Abstract
Purpose
To determine if long-term wear of a fluid-filled scleral lens alters basal tear production, corneal sensation, corneal nerve density and corneal nerve morphology in two disease categories.
Methods
Patients recruited from the Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) treatment program at Weill Cornell Medical College were categorized into two groups: distorted corneas (DC) or ocular surface disease (OSD). We measured tear production, central corneal sensation, sub-basal nerve density and tortuosity, and stromal nerve thickness before and after long-term wear of the prosthetic device used in PROSE treatment, defined as at least 60 days of wear for a minimum of eight hours a day.
Results
Twenty patients were included in the study. After long-term wear of the prosthetic device, tear production decreased in patients with DC (21.2±8.5 mm to 10.4±4.6 mm; P < 0.0001), but did not change in patients with OSD (7.5±5.2 mm to 8.7±7.2 mm; P = 0.71). Corneal sensation increased in the DC group (45.6±9.2 mm to 55.0±5.6 mm; P < 0.05). There was no significant change in sensation in patients with OSD (45.0±8.7 mm to 49.1±14.8 mm; P = 0.37). Sub-basal nerve density, sub-basal nerve tortuosity, and stromal nerve thickness remained unchanged in both DC and OSD groups after long-term wear (P > 0.05)
Conclusions
Patients with DC had significantly reduced basal tear production and increased corneal sensation after long-term wear of the scleral lens, but patients with OSD did not show any changes in tear production or corneal sensation.
Keywords: scleral lens, PROSE, corneal nerves, lacrimal functional unit, ocular surface disease, corneal ectasia, tear production, corneal sensation, confocal
Introduction
The prosthetic device used in the prosthetic replacement of the ocular surface ecosystem (PROSE) treatment program (BostonSight™, Boston, MA, USA) is a custom-designed scleral lens which provides a fluid-filled reservoir that protects the corneal surface from lid- or lash-related microtrauma, prevents corneal desiccation, and neutralizes corneal irregularities. Each prosthetic device is custom fit for each patient’s eye. The device is generally worn during waking hours and removed for sleep, with fresh saline placed in the reservoir every morning. Current literature supports the use of PROSE to treat a wide variety of ocular diseases with the majority of indications falling into two broad categories: distorted corneas (DC) such as keratoconus, pellucid marginal degeneration, irregular astigmatism, or post-laser vision correction ectasia; and ocular surface disease (OSD) such as severe dry eye syndrome, ocular graft-versus-host disease, or Stevens-Johnson syndrome.1–5
Despite the widespread use and extensive clinical indications for PROSE treatment, there have been few studies on the effects of long-term scleral lens wear on corneal physiology, and in particular, the effects on the lacrimal functional unit (LFU). The LFU is a conceptualized, integrated unit comprised of the physical surface of the eye, tear-secreting glands, corneal nerves, and neuroendocrine factors. It regulates the tear film in response to both exogenous environmental influences and to endogenous endocrine influences. Damage to any component of the LFU alters the tear film, leading to eye desiccation. 6–8
Traditional contact lens wear has numerous effects on the LFU such as mechanically disrupting the tear film structure, and decreasing tear evaporation rate.6,9,10 Contact lens wear has also been shown to decrease corneal sensation.11,12 The mechanism is unknown, but may be due to neural adaptation to the continuous presence of a contact lens11, or to changes in the corneal sub-basal nerve density.13–15 This change in the functional and/or anatomic status of the corneal nerves in turn affects the afferent limb of the lacrimation (tearing) reflex, serving to decrease tear secretion.
We hypothesize that a PROSE prosthetic device, which maintains a fluid layer over the cornea, may also affect the LFU through changes in corneal innervation and alteration of lacrimation. We further hypothesize that wearing the prosthetic device may differentially affect patients with OSD, who may have preexisting LFU dysfunction, compared to DC patients, who presumably have a relatively normal LFU at baseline.
In order to evaluate these hypotheses, we conducted a prospective study which measured components of the LFU (basal tear production, corneal sensation, corneal sub-basal nerve density and tortuosity, and corneal stromal nerve thickness) in a cohort of PROSE patients with either OSD or DC before device fitting had commenced and after two months of wearing the device had been achieved.
