Introduction
Protein kinase C delta (PKCδ) has been cloned over 25 years ago [1] and many studies have identified diverse roles and substrates for this member of the family of PKC enzymes in normal and in pathological conditions. Although PKCδ has been implicated in diverse human diseases, including cancer [2], stroke [3], ischemic heart disease [4, 5], diabetes [6, 7], neurodegenerative diseases [8, 9], sepsis [10], etc., there are no approved drugs affecting this isozyme. In this brief review, we will discuss some of the challenges in drug development that will regulate the multi-functional, PKCδ.
Technical Approaches to Identifying The Role of PKCδ
Over the years, a variety of tools were used to determine the role of PKCδ in normal and disease states. They include pharmacological and genetic tools and they are described briefly in the following.
1. Competitive inhibitors of the catalytic site or the regulatory site of the enzyme
PKCδ is a member of the highly homologous, diacylglycerol-dependent Ser and Thr protein kinase C family (PKC) (Fig. 1). Because the catalytic domain of PKC is highly similar to many of the other protein kinases, in contrast to early reports, most of the competitive inhibitors of the ATP-binding site were found to be non-selective [11]. A common compound that was used to study the role of PKCδ is rottlerin [12]. However, rottlerin is a potent large conductance potassium channel opener, and it affects multiple other protein kinases [13]. Due to their non-selective nature, competitive inhibitors of the catalytic site are not optimal as tools to identify the role of PKCδ.
Figure 1.
PKCδ is a member of the family of highly homologous PKC isozymes. A scheme identifying the common, highly homologous regions C1, C2, C3 and C4, and the short intervening less homologous regions, V1, V2, V3, V4 and V5.
Another target for PKC regulators is focused on the regulatory domain of the enzyme. Initial studies used phorbol esters that bind to the diacylglycerol-binding site in the C1 domain (Fig. 1), at high (nM) affinity [14]. However, phorbol esters activate all the classical and novel PKC isozymes (Fig. 1) as well as some other non-PKC C1-containing proteins [15]. Another approach focused on generating isozyme-selective synthetic analogs of bryostatin, a family of natural products from the marine bryozoan, Bugula neritina; one analog improved selectivity for PKCδ [16]. However, activation by bryostatin is much longer lasting and subcellular localization of the activated isozyme may be different than when activated with diacylglycerol or other endogenous stimuli [17]. Therefore, in general, it has proven difficult to selectively activate PKCδ by focusing on the diacylglycerol site [18].
2. Genetic tools
Some of the data regarding the role of PKCδ were generated using genetic tools in which the enzyme was knocked out or over-expressed. However, conflicting data were obtained, even in the same or similar systems. For example, in one study, mice over-expressing PKCδ in basal epidermal keratinocytes exhibited attenuated progression of papilloma to squamous cell carcinoma (SCC) [19]. In contrast, another study showed that over-expression of PKCδ in human SCC lines and in mouse epidermis induced apoptosis and suppressed tumorigenicity, suggesting that PKCδ activation selectively inhibits SCC [20]. Notably, PKCδ−/− mice develop normally, and only under specific conditions exhibit a phenotype different from wild type, PKCδ+/+ mice. For example, PKCδ−/− have increased arteriosclerotic lesions in vein grafts as compared to wild-type mice [21] and PKCδ−/− smooth muscle cells are resistant to apoptosis by a variety of stimuli (UV, TNF-α, IL-1β, IFN-γ, and H2O2; [21]). Further, under ischemic conditions, the levels of enzymes related to energy and metabolism are altered in PKCδ−/− leading to a shift from glucose to lipid metabolism in the hearts of PKCδ−/− mice [22]. Therefore, the lack of severe phenotype in PKCδ null mice likely reflects compensation for the loss of this enzyme by other protein kinases, limiting the ability to assess the role of this enzyme in human diseases using genetic tools.
