Abstract
Neurofibromatosis 1 is a hereditary syndrome characterized by the development of numerous benign neurofibromas, a small subset of which progress to malignant peripheral nerve sheath tumors (MPNSTs). To better understand the genetic basis for MPNSTs, we performed genome-wide or targeted sequencing on 50 cases. Sixteen MPNSTs but none of the neurofibromas tested were found to have somatic mutations in SUZ12, implicating it as having a central role in malignant transformation.
Neurofibromatosis 1 (NF1), also known as von Recklinghausen disease, is an autosomal dominant hereditary cancer syndrome that affects ∼1 in 3,000 individuals1. It is characterized by features including café-au-lait spots, Lisch nodules, axillary freckling, optic pathway gliomas, skeletal anomalies and neurofibromas. Neurofibromas are benign tumors that can be broadly classified on the basis of their location (as dermal, spinal or plexiform) or on the basis of their morphology (as localized, plexiform or diffuse)2. Some neurofibromas, typically those that are deep-seated and plexiform, progress to MPNSTs3–5. An individual with NF1 has a 10–15% chance of developing an MPNST, and ∼50% of all MPNSTs arise in the setting of NF1. MPNSTs are sarcomas that can arise anywhere in the body and confer a 5-year survival rate as low as 20%, despite treatment through radical surgical resection followed by radiation and chemotherapy6.
Previous studies have demonstrated that neurofibromas in individuals with NF1 develop through the acquisition of a somatic, inactivating mutation in the NF1 gene inherited from the unaffected parent7. This finding is in accordance with the two-hit hypothesis for tumor-suppressor genes8. However, the genetic alterations responsible for progression to much rarer MPNSTs are not known. To address this issue, we performed whole-genome sequencing on four MPNSTs, each from a different unrelated individual with NF1. We also performed whole-genome sequencing on leukocytes derived from the same individuals to determine whether the alterations identified were somatic. High-quality coverage averaged approximately 59-fold (range of 55.1- to 64.4-fold) in the tumor genomes and approximately 42-fold (range of 40.2- to 46.3-fold) in the normal genomes (Supplementary Table 1). After processing the sequencing data using a series of bioinformatics algorithms and stringent base calling filters that have previously been described9, we identified a median of over 60 somatic nonsynonymous point mutations or small insertion-deletions (indels) (range of 14 to 208) in the coding regions of these tumors (Supplementary Fig. 1 and Supplementary Table 1). Of the 261 total alterations, 64.4%, 24.1%, 6.5% and 5% were missense, small indel, truncating or splice-site mutations, respectively. The most surprising and statistically significant of these somatic alterations were in the SUZ12 gene encoding zeste homolog 12 (Drosophila), a chromatin-modifying protein. Unequivocal inactivating mutations of SUZ12 occurred in each of the four MPNSTs studied (Supplementary Tables 2 and 3). All four mutations were confirmed through deep sequencing of PCR products from the altered regions, showing high fractions of mutant alleles in all cases (Table 1).
Table 1. Somatic mutations in the MPNST discovery set.
| Sample |
NF1 germline mutation |
NF1 somatic mutation |
NF1 MAF (%) |
SUZ12 somatic mutation |
SUZ12 MAF (%) |
Somatic deletions on chromosome 17 |
Other chromatin- modifying gene somatic mutations |
Other driver gene mutations of interest |
|---|---|---|---|---|---|---|---|---|
| MPNST 01 | NC_000017.10: g.26327000_33008000del | |||||||
| MPNST 02 | NA | NM_003797.3 (EED):c.134delA | NM_000268.3 (NF2): c.552G>A | |||||
| MPNST 04 | NM_000267.3: c.7806+2T>C | 85 | NM_015355.2: c.252delG | 100 | NC_000017.10: g.57703000_8211000del | |||
| MPNST 05 | NM_000267.3: c.5242C>T | 88 | NM_015355.2: c.1995_1996insC | 79 | ||||
| MPNST 06a | NM_000267.3: c.6362delG | 66 | NM_015355.2: c.[333delG(;)334C>A] | 100 | ||||
| MPNST 08 | NA | NM_000267.3: c.6580–19delAGA GTATCCCCTTTTTTA | 12 | NM_001272004.1 (EPC1): c.330delT | NM_000489.4 (ATRX): c.1230delA | |||
| MPNST 09 | NA | NM_001273.3 (CHD4): c.1358G>A | ||||||
| MPNST 10 | NM_000267.3: c.2027_2028insC | NM_015355.2: c.1940delTAAT | 23 | NC_000017.10: g.25533000 30513000del |
MAF, mutant allele fraction; NA, not applicable.
