Abstract
For migratory marine animals, like sea turtles, effective conservation can be challenging because key demographic information such as duration of life stages and exposure to spatially explicit threats in different habitats are often unknown. In the eastern Pacific near the Baja California Peninsula (BCP), Mexico, tens of thousands of endangered North Pacific loggerhead sea turtles (Caretta caretta) concentrate at a foraging area known to have high rates of fishery bycatch. Because stage survivorship of loggerheads in the BCP will vary significantly depending on the number of years spent in this region, we applied skeletochronology to empirically estimate residency duration in this loggerhead hotspot. The observed age distribution obtained from skeletochronology analysis of 146 dead-stranded loggerheads ranged from three to 24 years old, suggesting a BCP residency of >20 years. Given the maximum estimated age and a one-year migration to western Pacific nesting beaches, we infer age-at-maturation for BCP loggerheads at ~25 years old. We also examine survivorship at varying BCP residency durations by applying our findings to current annual mortality estimates. Predicted survivorship of loggerheads spending over 20 years in this BCP foraging habitat is less than 10%, and given that ~43,000 loggerhead turtles forage here, a significant number of turtles are at extreme risk in this region. This is the first empirical evidence supporting estimated age-at-maturation for BCP North Pacific loggerheads, and the first estimates of BCP stage survivorship. Our findings emphasize the urgent need for continued and effective international conservation efforts to minimize bycatch of this endangered species.
Keywords: Endangered species, demography, survivorship, sink habitat, sea turtle, age-at-maturation
Introduction
Animals with complex life histories, particularly those that are long-lived and migratory, present unique conservation challenges (Wilson et al. 2006, Martin et al. 2007). For many species, important life stages are poorly understood and key conservation questions remain unknown, such as: How long do certain stages last? Where do animals go during cryptic life stages? Which life stages should be targeted for conservation to maximize potential reproductive value and best influence recovery of a threatened population? Effective management of migratory species depends on a solid, foundational understanding of the species’ ecology and life history (Webster et al. 2002, Marra et al. 2010). This includes identifying key habitats and resources, and elucidating the timing and frequency of migrations and ontogenetic shifts (Sutherland 1998, Fahrig 2001, Gerber et al. 2005). Given that different spatially explicit threats exist among locations, species survival rates may vary drastically as they migrate among distinct habitats. By determining where animals live during each life stage, and how long they inhabit each location, managers can better assess threats and prioritize management efforts.
One approach to conservation prioritization is to identify distinct habitats used during various life stages that have significantly different survival rates (Brooks et al. 2006, Wallace et al. 2011). Two contrasting categories for habitats are “source” or “sink” (Pulliam 1988). A source habitat, characterized by high survival rates and typically inhabited by individuals of high reproductive value, contributes to positive population growth (Pulliam 1988). Conversely, a sink habitat, characterized by individuals of high reproductive value facing low survival rates, contributes to population decline (Pulliam 1988, Dias 1996). Habitats identified as population sinks, including those habitats considered ecological traps, are a primary focus for managing species of conservation concern (Battin 2004, Kappel 2005). This includes habitats with high resource quality but where the presence of anthropogenic impacts degrades overall habitat quality and species’ health and survival. Since Pulliam (1988), many examples of population sinks, and the potential consequences on protected species, illustrate the conservation impacts and management challenges sink habitats present for a wide range of taxa, including marine species (anadromous fish, Hickford and Schiel 2011; marine fish, Dayton et al. 1995; and marine megafauna (marine mammals, seabirds, and sea turtles), Lewison et al. 2004, 2014).
Sea turtles are an example of megafauna that exhibit complex life histories, undergo ontogenetic shifts in habitat use, and occupy a wide variety of habitats and ocean regions throughout their life cycle (Wyneken et al. 2013). As a result, many sea turtle populations will reside in a sink habitat during at least one of their life stages, increasing their conservation risks. Determining the duration of time and the specific life stage spent in such habitats for threatened or endangered sea turtles is a top priority for managers of these species (Hamann et al. 2010, NRC 2010).
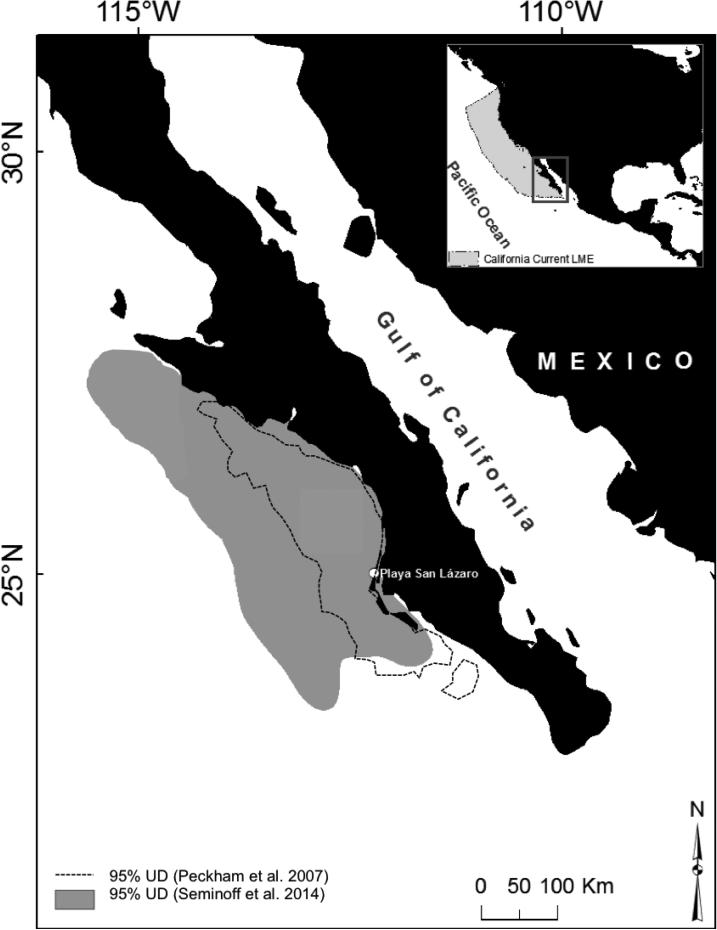
The North Pacific loggerhead (Caretta caretta), declared a Distinct Population Segment in 2011 by the USFWS and NMFS (NMFS and USFWS 2011), is an endangered population known to suffer high juvenile mortality at a developmental foraging hotspot in the eastern Pacific off the western coast of Mexico's Baja California Peninsula (BCP; Peckham et al. 2008, Koch et al. 2013; Fig. 1). All nesting for North Pacific loggerheads occurs in the western Pacific – largely, if not exclusively, in Japan – and hatchlings undergo lengthy developmental migrations to foraging areas in the central North Pacific (CNP) and eastern Pacific, including in the BCP (Bowen et al. 1995, Kobayashi et al. 2008, Abecassis et al. 2013). Upon reaching maturity, turtles migrate back to their natal beaches in the western Pacific for reproduction and foraging around the Japanese Archipelago (Nichols et al. 2000, Hatase et al. 2002, Kamezaki et al. 2003).
