Abstract
Prostaglandin E2 (PGE2) is a pro-inflammatory lipid mediator that promotes cancer growth. The 15-hydroxyprostaglandin dehydrogenase (15-PGDH) catalyzes oxidation of the 15(S)-hydroxyl group of PGE2, leading to its enzymatic inactivation. Therefore, 15-PGDH induction may offer a strategy to treat cancers that are driven by PGE2, such as human cholangiocarcinoma. Here we report that omega-3 polyunsaturated fatty acids (omega-3 PUFA) upregulate 15-PGDH expression by inhibiting miR26a and miR26b, thereby contributing to omega-3 PUFA-induced inhibition of human cholangiocarcinoma cell growth. Treatment of human cholangiocarcinoma cells (CCLP1 and TFK-1) with ω-3 PUFA (DHA) or transfection of these cells with the Fat-1 gene (encoding Caenorhabditis elegans desaturase which converts ω-6 PUFA to ω-3 PUFA) significantly increased 15-PGDH enzymes levels, but with little effect on the activity of the 15-PGDH gene promoter. Mechanistic investigations revealed that this increase in 15-PGDH levels in cells was mediated by a reduction in the expression of miRNA26a and miRNA26b, which target 15-PGDH mRNA and inhibit 15-PGDH translation. These findings were extended by the demonstration that overexpressing miR26a or miR26b decreased 15-PGDH protein levels, reversed omega-3 PUFA-induced accumulation of 15-PGDH protein and prevented omega-3 PUFA-induced inhibition of cholangiocarcinoma cell growth. We further observed that omega-3 PUFA suppressed miRNA26a and miRNA26b by inhibiting c-myc, a transcription factor that regulates miR-26a/b. Accordingly, c-myc overexpression enhanced expression of miRNA26a/b and ablated the ability of omega-3 PUFA to inhibit cell growth. Taken together, our results reveal a novel mechanism for omega-3 PUFA-induced expression of 15-PGDH in human cholangiocarcinoma and provide a preclinical rationale for the evaluation of omega-3 PUFA in treatment of this malignancy.
Keywords: ω-3 PUFA, 15-PGDH, miR-26, cholangiocarcinoma
INTRODUCTION
Cholangiocarcinoma (CCA) is a highly malignant cancer of the biliary tract with poor prognosis(1–12). The incidence and mortality of cholangiocarcinoma is rising worldwide and currently there is no effective chemoprevention or treatment. The tumor often arises from background conditions that cause long-standing inflammation, injury and reparative biliary epithelial cell proliferation, such as primary sclerosing cholangitis (PSC), clonorchiasis, hepatolithiasis or complicated fibropolycystic diseases. Intrahepatic cholangiocellular carcinoma is also associated with hepatitis C viral infection and liver cirrhosis secondary to other nonbiliary causes. The carcinogenic processes involve a series of sequential events including chronic inflammation, cholangiocyte proliferation, dysplasia and ultimately malignant transformation.
Consistent with the strong association between bile duct chronic inflammation and cholangiocarcinogenesis, compelling evidence(10, 13–28) have shown that mediators of inflammation, such as prostaglandins (PGs), play an important role in cholangiocarcinogenesis. For example, elevated expression of COX-2 has been documented in CCA cells and pre-cancerous bile duct lesions but not in normal bile duct epithelial cells(21–25); the expression of COX-2 is induced by proinflammatory cytokines as well as bile acids(29, 30). Overexpression of COX-2 in human CCA cells enhances PGE2 production and promotes tumor growth, whereas depletion of COX-2 reduces PGE2 production and prevents growth(14, 15). PGE2 treatment is known to increase CCA cell growth and prevent apoptosis(13–15, 26–28). Accordingly, inhibition of COX-2 by molecular and pharmacological approaches prevents the growth and invasion of CCA cells in vitro and in animal models(10, 14, 15, 22, 25, 27, 28). These findings provide important preclinical evidence for targeting COX-2 in prevention and treatment of human CCA. However, as some COX-2 inhibitors are known to be associated with increased cardiovascular side effect(31–34), there is an urgent and practical need to identify COX-2 downstream target for effective anti-CCA therapy with fewer side effects.
The amount of biologically active PGE2 in the inflammatory and tumor microenvironment is regulated by the balance between PGE2 synthesis and degradation. While previous studies have focused on the role of COX-2 in carcinogenesis, the role of PGE2 degradation enzyme, the NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH), has not been recognized until recently. 15-PGDH catalyzes oxidation of the 15(S)-hydroxyl group of PGE2, converting PGE2 into 15-keto-PGE2; this enzymatic reaction leads to reduction of the pro-inflammatory and pro-tumorigenic PGE2(35). Indeed, accumulating evidence suggests that 15-PGDH is an important tumor suppressor in a number of human cancers including cholangiocarinoma(36).
While the pro-inflammatory and pro-carcinogenic PGs are synthesized from arachidonic acid (AA), a ω-6 PUFA; this process is competitively inhibited by É-3 polyunsaturated fatty acids (É-3 PUFAs). The lipid mediators derived from ω-6 and ω-3 PUFA are metabolically distinct and often have opposing physiological and pathological functions; for example, ω-6 PUFA-derived eicosanoids tend to promote inflammation and carcinogenesis, while ω-3 PUFA-derived lipid mediators largely inhibit inflammation and prevent carcinogenesis (or less promotional for inflammation and proliferation). In the current study, we report that ω-3 PUFA (but not ω-6 PUFA) up-regulates the expression of 15-PGDH by inhibiting miR26a and miR26b in human cholangiocarcinoma cells. We show that 15-PGDH is a bona fide target of miR26a and miR26b. Our findings provide novel evidence for ω-3 PUFA-regulated miR26a/b and 15-PGDH cascade and support ω-3 PUFA as a non-toxic therapeutic agent for the treatment of human cholangiocarcinoma.
MATERIALS AND METHODS
Materials
Docosahexaenoic acid (DHA) and arachidonic acid (AA) were purchased from Cayman Chemical (Ann Arbor, MI). miR26a and miR26b lentiviral particles were purchased from GeneCopoeia (Rockville, MD). 15-PGDH 3’UTR-luciferase reporter was obtained from ORIGENE (Rockville, MD). Rabbit polyclonal antibody against 15-PGDH was purchased from Cayman chemical (Ann Arbor, MI). Rabbit polyclonal antibody against c-myc was purchased from Santa Cruz Biotechnology (Dallas, TX). Mouse monoclonal antibodies against CTDSPL and CTDSP1 were purchased from Abcam (Cambridge, MA). Mouse monoclonal antibodies against β-actin were purchased from Sigma-Aldrich (St. Louis, MO). siRNA against 15-PGDH was synthesized by ORIGENE (Rockville, MD). NOD CB17-prkdc/SCID mice were purchased from Jackson lab (Bar Harbor, Maine) and maintained in Tulane transgenic mice facility according to the protocol approved by the American Association for Accreditation of Laboratory Animal Care. All primers used in this study were synthesized by Integrated DNA Technologies (IDT, Coralville, IA) (Supplementary Table 1). All chemical reagents were analytical grades (Sigma, St. Louis, MO).
Cell culture
Two human cholangiocarcinoma cell lines, CCLP1 and TFK-1, were used in this study. TFK-1 cells (37) were obtained from the Japanese Cancer Research Resources Bank where the cell line was tested and authenticated by DNA fingerprinting, isozyme detection, cell vitality assay and mycoplasma detection. Authentication of CCLP1 cells (38) was based on morphologic features, immunohistochemistry, ultrastructural analysis and cytogenetic analysis, and the cells were cultured as we previously described (14, 17, 19). The TFK-1 and CCLP1 were maintained in RPMI-1640 and DMEM culture medium, respectively. All culture medium were supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin and streptomycin. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. All cell lines were used at low passage number not exceeding 30 passages.