3. PKCδ-selective regulators of protein-protein interactions
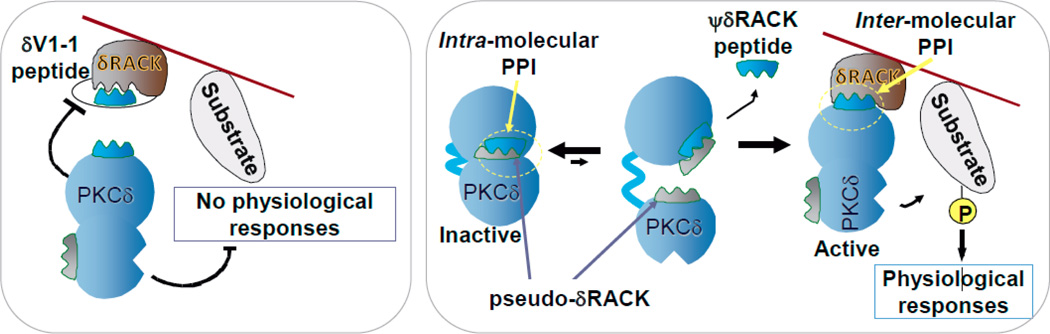
In 1991, we described the rational design and the application of an isozyme-selective inhibitor and an isozyme-selective activator of PKCδ [4]. These PKCδ-selective regulators are short peptides, derived from PKCδ; they inhibit or induce the activation and translocation of this isozyme, by selectively competing with PKCδ-selective protein-protein interactions (PPIs). The peptide inhibitor, δV1-1, inhibits a PPI between PKCδ and its anchoring protein, RACKδ. The peptide activator, ψδRACK, inhibits intramolecular PPI, which keeps PKCδ in the inactive form, thus leading to selective activation of PKCδ (Fig. 2; [23, 24]). δV1-1 corresponds to amino acids 8–17 in the C2 domain of PKCδ whereas ψδRACK corresponds to amino acids 74–81 in the C2 domain of PKCδ [4]; these sequences are unique to PKCδ, are conserved in evolution and are absent in the other PKC isozymes.
Figure 2.
A scheme describing PPIs as targets for selective regulators of PKCδ. Left: A scheme of a PPI peptide inhibitor of PKCδ interaction with its anchoring molecule, RACK. The peptide that corresponds to the RACK-binding site in the enzyme, competes with association of the activated PKCδ close to its substrate, thus leading to a loss of the physiological responses of PKCδ. Right: A scheme of a PPI peptide activator of PKCδ. The peptide, derived from a site on PKCδ that mimics the PKCδ-binding site on its RACK, is thus called pseudo-RACK site or ψRACK site. A peptide corresponding to ψRACK site competes with the intramolecular inhibitory PPI within PKCδ, leading to its activation. As the affinity of the RACK for PKCδ is greater, the peptide is replaced by the RACK, which brings the activated PKCδ near its substrate. Phosphorylation of that substrate leads to physiological responses. Many studies demonstrated the selectivity of these peptides for the given isozyme. Also reviewed in [23, 24, 31].
Although peptides are not cell permeable, cross-linking them to HIV TAT protein-derived cell permeable peptide, TAT47–57, enables the delivery of these peptides into cells when used in cells in culture as well as in vivo [25]. Effective delivery is observed following intraperitoneal injection (for acute treatment) or subcutaneous delivery, using an Alzet pump, for sustained delivery (e.g.3] and [9], respectively). The peptides are well tolerated at doses greater than 3 mg/Kg/day, even when delivered for many weeks, and can access all tissues, including across the blood-brain barrier [9].
Many PKCδ functions have been identified using these peptide regulators in our laboratory as well as in other laboratories. Here we focus on some of the PKCδ functions that were identified in our laboratory.
In the first study with these short peptide regulators, we showed that PKCδ increases damage induced by cardiac ischemia (heart attack) in vitro and in vivo [4]. These studies in rats and mice were subsequently confirmed also in a porcine model of heart attack [5]; both acute 70% reduction in infarct size and a sustained improvement in cardiac functions were observed. A statistically significant benefit was also observed in a small human study of patients with myocardial infarction when the peptide inhibitor, δV1-1, was delivered at reperfusion and directly through the previously occluded coronary artery, as in the porcine model [26]. We then showed that injury induced by PKCδ activation during reperfusion is not selective to cardiac myocyes. PKCδ activation in the microvasculature impairs blood flow in the infarcted tissue after opening the occluded artery due to induced swelling and apoptosis of the endothelial cells in the microvasulature [27]. Finally, one of the major benefits of PKCδ inhibition during reperfusion injury following myocardial infarction was found to be the increase in ATP regeneration; whereas in control-treated hearts ATP levels remained at 20% of basal, 30 minutes after treatment with δV1-1, ATP levels were at 70% of basal [5]. Therefore, an inhibitor of PKCδ reduces the overall damage to the myocardium and tissue necrosis following myocardial infarction by increasing ATP regeneration as well as keeping the microvascular patent during the reperfusion period.
