Abstract
Gln3p is one of two well characterized GATA family transcriptional activation factors whose function is regulated by the nitrogen supply of the cell. When nitrogen is limiting, Gln3p and Gat1p are concentrated in the nucleus where they bind GATA sequences upstream of nitrogen catabolite repression (NCR)-sensitive genes and activate their transcription. Conversely, in excess nitrogen, these GATA sequences are unoccupied by Gln3p and Gat1p because these transcription activators are excluded from the nucleus. Ure2p binds to Gln3p and Gat1p and is required for NCR-sensitive transcription to be repressed and for nuclear exclusion of these transcription factors. Here we show the following. (i) Gln3p residues 344–365 are required for nuclear localization. (ii) Replacing Ser-344, Ser-347, and Ser-355 with alanines has minimal effects on GFP-Gln3p localization. However, replacing Gln3p Ser-344, Ser-347, and Ser-355 with aspartates results in significant loss of its ability to be concentrated in the nucleus. (iii) N and C termini of the Gln3p region required for it to complex with Ure2p and be excluded from the nucleus are between residues 1–103 and 301–365, respectively. (iv) N and C termini of the Ure2p region required for it to interact with Gln3p are situated between residues 101–151 and 330–346, respectively. (v) Loss of Ure2p residues participating in either dimer or prion formation diminishes its ability to carry out NCR-sensitive regulation of Gln3p activity.
Saccharomyces cerevisiae is increasingly used as a model to identify the functions of mammalian proteins as well as how their production and activities are regulated and integrated. One of the gene families shared by S. cerevisiae and higher eukaryotes is the GATA family of DNA-binding proteins. In animal cells, GATA family proteins were originally shown to be responsible for regulating globin gene expression (1). However, they are now known to regulate a diverse set of developmental functions (2, 3). In yeast, the GATA family proteins Gln3p, Gat1p/Nil1p, Dal80p, Deh1p/Gzf3p have been studied as the main regulators of nitrogen catabolic gene expression (4–6), and recently their regulatory functions also been shown to be far more diverse (7, 8). Gln3p and Gat1p are transcriptional activators, whereas Dal80p and Deh1p repress transcription by competing with these activators for binding to their target GATA sequences (4–6, 9–11).
Gln3p and Gat1p function is also dramatically regulated by the nitrogen supply of the cell. When nitrogen is limiting, Gln3p and Gat1p are concentrated in the nucleus, bind to the GATA sequences of their target promoters, and activate transcription (8, 9, 12–15). On the other hand, when nitrogen is in excess, Gln3p and Gat1p are excluded from the nucleus, and nitrogen catabolite repression (NCR)1-sensitive gene expression is repressed. Nuclear exclusion of Gln3p and Gat1p requires Ure2p, the first NCR regulator identified (16, 17) and recently much studied as a prion precursor (18–27). Nuclear exclusion also correlates with hyperphosphorylation of Gln3p (8, 12–14), a process that requires the phosphatidylinositol kinase-3 homologous Tor1/2p, and is inhibited by the immunosuppressive, antifungal, antineoplastic drug rapamycin (8, 12–14). The mechanistic details of the signal transduction pathway associated with Gln3p and Gat1p controls, however, are not fully understood. Some investigators (12) report Sit4p phosphatase is required for dephosphorylation of Gln3p, its nuclear entry, and NCR-sensitive gene expression, whereas others (8, 14) cannot demonstrate Sit4p participation in Gln3p phosphorylation, localization, and control. Similarly, Cardenas et al. (14) and Hardwick et al. (8) find Ure2p to be phosphorylated, whereas Beck and Hall do not (12). Although all in the field agree that Ure2p complexes with Gln3p and Gat1p, the cause-effect relationships of Gln3p phosphorylation, Ure2p-Gln3p complex formation, and the preeminent determinant of Gln3p nuclear exclusion remain to be established.
Establishing the cause-effect relationships associated with Ure2p control of Gln3p and Gat1p localization require a more detailed understanding of these proteins. Ure2p structure has been studied in detail (26–28). The N-terminal asparagine-rich prion domain (amino acids 1–65) is primarily, although not exclusively, required for prion formation, whereas the C-terminal region (amino acids 66–354) complements a ure2 null mutation for growth requiring ureidosuccinate uptake by an NCR-sensitive permease (19). For Gln3p, three functional regions have been identified as follows: a C2X17C2 zinc finger motif (residues 306–330) that binds to GATA sequences (29), a predicted α-helical motif that mediates transcriptional activation (residues 126–138) (30), and a large C-terminal region (residues 510–720) that associates with Tor1p (13).