Materials and Methods
Patients
This single-center, prospective, study was conducted in the Department of Ophthalmology at Weill Cornell Medical College. The study protocol was approved by the Weill Cornell Medical College Institutional Review Board and carried out in compliance with the Health Insurance Portability and Accountability Act regulations. Written informed consent was obtained from all patients.
Patients were recruited from the PROSE treatment program at the Weill Cornell Department of Ophthalmology between July 1st, 2012 and April 1st, 2013. Only patients who achieve long-term wear, defined as wearing the prosthetic device for at least eight hour a day for a period of at least 60 days, were included. The 60 day time point was chosen, as this represented a typical time for stabilization of wear and for likely stabilization of ocular surface parameters. Patients who had been fit previously with any scleral lenses were excluded. A single, trained provider of PROSE prosthetic devices conducted the fitting process by assessing and customizing the device fit. Patients were evaluated approximately every two to four weeks over a six-month period until optimal fit and comfort were achieved. At each visit, daily wear time was surveyed. Baseline measurements for tear production, corneal nerve sensation, and corneal nerve imaging (described below) were obtained prior to device fitting and wear. Repeat measurements were obtained after the patients reached long-term wear. Some patients deferred one or more measurements and were included in the analysis only for the tests with completed measurements. All measurements were obtained after removal of the prosthetic device.
Categorization of diagnoses
Patients were categorized into two groups based on indication for PROSE treatment: 1) Distorted corneas (DC), including ectatic corneal diseases such as keratoconus, post-refractive surgery irregularity, pellucid marginal degeneration, and Terrien’s marginal degeneration; and 2) ocular surface disease (OSD), including primary dry eye syndrome, Sjögren’s syndrome, ocular graft-versus-host-disease, post-refractive surgery dry eye syndrome, dry eye syndrome associated with blepharitis, and exposure keratopathy. Those patients with irregular astigmatism secondary to a diseased ocular surface were placed in the OSD category.
Tear Production
Schirmer’s type I with anesthesia was conducted to measure tear production. Patients were administered one drop of anesthetic, 0.5% proparacaine hydrochloride solution (Akorn, Inc., Lake Forest, IL, USA), in each eye. A Schirmer’s strip was placed under the lower lid for five minutes with the patients’ eyes closed. Tear production was measured in millimeters of wetted strip.
Central corneal sensation
Central corneal sensation was measured using a Cochet-Bonnet aesthesiometer following the manufacturer’s instructions. The tip of the nylon monofilament, starting at full length of 60 mm, was touched to the patient’s central cornea until enough force was exerted that the filament bent. The filament was shortened by five millimeters increments until the patient felt contact at least three out of five touches, and that length was recorded. The maximum filament length is 60 mm, which exerts 11mm/gm of pressure on the cornea.
Confocal Microscopy
Corneal images were obtained using a ConfoScan 4 (Nidek, Inc., Freemont, CA, USA) confocal scanning microscope, equipped with a 40X immersion objective on which GenTeal Gel (Novartis Ophthalmics, Basel, Switzerland) was applied. Patients were administered one drop of 0.5% proparacaine hydrochloride solution in each eye. The lens was advanced until the gel contacted the cornea and the image was focused on the central cornea. 350 images of a 440 um × 330 um area were taken starting from the endothelial layer, with five micrometer steps between images. Each exam typically included three scans proceeding from the posterior to anterior cornea. A single best image showing nerves in the sub-basal nerve plexus and stromal nerves was chosen from each examination. If no images of corneal nerves were obtained in the exam, the exam was excluded. Images were analyzed for sub-basal corneal nerve fiber length and tortuosity, and stromal nerve trunk thickness using NeuroLucida Software (MBF Bioscience, Williston, VT, USA). Sub-basal epithelial nerve density was calculated by dividing the total length of nerve fibers in each image by the area photographed. Tortuosity is defined as the ratio of the length of the nerve segment divided by the distance between the endpoints. Triplicate measurements of the stromal nerve thickness were taken.
Statistical Analysis
We used a two-way ANOVA with repeated measures to determine statistical significance for each measurement. We compared the data at baseline and after long-term device wear between patients with DC and OSD. Sidek’s multiple comparisons test was used for post-hoc tests to compare means between groups. A significance level of P < 0.05 was used for all tests. All statistical tests were conducted using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA, USA).