Stable cell lines
CCLP1 and TFK-1 cells were transfected with Fat-1 expression plasmid or control vector pcDNA3 and then maintained in complete culture medium with 0.2 ug/ml puromycin (life technology, Grand Island, NY). CCLP1 and TFK-1 cells were also infected with miR26a/b lentivirus or miRNA-scramble control and the cells were maintained in culture medium with 0.2 mg/ml Geneticin (life technology, Grand Island, NY). Medium is replaced every 3 days for 2–4 weeks until outgrowth of resistant cells. The resistant cells were harvested and maintained in culture media with selection agents for further use.
Gene expression analysis
Total RNA was extracted according to TRIzol® Reagent method (life technology, Grand Island, NY). mRNA levels were quantified by using RT2 SYBR® Green qPCR kit (QIAGEN, Germantown, MD); GAPDH is measured as reference gene. miRNA levels were quantified by using miScript Primer Assays kit (QIAGEN, Germantown, MD); U6 is measured as reference gene. Primers used are listed in Supplementary Table 1. For Western blotting analyses, whole cell lysate were prepared by using RIPA lysis buffer with protease inhibitor cocktail tablets (Roche Diagnostics, Indianapolis, Indiana). Cellular proteins were separated by SDS-PAGE electrophoresis and transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was blocked by PBS-T (0.5% Tween 20 in PBS) containing 5% nonfat milk for 1h room temperature, and then incubated with individual primary antibodies in PBS-T containing 5% nonfat milk for 2–5h at room temperature with the dilutions specified by the manufacturers. Following three washes with PBS-T, the membranes were incubated with IRDye 680LT/IRDye 800CW secondary antibodies (LI-COR Biosciences, Lincoln, NE) in PBS-T for 1 h room temperature. The membranes were then washed with PBS-T and the protein bands were visualized by using the ODYSSEY infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Dual-Luciferase Reporter Assay
Cells were co-transfected with luciferase reporter (15-PGDH promoter-luciferase, 15-PGDH 3’UTR-luciferase) and pRL-TK (Promega, Madison, WI). pRL-TK provides the constitutive expression of Renvilla luciferase that was used as an internal control. 72h after transfection, cells were collected and passively lysed. Luciferase activities in the extracts were measured by DLReady Centro XS3 LB960 luminometer with the use of Dual-Luciferase Reporter (DLR™) Assay kit (Promega, Madison, WI). Luciferase activity was measured against Renilla luciferase activity for transfection efficiency.
ChIP Assay
Cells were cross-linked by 1% formaldehyde for 10min. Chromosome DNA was extracted according to the protocol provided by SimpleChIP Assay Kits (Cell signaling, Danvers, MA) and precipitated by using specific c-myc Rabbit polyclone antibody. Rabbit polyclone antibody Histone 3 was used as positive control while Rabbit IgG was used as negative control. Regular PCR procedure (5min at 94°C, followed by 30 cycles of 30s at 94°C, 30s at 55°C, 30s at 72°C, ended by 10min at 72°C) was adopted to amplify c-myc binding site sequence. Primers were listed in Supplementary Table 1.
Cell proliferation assay
5 × 103 cells were plated in each well of 96-well plates and synchronized in G0 phase by serum deprivation. Growth arrest was released by adding 2% serum. WST-1 reagent (Roche Diagnostics, Indianapolis, Indiana) was used to detect cell proliferation rate according to the manufacturer’s instructions. Each point in cell growth curve represents the mean of three independent normalized OD450 reads.
Colony forming Assay
1 × 103 cells were plated in 10-cm dish and allowed to grow for 14 days. The colonies were stained with crystal violet (Amersco, Solon, OH). The colonies in each dish were counted.
TUNEL assay
Cells (1×104 per well) were seeded in 8 well-chamber slide and cultured overnight. Then, the cells were fixed by 4% formaldehyde in PBS for 25 min. The cell apoptosis on the slide was detected according to the protocol of Deadend™ colorimetric TUNEL system (Promega, Madison, WI).
Xenograft tumor study in SCID mice
SCID mice were injected subcutaneously at the axillary area with indicated groups of CCLP1 cells (1×107 cells in 100µl of PBS). The mice were closely monitored for tumor growth and sacrificed 35 days post inoculation to recover the tumors. The tumor volume was measured and calculated by using the formula: larger diameter × (smaller diameter)2/2. RNA was extracted from recovered tumor tissues using TRIzol® Reagent (life technology, Grand Island, NY) to measure the level of miR-26a. Proteins from the tumor tissues were extracted by using NP-40 lysis buffer for Western blotting analysis.
Intrahepatic tumor growth via splenic injection
General anesthesia in mice was induced by Fluriso (Vetone, BOISE, ID). The abdominal cavity was opened by a 0.5 cm left sided transverse laparotomy. The spleen was identified, and 1 × 106 cells (with or without 15-PGDH knockdown) in a total volume of 100µl PBS were injected into the spleen. After tumor cell inoculation, the spleen was resected and the abdominal cavity was closed by a running 3/0 braided silk suture (CP medical, Portland, OR). The mice were intraperitoneally injected with 200µl DHA (0.5mg/ml, dissolved in BSA solution) or BSA control every 2 days (starting 2 days after surgery). Five weeks after DHA treatment, the mice were sacrificed and the livers were removed to document tumor growth parameters (tumor volume was calculated by using the formula: larger diameter × [smaller diameter]2/2).
Statistics
Results are presented as mean ± standard error from a minimum of 3 replicates. Difference between groups was evaluated by SigmaPlot statistical software with unpaired analysis of student’s t-test and one-way analysis of variance. P<0.05 was considered as statistically significant.
RESULTS
ω-3 PUFAs induces 15-PGDH expression in human cholangiocarcinoma cells
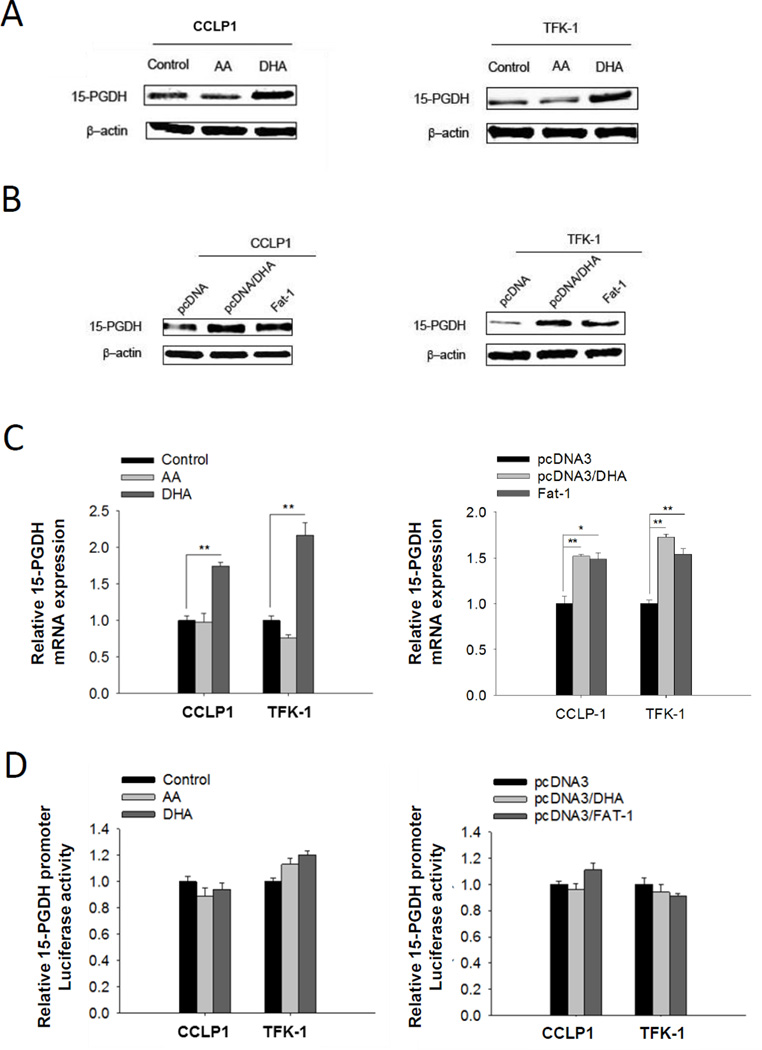
We compared the effect of ω-3 PUFA (DHA) versus ω-6 PUFA (AA) on 15-PGDH expression in human cholangiocarcinoma cell lines (CCLP1 and TFK-1). While DHA treatment increased the level of 15-PGDH protein, AA treatment exhibited no effect (Figure 1A and Supplementary Figure S1). In separate experiments, we stably transfected CCLP1 and TFK-1 cells with vector expressing the Fat-1 gene (which encodes a C. elegans ω-3 fatty-acid desaturase converting ω-6 to ω-3 fatty acids (39)). Overexpression of the Fat-1 gene was also found to increase 15-PGDH protein expression; the effect of Fat-1 gene transfection on 15-PGDH is comparable to DHA treatment of control cells (Figure 1B). Additionally, the levels of 15-PGDH mRNA were also elevated in DHA treated or Fat-1 overexpressed cells (Figure 1C). DHA treatment or Fat-1 expression did not alter 15-PGDH promoter activity, as reflected by the 15-PGDH promoter-luciferase assay (Figure 1D).