Our purpose here is to identify sequences required: (i) for nuclear-import of Gln3p, i.e. the nuclear localization signal (NLS); (ii) for Gln3p to interact with Ure2p; and (iii) for Ure2p to interact with Gln3p.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions
The strains we used are listed in Table I. Cultures were grown overnight in YNB medium (0.17% YNB, with casamino acids, 0.5% ammonia, and 2% raffinose (GYC86) or glucose (TCY57)) to A600 = 0.25–0.8 for microscopy. Auxotrophic requirements were supplemented as necessary. Transformants of GYC86 were induced by adding galactose (final concentration, 4%). Transformants of TCY57 were induced by transferring washed cells to YNB galactose (4%), ammonia (0.5%) medium. Cultures were induced for 3 or more hours. For β-galactosidase assays YNB (0.17% YNB without casamino acids, 2% glucose, and 0.1% appropriate nitrogen source) medium was used.
Table I.
Strains and plasmids used in this work
| Strain | Genotype |
|---|---|
| S. cerevisiae | |
| GYC86 | MATa his3 leu2 trp1 ura3/MATα his3 leu2 trp1 ura3 |
| TCY57 | Matα lys2 ura3 trp1::hisG leu2::hisG GAL1,10-URE2 |
| RR912 | Matα lys2 ura3 gln3Δ::hisG trp1Δ::hisG his3Δ::hisG |
| STCY32 | MATa lys5 ura3 trp1Δ::hisG his3Δ::hisG |
| BY4742 | MATα his3D1 leu2D0 lys2D0 ura3D0 |
| E. coli | |
| DH5α | [F/endA1 hsdr17(rK− mK+) supE44 thi1 recA1 Δ(lacZYA-argF)U169 (m80lacZΔ1M15)] |
Plasmid Constructions
DNA cloning was performed using standard methods (31). Oligonucleotides used in this work are listed in the Table II. GFP-Gln3p deletion inserts were cloned into the NdeI and HindIII sites of vector pNVS2. PCRs were performed using pRR312 (32), containing the full-length Gln3p coding sequence with an internal NdeI site destroyed, as template. LexA-Ure2p inserts were cloned finally into the sites of vector pEG202. Inserts were generated by standard PCR, using pRD17 as a template (31), and cloning techniques. All constructs were verified by DNA sequence analyses.
Table 2.
| Plasmid | Characteristics |
|---|---|
| pKA10 | GFP-Gln3p-(1–301) |
| pKA14 | GFP-Gln3p-(1–365) |
| pKA42 | GFP-Gln3p-(1–487) |
| pKA17 | GFP-Gln3p-(1–542) |
| pKA24 | GFP-Gln3p-(1–680) |
| PKA 21 | GFP-Gln3p-(1–710) |
| pRR482 | GFP-Gln3p-(1–730) (full length) (15) |
| pKA26 | GFP-Gln3p-(103–493) |
| pKA27 | GFP-Gln3p-(187–493) |
| pKA30 | GFP-Gln3p-(256–493) |
| pKA34 | GFP-Gln3p-(297–493) |
| pKA36 | GFP-Gln3p-(344–493) |
| pKA38 | GFP-Gln3p-(384–493) |
| pKA52 | GFP-Gln3p-(344–493) S344A, S347A, S355A |
| pKA56 | GFP-Gln3p-(344–493) S344D, S347D, S355D |
| pKA53 | GFP-Gln3p-(344–493) K352E, R353E, K357E, R358E |
| pRR312 | Containing coding region of Gln3p, AmpR |
| pNVS2 | GAL1,10 promoter eGFP, URA3, AmpR (33) |
| pHS18–34 | LexA-lacZ reporter plasmid (33, 44) |
| pEG202 | LexA 2 μm plasmid, HIS3, AmpR (33, 44) |
| pAA15 | LexA-Ure2p-(1–354) (full length) |
| pAA107 | LexA-Ure2p-(1–346) |
| pAA113 | LexA-Ure2p-(1–330) |
| pAA112 | LexA-Ure2p-(1–315) |
| pAA106 | LexA-Ure2p-(1–300) |
| pAA105 | LexA-Ure2p-(1–275) |
| pAA104 | LexA-Ure2p-(1–250) |
| pAA103 | LexA-Ure2p-(1–200) |
| pAA102 | LexA-Ure2p-(1–150) |
| pAA101 | LexA-Ure2p-(1–100) |
| pAA100 | LexA-Ure2p-(1–65) |
| pAA110 | LexA-Ure2p-(66–354) |
| pAA114 | LexA-Ure2p-(101–354) |
| pAA115 | LexA-Ure2p-(151–354) |
| pAA116 | LexA-Ure2p-(201–354) |
| pAA117 | LexA-Ure2p-(201–354) |
| pAA118 | LexA-Ure2p-(276–354) |
| pRD17 | Containing upstream sequence and coding region of Ure2p (53) |
Fluorescence Microscopy
Samples were prepared for fluorescence microscopy as described earlier (9, 15, 33). Images were irradiated with a combination of white and/or epi-fluorescent light, viewed using the 63× objective of a Zeiss Axiophot microscope equipped with a GFP filter set and collected with a Zeiss Axiocam digital camera using AxioVision 2.0.5.3 software. Photographs were imported into Photoshop 4.0.