Results
Baseline characteristics
Twenty patients met the inclusionary criteria for the study. Reported wear times ranged from 8–13 hours per day for a period of 61 – 190 days. The patient demographics and indication for PROSE treatment are presented in Table 1. The number of patients included for each analysis is as follows: tear production, 18 patients (34 eyes); corneal sensitivity, 14 patients (20 eyes); sub-basal density, 12 patients (21 eyes); sub-basal tortuosity, 12 patients (21 eyes); and stromal thickness, 11 patients (17 eyes). Two eyes were excluded from the analysis of tear production due to an initial Schirmer’s test measurement of zero, indicating no measureable tear production. Fifteen eyes were excluded from the analysis of corneal sensation because of a baseline sensation at the maximal filament length of 60 mm.
Table 1.
Gender, age and disease category of patients.
| Demographics | N or mean ± SD |
|---|---|
| Male | 13 |
| Female | 7 |
| Age (years) | 54 ± 13 |
| Distorted Corneas, total | 11 |
| Keratoconus | 6 |
| Post-refractive surgery | 2 |
| Pellucid Marginal Degeneration | 2 |
| Terrien's Marginal Degeneration | 1 |
| Ocular surface disease, total | 9 |
| Primary dry eye syndrome | 2 |
| Sjögren’s syndrome | 2 |
| Graft-versus-host-disease | 2 |
| Dry eye syndrome post-refractive surgery | 1 |
| DES secondary to blepharitis | 1 |
| Exposure keratopathy | 1 |
| Total patients | 20 |
Tear production
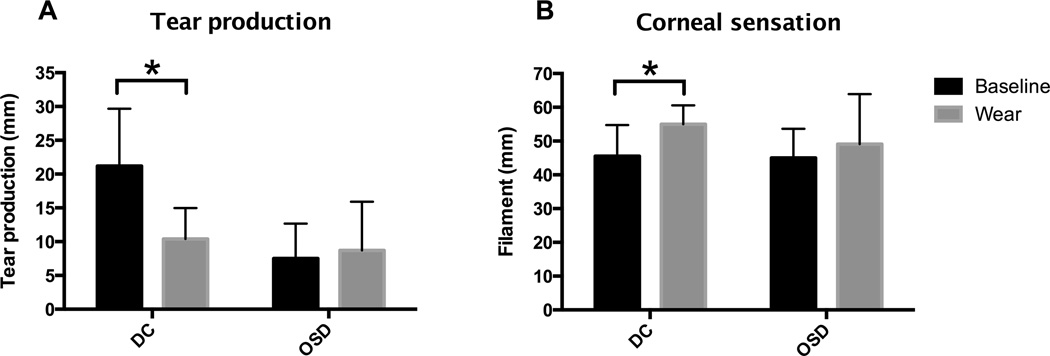
Basal tear production in patients with DC significantly decreased after long-term wear of the PROSE prosthetic device (21.2±8.5 mm to 10.4±4.6 mm; P < 0.0001). In contrast, tear production did not change in OSD patients after long-term wear (7.5±5.2 mm to 8.7±7.2 mm; P = 0.71). The statistical interaction of main effects between disease group and tear production over time was significant (P < 0.0001; Figure 1A).
Figure 1.
Corneal function as assessed by tear production and corneal sensation. Patients are grouped as distorted corneas or ocular surface disease, and tear production and sensation are compared at baseline to after long-term device wear of at least 60 days of eight hours wear per day. * indicates P < 0.05.
Corneal sensation
Corneal sensation in patients with DC was significantly increased after long-term wear of the prosthetic device (45.6±9.2 mm to 55.0±5.6 mm; P < 0.05). However, corneal sensation in OSD patients was similar at baseline (45.0±8.7 mm) and after long term wear (49.1±14.8 mm; P = 0.37). The statistical interaction of main effects between disease group and sensitivity over time was not significant (P = 0.26; Figure 1B).
