013–0.999) | 0.053 |
| HA-BSIa | 5.45 (4.45–6.46) | 113/95 | 5.0 (4.0–6.0) | 96/80 | −0.45 (−1.87–0.97) | 0.53 |
| Blood culture contaminationa | 5.45 (4.45–6.46) | 113/96 | 4.84 (3.86–5.83) | 93/73 | −0.61(−2.02–0.80) | 0.40 |
| Clinical cultures positive for MDROsa | 5.41 (4.40–6.41) | 112/85 | 4.84 (3.86–5.83) | 93/79 | −0.57 (−1.97–0.83) | 0.43 |
| ICU LOS (days)b | 2.39 (1.21–4.95) | 2.56 (1.24–5.09) | 0.169 (−0.01–0.321) | 0.12 | ||
| Hospital LOS (days)b | 5.0 (2.0–9.0) | 5.0 (2.0–9.0) | 0 (0–0) | 0.38 | ||
| In-hospital mortality (%)c | 449 (9.25) | 449 | 367 (8.18) | 367 | −1.07 (−2.2–0.07) | 0.066 |
| In-hospital mortality adjustedd | 0.32 | |||||
| As treated | Control | Chlorhexidine |
Difference (CI) |
P value |
||
| No. | 5091 | 4253 | ||||
| Patient-days | 21507.5 | 18464.4 | ||||
| Infections per 1000 patient-days |
Rate (CI) | No. events / no. patients |
Rate (CI) | No. events / no. patients |
||
| Composite primary outcomea | 2.84 (2.12–3.55) | 61/59 | 2.98 (2.19–3.77) | 55/52 | 0.14 (−0.92–1.20) | 0.79 |
| CLABSIa | 0.19 (0.004–0.37) | 4/4 | 0.22 (0.004–0.43) | 4/4 | 0.03 (−0.25–0.31) | 0.83 |
| CAUTIa | 1.53 (1.01–2.06) | 33/32 | 1.14 (0.65–1.62) | 21/20 | −0.39 (−1.11–0.33) | 0.28 |
| C. difficilea | 0.74 (0.38–1.11) | 16/16 | 0.70 (0.32–1.09) | 13/13 | −0.04 (−0.57–0.49) | 0.88 |
| VAPa | 0.37 (0.11–0.63) | 8/8 | 0.92 (0.48–1.36) | 17/17 | 0.55 (0.05–1.05) | 0.035 |
| HA-BSIa | 5.35 (4.37–6.32) | 115/97 | 4.93 (3.92–5.94) | 91/76 | −0.42 (−1.83–0.99) | 0.56 |
| Blood culture contaminationa | 5.25 (4.29–6.22) | 113/96 | 4.82 (3.82–5.82) | 89/70 | −0.43 (−1.82–0.96) | 0.54 |
| Clinical cultures positive for MDROs | 5.35 (4.37–6.32) | 115/88 | 5.03 (4.01–6.06) | 93/79 | −0.31 (−1.72–1.10) | 0.67 |
| ICU LOS (days)a | 2.36 (1.20–4.89) | 2.61 (1.28–5.22) | 0.247 (.102 – 0.394) | 0.0043 | ||
| Hospital LOS (days)b | 5.0 (2.0–9.0) | 5.0 (2.0–9.0) | 0 (0 – 0) | 0.92 | ||
| In-hospital mortality (%)c | 474 (9.31) | 474 | 346 (8.14) | 346 | −1.17 (−2.3–−0.03) | 0.046 |
| In-hospital mortality adjustedd | 0.051 | |||||
CI, confidence interval; CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, probable and possible ventilator-associated pneumonia; HA-BSI, healthcare-associated bloodstream infection; blood culture contamination expressed as number of contaminated blood cultures per 1000 patient-days; MDROs, multi-drug resistant organisms expressed as clinical cultures positive for MDROs per 1000 patient-days; LOS, length of stay expressed as mean (CI);