Fig. 1.
Study site off the Pacific coast of the Baja California Peninsula (BCP), Mexico. Samples were collected at Playa San Lázaro. Shaded area shows 95% kernel density estimator utilization distribution (UD), core area of distribution, of loggerhead sea turtles for 2005-2007 during aerial surveys (Seminoff et al. 2014). Dashed lines show 95% UD contours of 30 satellite tracked loggerhead turtles (Peckham et al. 2007).
The BCP is the largest known foraging hotspot in the eastern Pacific for this endangered loggerhead population and exhibits high mortality of loggerheads (Peckham et al. 2007, INAPESCA 2012). For example, in the summer of 2012 alone, over 500 loggerheads stranded along a 44.3-km beach near the foraging area (PROFEPA 2012, Peckham et al. 2013). The loggerhead mortality in this region resulted in Mexico being identified under the High Seas Driftnet Moratorium Protection Act for not adopting a regulatory program that is comparable to the United States to end or reduce bycatch, taking into account differing conditions (NOAA-Fisheries 2013). To fully understand the biological and management implications of this fishery interaction, a thorough understanding of the age of turtles affected by this mortality, and the length of time turtles spend in this high-mortality area, is necessary.
Skeletochronology, the study of regular growth increments in bones, has proven useful for studying age and growth rates of many species, including sea turtles. Multiple studies have validated the annual formation of growth layers in sea turtle bones, especially for populations that inhabit ocean regions that experience seasonal variability in water temperature, food source, and overall productivity (e.g. Snover and Hohn 2004, Goshe et al. 2010, Snover et al. 2011). Skeletochronology has been applied to estimate age, regional demographics, and growth patterns for multiple sea turtle species in a variety of regions (e.g. Zug and Glor 1998, Bjorndal et al. 2003, Avens et al. 2012, Petitet et al. 2012).
Here we apply skeletochronology to sea turtle humerus bones to generate age estimates for 146 dead-stranded loggerhead turtles collected in the BCP. We used the estimated age ranges of these turtles to determine residency duration and calculate age-at-maturation, thereby facilitating estimates of survivorship for loggerheads in the BCP. In applying estimated stage duration to existing annual survivorship rates, we predict the likelihood of juvenile turtles inhabiting this foraging area surviving to maturity. Our study provides greater context for previous estimates of stage duration and age-at-maturation for this population (Van Houtan and Halley 2011, Seminoff et al. 2014), and further underscores the demographic and conservation implications of the BCP as a sink habitat.
Methods
1.1 Study site
Tens of thousands of juvenile loggerheads congregate in the BCP (Fig. 1), an eastern Pacific hotspot known for high productivity and abundant food resources for sea turtles and other marine vertebrates (Etnoyer et al. 2006, Wingfield et al. 2011). In the BCP, which is located within the California Current Large Marine Ecosystem, loggerheads forage upon swarms of pelagic red crab (Pleuroncodes planipes), pelagic and benthic invertebrates, and fish species discarded by fisheries (Aurioles-Gamboa 1992, Peckham et al. 2011, S.H. Peckham, pers. comm.). Seasonal upwelling supports industrial, and especially artisanal, fishing efforts that target a variety of species and use multiple gear types that impact turtles including bottom-set gillnets and longlines (Peckham et al. 2007, 2008, Ramírez-Rodríguez & Ojeda-Ruíz 2012, Wallace et al. 2013). The overlap of high turtle numbers and intense fishing effort in the BCP results in significant sea turtle mortality rates and makes this foraging area a sink habitat (Peckham et al. 2007, Koch et al. 2013, Lewison et al. 2014).
1.2 Sample collection and preparation
We collected humerus bones from 146 dead-stranded loggerhead turtles from 2003 to 2011 in the BCP. All samples were collected along a 44.3-km stretch of beach, Playa San Lázaro, in Baja California Sur, Mexico, just north of Bahía Magdalena and immediately adjacent to the Gulf of Ulloa (Fig. 1). Humeri were collected as part of the long term index shoreline stranding survey that Grupo Tortuguero de las Californias and Proyecto Caguama have conducted on Playa San Lázaro since 2003 (Peckham et al. 2008). We extracted humerus bones from the front flippers of dead-stranded turtles, removed the flesh, air dried the bones, then stored them at room temperature prior to processing for skeletochronology. We also reprocessed and analyzed an additional 11 bones from small juvenile North Pacific loggerhead turtles captured in the CNP between 1991 and 1992 and previously analyzed in Zug et al. (1995) (see Zug et al. 1995 and Wetherall et al. 1993 for additional details on these samples). These archived, dried, and unprocessed bones from the 11 juvenile CNP turtles underwent the same skeletochronology processing, at the same time, as the more recently collected bones from Mexico.
We recorded curved carapace length (CCL) from the nuchal notch to the posterior marginal tip for most of the turtles (n = 107); however, CCL was not recorded at the time of bone collection for 50 turtles. For these animals, we estimated the CCL at stranding based on the humerus diameter (HD, mm), a measurement made distal to the insertion scar, according to the equation
derived from turtles measured at Playa San Lázaro, as well as the CNP, and used in this study (n = 107, r2 = 0.84, p < 0.001). Any carapace lengths recorded as straight carapace length (SCL) instead of CCL were converted using equation
from Peckham et al. (2008). All CCL data were rounded to the nearest cm.
1.3 Skeletochronology
Bones were measured, cross-sectioned, decalcified, stained, and imaged according to Goshe et al. (2009) and Avens et al. (2012). Most of the humeri from the BCP (n = 100), and all CNP humeri, were decalcified using Cal-Ex II (Fisher Chemical), a decalcifying agent commonly used for sea turtle skeletochronology processing due to its multifunction as both a fixative and decalcifier. The remaining 46 bones were processed using a different decalcifier, RDO (Apex Engineering), as we found that RDO yielded higher quality sectioning and images for the remaining sea turtle bones from the BCP. These bones were separately fixed in 10% formalin prior to decalcification. Upon final processing, humerus sections were photographed and then digitized into high-resolution images for aging analysis (Goshe et al. 2010).