Sections of this tumor were available for immunohistochemistry and were shown to be negative for staining with antibodies to both SUZ12 and H3K27me3.
Subsequently, we were able to obtain and analyze four additional frozen tumors (from four different individuals) via either whole-genome or whole-exome sequencing (Table 1 and Supplementary Tables 2 and 3). One of these additional individuals had neurofibromatosis, and her tumor contained an inactivating mutation of SUZ12. The other three MPNSTs were from individuals without a personal or family history of neurofibromatosis. The sequencing coverage of the cancer and normal genomes was similar to that for the hereditary cases, as was the number of mutant genes (excluding MPNST 04, which had 208 alterations in the setting of previous radiation and chemotherapy) (Supplementary Tables 1 and 3). No SUZ12 mutations were observed in the sporadic MPNSTs. However, all three had inactivating mutations in other chromatin-modifying genes. One tumor had a mutation of the EED gene, whose gene product is a member of Polycomb repressor complex 2 (PRC2), which includes the protein encoded by SUZ12. The two other nonhereditary cases had mutations in EPC1 (encoding enhancer of Polycomb homolog 1 (Drosophila)) or CHD4 (encoding chromodomain helicase DNA-binding protein 4) (Table 1). The proteins encoded by SUZ12, EED, EPC1, AEBP2, EZH2 and RBBP7 all have pivotal roles in chromatin homeostasis and interact with PRCs. PRCs have critical roles in histone maintenance whereby they regulate methylation at lysine 9 and lysine 27 of histone H3. Methylation at these positions results in transcriptional repression of target genes. There is a rapidly growing body of evidence showing that genes involved in chromatin homeostasis, particularly those regulating methylation of histone H3, drive neoplastic development in a number of different tumor types10.
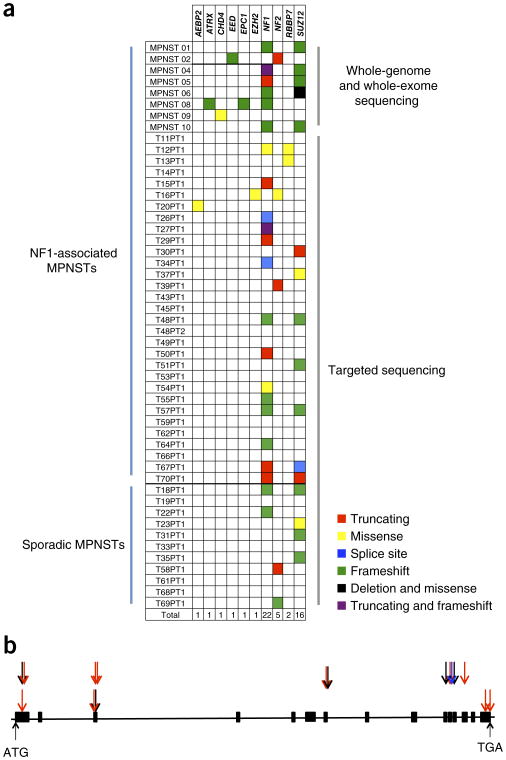
To validate these genetic data, we analyzed an independent collection of 42 paraffin-archived MPNST specimens. Thirty-one of these were from individuals with neurofibromatosis, and 11 were from sporadic cases. In 11 of the hereditary neurofibromatosis cases, a neurofibroma and an MPNST were available for comparison. Illumina-type libraries were constructed for all specimens, and library DNA was captured with probes corresponding to NF1, NF2 and six additional chromatin-modifying genes, including SUZ12, suspected of having a role in MPNST on the basis of the results from the discovery screen. Fifteen MPNSTs harbored a somatic mutation in a chromatin-modifying gene, distributed in a mutually exclusive fashion (Fig. 1 and Supplementary Tables 4 and 5). The gene most commonly affected by these mutations, by far, was SUZ12, altered in 11 tumors (26%). The fraction of MPNSTs harboring a SUZ12 mutation is likely an underestimate given the archival nature of these specimens and our inability to detect larger indels or translocations through the targeted sequencing approach with partially degraded DNA. Even so, of the 11 mutations detected, 9 were indels or truncating mutations that would be expected to abolish protein function (Fig. 1a,b). Seven of the SUZ12 mutations were from MPNSTs occurring in individuals with NF1, and the remaining four were from sporadic cases. None of the 11 neurofibromas tested had mutations in SUZ12 (Supplementary Table 4). To determine