Figure 1. ω-3 PUFAs induces 15-PGDH expression in human cholangiocarcinoma cells.
(A) 15-PGDH protein level increased in cholangiocarcinoma cells cultured with ω-3 PUFA DHA but not with ω-6 PUFA AA. CCLP1 or TFK-1 cells were synchronized by serum deprivation, and then maintained in serum-free medium containing 50 µM DHA or AA for 12 h; (B) 15-PGDH protein level increased in cholangiocarcinoma cells expressing Fat-1 which converts ω-6 PUFA to ω-3 PUFA. CCLP1 or TFK-1 cells stably transfected with Fat-1 expression vector or control vector were synchronization by serum deprivation. The cells weer treated with or without 50 µM DHA in serum-free medium for 12 h. Total protein was analyzed by Western blotting with 15-PGDH antibody. β-actin was measured as a reference gene. (C) DHA treatment or Fat-1 gene expression increases 15-PGDH mRNA level in cholangiocarcinoma cells. 15-PGDH mRNA was measured by real-time PCR. Results were normalized to control group; the data were shown with mean±SE. *P<0.05, **P<0.01; (D) 15-PGDH promoter activity was not influenced by DHA treatment or Fat-1 expression in cholangiocarcinoma cells. CCLP1 or TFK-1 cells were co-transfected with 15-PGDH promoter-luc3 and pRL-TK plasmid which code Renilla luciferase. 72h after transfection, Luciferase activity was measured. Results were normalized to control group. Data were shown with mean±SE.
ω-3 PUFAs suppress miR26a/b and prevent their targeting of 15-PGDH in cholangiocarcinoma cells
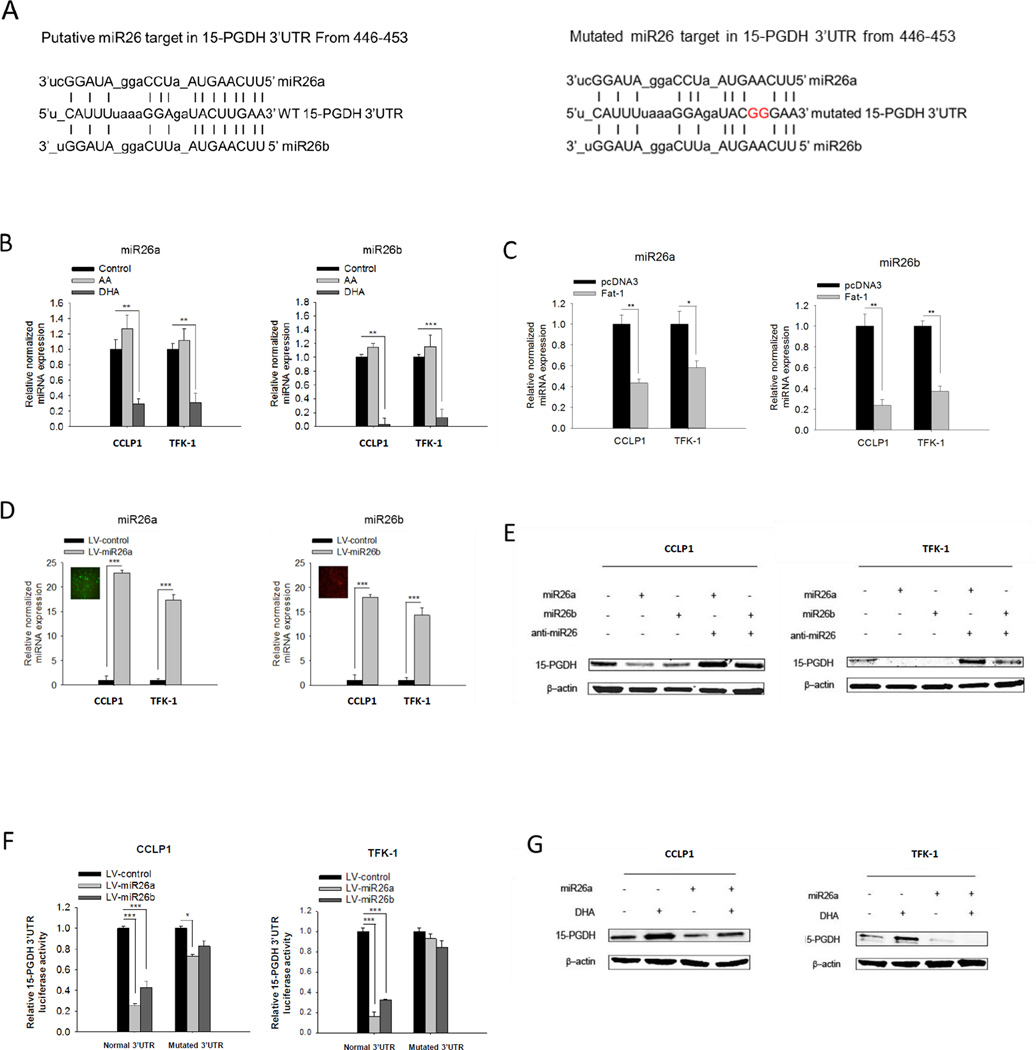
As ω-3 PUFA increased 15-PGDH protein and mRNA levels without induction of 15-PGDH promoter activity, we reasoned that ω-3 PUFA might regulate 15-PGDH gene expression through post-transcriptional mechanism. We directed out attention to microRNAs which could potentially bind to the 15-PGDH 3’UTR. Sequence analysis identified four conserved microRNAs (miR26a, miR26b, miR1297, miR4465) that are complementary to the 15-PGDH 3’UTR (Figure 2A and Supplementary Figure S2). Among these 4 microRNAs, miR26a and miR26b were found to be highly expressed in cholangiocarcinoma cells relative to the other two (miR1297, miR4465) (Supplementary Figure S2). We next performed qRT-PCR analysis to determine whether ω-3 PUFAs might alter the expression of these miRNAs. As shown in Figure 2B and 2C, DHA treatment or Fat-1 overexpression decreased the levels of miR26a and miR26b, but not the other two miRNAs (miR1297 and miR4465). These findings suggest that ω-3 PUFA may induce 15-PGDH expression through alteration of miR26a and/or miR26b.
Figure 2. ω-3 PUFAs suppress miR26a/b and prevent their targeting of 15-PGDH in cholangiocarcinoma cells.