1.4 Age Estimation
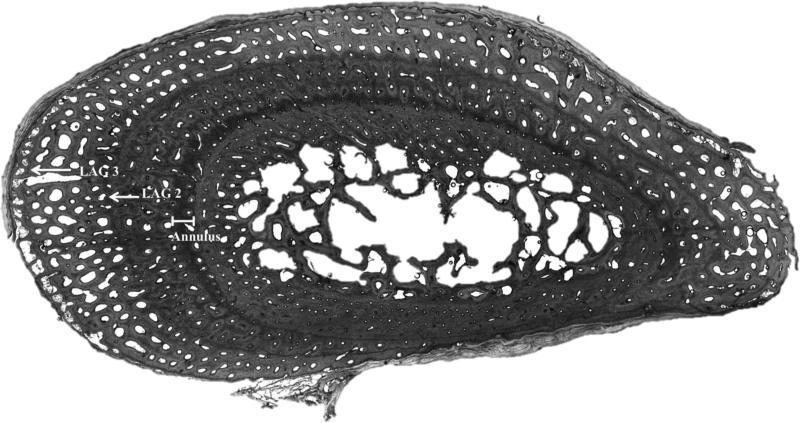
Images of all humerus cross sections were independently assessed by at least two of the authors (CTT, LG, LA, KB), and the location and number of observed lines of arrested growth (LAGs) were determined as described in Goshe et al. (2009, 2010). For each bone, the total number of LAGs was counted and each LAG diameter was measured (e.g. Snover & Hohn 2004, Goshe et al. 2010, Piovano et al. 2011). We assumed annual LAG deposition based on the results of validation studies of loggerheads in the Atlantic (Klinger & Musick 1992, Coles et al. 2001, Snover & Hohn 2004, Snover et al. 2007, Avens et al. 2013), and green turtles (Chelonia mydas) in the Pacific (Snover et al. 2011). Any bones containing a distinctive diffuse mark that is characteristic of the first-year annulus, marking the first year of a turtle's life (Snover & Hohn 2004), we interpreted similarly and categorized as “directly aged” samples (n = 14) (e.g Avens et al. 2013; Fig. 2). Any bones without the first-year annulus mark (n = 143) were assumed to have resorbed some LAGs during bone growth, requiring application of a correction factor to estimate the number of LAGs lost as described in Goshe et al. (2010) and Avens et al. (2012). Two correction factors were used for these bones, depending on whether the diameter of the innermost LAG was larger or smaller than the largest LAG from the directly aged bones (18.5 mm). First, for bones without a first-year annulus but with an innermost LAG diameter less than or equal to 18.5 mm, we used one correction factor, termed the “first order correction factor.” For larger bones without a first-year annulus but with the smallest retained LAG larger than 18.5 mm, we used a different correction factor, termed the “second order correction factor.” The estimated age-at-stranding of each turtle was then calculated by summing together the total number of observed LAGs with the calculated number of LAGs lost.
Fig. 2.
Image of a humerus cross section from a loggerhead stranded in BCP that retained the annulus and for which age was determined to be three years.
Similar to the process described in Avens et al. (2012, 2013), age was adjusted to account for partial-year age and growth. This is required, for example, when a turtle hatched during a summer month dies during a different time of the year. LAGs form during periods of slower growth, and for ectothermic reptiles in the northern hemisphere, we assume this would typically occur during the winter and spring as was concluded by Snover et al. (2011) for green turtles in the North Pacific, and was observed by Snover & Hohn (2004) for Kemp's ridley (Lepidochelys kempii). Following LAG deposition, a period of more rapid growth occurs during the warmer, more productive summer and fall months and is observed as the diffuse space in bones that exists between LAGs (Zug et al. 1986, Castanet et al. 1993, Snover et al. 2011).
Mean hatching period for loggerheads in Japan is during the summer months (June, July, August), therefore, loggerhead turtles stranded in the BCP during these same summer months received no age adjustment. However, for BCP strandings that occurred during the fall (September, October, November), age was adjusted by +0.25 year. For winter and spring strandings, those that occurred during the presumed formation of LAGs, age was adjusted depending on whether growth was observed beyond the outermost LAG or not. If growth was observed beyond the outermost LAG, age was adjusted by +0.5 year for winter strandings (December, January, February), and by +0.75 year for spring strandings (March, April, May). If no growth was observed beyond the outermost LAG, it was assumed that the LAG was newly deposited that winter/spring and age was adjusted by -0.5 year for winter strandings and -0.25 for spring strandings.
Results
2.1 Size distribution
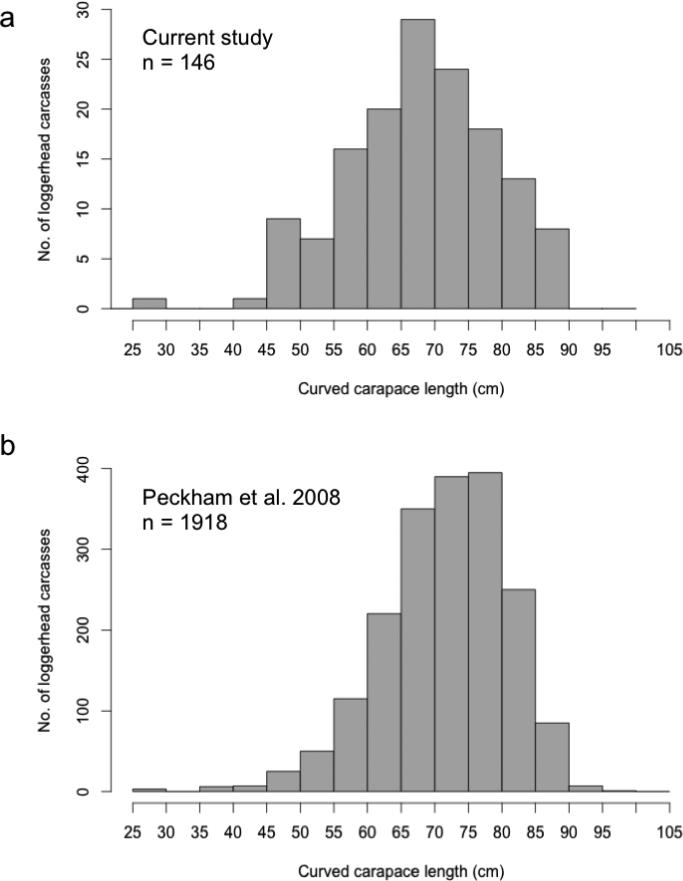
The CCL of the 146 BCP turtle humerus bone samples collected between 2003 and 2011 ranged from 29 to 90 cm. Of these, the mean (± SD) CCL was 69 ± 11 cm. All bone samples used in this study were from juvenile turtles with CCL smaller than the mean nesting size of adult loggerheads in Japan, < 91 cm (Hatase et al. 2004; Fig. 3a). Of the 14 directly aged turtles, the body sizes for the 11 bones from the CNP ranged from 15 to 47 cm CCL with an average of 29 ± 12 cm and the three from the BCP were 29, 50 and 58 cm CCL.
Fig. 3.
Sample size distribution for stranded loggerhead turtles collected at Playa San Lázaro a) used in the current study and b) from Peckham et al. 2008.