p value derived using Poisson regression;
p-value derived using Mann-Whitney U test;
p-value derived using uncorrected Pearson’s Chi-square test,
P value calculated after adjusting for UHC expected mortality in logistic regression model
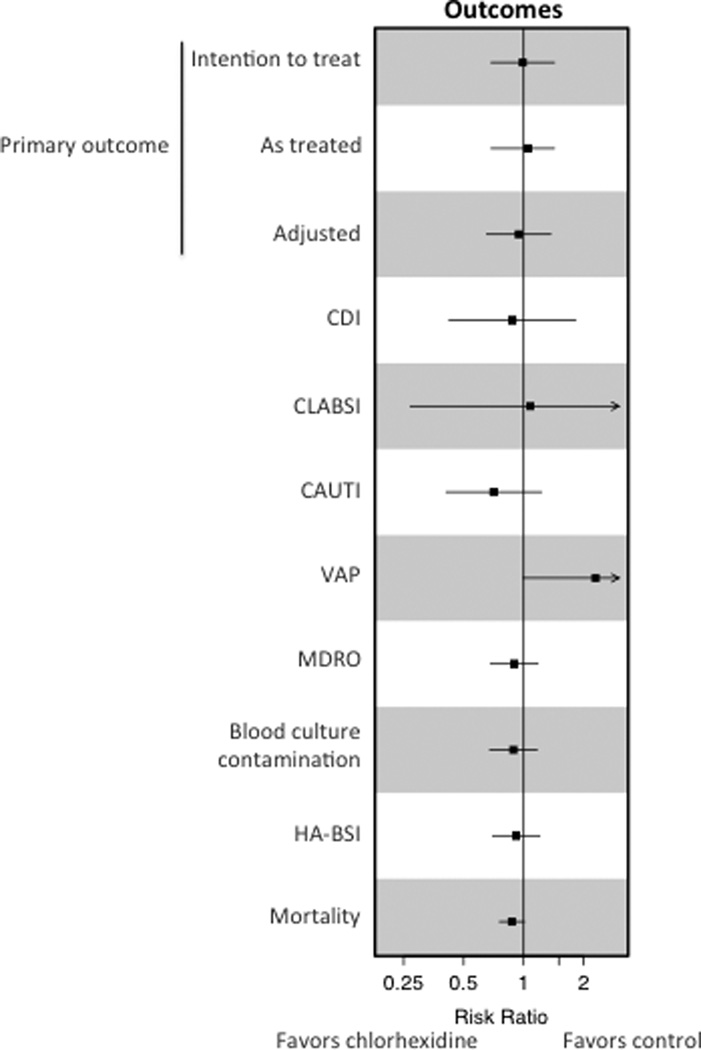
Figure 2. Effect of Chlorhexidine Bathing on Outcomes.
The chlorhexidine effect on intention to treat, as-treated, and adjusted analyses of the primary outcome of the composite rate of CLABSI, CAUTI, VAP, and C. difficile are shown. Intention to treat analyses of secondary outcomes are shown. Boxes indicate the risk ratios with horizontal bars representing confidence intervals. The vertical line depicts a risk ratio of one. CDI, Clostridium difficile infection; CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, probable and possible ventilator-associated pneumonia; HA-BSI, healthcare-associated bloodstream infection; MDROs, multi-drug resistant organisms.
Secondary Outcomes
No significant difference in the rate of healthcare-associated bloodstream infections was seen between the chlorhexidine and control periods (5.00 and 5.45, respectively; rate difference, −0.45; 95% CI, −1.87 to 0.97; P=0.53)(Table 2, Figure 2). In addition, no significant differences in the rates of blood culture contamination (4.84 per 1000 patient-days and 5.45 per 1000 patient-days; rate difference, −0.61; 95% CI, −2.02 to 0.80; P=0.40) or clinical cultures positive for multi-drug resistant organisms (4.84 and 5.41 per 1000 patient-days; rate difference, −0.57; 95% CI, −1.97 to 0.83; P=0.43) were found between the chlorhexidine and control periods, respectively (Tables 2 and S3, Figure 2). When analyzed independently, the individual infections comprising the primary outcome were not significantly different between intervention and control bathing periods and no difference in ICU or hospital length of stay was observed (Table 2). In-hospital mortality was 8.18% in the chlorhexidine bathing periods and 9.25% in the control periods (difference in percent, −1.07%; 95% CI, −2.22% to 0.07%; P=0.066).