Corneal nerve morphology
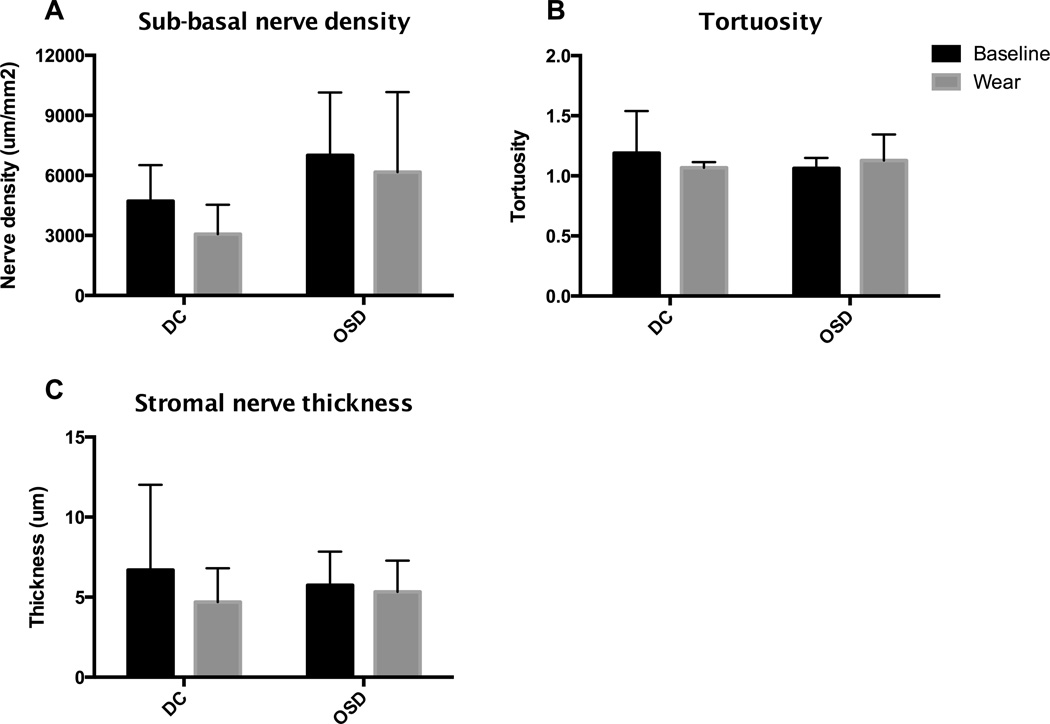
Representative confocal images of sub-basal density, sub-basal tortuosity, and stromal nerve thickness are shown in Figure 2. Our testing did not demonstrate a significant change in sub-basal nerve density in either OSD or DC patient groups. Sub-basal nerve density in patients with corneal irregularity was 4719±1793 um/mm2 at baseline and 3066±1473 um/mm2 after long-term wear (P = 0.47). Sub-basal density in OSD patients was 7002±3137 um/mm2 at baseline and 6166±3998 um/mm2 after long-term wear (P = 0.72). However, the between-subjects analysis showed that patients with DC have lower overall sub-basal nerve density compared to patients with OSD (P < 0.05). The statistical interaction of main effects between the diagnosis group and sub-basal nerve density over time was not significant (P = 0.66; Figure 3A).
Figure 2.
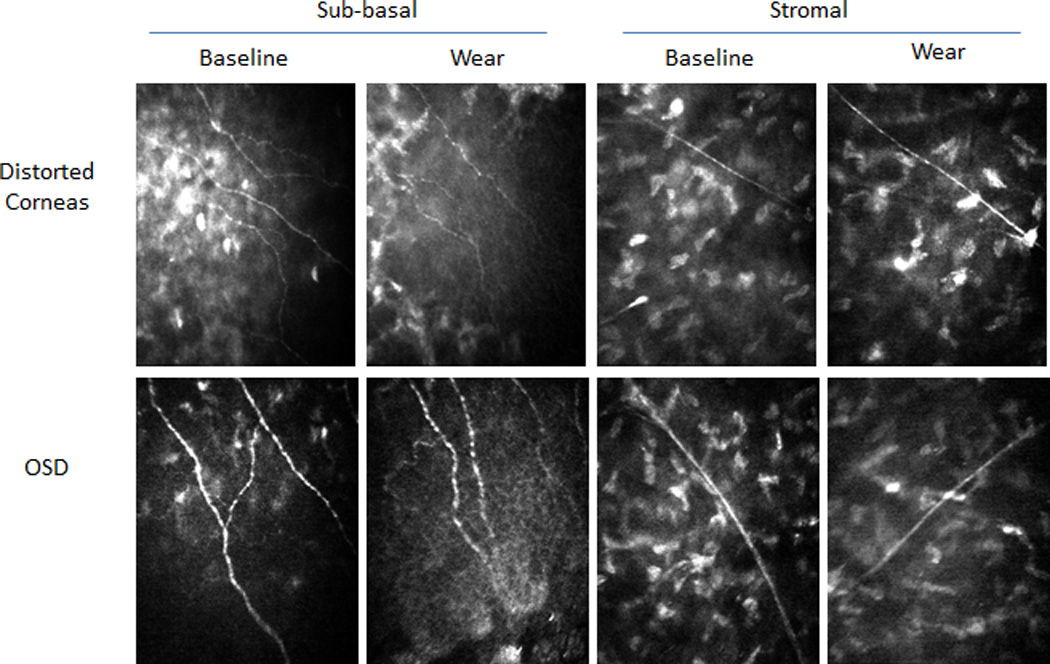
Confocal images of sub-basal and stromal nerves from representative patients at baseline and after long-term device wear.