2.2 Age Estimation
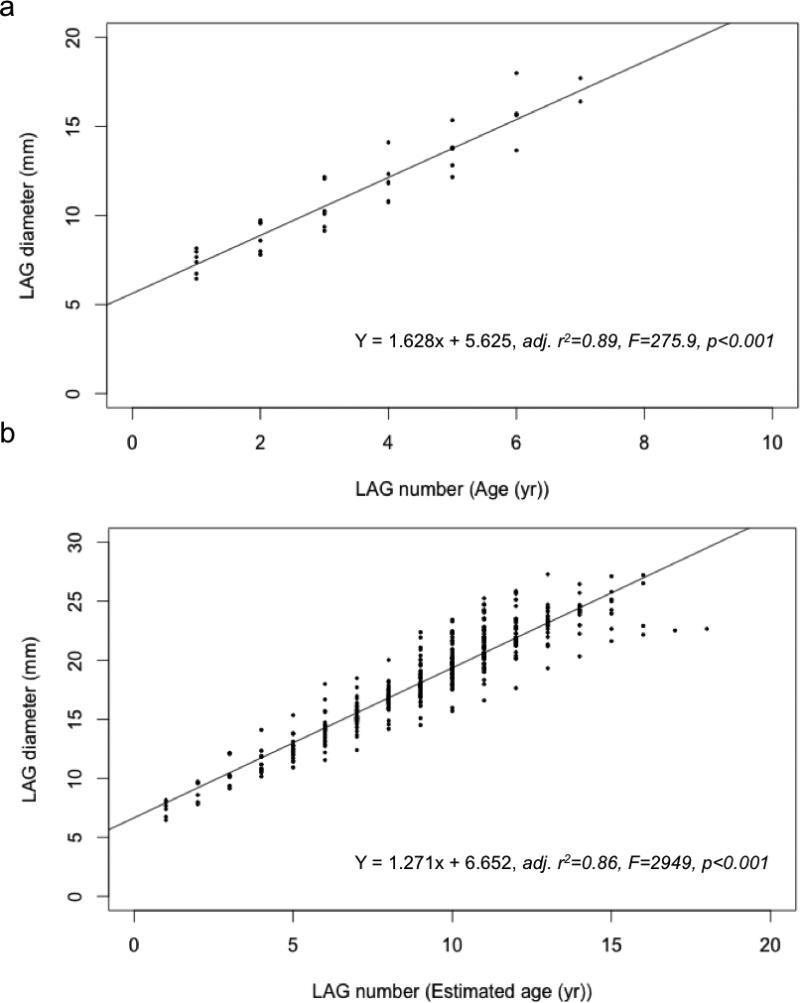
The age of the 14 directly aged turtles, based on direct LAG count, ranged from zero to six years old. These humeri retained a total of 37 LAGs, and the LAG number and LAG diameter measurements were positively correlated (p < 0.001, adj. r2 = 0.89) and described by the following regression equation (Fig. 4a):
We used this equation as the first order correction factor to estimate the number of LAGs lost (3 to 8) within the 72 larger bones that did not retain an annulus or an innermost LAG exceeding 18.5 mm (Fig. 4a). The LAG numbers and LAG diameters from the directly aged group (n = 37 LAGs), and from the bones upon which the first order regression equation was applied (n = 427 LAGs) were combined (total: 86 bones, n = 464 LAGs) in order to generate the linear regression equation (p = 0.001, adj. r2 = 0.86) used for the second order correction factor (Fig. 4b):
This second order correction factor was used to estimate the LAGs lost (9 to 18) for the remaining 71 bones. Ages were estimated by adding the number of observed LAGs to the calculated number of LAGs lost.
Fig. 4.
The linear relationships between line of arrested growth (LAG) diameter and LAG number. The linear regression equations from these relationships were used for a) the first order correction applied to 72 bones, (humerus diameter (HD) range 17.3 to 27.5 mm, and CCL range 45 to 81 cm); and b) second order correction applied to 71 bones (HD range 20.3 to 33.6 mm, and CCL range 51 to 90 cm).
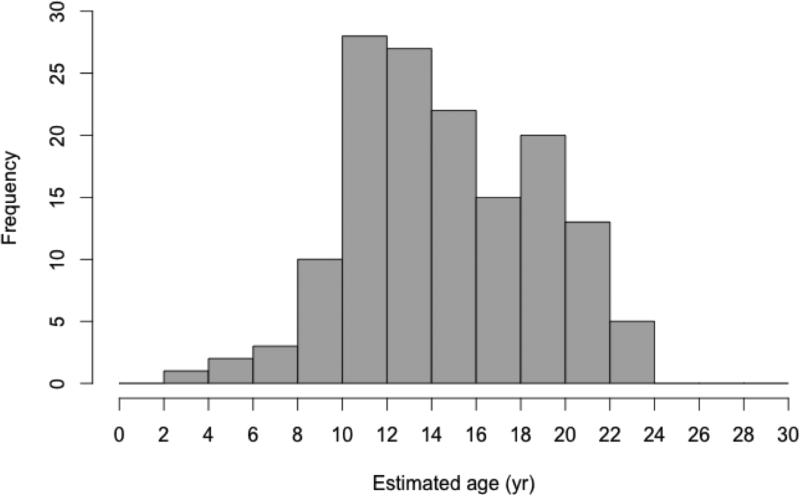
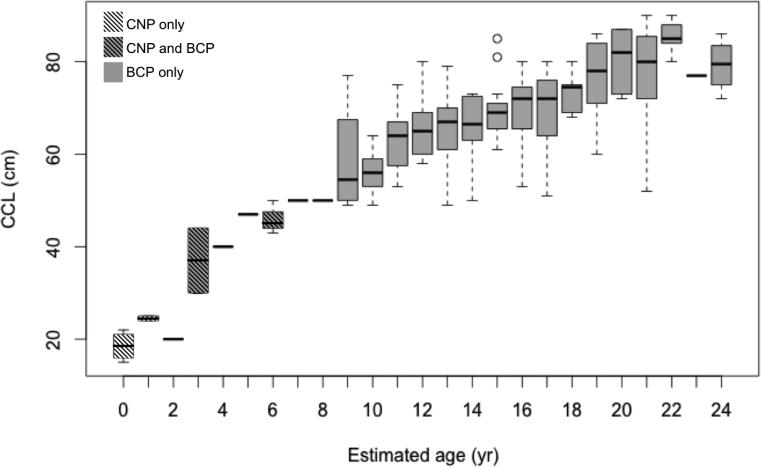
Age estimates were rounded to the nearest whole number, and were adjusted for stranding date. The majority of BCP samples (81.5%) were collected during the summer months and required no adjustment, whereas 29 bones (18.5%) required age adjustment. The final age estimates of the 146 juvenile turtles from the BCP ranged from three to 24 years (mean ± SD: 15 ± 4.2 years; Fig. 5). There was no difference between average age ± SD when the age adjustment was applied, compared to unadjusted ages (15 ± 4.2 years). Of the 146 turtles, 50% were between the ages of 12 and 19 years old, and between 62 and 76 cm CCL in size. While the oldest turtles aged in this study (age 24, n = 4) were not necessarily the largest turtles, there was an overall trend of increasing age with increasing body size (n = 157, adj. r2=0.69, p < 0.001, F1,155: 353.1) (Fig. 6).
Fig. 5.
Estimated age distribution using skeletochronology analysis of 146 loggerhead turtles stranded on Playa San Lázaro. Estimated age is equal to the sum of the number of retained LAGs and the number of resorbed LAGs calculated by applying correction factor equations described in the text.
Fig. 6.
Range of curved carapace length (CCL, cm) for estimated age of stranded loggerhead turtles from the central North Pacific (CNP) (n = 11) and the Baja California Peninsula (BCP) (n = 146).