(A) Putative miR26a and miR26b binding site in normal 3′-UTR or mutated 3′-UTR of 15-PGDH mRNA. (B) DHA, but not AA, decreases the levels of miR26a and miR26b in cholangiocarcinoma cells. CCLP1 or TFK-1cells were synchronized by serum deprivation, and then maintained in serum-free medium containing 50 µM DHA or AA for 12 h; (C) Fat-1 expression decreased miR26a and miR26b levels in cholangiocarcinoma cells. (D) The levels of miR26a or miR26b in cholangiocarcinoma cells infected with respective lentiviral vectors. CCLP1 or TFK-1 cells were infected with miR26a or miR26b lentivirus and then subject to Geneticin selection. miR26a and miR26b were measured by real-time PCR. Results were normalized to control group. Data were shown with mean±SE. ***P<0.001. (E) miR26a or miR26b suppressed 15-PGDH expression. CCLP1 or TFK-1 cells overexpressing miR26a or miR26b were transfected with anti-miR26 or scramble control. Total protein was analyzed 72h after transfection by Western blotting using 15-PGDH antibody. β-actin was measured as a reference gene. (F) miR26a or miR26b target 15-PGDH mRNA 3’UTR. CCLP1 and TFK-1 cells overexpressing miR26a or miR26b were transfected with 15-PGDH 3’UTR luciferase reporter vector or mutated construct. 72 hours after transfection, the cell lysates were obtained to measure luciferase activity. The results were normalized to control group and the data were presented as mean±SE. *P<0.05, ***P<0.001. (G) miR26a expression prevented ω-3 PUFA-induced 15-PGDH expression. CCLP1 and TFK-1 cells overexpressing miR26a were treated with or without 50 µM DHA for 12 h. Cellular proteins were analyzed by Western blotting using 15-PGDH antibody (β-actin was measured as a reference gene).
To further determine the effect of miR26a and miR26b on 15-PGDH, we infected human cholangiocarcinoma cells with lentivirus particles carrying miR26a (green) or miR26b gene (red) (Figure 2D); these cells were then analyzed for 15-PGDH protein expression. As shown in Figure 2E, overexpression of miR26a or miR26b significantly reduced 15-PGDH protein in both CCLP1 and TFK-1 cells and the effects were reversed by anti-miR26. We next measured the 15-PGDH 3’UTR-luciferase reporter activities in miR26a or miR26b overexpressed or control cells. As shown in Figure 2F, miR26a or miR26b overexpression decreased the 15-PGDH 3’UTR luciferase reporter activity; this effect was abolished when the miR26a/b binding sites were mutated. These results establish 15-PGDH as a direct target of miR26a/b. Accordingly, we observed that overexpression of miR26a or miR26b prevented DHA-induced 15-PGDH protein accumulation (Figure 2G).
Taken together, our findings suggest that ω-3 PUFAs induce 15-PGDH protein accumulation through suppression of miR26a/b in human cholangiocarcinoma cells.
C-myc is implicated in ω-3 PUFA-induced suppression of miR26a/b
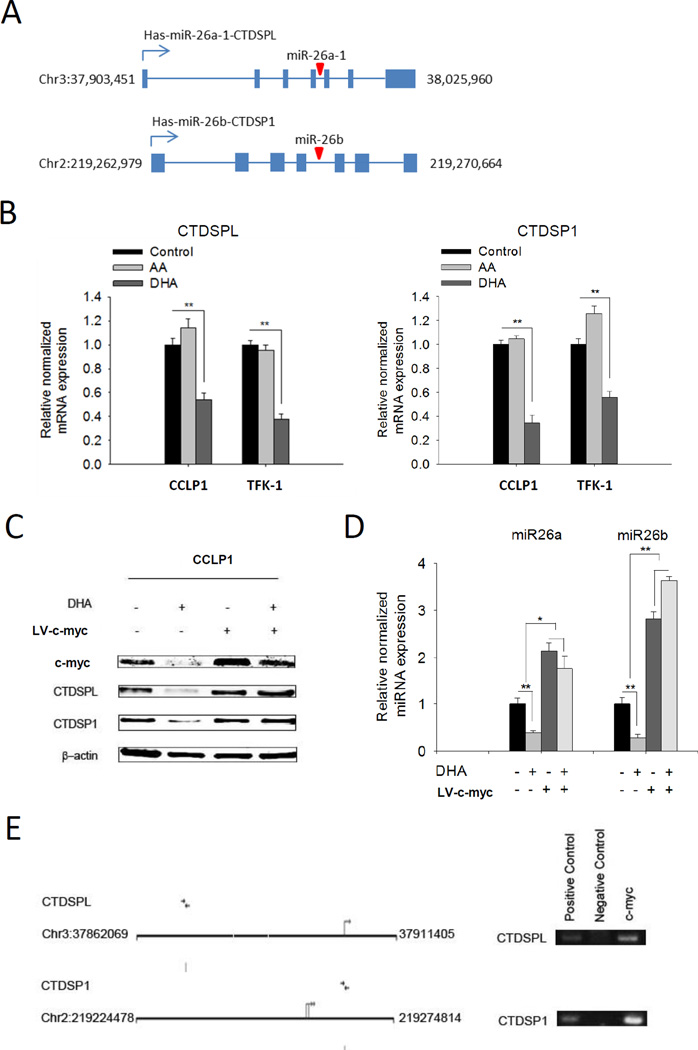
miR26a/b are located in the introns of CTDSPs (carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase) gene family (40, 41) (illustrated in Figure 3A). Given that the expression of miR26a/b is reported to be concomitant with their host genes (40), we measured the mRNA level of CTDSPs (CTDSPL and CTDSP1) in cholangiocarcinoma cells treated with ω-3 PUFA. Our data showed that the ω-3 PUFA DHA suppressed the expression of both CTDSPL and CTDSP1, whereas the ω-6 PUFA AA had no effect (Figure 3B). The pattern of CTDSPL and CTDSP1 alterations appears to be similar to their intronic microRNAs, suggesting that miR26a/b and their host genes are co-regulated by ω-6 PUFA in cholangiocarcinoma cells.
Figure 3. C-myc is implicated in ω-3 PUFA-induced suppression of miR26a/b.
(A) Schematic representation of gene map for miR26a/b and their host gene CTDSPL and CTDSP1; (B) DHA, but not AA, decreases the mRNA levels of miR26a/b host genes CTDSPL/CTDSP1 in cholangiocarcinoma cells. CCLP1 or TFK-1 cells were synchronized by serum deprivation, and then maintained in serum-free medium containing 50 µM DHA or AA for 12 h. CTDSPL or CTDSP1 mRNA was measured by real-time PCR. Results were normalized to control group; the data were shown with mean±SE. **P<0.01. (C) c-myc overexpression prevents DHA induced inhibition of CTDSPL and CTDSP1. CCLP1 cells infected with c-myc lentivirus or scramble control were maintained in serum free culture medium with or without 50 µM DHA for 12 h. Cellular proteins were analyzed by Western blotting with antibody against c-myc, CTDSPL and CTDSP1, respectively. β-actin were measured as reference gene. (D) c-myc overexpression prevents DHA induced inhibition of miR26a or miR26b. CCLP1 cells infected with c-myc lentivirus or scramble control were treated with or without 50 µM DHA for 12 h. The levels of miR26a and miR26b were measured by real-time PCR. Results were normalized to control group; data were shown with mean±SE. *P<0.05, **P<0.01; (E) c-myc binds to CTDSPL and CTDSP1 promoter region. Putative c-myc binding sites of CTDSPL and CTDSP1 promoter are shown. ChIP assay was performed by using antibody against c-myc to precipitate chromosome; antibody against Histone 3 was used as a positive control and Rabbit IgG as a negative control. Purified precipitating DNA was analyzed by PCR with primers amplifying c-myc binding regions in CTDSPL and CTDSP1 gene promoters.