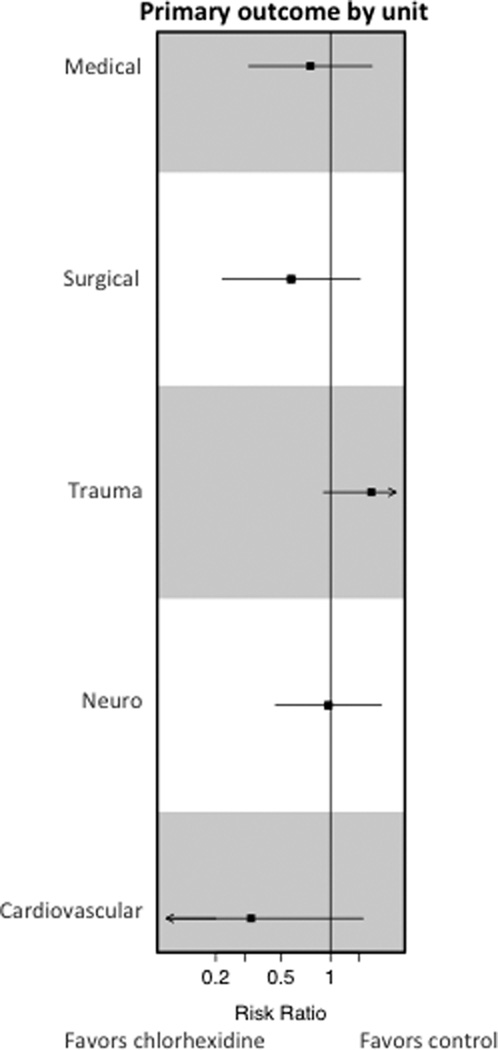
In a pre-specified subgroup analysis by ICU, no difference in the rate of the primary outcome was detected in any individual ICU in the chlorhexidine bathing and control periods (Table 3, Figure 3). A significant reduction in blood culture contamination (2.37 and 8.25 per 1000 patient-days during chlorhexidine and control periods, respectively; rate difference, −5.88; 95% CI, −9.41 to −2.35; P=0.0031) was detected in the cardiovascular ICU during periods of chlorhexidine bathing without a significant reduction in healthcare-associated bloodstream infections (2.71 and 4.42 per 1000 patient-days during chlorhexidine and control periods, respectively; rate difference, −1.71; 95% CI, −4.63 to 1.21; P=0.26). The rates of healthcare-associated bloodstream infections, blood culture contamination, or clinical cultures positive for multi-drug resistant organisms did not differ between intervention and control periods in any other unit. Although infection-related outcomes did not differ, the trauma ICU had a significant reduction in in-hospital mortality during periods of chlorhexidine bathing (6.17% versus 8.58%; difference in percent, −2.41%; 95% CI, −4.64% to −0.19%; P=0.033). After adjusting for the UHC expected mortality rate the adjusted OR was 0.85, 95% CI 0.51–1.39, P=0.51).
Table 3.
Primary and Secondary Outcomes for Individual ICUs.