Discussion
Here we present the first empirical evidence for residency duration and age distribution for endangered North Pacific loggerheads foraging in the BCP. Skeletochronology allowed us to estimate the age range of turtles that lived and then dead-stranded in the BCP loggerhead hotspot. These ages provide an estimate of the length of time turtles spend in this important foraging area, critical information for conservation managers. Our results demonstrate the value of analyzing anatomical aspects of deceased animals to better address questions regarding conservation ecology, life history, and the variable impact of threats experienced throughout the lifetime of a species. In addition, it would not be possible to obtain the results presented here from the study of living specimens alone.
3.1 Age distribution and population representation
Age estimates for turtles in this study were normally distributed, with no evidence of bimodal age distribution (Fig. 5), suggesting that the likelihood of experiencing fishery interaction is not age-dependent. The observed variation in turtle body size at a given age (Fig. 6) reflects individual differences which could be based on a number of factors, such as variable foraging patterns or habitats, genetic plasticity, compensatory growth, and or environmental stochasticity. Additionally, the number of LAGs in individual humeri and the variation in size-at-age observed in this study are comparable to findings from other skeletochronology studies (see review in Avens & Snover 2013).
To ensure that the length frequency of turtles used in this study is representative of turtles found in the BCP, we compared the distribution of turtle sizes used in this study to the size distribution of previous studies with larger sample sizes. The distribution of body sizes (CCL) for the BCP turtles used in this study is similar to the normal size distribution presented in Peckham et al. (2008), which assessed length frequency of nearly 2,000 loggerheads stranded in Baja California from 2003 to 2007 (Peckham et al. 2008 mean 78 ± 9 cm CCL vs. this study mean 69 ± 11 cm CCL; Fig. 3). As expected for turtles nearing reproductive maturity in the BCP, the CCL of the largest turtles aged in this study (maximum 90 cm) approach the average size of nesting females observed in Japan (91 cm; Hatase et al. 2004), and Peckham et al. (2008) found only 9 (of ~2,000) turtles in the BCP larger than 91 cm.
Further, the turtles aged in the present study were largely, if not exclusively, composed of loggerheads that had interacted with the local artisanal fishery, a primary conservation concern for this population (Peckham et al. 2008). Fishery interaction is likely as there were no obvious signs of other injuries, illness, or impact from pollution on carcasses. There was no indication of a size-based bias in turtles dead-stranding at this site that would be related to body size (net escape ability, fishing gear size, etc.) or behavior (foraging location, depth, prey preference, net detection or avoidance, etc.). We acknowledge that if behavioral differences that affect bycatch rates exist among turtle size classes (e.g. shallower dive depths of smaller turtles) there could be an underrepresentation of certain size classes. However, to date, no published data exists supporting such size-based differences, but warrants further study. Therefore, we believe the approach applied in this study is appropriate to investigate the demography of loggerheads in this BCP sink habitat.
3.2 Loggerhead residency duration
Our skeletochronology results indicate that North Pacific loggerheads may remain in the bycatch hotspot of the BCP for at least 20 years. The youngest and smallest BCP turtles included in this study (three to six years old and 29-45 cm CCL, respectively) represent the earliest age and smallest size at which loggerheads are likely to recruit to nearshore foraging habitats in the eastern Pacific. Likewise, the oldest and the largest turtles (24 years old and 90 cm CCL, respectively) encountered in this study represent the oldest age and largest size at which loggerheads are likely to remain in the east Pacific. Thus, if we subtract the minimum age at recruitment to the BCP from the maximum age observed in the BCP, we can estimate the maximum residency duration.
Duration estimates may differ according to variations in individual behavior, climatic conditions, and fishing effort. For example, actual BCP duration may be longer than 20 years given that all turtles in this study were stranded, and therefore had died prior to having the opportunity to emigrate back to the western Pacific. In addition, the largest turtles included in this study were not the largest North Pacific loggerheads ever found in this region (90 cm CCL this study vs. 98 cm CCL; Peckham et al. 2007). Similarly, immigration and emigration may vary among individuals, with some arriving in the eastern Pacific at an older age or larger size, and some leaving the eastern Pacific at a younger age or smaller size, thereby potentially reducing the estimated residency duration.
3.3 Age-at-maturation
The oldest BCP turtle aged in this study was ~24 years old. Based on data from satellite and flipper-tagged large turtles from the BCP (Resendiz et al. 1998, Nichols et al. 2000, Peckham et al. 2007), the migration duration from the eastern Pacific back to nesting beaches in the western Pacific is approximately one year. Therefore, we estimate that reproductive maturity and initial nesting in the western Pacific begins at >25 years of age. Previous age-at-maturation studies have yielded similar findings and were based on body size at first nesting, remigration intervals, and climate forcing models (Kamezaki et al. 1995, Hatase et al. 2004, Van Houtan and Halley 2011).
Our results support current knowledge on general sea turtle life history patterns and age-at-maturity estimates. For Atlantic loggerheads, age-at-maturity has been approximated at ~10 to 40 years by a variety of methods including mark recapture, length frequency, and skeletochronology studies (see reviews: Heppell et al. 2003, Avens and Snover 2013). Estimates for age-at-maturity for Pacific loggerheads include ~36 years for the South Pacific population using mark-recapture techniques (Frazer et al. 1994), and ~25 years for the North Pacific population using climate forcing models that incorporated observed Japan nesting numbers and trends (Van Houtan and Halley 2011).
3.4 Implications for demographic impacts and conservation management
The application of loggerhead turtle BCP residency duration to key sea turtle demographic parameters indicate that the population-level impact of this loggerhead mortality is extreme. Current estimates for annual mortality rate in this region are ~11% (Seminoff et al. 2014). Using this value, we can estimate survivorship for juveniles foraging in the BCP at varying residency durations (Table 1). North Pacific loggerheads spending 20 years in this area have a predicted survivorship rate of ~10%. Survivorship increases to ~30% if turtles spend half as many years (10 years) in the BCP. However, if turtles remain in this region for 25 to 30 years, predicted survivorship drops below 5%.
Table 1.
Survivorship of loggerhead sea turtles at varying residency duration times off the Pacific coast of the Baja California Peninsula (BCP) based on 11% annual mortality rate (Seminoff et al. 2014). Stage survivorship = Annual survivorship ^ Residency duration.
| Residency duration (years) | Annual survivorship | Stage survivorship |
|---|---|---|
| 5 | 0.89 | 0.56 |
| 10 | 0.89 | 0.31 |
| 20 | 0.89 | 0.10 |
| 25 | 0.89 | 0.05 |
| 30 | 0.89 | 0.03 |
These are bleak odds for the tens of thousands of juvenile loggerheads known to inhabit this eastern Pacific developmental foraging area. Future research that quantifies the proportion of loggerheads from the entire North Pacific population utilizing the BCP foraging grounds will better elucidate the full impact that this regional bycatch-related mortality has on the recovery ability of the population. Yet even without the quantification of this value, a long, multi-decadal residency in this region of low survivorship will significantly impact the recovery ability of North Pacific loggerheads.