Figure 3.
Corneal nerve structure and morphology assessed by sub-basal nerve density, tortuosity and stromal nerve thickness. Measurements at baseline and after long-term device wear were compared and categorized by patient diagnosis. There was no change in sub-basal nerve density, sub-basal tortuosity, or stromal nerve thickness over time for either diagnosis group, but DC patients showed a lower density of sub-basal nerves compared to OSD patients.
Additional analysis of nerve morphology did not reveal any differences in critical nerve morphologic indices as a result of long-term wear of the PROSE prosthetic device. Sub-basal nerve tortuosity in DC patients was 1.19±0.35 at baseline and 1.07±0.05 after long-term wear (P = 0.41). OSD patients had sub-basal nerve tortuosity of 1.06±0.09 at baseline and 1.13±0.21 after long-term wear (P = 0.65). The statistical interaction of main effects between diagnosis group and tortuosity over time was not significant (P = 0.15; Figure 3B). Patients with DC had a stromal nerve thickness of 6.69±5.32 um at baseline, and 4.69±2.11 um after long-term wear (P = 0.35). Stromal nerve thickness in OSD patients was 5.74±2.10 um at baseline, and 5.33±1.95 um after long-term wear (P = 0.89). There was no statistical interaction of main effects between diagnosis and stromal nerve thickness over time (P = 0.38; Figure 3C).
Discussion
Our study is the first to show that long-term wear of an ocular surface prosthetic device can change corneal nerve function. During wear of the PROSE prosthetic device, the corneal surface is submerged in an aqueous reservoir which prevents the corneal nerves from sensing the true ocular surface environment. Instead, the corneal nerves sense a wet saline environment devoid of the normal neuroendocrine and mechanical factors that provide feedback to regulate tear production. Furthermore, scleral lens application prevents evaporative tear loss which abrogates evaporation-mediated cooling. This cooling is required to trigger cold receptors that set the basal tearing rate.16,17 Thus, the scleral lens interrupts chemical, mechanical and thermal feedback mechanisms, resulting in a likely decreased stimulus for basal tear secretion.
Importantly, the LFU changes were observed in patients with DC but not in patients with OSD. The difference in disease pathophysiology between DC and OSD may provide insight for why these groups responded differently to long-term device wear. In patients with OSD, chronic dryness leads to lacrimal gland and ocular surface inflammation. Chronic inflammation causes release of cytokines and other inflammatory mediators that further damage the ocular surface, including the corneal nerves.6,8,18 Thus, in OSD the LFU components may be impaired, leading to a blunted response to long-term PROSE wear. However, patients with DC have primarily non-inflammatory corneal disease with normal feedback mechanisms and thus, may respond to long-term device wear by decreasing baseline tear production.
We also found that long-term device wear caused increased corneal sensation in patients with DC but not in those with OSD. Improvement of corneal sensation has important implications for patients with DC, and in particular, patients with keratoconus. Decreased corneal sensation is a hallmark of keratoconus, and corneal nerve function may be involved in the pathogenesis of the disease. 19–22 Several studies have correlated decreased sensation to disease severity in patients with keratoconus.23 Contact lenses, which are commonly worn by keratoconus patients, further decrease sensation.20 PROSE treatment appears to improve sensation and may provide added benefit for patients with keratoconus and other corneal irregularities. Currently, PROSE treatment is generally considered after failure to tolerate standard corneal contact lenses due to the added difficulty of prosthetic device insertion and added cost. Our study suggests that earlier implementation of PROSE treatment might be considered as it may have less undesired effects on the corneal innervation compared to corneal contact lenses.