the functional consequence of the SUZ12 mutations, we performed immunohistochemistry for SUZ12 expression on 25 MPNST samples (in the other cases, insufficient amounts of high-quality tissue were available). In the 5 cases tested with SUZ12 alterations, no protein expression was detected. In addition, 1 tumor in which a mutation was not found also lacked SUZ12 expression, whereas the other 19 cases without evident SUZ12 mutations expressed SUZ12. Moreover, in all six cases in which SUZ12 was undetectable, trimethylation of lysine 27 of histone H3 (H3K27me3), a known downstream target of SUZ12, was not discernible (Table 1, Supplementary Fig. 2 and Supplementary Table 4). Adjacent normal cells provided unequivocal internal controls for staining in all cases (Supplementary Fig. 2).
Figure 1.
Somatic mutations in MPNSTs. (a) Distribution of somatic mutations in NF1, NF2 and genes involved with the PRC2 complex. (b) Schematic of the distribution of SUZ12 mutations along the gene. Black arrows represent nonsynonymous mutations, blue arrows represent splice-site alterations and red arrows represent indels. The arrows immediately above the schematic represent mutations observed in the samples that underwent whole-genome or whole-exome sequencing, whereas those in the second row above the schematic represent mutations observed in the samples having undergone targeted sequencing.
Most individuals with NF1 carry a causative germline point mutation or small indel within the NF1 gene. However, 5–10% of individuals with NF1 have a larger deletion on chromosome 17q11.2 that involves the entire NF1 locus as well as a variable number of adjacent genes. There are two classes of deletion, called type 1 and type 2. Type 1 and type 2 deletions are thought to arise via nonallelic homologous recombination involving the NF1–REP A and NF1–REP C and the SUZ12 and SUZ12P regions, respectively11,12. Both deletion types incorporate the SUZ12 gene. The germline deletions of SUZ12 and other genes in these regions were assumed to be passenger events in tumorigenesis, occurring only coincidentally owing to the adjacency of these regions to the target NF1 locus. Our discovery of the consistent presence of inactivating somatic mutations in SUZ12 in hereditary MPNSTs leaves little doubt that these mutations have a major role in the pathogenesis of this tumor type.
Recurrent, inactivating SUZ12 mutations have not previously been reported in any tumor type, although a small fraction of hematological malignancies have been shown to harbor missense mutations7,13–15. However, SUZ12 has been definitively implicated in the pathogenesis of endometrial stromal sarcoma, where more than 50% of tumors have a t(7;17)(p15;q21) translocation resulting in the JAZF1-SUZ12 gene fusion16. The fusion gene is thought to promote cell proliferation and protects against hypoxia-induced apoptosis17. Interestingly, this fusion likely activates the SUZ12 gene, endowing it with oncogenic functions17. Expression-based analyses have also suggested that SUZ12 might have a role in non–small-cell lung, colon and gastric cancers7,8,18. In contrast, the mutations we identified in MPNSTs inactivate SUZ12, indicating that this gene is a tumor suppressor. This situation is reminiscent of the dual roles of NOTCH1, in which activating mutations occur in lymphomas and inactivating mutations are characteristic of squamous cell cancers19.