The most recent abundance estimate of ca. 43,000 BCP foragers, compared with population estimates based on annual nesting abundance in Japan, suggests that a significant proportion of juvenile North Pacific loggerheads spend time foraging in the BCP region (Seminoff et al. 2014). Furthermore, evidence from the present study shows these turtles could spend >20 year in the BCP, a substantial amount of time to be exposed to high rates of bycatch. Further, the majority of turtles in this region are large juveniles, a life stage known to have high reproductive value as well as high sensitivity and therefore impact on the overall population growth rate (Crouse et al. 1987, Crowder et al. 1994). High mortality rates experienced by juvenile turtles are known to contribute to declining population levels (Crowder et al. 1994), as have been observed for this loggerhead population over the past several decades (Kamezaki et al. 2003, Peckham et al. 2007, NMFS & USFWS 2011).
Compounding the already difficult situation for loggerheads in this region, an unprecedented 841 turtles were observed dead-stranded during 2012 (PROFEPA 2012), a stark contrast with the annual average of 477 strandings (January through December; 2003-2007) (Peckham et al. 2008). The January 2013 identification of Mexico by the US Government under the High Seas Driftnet Moratorium Protection Act for a failure to adopt a regulatory program that is comparable to the United States to end or reduce bycatch taking into account differing conditions emphasizes the international urgency to address this conservation issue (NMFS 2013). Wildlife conservation efforts are often most effective when focused on habitats where there is a large impact on the population's survival rate and reproductive value, and these areas of high conservation priority are frequently identified sink habitats. The BCP foraging ground in the eastern Pacific represents a sink habitat for the endangered North Pacific population of loggerhead sea turtles and continued international management is necessary to ensure its recovery.
Highlights.
Loggerhead demographic data off Baja, Mexico are important for turtle management.
We apply skeletochronology to estimate residency duration and age-at-maturation.
Dead-stranded juvenile turtles at this foraging site ranged from 3 to 24 years old.
We estimate residency duration > 20 years, and age-at-maturation ~25 years old.
Ongoing management to reduce fishery bycatch at this foraging hotspot is critical.
Acknowledgements
We acknowledge the many partners in Mexico for multiple years worth of sample collection and permitting by members of Grupo Tortuguero de las Californias and Proyecto Caguama, especially Victor and Vladimir de la Toba and Jesus Lucero. We also thank Erin LaCasella, Robin LeRoux, Joel Schumacher, Camryn Allen, and Matt Leslie for assistance with sample permitting, processing and publication prep, as well as Kerri Danil, Nicky Beaulieu, Michelle McCartha, Anji Shakya, Emily Chou, and Rosario Marroquin Flores for assistance in the lab. This work was supported by NOAA Fisheries, and C.T.T. was supported by the University of California San Diego (UCSD), NIH T32 GM007240 Cell and Molecular Genetics Training Program, Jeanne Messier Memorial Fellowship, ARCS Foundation Scholarship, and a Hearts de Vite Research Grant. The shoreline stranding survey at Playa San Lázaro was funded from 2003-2011 with support from the US Fish & Wildlife Service, NMFS-SWFSC, Western Pacific Fisheries Management Council (NOAA Grant FNA05NMF4411092), the David and Lucile Packard Foundation. Fieldwork and sample processing by Grupo Tortuguero de las Californias was also supported by C. & H. Shea, B. & S. Turner, D. & C. Yohn, T. & N. Tomaszewicz, M. Tomaszewicz, T. Dunbar, J. & E. Paisley, K. & E. Roeland, S. Baird, F. & D. O'Cathain, S. Bakeman, H. & M. Diller, and C. Kopp. All research activities and permits were authorized by the Mexican government through SEMARNAP and SEMARNAT permits 150496-213- 03, 280597-213-03, 190698-213-03, 280499-213-03, 280700- 213-03, SGPA/DGVS/002 4661, SGPA/DGVS/10358, SGPA/ DGVS/03501/06, SGPA/DGVS/03406/07, SGPA/DGVS/03481/09, SGPA/DGVS/04990/10, and SGPA/DGVS/04568/11. All exported and imported CITES regulated samples were authorized by the Mexican government through export permit numbers MX-58124 and MX-64301, and the United States government through import permit numbers 11US844694/9 and 12US844694/9.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecassis M, Senina I, Lehodey P, Gaspar P, Parker D, Balazs G, Polovina J. A Model Of Loggerhead Sea Turtle (Caretta Caretta) Habitat And Movement In The Oceanic North Pacific. Plos One. 2013;8:e73274. doi: 10.1371/journal.pone.0073274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurioles-Gamboa D. Inshore-offshore movements of pelagic red crabs Pleuroncodes planipes (Decapods, Anomura, Galatheidae) off the Pacific Coast of Baja California Sur, Mexico. Crustaceana. 1992;62:71–84. [Google Scholar]
- Avens L, Goshe LR, Harms CA, Anderson ET, et al. Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Marine Ecology Progress Series. 2012;458:213–229. [Google Scholar]
- Avens L, Goshe LR, Pajuelo M, Bjorndal KA, MacDonald BD, Lemons GE, Bolten AB, Seminoff JA. Complementary skeletochronology and stable isotope analysis offer new insight into juvenile loggerhead sea turtle oceanic stage duration and growth dynamics. Marine Ecology Progress Series. 2013;491:235–251. [Google Scholar]
- Avens L, Snover ML. Age and age estimation in sea turtles. In: Wyneken J, Lohmann KJ, Musick JA, editors. The Biology of Sea Turtles. III. CRC Press; Boca Raton, FL: 2013. pp. 97–134.pp. 475 [Google Scholar]
- Battin J. When Good Animals Love Bad Habitats: Ecological Traps and the Conservation of Animal Populations. Conservation Biology. 2004;18:1482–1491. doi: 10.1111/j.1523-1739.2004.00417.x. [Google Scholar]
- Bjorndal KA, Bolten AB, Dellinger T, Delgado C, Martins HR. Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecology. 2003;84:1237–1249. [Google Scholar]
- Brooks M, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. Global biodiversity conservation priorities. Science. 2006;313:58–61. doi: 10.1126/science.1127609. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- Bowen BW, Abreu-Grobois FA, Balazs GH, Kamezaki N, Limpus CJ, Ferl RJ. Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial-DNA markers. Proc Natl Acad Sci USA. 1995;92:3731–3734. doi: 10.1073/pnas.92.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanet J, Francillon-Vieillot H, Meunier F, De Ricqles A. Bone and individual aging. In: Hall BK, editor. Bone, Volume 7: Bone growth. CRC Press; Ann Arbor, Michigan: 1993. pp. 245–283.pp. 368 [Google Scholar]