Unlike patients with DC, patients with OSD showed no significant change in corneal sensation in response to long-term wear of the device used in PROSE treatment. Changes in corneal sensation are more variable in patients with OSD compared to patients with corneal irregularities. Studies have reported both hyperesthesia and hypoesthesia in dry eye and OSD patients, varying by etiology. For example, primary dry eye disease is associated with decreased corneal sensation24–26, whereas Sjögren’s syndrome is associated with increased sensation27,28. The poorly understood effects of inflammation which accompanies OSD on corneal nerve function may explain the divergent responses of corneal sensation to OSD. We did not study each etiology of OSD separately, and thus cannot comment on specific effects of the prosthetic devices in the various causes of OSD.
Our examination of corneal nerve morphology did not find any changes after long-term device wear in either disease group. The clinical significance of sub-basal nerve density is that decreased sub-basal nerve density has been correlated to corneal sensation in a study of keratoconus patients,20 and studies on post-refractive surgery patients also concluded strong positive correlations between sub-basal nerve density and corneal sensation.29 In contrast, our results found that patients with corneal irregularity had improved corneal sensation after long-term wear but did not show any change in sub-basal nerve density. However, we did find that patients with DC overall had a significantly lower sub-basal nerve density compared to patients with OSD (between-subjects effect). It has previously been reported that patients with keratoconus have a lower sub-basal nerve density than patients with normal corneas.19,22 In contrast, reports on sub-basal nerve density in OSD have been varied.24,25
We did not find any significant change in sub-basal tortuosity or stromal nerve thickness with long-term device wear in either patients with DC or OSD. Sub-basal corneal nerve tortuosity has been previously shown to increase in some disease states, such as Sjögren’s syndrome28, and corneal stromal nerve thickness has previously been reported to be decreased in keratoconus 30,31 and Sjögren’s syndrome.28 One limitation to this analysis is that there is no standardized method for calculating tortuosity. Previously published methods include a scheme of grading tortuosity on a scale of one to four, various approaches to calculating tortuosity from hand-traced nerve drawings, and grading with automated tracing of nerves.32–34 The variation in methods make comparisons between studies problematic. In addition, there is debate on the accuracy of quantitative analysis of stromal nerve thickness, due to the complexities of confocal imaging, making it difficult to obtain a reliable measurement.29
This is the first study to evaluate the function and structure of the corneal nerves over the course of PROSE treatment; however, this study was limited by the relatively short follow-up time of 61 to 190 days. It is possible that the patients with OSD may require longer wear times to reverse the inflammatory nature of the disease, and a longer study period is needed to detect any changes in LFU function in this disease group. Future studies that follow patients for longer periods of time may allow for more accurate assessment, particularly regarding changes in corneal nerve morphology.
In summary, our study showed that long-term wear of the prosthetic device used in PROSE treatment has significant effects on corneal nerve function in patients with corneal distortions. Since patients with DC have a healthier ocular surface, the intact lacrimal functional unit responds to the constant saline exposure by reducing the basal tear production and increasing corneal sensation, which are possible signs of improvement in corneal disease. However, patients with ocular surface disease did not have similar alterations in LFU function possibly due to ongoing inflammatory processes disrupting the lacrimal functional unit. As scleral lenses become more widely used as long-term therapies, the changes these devices confer on corneal neurobiology should be considered.
Acknowledgements
This investigation was supported by an unrestricted grant from Research to Prevent Blindness, and grants from the National Institutes of Health: UL1TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College, and R01EY018594 from the National Eye Institute.
Funding: This investigation was supported by an unrestricted grant from Research to Prevent Blindness, and grants from the National Institutes of Health: UL1TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College, and R01EY018594 from the National Eye Institute.