We posit that hereditary MPNSTs develop from benign neurofibromas by virtue of germline NF1 mutation (first hit) combined with somatic inactivation of the NF1 allele from the non-affected parent (second hit). In those neurofibromas in which the first or second hit is a deletion that coincidentally affects SUZ12, the stage is set for a subsequent mutation of the remaining SUZ12 allele (third hit). This third hit would convert the neurofibroma to an MPNST and would emerge when the first hit is a type 1 or type 2 microdeletion or when the second hit involves loss of a large region on chromosome 17q including both NF1 and SUZ12. This model is supported by the fact that three of the five NF1-associated MPNSTs that underwent either whole-exome or whole-genome sequencing had a somatic deletion on chromosome 17q that involved both NF1 and SUZ12 (Table 1). With only one allele of SUZ12 remaining, the probability of a single additional hit in SUZ12 is much greater than the probability of two independent mutations (two additional hits) occurring in another chromatin-modifying gene. Although our explanation is speculative, it is consistent with the absence of SUZ12 mutations in neurofibromas (Supplementary Table 4) and the fact that individuals with germline microdeletions of the NF1 locus are at greater risk for the development of MPNSTs than individuals with NF1 who have point mutations of NF1 (ref. 2). Future studies on larger cohorts of individuals with NF1, as well as functional studies, will be necessary to fully elucidate the precise role of SUZ12 in MPNST.
Online Methods
Sample acquisition
Fresh-frozen or paraffin-embedded tumor and matched blood were obtained from patients under an institutional review board (IRB)-approved protocol at the Johns Hopkins Hospital. When matched blood was collected, informed consent from each patient was obtained. The diagnosis of each specimen underwent central pathological review and was verified by an independent group of pathologists. Tumor tissue was analyzed to assess neoplastic cellularity. Tumors were macrodissected to remove residual normal tissue and enhance neoplastic cellularity.
Whole-exome and whole-genome sequencing
Genomic DNA libraries were prepared and captured according to the protocol suggested by Illumina and as previously described9,20. DNA libraries were sequenced with the Illumina HiSeq Genome Analyzer, yielding 100 bp of sequence from the final library fragments for whole-exome and whole-genome analyses.
Targeted capture
A DNA-targeted capture chip was designed to capture all the exons of eight genes: NF1 (CCDS11264.1, CCDS42292.1, CCDS45645.1), NF2 (CCDS13865.1, CCDS54516.1), EED (CCDS8273.1, CCDS8274.1), SUZ12 (CCDS11270.1), EZH2 (CCDS5892.1, CCDS56518.1), RBBP4 (CCDS366.1, CCDS44106.1), RBBP7 (CCDS14179.1, CCDS56598.1) and AEBP2 (CCDS44842.1, CCDS58215.1). The total coverage of the chip was 70,583 bp. Capture was performed using the Agilent SureSelect Target Enrichment kit (Agilent Technologies).
Bioinformatics analysis
Bioinformatics analyses were performed at Personal Genome Diagnostics. Whole-genome and whole-exome sequencing data for matched tumor and normal samples were analyzed to identify tumor-specific mutations. Tumor and normal sequences were aligned to the human reference genome (hg19) using Illumina CASAVA and ELAND. Alignments were analyzed to identify somatic point mutations and small insertions and deletions present in tumor but not in matched normal samples. The functional consequence of each mutation was predicted using gene annotations from the CCDS, RefSeq and Ensembl databases. Mutations were filtered for sequencing quality, depth of coverage in the tumor and normal samples, and mutant fraction. Known polymorphisms recorded in dbSNP were removed from the analysis. Mutant reads that matched the reference genome in multiple locations were excluded as possible alignment artifacts. Potential somatic mutations were visually inspected as described previously20. Copy number alterations were identified by comparing the normalized average per-base coverage for a particular gene in a tumor sample to the normalized average per-base coverage in a matched normal sample for the patient.
Immunohistochemistry
Immunohistochemistry was performed on 40 tumor cases. Immunohistochemistry was performed on 4-μm formalin-fixed, paraffin-embedded sections using an automated slide stainer (Benchmark Ultra, Ventana Medical System). Positive and negative controls (HeLa cells and tonsil tissue) were tested in parallel. For staining, sections were deparaffinized and hydrated, and an antigen retrieval step was performed using a high-pH buffer. Antibodies to histone H3 (trimethylated at lysine 27) (mabcam 6147, Abcam) and SUZ12 (SUZ220A) (ab126577, Abcam) were applied using optimal dilutions (10 μg/ml), and reactions were developed using Opti-View DAB detection (Ventana Medical System). Nuclear labeling was scored for all MPNST and neurofibroma cases. Tumors were scored as positive when nuclear staining was intact and were scored as lost when tumor cells were negative for immunostaining. Staining of lymphocytes and endothelium was used as internal controls.