β-Galactosidase Assay
Transformants were prepared and assayed, including numbers of independent transformants assayed and variation in observed results, as described (34).
Northern Blot Analysis
Total RNA was prepared (11), and hybridization reactions were performed as described (11). Hybridization probes were generated using PCR products (7).
RESULTS
Gln3p Sequence Required for Nuclear Localization
Intra-cellular localization is the preeminent control of Gln3p activity. To identify the Gln3p NLS, we constructed GFP-Gln3 C-terminal truncation proteins (Fig. 1, upper portion). To ensure GFP-Gln3p retained normal function, a gln3Δ was transformed with GFP-GLN3 pRR482. Complementation of the gln3Δ, measured as the ability to support DAL5 expression, was efficient even when Gln3p was expressed at very low levels (Fig. 2, lane A). A minor species migrates just ahead of the DAL5 transcript. We don’t know its identity for sure, but its presence in both lanes eliminates the possibility that it is DAL5 mRNA because DAL5 expression does not occur in a gln3Δ (35) (Fig. 2, lane B).
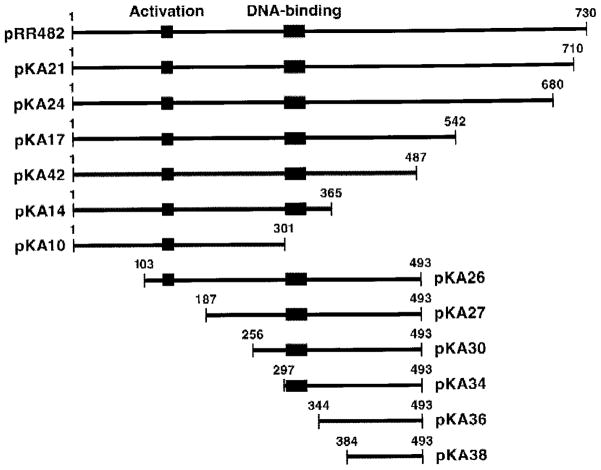
Fig. 1. Gln3p truncations used in this work.
5′ and 3′ gln3 deletions generated by PCR-based cloning in the vector pNVS2. Bars indicate Gln3p residues remaining.
Fig. 2. Complementation of a gln3Δ by EGFP-GLN3.

Cultures of strain RR912 (gln3Δ), transformed with either vector pNVS2 or GFP-GLN3 pRR482, were grown in minimal glucose/proline medium. Total RNA from these cultures was prepared and resolved in a Northern blot. A full-length DAL5 gene was used as probe.