The expression of CTDSPs is well-known to be associated with the transcription factor c-myc. Given that c-myc is a downstream gene of Wnt signaling (42–44) and that ω-3 PUFA suppresses Wnt pathway (45, 46), we sought to further examine whether c-myc might be implicated in ω-3 PUFA-mediated suppression of CTDSPs/miR26s. Our data showed that DHA treatment decreased c-myc along with reduction of CTDSPs/miR26s (Figure 3C, 3D). Importantly, DHA-induced reduction of CTDSPs/miR26s was partially revered by overexpression of c-myc (Figure 3C, 3D). ChIP assay showed that c-myc was associated with the promoters of the CTDSPL/miR26a and CTDSP1/miR26b gene clusters (Figure 3E). These findings suggest that ω-3 PUFA regulates the expression of miR26a/b at least in part through c-myc.
Overexpression of miR26a prevents Fat-1-induced inhibition of cholangiocarcinoma growth
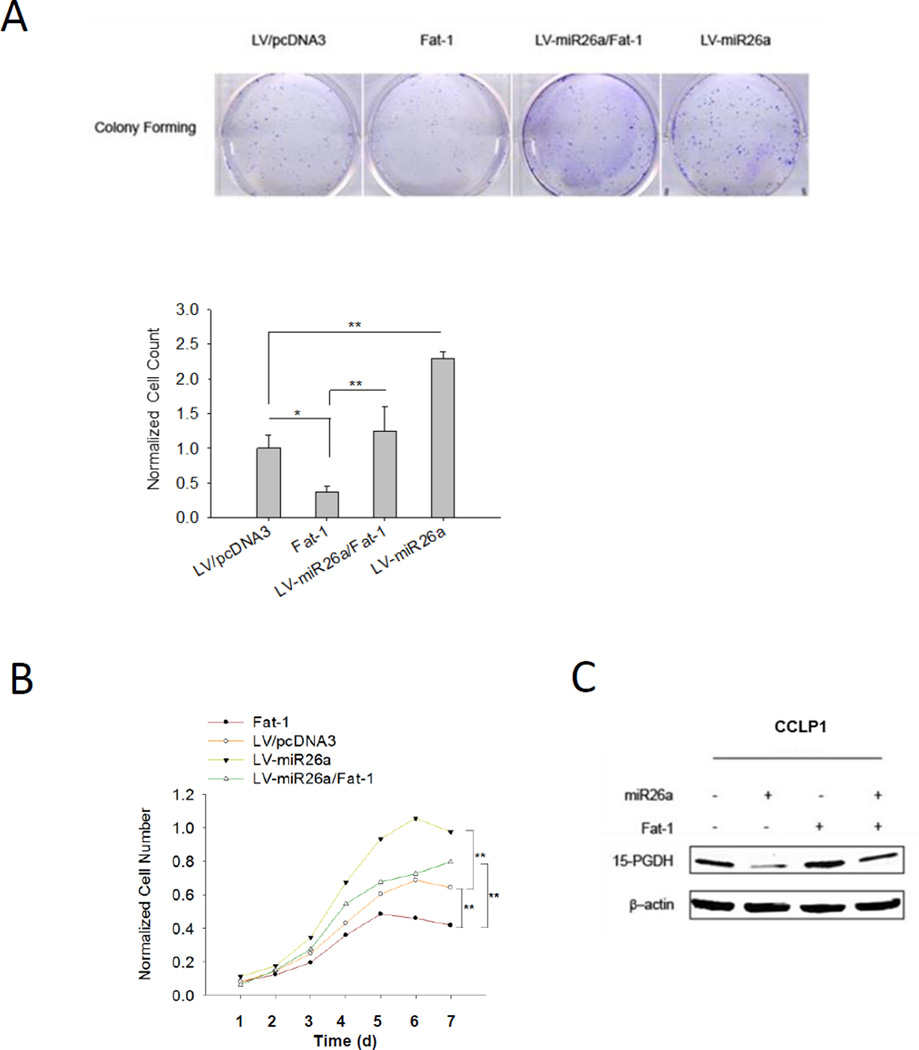
To further determine the role of miR26/15-PGDH in ω-3 PUFA-induced inhibition of cholangiocarcinoma cell growth, we evaluated the growth parameters of tumor cells overexpressing Fat1 and/or miR26a. As shown in Figure 4A and 4B, overexpression of miR26a abolished Fat-1-induced inhibition of CCLP1 cell growth and colony formation, in vitro. TUNEL assay showed that Fat-1-induced CCLP1 cell apoptosis was partially reversed by miR26a overexpression (Supplementary Figure 3) (this result is consistent with our previous report that ω-3 PUFAs inhibit cholangiocarcinoma predominantly through induction of apoptosis(19)). Our further Western blotting analysis confirmed that miR26a overexpression attenuated Fat-1-induced induction of 15-PGDH (Figure 4C).
Figure 4. Overexpression of miR26a prevents Fat-1-induced inhibition of cholangiocarcinoma growth in vitro.
Different groups of CCLP1 cells (Fat-1 expression, miR26a overexpression, Fat-1/miR26a co-expression and control) were analyzed for cell proliferation by WST-1 assay and by colony formation assay. (A) Overexpression of Fat-1 inhibited CCLP1 colony forming ability; this effect was reversed by overexpression of miR-26a. Overexpression of miR26a alone was found to enhance CCLP1 colony formation efficiency. Representative of three independent experiments were showed in upper panel. Quantified results were normalized to control group and presented as mean±SE in lower panel; *P<0.05; **P<0.01; (B) Overexpression Fat-1 inhibited CCLP1 growth in vitro; this effect was reversed by miR26a overexpression. Overexpression miR26a alone significantly enhanced CCLP1 growth. The data are presented as mean±SE from 3 independent experiments. **P<0.01; (C) The levels of 15-PGDH protein in CCLP1 cells with Fat-1 overexpression, miR26a overexpression, Fat-1/miR26a co-expression, or control vector cells. Total protein was analyzed by Western blot with 15-PGDH antibody. β-actin were measured as a reference gene.
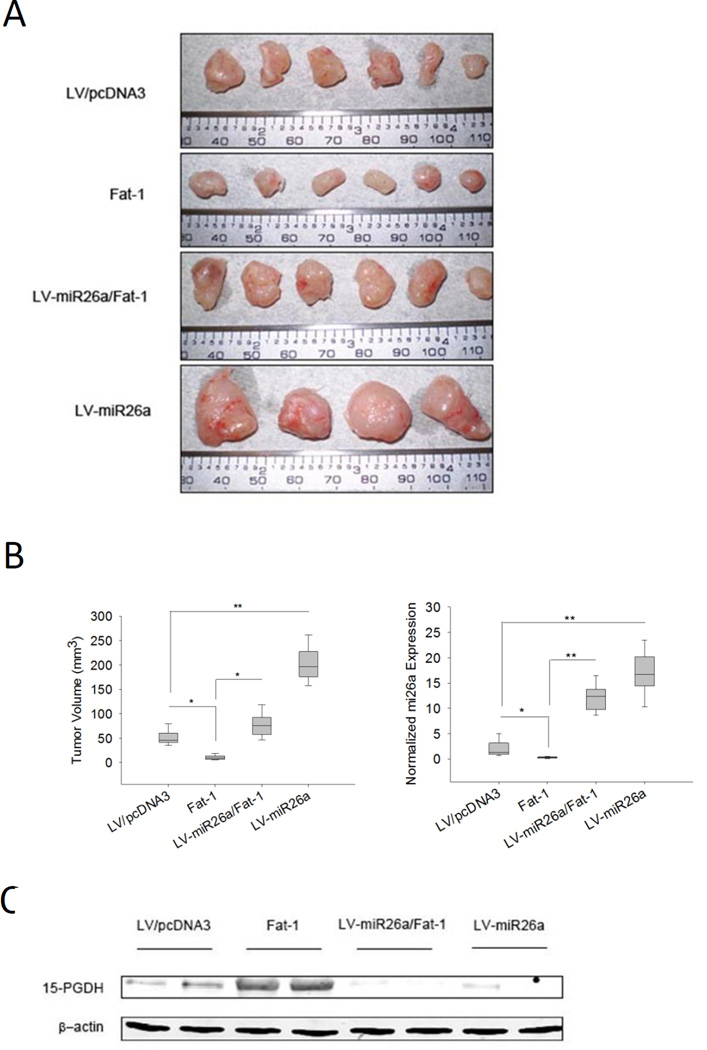
We then inoculated CCLP1 cells with or without Fat-1 and/or miR26a overexpression subcutaneously into SCID mice to monitor tumor growth in vivo. While Fat-1 expression inhibited xenograft tumor growth, overexpression of miR26a enhanced tumor growth and offset the inhibitory effect of Fat-1 (Figure 5A and 5B). Western blotting analysis using the recovered xenograft tumor tissues confirmed that miR-26a overexpression attenuated Fat-1-induced induction of 15-PGDH in vivo (Figure 5C).