| Cardiovascular | ||||||
| Infections per 1000 patient-days |
Control |
No. events / no. patients |
CHG |
No. events / no. patients |
CI Rate Diff |
P value |
| No. | 986 | 906 | ||||
| Patient-days | 3392.3 | 2954.8 | ||||
| Primary outcomea | 2.06 (0.53 – 3.59) | 7/6 | 0.68 (0 – 1.61) | 2/2 | −1.38 (−3.17 – 0.41) | 0.16 |
| CLABSIa | 0.59 (0 – 1.41) | 2/2 | 0.34 (0 – 1.00) | 1/1 | −0.25 (−1.30, 0.80) | 0.65 |
| CAUTIa | 1.18 (0.02 – 2.33) | 4/4 | 0 (NA) | 0/0 | −1.18 (−2.34 – −0.024) | NA |
| C. difficilea | 0 (NA) | 0/0 | 0.34 (0 – 1.00) | 1/1 | 0.34 (−0.32 – 1.00) | NA |
| VAPa | 0.29 (0 – 0.87) | 1/1 | 0 (NA) | 0/0 | −0.29 (−0.87 – 0.29) | NA |
| HA-BSIa | 4.42 (2.18 – 6.66) | 15/12 | 2.71 (0.83 – 4.58) | 8/7 | −1.71 (−4.63 – 1.21) | 0.26 |
| Blood culture contaminationa | 8.25 (5.20 – 11.31) | 28/21 | 2.37 (0.61 – 4.12) | 7/5 | −5.88 (−9.41, −2.35) | 0.0031 |
| Clinical cultures positive for MDROsa | 3.24 (1.33 – 5.16) | 11/9 | 1.69 (0.21 – 3.18) | 5/4 | −1.55 (−3.97 – 0.87) | 0.23 |
| In-hospital mortality N(%)b | 81 (8.22) | 81 | 57 (6.29) | 57 | −1.93 (−4.36 – 0.41) | 0.11 |
| In-hospital mortality adjustedc | 0.87 | |||||
| Medical | ||||||
| Infections per 1000 patient-days |
Control |
No. events / no. patients |
CHG |
No. events / no. patients |
CI Rate Diff |
P value |
| No. | 1215 | 1112 | ||||
| Patient-days | 4575.5 | 4544.8 | ||||
| Primary outcomea | 2.62 (1.14 – 4.11) | 12/12 | 1.98 (0.69 – 3.27) | 9/9 | −0.64 (−2.61 – 1.33) | 0.52 |
| CLABSIa | 0.22 (0 – 0.65) | 1/1 | 0 (NA) | 0/0 | −0.22 (−0.64 – 0.21) | NA |
| CAUTIa | 0.87 (0.02 – 1.73) | 4/4 | 1.32 (0.26 – 2.38) | 6/6 | 0.45 (−0.91 – 1.81) | 0.52 |
| C. difficilea | 1.31 (0.26 – 2.36) | 6/6 | 0.44 (0 – 1.05) | 2/2 | −0.87 (−2.08 – 0.34) | 0.18 |
| VAPa | 0.22 (0 – 0.65) | 1/1 | 0.22 (0 – 0.65) | 1/1 | 0 (−0.61 – 0.61) | 1 |
| HA-BSIa | 8.31 (5.66 – 10.95) | 38/31 | 5.72 (3.52 – 7.92) | 26/20 | −2.59 (−6.03 – 0.85) | 0.14 |
| Blood culture contaminationa | 10.71 (7.71 – 13.71) | 49/41 | 9.02 (6.26 – 11.78) | 41/31 | −1.69 (−5.77 – 2.39) | 0.42 |
| Clinical cultures positive for MDROsa | 7.43 (4.93 – 9.93) | 34/28 | 7.48 (4.97 – 10.00) | 34/31 | 0.05 (−3.49 – 3.59) | 0.98 |
| In-hospital mortality N(%)b | 186 (15.31) | 186 | 159 (14.3) | 159 | −1.01 (−3.90 – 1.88) | 0.49 |
| In-hospital mortality adjustedc | 0.33 | |||||
| Neurological | ||||||
| Infections per 1000 patient-days |
Control |
No. events / no. patients |
CHG |
No. events / no. patients |
CI Rate Diff |
P value |
| No. | 925 | 798 | ||||
| Patient-days | 4622.8 | 4123.6 | ||||
| Primary outcomea | 3.24 (1.60 – 4.89) | 15/14 | 3.15 (1.44 – 4.87) | 13/11 | −0.09 (−2.46 – 2.28) | 0.94 |
| CLABSIa | 0 (NA) | 0/0 | 0 (NA) | 0/0 | 0 (NA) | NA |
| CAUTIa | 3.24 (1.60 – 4.89) | 15/14 | 2.18 (0.76 – 3.61) | 9/8 | −1.06 (−3.23 – 1.11) | 0.35 |
| C. difficilea | 0 (NA) | 0/0 | 0.97 (0.02 – 1.92) | 4/4 | 0.97 (0.02 – 1.92) | NA |
| VAPa | 0 (NA) | 0/0 | 0 (NA) | 0/0 | 0 (NA) | NA |
| HA-BSIa | 6.06 (3.81 – 8.30) | 28/24 | 5.82 (3.49 – 8.15) | 24/18 | −0.24 (−3.47 –2.99) | 0.89 |