In other studies, activation of PKCδ in skeletal muscle cells was found necessary for cell spreading in a mechanism mediated by the phosphorylation of MARCKS, and the reorganization of the actin cytoskeleton [28]. Selective activation of PKCδ with ψδRACK promoted attachment of these muscle cells to fibronectin, suggesting a role for this isozyme in muscle response to injury [28]. We also found that a brief treatment with the PKCδ inhibitor, δV1-1, prevents accelerated coronary artery disease in transplanted hearts in vivo in a murine cardiac allograft transplantation model [29]; cardiomyocyte apoptosis and caspase-3 and caspase-9 (but not caspase-8) activities were significantly reduced following δV1-1 treatment. PKCδ activity also inhibits proliferation of rat cardiac fibroblasts [30]; treatment with δV1-1 increased basal proliferation, but did not affect TGFβ1-induced cell proliferation, suggesting a role for this isozyme in tissue repair following injury. Together, these and other studies indicate potential use of PKCδ-selective agonists and antagonists as treatments for a variety of human diseases, including myocardial infarction, heart transplantation and the development of heart failure [31].
Other Challenges in Generating Regulators of PKCδ
1. PKCδ is activated by a variety of mechanisms
A challenge in assessing the functions of PKCδ is that in addition to its activation by the lipid-derived diacylglycerol [32], the function of PKCδ has been shown to be regulated by proteolysis, tyrosine phosphorylation, intracellular localization, oxidation and other protein modifications (reviewed in [33]; [34]). Further, PKCδ activation can be affected by cell type, the form and extent of different PKCδ protein modifications, as well as by co-stimulation with other signaling events [33]. It is likely that the different forms of activated PKCδ will not be equally sensitive to pharmacological agents targeting this enzyme. For example, PKCδ inhibitors or activators that bind to the regulatory domain of the enzyme will not affect the catalytic fragment of PKCδ, often found in cancer. Likewise, protein modifications may alter the interaction of the pharmacological agent with PKCδ. Therefore, it may be necessary to screen for PKCδ regulators using the enzyme in the specific state of modification (e.g.Tyr phosphorylation or oxidation) that is specific for the target pathology (e.g.cancer vs. ischemic injury in the heart).
2. PKCδ has multiple protein substrates
The use of the PKCδ-specific inhibitor and activator enabled the identification of PKCδ-specific substrates. PKCδ substrates were identified in a variety of subcellular sites, including the cytosolic M2 pyruvate kinase [35], the cytoskeletal proteins, MARCKS [28] and troponin [36], the nuclear protein, DNA-dependent protein kinase (DNA-PK) [37], the mitochondrial surface proteins, dynamin-related protein 1 [8] and BAD [38], and the mitochondrial matrix enzyme, pyruvate dehydrogenase reactivation [39].
By phosphorylating such diverse substrates at different subcellular locations, PKCδ was shown to be directly involved in diverse responses even within the same cell type subjected to the same stimulus. For example, we found that after myocardial infarction, activated PKCδ triggers cardiac myocyte apoptosis, possibly by affecting BAD phosphorylation (directly or indirectly) [38]. As discussed above, we also found that PKCδ activation leads to necrosis by inhibiting ATP regeneration; this occurs by direct phosphorylation and activation of pyruvate dehyderogenase kinase (PDK) [39]. Activated pyruvate dehydrogenase kinase, in turn, phosphorylates and inactivates pyruvate dehydrogenases, thus shutting off the Krebs cycle [39]. (Direct phosphorylation of PDK was observed in vitro and in hearts subjected to ischemia and reperfusion and δV1-1 inhibited this phosphorylation [39]). PKCδ activation also triggers endoplasmic reticulum stress-induced response and apoptosis [40, 41] in a mechanism that is dependent on tyrosine phosphorylation of PKCδ and its physical association with c-Abl. However, what substrates are phosphorylated by activated PKCδ in the endoplasmic reticulum and how this phosphorylation results in endoplasmic reticulum-dependent apoptosis is not known. We also found that PKCδ activation following ischemia and reperfusion induces excessive mitochondrial fission, fragmentation and dysfunction by phosphorylating and activation of the pro-mitochondrial fission protein, dynamin-related protein 1 (Drp1) by phosphorylating Ser579 in the protein [8]. Selective inhibition of Drp1 activation (using P110, a novel inhibitor of Drp1 that we designed) reduces infarct size and prevents development of heart failure following myocardial infarction [42]. These data suggest a central role for PKCδ-mediated and Drp1-dependent excessive mitochondrial fragmentation in cardiac ischemic injury. Finally, we found that PKCδ activation inhibits removal of damaged mitochondria (mitophagy) by lysosomal-like structures following ischemia-induced injury; this effect is mediated by a direct phosphorylation of mitochondria-associated glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on Thr246 residue by PKCδ [43]. We also showed that expression of a GAPDH Thr246Ala mutant promoted mitochondrial uptake increased mitophagy and reduced apoptosis following ischemia. How critical is PKCδ-mediated inhibition of mitophagy to cardiac infarction has not yet been determined, as a selective inhibitor of mitophagy has not yet been identified.