Figure 5. ω-3 PUFAs induce 15-PGDH and inhibit cholangiocarcinoma growth in vivo.
CCLP1 cells (with Fat-1 overexpression, miR26a overexpression, Fat-1/miR26a co-expression, or control vector cells) were inoculated into SCID mice (n=6). Tumors growth was monitored and recovered 35 days later. Overexpression of Fat-1 inhibited tumor growth in vivo; this effect was reversed by overexpression of miR26a. Overexpression miR26a alone was found to enhance tumor growth in vivo. (A) Gross photograph of tumors recovered from SCID mice. (B) Bar graphs showing the average volume of recovered tumors and the average miR26a expression levels in the recovered tumors. The volume of tumor was calculated as described in the methods; the level of miR26a was measured by real-time PCR. Results were normalized to control group. Data was presented as mean mean±SE, *P<0.05, **P<0.01; (C) Representative Western blot for 15-PGDH in recovered tumor tissues. β-actin was measured as a reference gene.
Knockdown of 15-PGDH prevents ω-3 PUFA-induced inhibition of cholangiocarcinoma growth
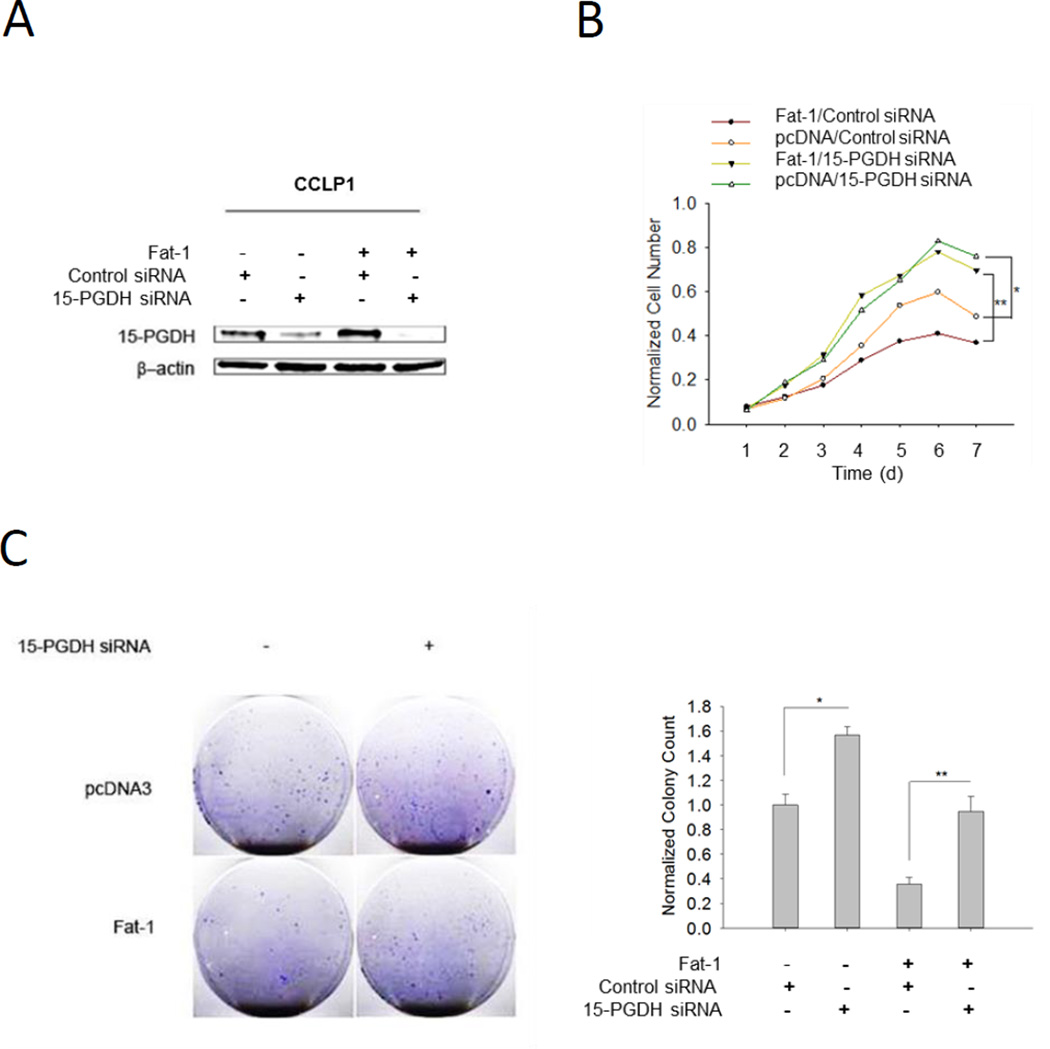
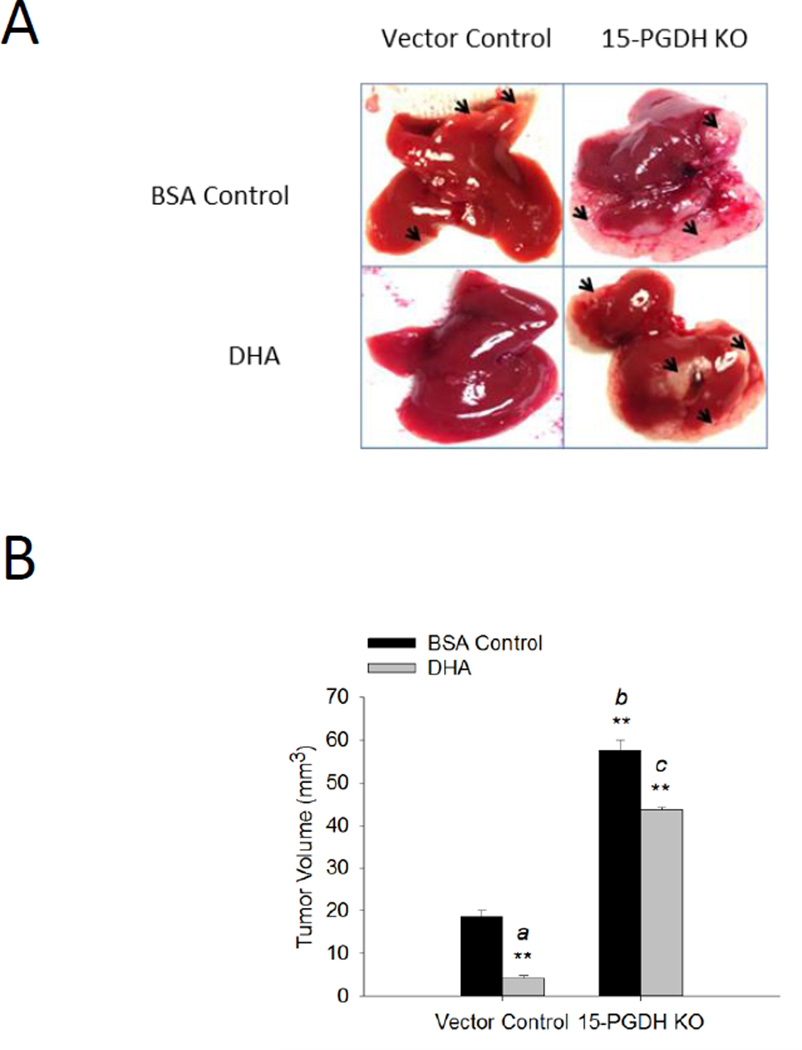
To further determine the role of 15-PGDH in ω-3 PUFA-induced inhibition of cholangiocarcinoma growth, we constructed cells with Fat-1 overexpressing plus 15-PGDH knockdown. By using siRNA approach, we were able to satisfactorily reduce 15-PGDH protein in normal or Fat-1 expressed CCLP1 cells (Figure 6A). We observed that knockdown of 15-PGDH revered Fat-1-induced inhibition of CCLP1 cell proliferation and colony formation, in vitro (Figure 6B and 6C). We next performed in vivo experiments to evaluate the effect of ω-3 PUFAs and 15-PGDH on cholangiocarcinoma growth in SCID mice. We observed that administration of exogenous DHA to SCID mice significantly decreased tumor growth when the mice were inoculated with control vector tumor cells and that 15-PGDH knockdown reversed DHA effect in vivo (Figure 7). These findings provide in vitro and in vivo evidences for an important role of 15-PGDH in ω-3 PUFA-induced inhibition of cholangiocarcinoma cell growth.