| Blood culture contaminationa | 4.11 (2.26 – 5.96) | 19/17 | 5.82 (3.49 –8.15) | 24/21 | 1.71 (−1.26 – 4.68) | 0.26 |
| Clinical cultures positive for MDROsa | 5.84 (3.64 – 8.04) | 27/19 | 3.40 (1.62 – 5.17) | 14/14 | −2.44 (−5.27 – 0.39) | 0.1 |
| In-hospital mortality N(%)b | 61 (6.59) | 61 | 54 (6.77) | 54 | 0.18 (−2.20 – 2.54) | 0.89 |
| In-hospital mortality adjustedc | 0.67 | |||||
| Surgical | ||||||
| Infections per 1000 patient-days |
Control |
No. events / no. patients |
CHG |
No. events / no. patients |
CI Rate Diff |
P value |
| No. | 654 | 618 | ||||
| Patient-days | 4343.0 | 3479.1 | ||||
| Primary outcomea | 2.99 (1.37 – 4.62) | 13/13 | 1.72 (0.34 – 3.10) | 6/6 | −1.27 (−3.40 – 0.86) | 0.26 |
| CLABSIa | 0.23 (0 – 0.68) | 1/1 | 0 (NA) | 0/0 | −0.23 (−0.68 – 0.22) | NA |
| CAUTIa | 0.69 (0 – 1.47) | 3/3 | 0.57 (0 – 1.37) | 2/2 | −0.12 (−1.24 – 1.00) | 0.84 |
| C. difficilea | 2.07 (0.72 – 3.43) | 9/9 | 0.29 (0 – 0.85) | 1/1 | −1.78 (−3.25 – −0.31) | 0.061 |
| VAPa | 0 (NA) | 0/0 | 0.86 (0 – 1.84) | 3/3 | 0.86 (−0.12 – 1.84) | NA |
| HA-BSIa | 4.61 (2.59 – 6.62) | 20/18 | 3.45 (1.50 – 5.40) | 12/12 | −1.16 (−3.97 – 1.65) | 0.43 |
| Blood culture contaminationa | 1.38 (0.28 – 2.49) | 6/6 | 2.30 (0.71 – 3.89) | 8/6 | 0.92 (−1.02 – 2.86) | 0.35 |
| Clinical cultures positive for MDROsa | 6.45 (4.06 – 8.84) | 28/17 | 6.32 (3.68 – 8.97) | 22/17 | −0.13 (−3.69 – 3.43) | 0.95 |
| In-hospital mortality N(%)b | 29 (4.43) | 29 | 32 (5.18) | 32 | 0.75 (−1.61 – 3.10) | 0.54 |
| In-hospital mortality adjustedc | 0.78 | |||||
| Trauma | ||||||
| Infections per 1000 patient-days |
Control |
No. events / no. patients |
CHG |
No. events / no. patients |
CI Rate Diff |
P value |
| No. | 1072 | 1054 | ||||
| Patient-days | 3787.0 | 4099.1 | ||||
| Primary outcomea | 3.43 (1.57 – 5.30) | 13/13 | 6.10 (3.71 – 8.49) | 25/24 | 2.67 (−0.36 – 5.70) | 0.093 |
| CLABSIa | 0 (NA) | 0/0 | 0.73 (0 – 1.56) | 3/3 | 0.73 (−0.10 – 1.56) | NA |
| CAUTIa | 1.58 (0.32 – 2.85) | 6/6 | 0.98 (0.02 – 1.93) | 4/4 | −0.6 (−2.19 – 0.99) | 0.45 |
| C. difficilea | 0.26 (0 – 0.78) | 1/1 | 1.22 (0.15 – 2.29) | 5/5 | 0.96 (−0.23 – 2.15) | 0.16 |
| VAPa | 1.58 (0.32 – 2.85) | 6/6 | 3.17 (1.45 – 4.90) | 13/13 | 1.56 (−0.58 – 3.70) | 0.16 |
| HA-BSIa | 3.17 (1.38 – 4.96) | 12/10 | 6.34 (3.90 – 8.78) | 26/23 | 3.17 (0.14 – 6.20) | 0.047 |
| Blood culture contaminationa | 2.90 (1.19 – 4.62) | 11/11 | 3.17 (1.45 – 4.90) | 13/10 | 0.27 (−2.16 – 2.70) | 0.83 |
| Clinical cultures positive for MDROsa | 3.17 (1.38 – 4.96) | 12/12 | 4.39 (2.36 – 6.42) | 18/15 | 1.22 (−1.49 – 3.93) | 0.38 |
| In-hospital mortality N(%)b | 92 (8.58) | 92 | 65 (6.17) | 65 | −2.41 (−4.64 – −0.19) | 0.03 |
| In-hospital mortality adjustedc | 0.51 | |||||
CHG, chlorhexidine; CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, probable and possible ventilator-associated pneumonia; HA-BSI, healthcare-associated bloodstream infection; blood culture contamination expressed as number of contaminated blood cultures per 1000 patient-days; MDROs, multi-drug resistant organisms expressed as clinical cultures positive for MDROs per 1000 patient-days.