Open Questions
Do all these PKCδ-mediated phosphorylations contribute equally to cardiac infarction? Therefore, would inhibition of all of these phosphorylations in non-ischemic tissue be safe? Clearly, inhibition of phosphorylation of some of these substrates is cardioprotective; e.g.inhibiting the phosphorylation of the mitochondrial pyruvate dehyderogenase kinase by PKCδ directly increases ATP regeneration and thus the repair capacity of the myocardium [5]. However, it is possible that phosphorylation of some of the PKCδ substrates is essential for normal cellular functions and that inhibition of the phosphorylation of these substrates will result in worse cardiac damage.
Taken together, it is possible that an inhibitor of the phosphorylation of all the protein substrates of PKCδ is less desired than inhibitors of a subset of phosphorylation events. Our current research focuses on generating such inhibitors, which we termed separation of function inhibitors or SoFIs. Whether such inhibitors can be identified and whether they will provide better therapeutic effects remains to be determined.
Acknowledgement
The work described in this review was supported by an NIH grant HL52141 to DM-R.
Footnotes
NQ and DMR have no current conflict to report.
References
- 1.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J. Biol. Chem. 1988;263:6927–6932. [PubMed] [Google Scholar]
- 2.Kim J, Koyanagi T, Mochly-Rosen D. PKCdelta activation mediates angiogenesis via NADPH oxidase activity in PC-3 prostate cancer cells. The Prostate. 2011;71:946–954. doi: 10.1002/pros.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J. Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heartin vivo . Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 6.Pereira S, Park E, Mori Y, Haber CA, Han P, Uchida T, Stavar L, Oprescu AI, Koulajian K, Ivovic A, Yu Z, Li D, Bowman TA, Dewald J, El-Benna J, Brindley DN, Gutierrez-Juarez R, Lam TK, Najjar SM, McKay RA, Bhanot S, Fantus IG, Giacca A. FFA-induced hepatic insulin resistance in vivo is mediated by PKCdelta, NADPH oxidase, and oxidative stress. Am J Physiol Endocrinol Metab. 2014;307:E34–E46. doi: 10.1152/ajpendo.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat. Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C delta under oxidative stress conditionsin vivo . Mol. Biol. Cell. 2011;22:256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of delta PKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J. Clin. Invest. 2008;118:173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilpatrick LE, Standage SW, Li H, Raj NR, Korchak HM, Wolfson MR, Deutschman CS. Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-delta (delta-PKC) J. Leukoc. Biol. 2011;89:3–10. doi: 10.1189/jlb.0510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias J-M, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotech. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 12.Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 13.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazanietz MG, Wang S, Milne GW, Lewin NE, Liu HL, Blumberg PM. Residues in the second cysteine-rich region of protein kinase C delta relevant to phorbol ester binding as revealed by site-directed mutagenesis. J. Biol. Chem. 1995;270:21852–21859. doi: 10.1074/jbc.270.37.21852. [DOI] [PubMed] [Google Scholar]
- 15.Caloca MJ, Garcia-Bermejo ML, Blumberg PM, Lewin NE, Kremmer E, Mischak H, Wang S, Nacro K, Bienfait B, Marquez VE, Kazanietz MG. beta2-chimaerin is a novel target for diacylglycerol: binding properties and changes in subcellular localization mediated by ligand binding to its C1 domain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11854–11859. doi: 10.1073/pnas.96.21.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wender PA, Loy BA, Schrier AJ. Translating Nature's Library: The Bryostatins and Function-Oriented Synthesis. Israel journal of chemistry. 2011;51:453–472. doi: 10.1002/ijch.201100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp. Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 18.Irie K, Nakahara A, Nakagawa Y, Ohigashi H, Shindo M, Fukuda H, Konishi H, Kikkawa U, Kashiwagi K, Saito N. Establishment of a binding assay for protein kinase C isozymes using synthetic C1 peptides and development of new medicinal leads with protein kinase C isozyme and C1 domain selectivity. Pharmacol. Ther. 2002;93:271–281. doi: 10.1016/s0163-7258(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 19.Jansen AP, Dreckschmidt NE, Verwiebe EG, Wheeler DL, Oberley TD, Verma AK. Relation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor–promotion susceptibilities of protein kinase Cα, -δ and -ϵ transgenic mice. Int. J. Cancer. 2001;93:635–643. doi: 10.1002/ijc.1395. [DOI] [PubMed] [Google Scholar]