- Coles WC, Musick JA, Williamson LA. Skeletochronology validation from an adult loggerhead (Caretta caretta). Copeia. 2001;1:240–242. [Google Scholar]
- Crouse DT, Crowder LB, Caswell H. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology. 1987;68:1412–1423. [Google Scholar]
- Crowder LB, Crouse DT, Heppell SS, Martin TH. Predicting the impact of turtle excluder devices on loggerhead sea turtle populations. Ecol Appl. 1994;4:437–445. [Google Scholar]
- Dayton PK, Thrush SF, Agardy MT, Hofman RJ. Environmental effects of marine fishing. Aquatic Conservation: Marine and Freshwater Ecosystems. 1995;5:205–232. [Google Scholar]
- Dias PC. Sources and sinks in population biology. Trends in Ecology & Evolution. 1996;8:326–330. doi: 10.1016/0169-5347(96)10037-9. [DOI] [PubMed] [Google Scholar]
- Etnoyer P, Canny D, Mate BR, Morgan LE, Ortega-Ortiz JG, Nichols W. Sea-surface temperature gradients across blue whale and sea turtle foraging trajectories off the Baja California Peninsula, Mexico. Deep Sea Research II. 2006;53:340–358. [Google Scholar]
- Fahrig L. How much habitat is enough? Biological Conservation. 2001;100:65–74. [Google Scholar]
- Frazer NB, Limpus CJ, Greene JL. Growth and estimated age at maturity of Queensland loggerheads. In: Bjorndal KA, Bolten AB, Johnson DA, Eliazar PJ, editors. Proceedings of the 14th Annual Symposium on Sea Turtle Biology and Conservation, NOAA Technical Memorandum NMFS-SEFSC-351. Hilton Head; SC.: 1994. pp. 42–45. [Google Scholar]
- Gerber LR, Heppell SS, Ballantyne F, Sala E. The role of dispersal and demography in determining the efficacy of marine reserves. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62(4):863–871. doi: 10.1139/F05-046. [Google Scholar]
- Goshe LR, Avens L, Bybee J, Hohn AA. An evaluation of histological techniques used in skeletochronological age estimation of sea turtles. Chelonian Conservation Biology. 2009;8:217–222. [Google Scholar]
- Goshe LR, Avens L, Scharf FS, Southwood AL. Estimation of age at maturation and growth of Atlantic green turtles (Chelonia mydas) using skeletochronology. Marine Biology. 2010;157:1725–1740. [Google Scholar]
- Hamann M, Godfrey MH, Seminoff JA, et al. Global research priorities for sea turtles: informing management and conservation in the 21st century. Endangered Species Research. 2010;11:245–269. [Google Scholar]
- Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T. Size related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Marine Ecology Progress Series. 2002;233:273–281. [Google Scholar]
- Hatase H, Matsuzawa Y, Sato K, Bando T, Goto K. Remigration and growth of loggerhead turtles (Caretta caretta) nesting on Senri Beach in Minabe, Japan: life-history polymorphism in a sea turtle population. Marine Biology. 2004;144:807–811. [Google Scholar]
- Heppell SS, Snover ML, Crowder LB. Sea turtle population ecology. In: Lutz PL, Musick JA, Wyneken J, editors. The biology of sea turtles. II. CRC Press; Boca Raton, FL: 2003. pp. 275–306.pp. 472 [Google Scholar]
- Hickford MJH, Schiel DR. Population sinks resulting from degraded habitats of an obligate life-history pathway. Oecologia. 2011;166:131–140. doi: 10.1007/s00442-010-1834-7. doi: 10.1007/s00442-010-1834-7. [DOI] [PubMed] [Google Scholar]
- INAPESCA (National Fisheries Science Institute) INAPESCA Technical Report, Technology Branch in the North Pacific. Mexico: 2012. A Biotechnological Evaluation of Alternative Fishing Methods in the Coastal Fishery of the Gulf Of Ulloa B. C. S. to Avoid Accidental Capture of Non-Target Species. Preliminary Actions. p. 29. [Google Scholar]
- Kamezaki N, Goto K, Matsuzawa Y, Nakajima Y, Omuta K, Sato K. Carapace length and width of the loggerhead turtle Caretta caretta nesting in the coast of Japan. Umigame Newsletter Japan. 1995;26:12–13. [Google Scholar]
- Kamezaki N, Matsuzawa Y, Abe O, et al. Loggerhead turtles nesting in Japan. In: Bolten AB, Witherington B, editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 210–218.pp. 352 [Google Scholar]
- Kappel CV. Losing pieces of the puzzle: threats to marine, estuarine, and diadromous species. Frontiers in Ecology and the Environment. 2005;3(5):275–282. [Google Scholar]
- Klinger RC, Musick JA. Annular growth layers in juvenile loggerhead turtles (Caretta caretta). Bulletin of Marine Science. 1992;51(2):224–230. [Google Scholar]
- Kobayashi DR, Polovina JJ, Parker DM, Kamezaki N, et al. Pelagic habitat characterization of loggerhead sea turtles, Caretta caretta, in the North Pacific Ocean (1997–2006): insights from satellite tag tracking and remotely sensed data. Journal of Experimental Marine Biology Ecology. 2008;356:96–114. [Google Scholar]
- Koch V, Peckham H, Mancini A, Eguchi T. Estimating at-sea mortality of marine turtles from stranding frequencies and drifter experiments. PLoS ONE. 2013;8:e56776. doi: 10.1371/journal.pone.0056776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewison RL, Freeman SA, Crowder LB. Quantifying the effects of fisheries on threatened species: the impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecology Letters. 2004;7:221–231. [Google Scholar]
- Lewison RL, Crowder LB, Wallace BP, Moore JE, Cox T, Zydelis R, McDonald S, DiMatteo A, Dunn DC, Kot CY, Bjorkland R, Kelez S, Soykan C, Stewart KR, Sims M, Boustany A, Read AJ, Halpin P, Nichols WJ, Safina C. Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. PNAS. 2014;111(14):5271–5276. doi: 10.1073/pnas.1318960111. doi/10.1073/pnas.1318960111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra PP, Studds CE, Webster M. Migratory connectivity. Encyclopedia of Animal Behavior. 2010;257(2):455–461. doi: 10.1016/B978-0-08-045337-8.00072. [Google Scholar]
- Martin TG, Chadès I, Arcese P, Marra PP, Possingham HP, Norris DR. Optimal conservation of migratory species. PLos ONE. 2007;2(8):e751. doi: 10.1371/journal.pone.0000751. doi:10.1371/journal.pone.0000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMFS & USFWS (National Marine Fisheries Service, US Fish and Wildlife Service) Endangered and threatened species; determination of nine distinct population segments of loggerhead sea turtles as endangered. Fed Reg. 2011;76:58868. [Google Scholar]
- NMFS (National Marine Fisheries Service) Sea turtle status assessment and research needs. Tech Memo NMFS-F/SPO. US Dept of Commerce, NOAA, Silver Spring; MD: 2013. [Google Scholar]