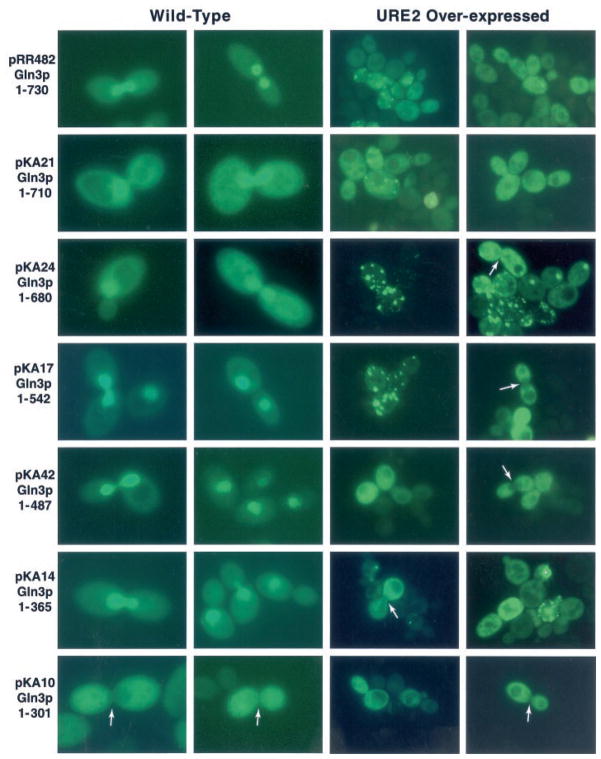
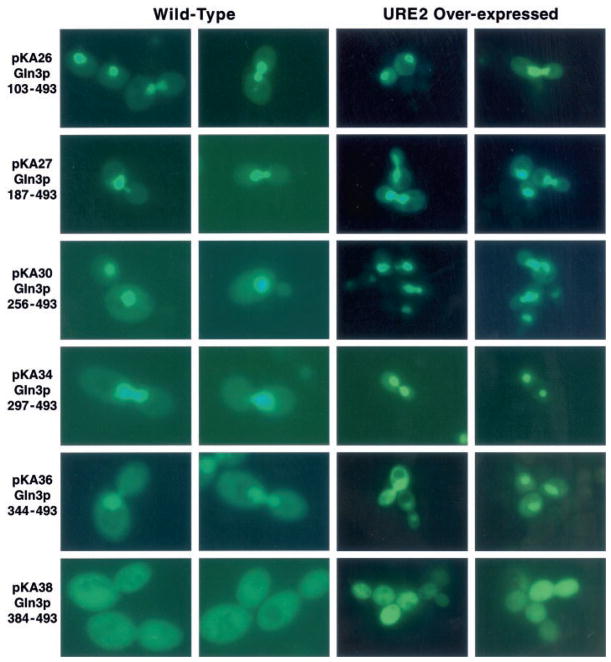
GFP-Gln3p truncation plasmids were transformed into wild type strain GYC86, and their intracellular distribution was evaluated using epifluorescence microscopy. GFP-Gln3 truncation proteins containing 365 or more N-terminal amino acids were concentrated in the nucleus (Fig. 3, left two columns). The transformants did not, however, behave identically. Greater cytoplasmic localization occurred with truncations GFP-Gln3p-(1–710) (pKA21) and GFP-Gln3p-(1–680) (pKA24) than with GFP-Gln3p-(1–542) (pKA17) and GFP-Gln3p-(1–487) (pKA42). The extent of cytoplasmic localization increased again with GFP-Gln3p-(1–365) (pKA14) even though nuclear concentration of GFP-Gln3p was clearly visible in all of the transformants just mentioned. Nuclear concentration of GFP-Gln3p was lost when residues between 301 (pKA10) and 365 (pKA14) were removed (Fig. 3, left two columns). In fact, fluorescent material can clearly be seen to be excluded from nuclei in cells where DNA is situated in the neck between mother and daughter (Fig. 3, left two columns, pKA10 arrows). These data argue the NLS C terminus is between Gln3p residues 301 and 365. A similar approach localized the N terminus of the Gln3p NLS (Fig. 1, lower portion). Fluorescent material was largely nuclear in Gln3p N-terminal truncations to residues 102 (pKA26), 186 (pKA27), 255 (pKA30), 296 (pKA34), and 343 (pKA36) (Fig. 4, left two columns). However, truncation to amino acid 383 (pKA38) resulted in loss of nuclear localization (Fig. 4, left two columns), localizing the N terminus of the Gln3p NLS between residues 344 and 384.
Fig. 3. Intracellular localization of C-terminally truncated EGFP-Gln3 proteins.
GAL1,10-EGFP-GLN3 deletion plasmids (Fig. 1) were transformed into WT GCY86 (left two columns of images) and TCY57 (right two columns of images), which expresses GAL1,10-URE2 at high levels. Transformants were viewed as described under “Experimental Procedures.”
Fig. 4. Intracellular localization of N-terminally truncated EGFP-Gln3 proteins.
GAL1,10-EGFP-GLN3 deletion plasmids (Fig. 1) were transformed into WT GCY86 (left two columns of images) and TCY57 (right two columns of images), which expresses GAL1,10-URE2 at high levels. Transformants were assayed as in Fig. 3.