p-value derived using Poisson regression,
p-value derived using uncorrected Pearson’s Chi-square test,
P value calculated after adjusting for UHC expected mortality in logistic regression model, p-values are not adjusted for multiple comparisons
Figure 3. Effect of Chlorhexidine Bathing by ICU.
The chlorhexidine effect on the primary outcome of the composite rate of CLABSI, CAUTI, VAP, and C. difficile in a prespecified subgroup of the intention to treat analysis by ICU is shown. Boxes indicate the risk ratios with horizontal bars representing confidence intervals. The vertical line depicts a risk ratio of one.
Three post-hoc analyses were performed; (i) an as-treated analysis to address a protocol violation in the cardiovascular ICU where 235 patients bathed with the incorrect cloths were analyzed according to the bathing treatment they received rather than the bathing treatment they were assigned (Table 2), (ii) an analysis where the 658 patients whose hospital stay spanned a crossover event and therefore received both bathing treatments were excluded (Table S1), and (iii) a group-level analysis performed on the unit clusters as opposed to the analyses of individual patients (Table S2). In each of these analyses, no difference between groups was detected for the primary outcome, healthcare-associated bloodstream infections, blood culture contamination, or clinical cultures positive for multi-drug resistant organisms. When the infections comprising the primary outcome were analyzed individually, a statistically significant increase in possible or probable VAP was detected during periods of chlorhexidine bathing in all post-hoc analyses (as-treated: 0.37 and 0.92 per 1000 patient-days in chlorhexidine and control bathing periods, respectively, rate difference 0.55, CI 0.05 to 1.05, P=0.035; analysis excluding patients that received both bathing treatments: 0.24 and 0.84 per 1000 patient days in chlorhexidine and control bathing periods, respectively, rate difference 0.6, CI 0.09 to 1.11, P=0.03; group-level analysis performed on the unit clusters: 0.41 and 0.95 per 1000 patient days in chlorhexidine and control bathing periods, respectively, rate difference 0.54, CI 0.02 to 1.06, P=0.047) (Tables 2, S1, and S2).
A non-significant reduction in-hospital mortality was present during chlorhexidine bathing periods in the primary intention to treat analysis (9.25% versus 8.18% during control and chlorhexidine bathing periods, respectively, rate difference −1.07, CI −2.2 to 0.07, P=0.066). In-hospital mortality was significantly reduced during chlorhexidine bathing periods in two post-hoc analyses (as-treated analysis, 8.14% and 9.31% in chlorhexidine and control periods, rate difference −1.17, CI −2.3 to −0.03, P=0.046; analysis excluding patients that received both bathing treatments, 7.99% and 9.24% in the chlorhexidine and control periods, CI −1.25 −0.02 to 0.001, P=0.040, Tables 2 and S1). This reduction in in-hospital mortality was not present after adjusting for baseline variables (As-treated analysis adjusted P=0.051, analysis excluding patients that received both bathing treatments adjusted P=0.31) (Tables S4, S5, and S6).