- NRC (National Research Council) Assessment of Sea-Turtle Status and Trends: Integrating Demography and Abundance. National Academies Press; Washington D.C.: 2010. p. 143. [Google Scholar]
- Nichols WJ, Resendiz A, Seminoff JA, Resendiz B. Transpacific loggerhead turtle migration monitored with satellite telemetry. Bull Mar Sci. 2000;67:937–947. [Google Scholar]
- NOAA-Fisheries . Improving international fisheries management: report to Congress pursuant to Section 403(a) of the Magnuson-Stevens Fishery Conservation and Management Reauthorization Act of 2006. US Department of Commerce; Washington, DC: 2013. [Google Scholar]
- Peckham SH, Maldonado D, Walli A, Ruiz G, Nichols WJ, Crowder L. Small-scale fisheries bycatch jeopardizes endangered Pacific loggerhead turtles. PLoS One. 2007 doi: 10.1371/journal.pone.0001041. doi:10.1371/journal.pone.0001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham SH, Maldonado-Diaz D, Koch V, Mancini A, Gaos A, Tinker MT, Nichols WJ. High mortality of loggerhead turtles due to bycatch, human consumption and strandings at Baja California Sur, Mexico, 2003 to 2007. Endang Species Res. 2008;5:171–183. [Google Scholar]
- Peckham SH, Maldonado-Diaz D, Tremblay Y, Ochoa R, et al. Demographic implications of alternative foraging strategies in juvenile loggerhead turtles Caretta caretta of the North Pacific Ocean. Mar Ecol Prog Ser. 2011;425:269–280. [Google Scholar]
- Peckham SH, Maldonado D, Senko J, Esliman A. Bycatch mass mortality of loggerhead turtles at NW Mexico.. Proceedings of the 33rd International Symposium on Sea Turtle Biology and Conservation; Baltimore, MD.. 2013. p. 110. NOAA Technical Memorandum NMFS-SEFSC-645. [Google Scholar]
- Petitet R, Secchi ER, Avens L, Kinas PG. Age and growth of loggerhead sea turtles in southern Brazil. Marine Ecology Progress Series. 2012;456:255–268. doi: 10.3354/meps09681. [Google Scholar]
- Piovano S, Clusa M, Carreras C, Giacoma C, Pascual M, Cardona L. Different growth rates between loggerhead sea turtles (Caretta caretta) of Mediterranean and Atlantic origin in the Mediterranean Sea. Marine Biology. 2011;158:2577–2587. doi: 10.1007/s00227-011-1759-7. [Google Scholar]
- PROFEPA (Procuraduria Federal de Protección al Ambiente) [5 May 2014];La Profepa ha mantenido vigilancia permanente en la zona de arribazón de la tortuga Caretta caretta, en Baja California Sur. 2012 Published online 22 November 2012 www.profepa.gob.mx/innovaportal/v/4745/1/mx/la_profepa_ha_mantenido_vigilancia_permanente_en_la_zona_de_arribazon_de_la_tortuga_caretta_caretta_en_ baja_california_sur.html.
- Pulliam RH. Sources, sinks, and population regulation. The American Naturalist. 1988;132(5):652–661. [Google Scholar]
- Ramírez-Rodríguez M, Ojeda-Ruíz MÅ Spatial management of small-scale fisheries on the west coast of Baja California Sur, Mexico. Marine Policy. 2012;36:108–112. [Google Scholar]
- Resendiz A, Resendiz B, Nichols WJ, Seminoff JA, Kamezaki N. First confirmed east-west transpacific movement of loggerhead sea turtle, Caretta caretta, released in Baja California, Mexico. Pacific Science. 1998;52:151–153. [Google Scholar]
- Seminoff JA, Eguchi T, Carretta J, Allen CD, Prosperi D, Rangel R, Gilpatrick JW, Forney K, Peckham HS. Loggerhead sea turtle abundance at a foraging hotspot in the eastern Pacific Ocean: implications for at-sea conservation. Endangered Species Research. 2014;24:207–220. [Google Scholar]
- Snover ML, Hohn AA. Validation and interpretation of annual skeletal marks in loggerhead (Caretta caretta) and Kemp’s ridley (Lepidochelys kempii) sea turtles. Fish Bull. 2004;102:682–692. [Google Scholar]
- Snover ML, Avens LA, Hohn AA. Back-calculating length from skeletal growth marks in loggerhead sea turtles Caretta caretta. Endangered Species Research. 2007;3:95–104. [Google Scholar]
- Snover ML, Hohn AA, Goshe LR, Balazs GH. Validation of annual skeletal marks in green sea turtles Chelonia mydas using tetracycline labeling. Aquatic Biology. 2011;12:197–204. doi: 10.3354/ab00337. [Google Scholar]
- Van Houtan KS, Halley JM. Long-term climate forcing in loggerhead sea turtle nesting. PLoS ONE. 2011;6:e19043. doi: 10.1371/journal.pone.0019043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BP, DiMatteo AD, Bolten AB, Chaloupka MY, Hutchinson BJ, et al. Global Conservation Priorities for Marine Turtles. PLoS ONE. 2011;6(9):e24510. doi: 10.1371/journal.pone.0024510. doi:10.1371/journal.pone.0024510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BP, Kot CY, DiMatteo AD, Lee T, Crowder LB, Lewison RL. Impacts of fisheries bycatch on marine turtle populations worldwide: toward conservation and research priorities. Ecosphere. 2013;4(3):40. doi:10.1890/ES12-00388.1. [Google Scholar]
- Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT. Links between worlds: unraveling migratory connectivity. TRENDS in Ecology & Evolution. 2002;15(2):76–83. [Google Scholar]
- Wetherall JA, Balazs GH, Tokunaga RA, Yong MYY. Bycatch of marine turtles in North Pacific high seas driftnet fishery and impacts on stock. In: Ito J (ed) INPFC symposium on biology, distribution and stock assessment of species caught in the high seas driftnet fishery in the North Pacific Ocean. Int N Pac Fish Comm. 1993:519–538. [Google Scholar]
- Wilson KA, McBride MF, Bode M, Possingham HP. Prioritizing global conservation efforts. Nature. 2006;440:337–340. doi: 10.1038/nature04366. doi:10.1038/nature04366. [DOI] [PubMed] [Google Scholar]
- Wingfield DK, Peckham SH, Foley DG, Palacios DM, et al. The making of a productivity hotspot in the coastal ocean. PLoS ONE. 2011;6:e27874. doi: 10.1371/journal.pone.0027874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyneken J, Lohmann KJ, Musick JA, editors. The Biology of Sea Turtles. III. CRC Press; Boca Raton, FL.: 2013. p. 457. [Google Scholar]
- Zug GR, Wynn AH, Ruckdeschel C. Age determination of loggerhead sea turtles, Caretta caretta, by incremental growth marks in the skeleton. Smithsonian Contributions to Zoology. 1986:34. [Google Scholar]
- Zug GR, Balazs GH, Wetherall JA. Growth in juvenile loggerhead seaturtles (Caretta caretta) in the North Pacific pelagic habitat. Copeia. 1995;1995:484–487. [Google Scholar]
- Zug GR, Glor RE. Estimates of age and growth in a population of green sea turtles (Chelonia mydas) from the Indian River lagoon system, Florida: a skeletochronological analysis. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1998;76(8):1497–1506. [Google Scholar]