Figure 6. Knockdown of 15-PGDH prevents Fat-1-induced inhibition of cholangiocarcinoma growth.
Fat-1 overexpressed or control CCLP1 cells were transfected with 15-PGDH siRNA and the cells were evaluated for proliferation (WST-1) and colony formation. (A) The levels of 15-PGDH protein in cells with or without 15-PGDH knockdown. The cells were lysed 72 h after 15-PGDH siRNA transfection; total protein was analyzed by western blot with 15-PGDH antibody (β-actin was measured as a reference gene). (B) Knockdown 15-PGDH enhances CCLP1 cell growth and prevents Fat-1 induced inhibition of growth. The data are presented as mean±SE from 3 independent experiments. *P<0.05; **P<0.01; (C) Knockdown of 15-PGDH in CCLP1 cells prevents Fat-1 induced inhibition of colony formation. Representative of three independent experiments were showed in the left panel. Quantified results were normalized to vector control group and presented as mean±SE in the right panel; *P<0.05; **P<0.01.
Figure 7. The effect of 15-PGDH and DHA on cholangiocarcinoma growth in vivo.
CCLP1 cells with or without 15-PGDH knockdown were inoculated into SCID mice via splenic injection and the animals were treated with DHA or BSA control as described in the Methods. (A) Representative gross images of the liver from each group of mice. The arrowheads denote areas of tumor growth. (B) Average tumor volume (**P<0.01; a compared to vector control group with BSA injection, b compared to vector control group with BSA injection, c compared to vector control group with BSA+DHA injection).
DISCUSSION
The current study provides the first evidence that ω-3 PUFAs up-regulate the expression of 15-PGDH by inhibiting miR26a and miR26b and that these effects contribute to ω-3 PUFA-induced inhibition of cholangiocarcinoma growth. Our findings support that ω-3 PUFA may be utilized as a non-toxic adjuvant therapeutic agent for the treatment of human cholangiocarcinoma. The significance of the study is further underscored by the fact that cholangiocarcinoma is a highly malignant human cancer currently with no effective therapy.
Previous studies have shown that ω-3 PUFAs inhibit the growth of tumor cells, while they are significantly less toxic toward normal cells (47, 48). For cells of biliary origin, we have shown that ω-3 PUFAs inhibit the growth of cholangiocarcinoma cells but not primary biliary epithelial cells (19). Thus, ω-3 PUFA may represent a non-toxic therapeutic agent for treatment of human cholangiocarcinoma. Several mechanisms for ω-3 PUFA as a cancer therapeutic agent have been documented (49). Our previous study has shown that ω-3 PUFAs inhibit cholangiocarcinoma cell growth in part through inhibition of Wnt/beta-catenin and COX-2 signaling pathways (19). The current study describes a separate novel mechanism, miR-26a/b-mediated regulation of 15-PGDH, in ω-3 PUFA-mediated inhibition of human cholangiocarcinoma. It is notable that 15-PGDH, a key enzyme that catalyzes PGE2 oxidation, is an important tumor suppressor regulated by ω-3 PUFA. Our results show that ω-3 PUFA up-regulates 15-PGDH expression in cholangiocarcinoma cells and this effect contributes to inhibition of cholangiocarcinoma growth, in vitro and in vivo.
The mechanisms for 15-PGDH-mediaed inhibition of cholangiocarcinoma growth include deactivation of PGE2, a pro-inflammatory and pro-tumorigenic lipid mediator which is known to promote tumor growth, invasion and angiogenesis(10). In parallel, 15-PGDH catalyzes the biotransformation of PGE2 to 15-keto-PGE2 which is known to activate peroxisome proliferator-activated receptor γ (PPARγ) and Smad2/3 leading to induction of TAp63 and inhibition of cholangiocarcinoma cell growth(36). It is possible that all of these mechanisms may be implicated in ω-3 PUFA-induced inhibition of cholangiocarcinoma cell growth.
Our findings present in the current study suggest that induction of 15-PGDH by ω-3 PUFA may represent an effective therapeutic target for CCA. Since the cardiovascular side effect associated with COX-2 inhibitors is largely due to inhibition of the antithrombotic prostacyclin (PGI2), induction or reaction of 15-PGDH is expected to block CCA growth without inhibiting PGI2 and thus incurring no significant side effect. In this context, the results presented in this study are expected to have significant impact for future management of CCA.
Another novel aspect of the current study is the illustration of miR26a/b as a key factor linking ω-3 PUFA to 15-PGDH. We show that ω-3 PUFA inhibits the expression of miR26a/b, thus leading to 15-PGDH protein accumulation. Direct targeting of 15-PGDH by miR26a/b was demonstrated by the observations that miR26a/b inhibits 15-PGDH 3’UTR luciferase reporter activity and that miR26a/b overexpression prevents ω-3 PUFA-induced 15-PGDH protein accumulation. We noted that knockdown of 15-PGDH did not reverse cholangiocarcinoma growth as potently as miR26a overexpression; this aspect may be explained by the facts that miR26s enhance Wnt/β-catenin signaling via inhibiting GSK-3β and that GSK-3β is another target of ω-3 PUFA(19, 50). Thus, the data presented in the current study, along with our previous findings, suggest that there are two targets, 15-PGDH and GSK-3β, which can be regulated by ω-3 PUFA/miR26s in human cholangiocarcinoma cells. The interplays between PGE2 and Wnt/β-catenin signaling pathways and their regulation by ω-3 PUFA are illustrated in Supplementary Figure S4.
While c-myc is a downstream oncogene of Wnt/β-catenin signaling, it is also the co-factor regulating the gene clusters formed by miR26a/b and their host CTDSPs genes(40–43). Our data presented in the current study suggest that ω-3 PUFA suppress miRNA26a and miRNA26b by inhibiting c-myc, through regulation of their host genes CTDSPs. The latter assertion is further supported by the ChIP assay showing that c-myc is associated with the promoters of the CTDSPL/miR26a and CTDSP1/miR26b gene clusters and by the observation that over-expression of c-myc prevents ω-3 PUFA-induced reduction of CTDSPs/miR26s.
In summary, the current study provides novel evidence for induction 15-PGDH by ω-3 PUFA via suppression of miR26s in human cholangiocarcinoma cells. Our findings further support the use of ω-3 PUFA as non-toxic adjuvant therapeutic agent for the treatment of human cholangiocarcinoma.
Supplementary Material
Acknowledgments
This works is supported by grants from NCI and NIDDK (R01 CA134568, R01 CA102325, R01 CA106280, and R01 DK077776 to T.W.).