DISCUSSION
In this single center, multi-ICU, cluster-randomized, crossover study, once-daily bathing with chlorhexidine did not reduce the rate of the composite primary outcome of infections including CLABSI, CAUTI, possible or probable VAP, or infection with C. difficile. Other infection-related secondary outcomes, including healthcare-associated bloodstream infections, blood culture contamination, and clinical cultures positive for multi-drug resistant organisms were also not improved by chlorhexidine. Chlorhexidine bathing is widely practiced in an effort to reduce healthcare-associated infections and has been incorporated into some expert guidelines9. Yet chlorhexidine use incurs a cost and exposure to chlorhexidine may increase microbial resistance10,11. Therefore, the finding that chlorhexidine bathing did not reduce infections in this study suggests that such bathing may not be necessary, resulting in cost saving and avoiding unnecessary exposure without adversely affecting clinical outcome.
In contrast to the findings of the current study, Climo et al. performed a multi-center, cluster-randomized, crossover trial of daily chlorhexidine bathing in 7727 patients admitted to 9 intensive care or bone marrow units and reported a significant reduction in MRSA and VRE (MDRO) acquisition, healthcare-associated bloodstream infections, and CLABSI with chlorhexidine bathing8. These studies differ in several ways. The duration of the chlorhexidine bathing intervention in the Climo study was 24 weeks compared to 10 weeks in the current study and it is possible that a longer intervention may have ecological consequences that reduce infectious outcomes. Climo et al. performed active surveillance for MRSA and VRE colonization, and included bone marrow transplant units, neither of which were done in this study. Since bone marrow transplantation places patients at high risk of infection, this may have altered outcomes. In addition, some of the infection rates were low in this study and a lower limit to the rates of infection may exist beyond which chlorhexidine bathing no longer provides detectable benefit. The reduction in healthcare-associated bloodstream infections in the Climo study was driven primarily by a reduction in positive blood cultures caused by the skin commensal coagulase-negative staphylococci and it is not clear if this observation was a result of blood culture contamination or true infection. Another recent study included chlorhexidine bathing as one of multiple interventions shown to reduce MRSA clinical isolates in a large cluster randomized trial of targeted versus universal decolonization of ICU patients14. The individual benefit from chlorhexidine bathing cannot be ascertained from this study, however.
In post-hoc, unadjusted analyses, in-hospital mortality was significantly reduced during periods of chlorhexidine bathing but not after adjustment for baseline variables (Tables 2, S1). This finding also does not account for multiple comparisons. Furthermore, this in-hospital mortality difference is partially explained by differences in the UHC expected mortality, which differ between bathing periods. Although it is possible that chlorhexidine bathing reduced the incidence of unmeasured infections, such as viral or surgical site infections, no clear mechanism for improved survival from chlorhexidine bathing exists in the absence of reduced infections.
This study has several strengths. The multiple crossover events allowed for assessment of two temporally separated intervention and control periods within each unit, which better accounts for intercluster variability while also controlling for seasonal variation in outcomes. The individual infections included in the primary outcome are rare events and a composite primary outcome was chosen to maximize the chance of detecting a difference between groups. Additionally, this study focused on patient-centered outcomes and tested the effect of chlorhexidine bathing on several infections other than BSI, CLABSI, and clinical cultures positive for multi-drug resistant organisms, including C. difficile infection, which has been impacted by chlorhexidine in a previous quasi-experimental study15. The limitations to this study include the inability to blind staff administering baths to the treatment group; however, personnel responsible for adjudicating infections were blinded to the treatment. Additionally, this is a single center study that included multiple ICUs encompassing a diverse patient population and a large sample size. Of the infections included in the Medicare Hospital Compare website (www.medicare.gov/hospitalcompare), Vanderbilt University Medical Center is similar to national benchmarks, suggesting these findings are generalizable to other medical centers. This trial was designed as an effectiveness rather than an efficacy trial whereby the interventions were performed as a component of routine patient care rather than by dedicated study personnel. Therefore, bathing compliance was not assessed and it is unclear if this may have affected outcomes. As noted above, active surveillance for multi-drug resistant organism acquisition is not routinely done in our ICUs and was not a component of this study but has been included as an outcome in previous studies8,15–19.