REFERENCES
- 1.Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007;26:1195–1199. doi: 10.1097/ICO.0b013e318155743d. [DOI] [PubMed] [Google Scholar]
- 2.Grey F, Carley F, Biswas S, Tromans C. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye. 2012;35:288–291. doi: 10.1016/j.clae.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134. doi: 10.1097/01.icl.0000152492.98553.8d. [DOI] [PubMed] [Google Scholar]
- 4.Baran I, Bradley JA, Alipour F, et al. PROSE treatment of corneal ectasia. Cont Lens Anterior Eye. 2012;35:222–227. doi: 10.1016/j.clae.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Stason WB, Razavi M, Jacobs DS, et al. Clinical benefits of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2010;149:54–61. doi: 10.1016/j.ajo.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mathers WD. Why the eye becomes dry: a cornea and lacrimal gland feedback model. CLAO J. 2000;26:159–165. [PubMed] [Google Scholar]
- 8.Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Craig JP, Willcox MDP, Argüeso P, et al. The TFOS International Workshop on Contact Lens Discomfort: Report of the Contact Lens Interactions With the Tear Film Subcommittee. Investigative Ophthalmology & Visual Science. 2013;54:123–156. doi: 10.1167/iovs.13-13235. [DOI] [PubMed] [Google Scholar]
- 10.Iskeleli G, Karakoç Y, Aydin O, et al. Comparison of tear-film osmolarity in different types of contact lenses. CLAO J. 2002;28:174–176. doi: 10.1097/01.ICL.0000024117.46518.A4. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton F, Marfurt CF, Golebiowski B, et al. The International Workshop on Contact Lens Discomfort: Report of the Subcomitee on Neurobiology. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.13-13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golebiowski B, Papas EB, Stapleton F. Corneal and conjunctival sensory function: the impact on ocular surface sensitivity of change from low to high oxygen transmissibility contact lenses. Invest Ophthalmol Vis Sci. 2012;53:1177–1181. doi: 10.1167/iovs.11-8416. [DOI] [PubMed] [Google Scholar]
- 13.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, McDermott AM, Miller WL. Elevated nerve growth factor in dry eye associated with established contact lens wear. Eye Contact Lens. 2009;35:232–237. doi: 10.1097/ICL.0b013e3181b3e87f. [DOI] [PubMed] [Google Scholar]
- 15.Hoşal BM, Ornek N, Zilelioğlu G, et al. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19:1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 16.Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 17.Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:3969–3976. doi: 10.1167/iovs.09-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemp MA, Baudouin C, Baum JM, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 19.Simo ML, Tromans C, O’Donnell C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Cont Lens Anterior Eye. 2005;28:185–192. doi: 10.1016/j.clae.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Patel DV, Ku JYF, Johnson R, et al. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye (Lond) 2009;23:586–592. doi: 10.1038/eye.2008.52. [DOI] [PubMed] [Google Scholar]
- 21.Zabala M, Archila EA. Corneal sensitivity and topogometry in keratoconus. CLAO J. 1988;14:210–212. [PubMed] [Google Scholar]
- 22.Spadea L, Salvatore S, Vingolo EM. Corneal sensitivity in keratoconus: a review of the literature. ScientificWorldJournal. 2013;2013:683090. doi: 10.1155/2013/683090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dogru M, Karakaya H, Ozçetin H, et al. Tear function and ocular surface changes in keratoconus. Ophthalmology. 2003;110:1110–1118. doi: 10.1016/S0161-6420(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 24.Labbe A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 25.Labbé A, Liang Q, Wang Z, et al. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54:5144–5150. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 26.Situ P, Simpson TL, Fonn D, et al. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008;49:2971–2976. doi: 10.1167/iovs.08-1734. [DOI] [PubMed] [Google Scholar]
- 27.Tuisku IS, Konttinen YT, Konttinen LM, et al. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Villani E, Galimberti D, Viola F, et al. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48:2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 29.Patel DV, McGhee CNJ. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 30.Efron N, Hollingsworth JG. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 2008;91:34–55. doi: 10.1111/j.1444-0938.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 31.Mocan MC, Yilmaz PT, Irkec M, et al. In vivo confocal microscopy for the evaluation of corneal microstructure in keratoconus. Curr Eye Res. 2008;33:933–939. doi: 10.1080/02713680802439219. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Kallinikos P, Berhanu M, O’Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–422. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 34.Scarpa F, Zheng X, Ohashi Y, Ruggeri A. Automatic evaluation of corneal nerve tortuosity in images from in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:6404–6408. doi: 10.1167/iovs.11-7529. [DOI] [PubMed] [Google Scholar]