Supplementary Material
Acknowledgments
We thank our patients for their courage and generosity. We also thank J. Ptak, N. Silliman, L. Dobbyn, J. Schaeffer and M. Papoli for expert technical assistance. The work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research, a Burroughs Wellcome Career Award for Medical Scientists, a Johns Hopkins Clinical Scientist Award and grant 2014107 from the Doris Duke Charitable Foundation.
Footnotes
Accession codes. Whole-genome and whole-exome sequencing data have been deposited in the European Genome-phenome Archive (EGA) under accession EGAS00001000974.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
Author Contributions: K.W.K., N.P., B.V. and C.B. designed and supervised the project and analyzed the data. M.Z., Y.W., S.J., M.S., K.M. and Q.W. performed the sequencing studies and bioinformatics analysis. A.J.B., K.C., G.L.G., Z.L.G., G.J.R., J.-P.W. and C.B. contributed clinical samples and information. R.S., L.D.W., E.A.M. and R.H.H. performed pathological review and the immunohistochemistry experiments. M.Z., Y.W., S.J., M.S., A.J.B., K.C., G.L.G., Z.L.G., G.J.R., J.-P.W., L.D.W., E.A.M., R.H.H., K.W.K., N.P., B.V. and C.B. wrote and edited the manuscript.
Competing Financial Interests: The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Carey JC, et al. Ann NY Acad Sci. 1986;486:45–56. doi: 10.1111/j.1749-6632.1986.tb48061.x. [DOI] [PubMed] [Google Scholar]
- 2.Pasmant E, Vidaud M, Vidaud D, Wolkenstein PJ. Med Genet. 2012;49:483–489. doi: 10.1136/jmedgenet-2012-100978. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, et al. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasmant E, et al. Hum Mutat. 2010;31:E1506–E1518. doi: 10.1002/humu.21271. [DOI] [PubMed] [Google Scholar]
- 5.Rahrmann EP, et al. Nat Genet. 2013;45:756–766. doi: 10.1038/ng.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou C, et al. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, et al. Tumour Biol. 2014;35:6073–6082. doi: 10.1007/s13277-014-1804-5. [DOI] [PubMed] [Google Scholar]
- 8.Benoit YD, Laursen KB, Witherspoon MS, Lipkin SM, Gudas LJ. J Cell Physiol. 2013;228:764–772. doi: 10.1002/jcp.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sausen M, et al. Nat Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen BT, Knoepfer PS. Cancer Cell. 2013;24:567–574. doi: 10.1016/j.ccr.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorschner MO, Sybert VP, Weaver M, Pletcher BA, Stephens K. Hum Mol Genet. 2000;9:35–46. doi: 10.1093/hmg/9.1.35. [DOI] [PubMed] [Google Scholar]
- 12.López Correa C, Brems H, Lazaro C, Marynen P, Legius E. Am J Hum Genet. 2000;66:1969–1974. doi: 10.1086/302920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, et al. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brecqueville M, et al. Blood Cancer J. 2011;1:e33. doi: 10.1038/bcj.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ntziachristos P, et al. Nat Med. 2012;18:298–301. doi: 10.1038/nm.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koontz JI, et al. Proc Natl Acad Sci USA. 2001;98:6348–6353. doi: 10.1073/pnas.101132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, et al. Proc Natl Acad Sci USA. 2007;104:20001–20006. doi: 10.1073/pnas.0709986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y, Chen J, He Z, Xiao Y. Cell Physiol Biochem. 2013;31:778–784. doi: 10.1159/000350095. [DOI] [PubMed] [Google Scholar]
- 19.Lobry C, Oh P, Mansour MR, Look AT, Aifantis I. Blood. 2014;123:2451–2459. doi: 10.1182/blood-2013-08-355818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettegowda C, et al. Oncotarget. 2013;4:572–583. doi: 10.18632/oncotarget.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.