Conclusions
In this pragmatic trial, daily bathing with chlorhexidine did not reduce the incidence of healthcare-associated infections including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, or C. difficile. These findings do not support daily bathing of critically ill patients with chlorhexidine.
Supplementary Material
Acknowledgements
Michael Noto and Henry Domenico had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Tom Talbot’s Spouse has received research funding from Sanofi Pasteur, Gilead, MedImmune and serves on the Advisory Board for Teva Pharmaceuticals. We would like to thank the following persons at Vanderbilt University for their assistance, none of whom received compensation for their role in this study; Amber Goldston BS, JeNelle Rivers, Addison May M.D., Chad Wagner M.D., Joseph Fredi M.D., John Barwise MB ChB, Oscar Guillamondegui, M.D., Marcella Woods PhD, Lixin Chen MS, Li Wang MS, John Newman M.D., and C. Buddy Creech M.D, as well as the Vanderbilt University Department of Infection Prevention. This work was supported by the National Institute of Health under Award Numbers UL1 TR000445 and 2T32HL087738-06 as well as funding through the Vanderbilt Institute for Clinical and Translational Research. The funding agencies had no input into design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
"Research reported in this publication was supported by the National Institute of Health under Award Numbers UL1 TR000445 and 2T32HL087738-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."
Footnotes
No other authors have conflicts to disclose.
References
- 1.Kaye KS, Marchaim D, Chen TY, et al. Effect of nosocomial bloodstream infections on mortality, length of stay, and hospital costs in older adults. J Am Geriatr Soc. 2014 Feb;62(2):306–311. doi: 10.1111/jgs.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006 Aug;34(8):2084–2089. doi: 10.1097/01.CCM.0000227648.15804.2D. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RR, Scott RD, 2nd, Hota B, et al. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care. 2010 Nov;48(11):1026–1035. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- 4.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007 Dec;35(10) Suppl 2:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002 Dec;23(12 Suppl):S3–S40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 6.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011 May;52(9):e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karki S, Cheng AC. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: a systematic review. J Hosp Infect. 2012 Oct;82(2):71–84. doi: 10.1016/j.jhin.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013 Feb 7;368(6):533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014 Jul;35(7):772–796. doi: 10.1086/676534. [DOI] [PubMed] [Google Scholar]
- 10.Suwantarat N, Carroll KC, Tekle T, et al. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line-associated bloodstream infections. Infect Control Hosp Epidemiol. 2014 Sep;35(9):1183–1186. doi: 10.1086/677628. [DOI] [PubMed] [Google Scholar]
- 11.Wang JT, Sheng WH, Wang JL, et al. Longitudinal analysis of chlorhexidine susceptibilities of nosocomial methicillin-resistant Staphylococcus aureus isolates at a teaching hospital in Taiwan. J Antimicrob Chemother. 2008 Sep;62(3):514–517. doi: 10.1093/jac/dkn208. [DOI] [PubMed] [Google Scholar]
- 12.CDC. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2014 http://www.cdc.gov/nhsn.
- 13.Consortium UH. About UHC. [Accessed December 10, 2014]; https:// http://www.uhc.edu/about. [Google Scholar]
- 14.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013 Jun 13;368(24):2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012 Nov;33(11):1094–1100. doi: 10.1086/668024. [DOI] [PubMed] [Google Scholar]
- 16.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009 Jun;37(6):1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 17.Kassakian SZ, Mermel LA, Jefferson JA, Parenteau SL, Machan JT. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011 Mar;32(3):238–243. doi: 10.1086/658334. [DOI] [PubMed] [Google Scholar]
- 18.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006 Feb 13;166(3):306–312. doi: 10.1001/archinte.166.3.306. [DOI] [PubMed] [Google Scholar]
- 19.Viray MA, Morley JC, Coopersmith CM, Kollef MH, Fraser VJ, Warren DK. Daily bathing with chlorhexidine-based soap and the prevention of Staphylococcus aureus transmission and infection. Infect Control Hosp Epidemiol. 2014 Mar;35(3):243–250. doi: 10.1086/675292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.