ABBREVIATIONS
- 15-PGDH
NAD+-dependent 15-hydroxyprostaglandin dehydrogenase
- ω-3 PUFA
ω-3 polyunsaturated fatty acids
- DHA
Docosahexaenoic acid
- AA
Arachidonic acid
- miR26s
microRNA26a and microRNA26b
- CTDSPL
Carboxy-terminal domain small phosphatase-like
- CTDSP1
Carboxy-terminal domain small phosphatase-1
- C-MYC
Cellular Myelocytomatosis Viral Oncogene Homolog
- ROS
reactive oxygen species
- GSK-3β
Glycogen synthase kinase 3-β
- COX-2
cyclooxygenase-2
- PGE2
Prostaglandin E2
- PPAR-γ
peroxisome proliferator-activated receptors-γ
- PTEN
Phosphatase and tensin homolog
- PKC
Protein kinase C
- MEKK
MAP kinase kinase kinase
- JNK
c-Jun N-terminal kinase
Footnotes
No conflict of interest to disclose
Contributions: T.W. conceived the project and L.Y., C.H and T.W. designed the experiments; L.Y. performed experiments and analyzed the data; K.S. and J.Z. assisted experiments; K.S., J.Z. and K. L. and C.H. helped with data discussions; L.Y. and T.W. wrote the paper; T.W. obtained funding.
REFERENCE
- 1.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 5.Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1–G9. doi: 10.1152/ajpgi.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirica A. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 9.Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127–137. doi: 10.1055/s-2004-828890. [DOI] [PubMed] [Google Scholar]
- 10.Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta. 2005;1755:135–150. doi: 10.1016/j.bbcan.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. doi: 10.1053/j.gastro.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 12.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu T, Han C, Lunz JG, 3rd, Michalopoulos G, Shelhamer JH, Demetris AJ. Involvement of 85-kd cytosolic phospholipase A(2) and cyclooxygenase-2 in the proliferation of human cholangiocarcinoma cells. Hepatology. 2002;36:363–373. doi: 10.1053/jhep.2002.34743. [DOI] [PubMed] [Google Scholar]
- 14.Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res. 2004;64:1369–1376. doi: 10.1158/0008-5472.can-03-1086. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer Ther. 2004;3:299–307. [PubMed] [Google Scholar]
- 16.Han C, Demetris AJ, Liu Y, Shelhamer JH, Wu T. Transforming growth factor-beta (TGF-beta) activates cytosolic phospholipase A2alpha (cPLA2alpha)-mediated prostaglandin E2 (PGE)2/EP1 and peroxisome proliferator-activated receptor-gamma (PPAR-gamma)/Smad signaling pathways in human liver cancer cells. A novel mechanism for subversion of TGF-beta-induced mitoinhibition. J Biol Chem. 2004;279:44344–44354. doi: 10.1074/jbc.M404852200. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006;281:33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Han C, Lim K, Wu T. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide synthase and cyclooxygenase-2. J Biol Chem. 2008;283:3077–3087. doi: 10.1074/jbc.M705709200. [DOI] [PubMed] [Google Scholar]
- 19.Lim K, Han C, Xu L, Isse K, Demetris AJ, Wu T. Cyclooxygenase-2-derived prostaglandin E2 activates beta-catenin in human cholangiocarcinoma cells: evidence for inhibition of these signaling pathways by omega 3 polyunsaturated fatty acids. Cancer Res. 2008;68:553–560. doi: 10.1158/0008-5472.CAN-07-2295. [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Han C, Wu T. Microsomal prostaglandin E synthase-1 inhibits PTEN and promotes experimental cholangiocarcinogenesis and tumor progression. Gastroenterology. 2011;140:2084–2094. doi: 10.1053/j.gastro.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi N, Yamamoto H, Hiraoka N, et al. Differential expression of cyclooxygenase-2 (COX-2) in human bile duct epithelial cells and bile duct neoplasm. Hepatology. 2001;34:638–650. doi: 10.1053/jhep.2001.28198. [DOI] [PubMed] [Google Scholar]
- 23.Chariyalertsak S, Sirikulchayanonta V, Mayer D, et al. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut. 2001;48:80–86. doi: 10.1136/gut.48.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–372. doi: 10.1046/j.1440-1746.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- 25.Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis. 2002;22:303–313. doi: 10.1055/s-2002-34507. [DOI] [PubMed] [Google Scholar]
- 26.Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology. 2002;35:552–559. doi: 10.1053/jhep.2002.31774. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004;39:1028–1037. doi: 10.1002/hep.20143. [DOI] [PubMed] [Google Scholar]
- 28.Lai GH, Zhang Z, Sirica AE. Celecoxib acts in a cyclooxygenase-2-independent manner and in synergy with emodin to suppress rat cholangiocarcinoma growth in vitro through a mechanism involving enhanced Akt inactivation and increased activation of caspases-9 and -3. Mol Cancer Ther. 2003;2:265–271. [PubMed] [Google Scholar]
- 29.Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732–738. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 30.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 31.Vanchieri C. Vioxx withdrawal alarms cancer prevention researchers. J Natl Cancer Inst. 2004;96:1734–1735. doi: 10.1093/jnci/96.23.1734. [DOI] [PubMed] [Google Scholar]
- 32.Couzin J. Clinical trials. Nail-biting time for trials of COX-2 drugs. Science. 2004;306:1673–1675. doi: 10.1126/science.306.5702.1673. [DOI] [PubMed] [Google Scholar]
- 33.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 35.Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev. 2011;30:409–417. doi: 10.1007/s10555-011-9314-z. [DOI] [PubMed] [Google Scholar]
- 36.Lu D, Han C, Wu T. 15-hydroxyprostaglandin dehydrogenase-derived 15-keto-prostaglandin E2 inhibits cholangiocarcinoma cell growth through interaction with peroxisome proliferator-activated receptor-gamma, SMAD2/3, and TAP63 proteins. J Biol Chem. 2013;288:19484–19502. doi: 10.1074/jbc.M113.453886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Saijyo S, Kudo T, Suzuki M, et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Demetris AJ, Gollin SM, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 39.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Lu Y, Zhang Q, et al. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2012;40:4615–4625. doi: 10.1093/nar/gkr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J, Denli AM, Gage FH. The enemy within: intronic miR-26b represses its host gene, ctdsp2, to regulate neurogenesis. Genes Dev. 2012;26:6–10. doi: 10.1101/gad.184416.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 43.Frenzel A, Loven J, Henriksson MA. Targeting MYC-Regulated miRNAs to Combat Cancer. Genes Cancer. 2010;1:660–667. doi: 10.1177/1947601910377488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sander S, Bullinger L, Wirth T. Repressing the repressor: a new mode of MYC action in lymphomagenesis. Cell Cycle. 2009;8:556–559. doi: 10.4161/cc.8.4.7599. [DOI] [PubMed] [Google Scholar]
- 45.Song KS, Jing K, Kim JS, et al. Omega-3-polyunsaturated fatty acids suppress pancreatic cancer cell growth in vitro and in vivo via downregulation of Wnt/Beta-catenin signaling. Pancreatology. 2011;11:574–584. doi: 10.1159/000334468. [DOI] [PubMed] [Google Scholar]
- 46.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begin ME, Ells G, Das UN, Horrobin DF. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. Journal of the National Cancer Institute. 1986;77:1053–1062. [PubMed] [Google Scholar]
- 48.Hardman WE, Barnes CJ, Knight CW, Cameron IL. Effects of iron supplementation and ET-18-OCH3 on MDA-MB 231 breast carcinomas in nude mice consuming a fish oil diet. British journal of cancer. 1997;76:347–354. doi: 10.1038/bjc.1997.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephenson JA, Al-Taan O, Arshad A, Morgan B, Metcalfe MS, Dennison AR. The multifaceted effects of omega-3 polyunsaturated Fatty acids on the hallmarks of cancer. J Lipids. 2013;2013:261247. doi: 10.1155/2013/261247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating beta-catenin. Gastroenterology. 2012;143:246–256 e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.