- 20.Yadav V, Yanez NC, Fenton SE, Denning MF. Loss of protein kinase C delta gene expression in human squamous cell carcinomas: a laser capture microdissection study. Am. J. Pathol. 2010;176:1091–1096. doi: 10.2353/ajpath.2010.090816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J. Clin. Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayr M, Chung YL, Mayr U, McGregor E, Troy H, Baier G, Leitges M, Dunn MJ, Griffiths JR, Xu Q. Loss of PKC-delta alters cardiac metabolism. Am J Physiol Heart Circ Physiol. 2004;287:H937–H945. doi: 10.1152/ajpheart.00877.2003. [DOI] [PubMed] [Google Scholar]
- 23.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat. Biotechnol. 1998;16:919–924. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 24.Qvit N, Mochly-Rosen D. Highly specific modulators of protein kinase C localization: applications to heart failure. Drug Discovery Today: Disease Mechanisms. 2010;7:e87–e93. doi: 10.1016/j.ddmec.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivoprotein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 26.Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N, Mochly-Rosen D, Roe M, Sadowski Z, Solomon S, Widimsky P. Direct Inhibition of delta-Protein Kinase C Enzyme to Limit Total Infarct Size in Acute Myocardial Infarction (DELTA MI) Investigators. Circulation. 2008;117:886–896. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- 27.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Impaired perfusion after myocardial infarction is due to reperfusion-induced delta PKC-mediated myocardial damage. Cardiovasc. Res. 2007;73:699–709. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disatnik MH, Boutet SC, Lee CH, Mochly-Rosen D, Rando TA. Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J. Cell Sci. 2002;115:2151–2163. doi: 10.1242/jcs.115.10.2151. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T, Mochly-Rosen D, Robbins RC. Suppression of graft coronary artery disease by a brief treatment with a selective epsilon PKC activator and a delta PKC inhibitor in murine cardiac allografts. Circulation. 2004;110:II194–II199. doi: 10.1161/01.CIR.0000138389.22905.62. [DOI] [PubMed] [Google Scholar]
- 30.Braun MU, Mochly-Rosen D. Opposing effects of delta- and zeta-protein kinase C isozymes on cardiac fibroblast proliferation: use of isozyme-selective inhibitors. J. Mol. Cell. Cardiol. 2003;35:895–903. doi: 10.1016/s0022-2828(03)00142-1. [DOI] [PubMed] [Google Scholar]
- 31.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nature Reviews Drug Discovery. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogita K, Miyamoto S, Yamaguchi K, Koide H, Fujisawa N, Kikkawa U, Sahara S, Fukami Y, Nishizuka Y. Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1592–1596. doi: 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Pal D. Two faces of protein kinase C delta: the contrasting roles of PKC delta in cell survival and cell death. ScientificWorldJournal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siwko S, Mochly-Rosen D. Use of a novel method to find substrates of protein kinase C delta identifies M2 pyruvate kinase. Int. J. Biochem. Cell Biol. 2007;39:978–987. doi: 10.1016/j.biocel.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jideama NM, Noland TA, Jr., Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J. Biol. Chem. 1996;271:23277–23283. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- 37.Bharti A, Kraeft SK, Gounder M, Pandey P, Jin S, Yuan ZM, Lees-Miller SP, Weichselbaum R, Weaver D, Chen LB, Kufe D, Kharbanda S. Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Mol. Cell. Biol. 1998;18:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J. Biol. Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 39.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of delta PKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 40.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. J. Mol. Cell. Cardiol. 2007;43:420–428. doi: 10.1016/j.yjmcc.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi X, Mochly-Rosen D. The PKCdelta -Abl complex communicates ER stress to the mitochondria - an essential step in subsequent apoptosis. J. Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- 42.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute Inhibition of Excessive Mitochondrial Fission After Myocardial Infarction Prevents Long-term Cardiac Dysfunction. Journal of the American Heart Association. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yogalingam G, Hwang S, Ferreira JC, Mochly-Rosen D. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) phosphorylation by protein kinase Cdelta (PKCdelta) inhibits mitochondria elimination by lysosomal-like structures following ischemia and reoxygenation-induced injury. J. Biol. Chem. 2013;288:18947–18960. doi: 10.1074/jbc.M113.466870. [DOI] [PMC free article] [PubMed] [Google Scholar]