Serine Residues in the NLS Are Not Absolutely Required for Nuclear Localization
The most likely NLS candidate in Gln3p region 301–384 is 343LSLKSDVIKKRISKKRAK360 which possesses significant similarity to other documented NLSs (36, 37). To establish whether this region was responsible for Gln3p nuclear localization, we assayed a peptide (344–493, pKA36) in which basic residues Lys-352, Arg-353, Lys-357, and Arg-358 were changed to glutamate (pKA53). Fluorescent material concentrates in the nuclei of wild type (pKA36) transformants, whereas it is cytoplasmic with mutant pKA53 (Fig. 5).
Fig. 5. Intracellular localization of EGFP-Gln3p amino acid substitution mutant proteins.
Upper four rows of images: mutant GFP-gln3 plasmids were generated and assayed as described under “Experimental Procedures.” Bottom image, EGFP-Gln3p fluorescence showing distinct foci (arrows) similar to those observed with EGFP-Dal80p and EGFP-Dal82p (45).
Work in several well studied nuclear transport systems suggests NLS function can be regulated by phosphorylation (38–42). Since increased Gln3p phosphorylation correlates with its nuclear exclusion, and Gln3p-(344–365) contains a potential PKA-modification site, we first determined whether Ser-344, Ser-347, and Ser-355 were themselves required for nuclear localization by changing them to alanine (pKA52). Nuclear localization of Gln3pS344A,S347A,S355A is diminished minimally if at all (Fig. 5). If, on the other hand, these serines are changed to negatively charged aspartates (pKA56), nuclear localization is barely detectable (Fig. 5).
Gln3p Sequences Required for Interaction with Ure2p
It is well established that Gln3p/Gat1p and Ure2p form a complex in vivo and in vitro (8, 9, 12–15). In addition, overproduction of Ure2p results in loss of NCR-sensitive gene expression, nuclear exclusion of GFP-Gln3p and GFP-Gat1p, with the concomitant formation of highly punctate fluorescent foci identical to those seen with GFP-Ure2p (9, 15, 22). These observations provide a useful assay with which to identify Gln3p regions required for interaction with Ure2p. We transformed C-terminal GFP-Gln3p deletion plasmids described in the left two columns of Fig. 3 into GAL1,10-URE2 strain TCY57 (9, 15). In contrast to data obtained with the wild type strain, none of the truncated GFP-Gln3 proteins were concentrated in the nucleus; all localized to the cytoplasm (Fig. 3, right two columns). In fact, GFP-Gln3p was clearly excluded from many nuclei situated in the neck between mother and daughter cells (Fig. 3, right two columns, arrows). Furthermore, punctate foci similar to those previously seen with GFP-Ure2p or GFP-Gln3p in the presence of high URE2 expression (9, 15, 22) are clearly evident in many micrographs (Fig. 3, right two columns), arguing that sequences N-terminal to residue 301 are sufficient for Gln3p-Ure2p interaction and GFP-Gln3p exclusion from the nucleus.
To localize further the Ure2p interaction site of Gln3p, we transformed TCY57 with the N-terminal Gln3p deletion plasmids described in the left two columns of Fig. 4 (Fig. 4, right two columns). GFP-Gln3p was nuclear in all cases except pKA38 which lacked the NLS. Therefore, N terminus of the Gln3p region required for its interaction with Ure2p is between amino acids 1 and 103 and the C terminus is N-terminal to 301.
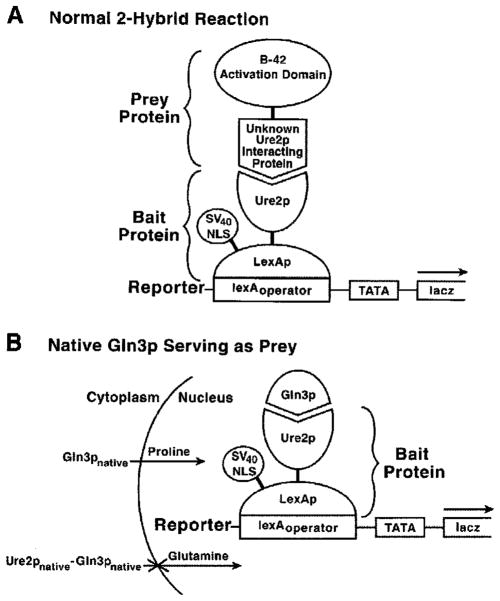
Two-hybrid Assays with Ure2p as Bait
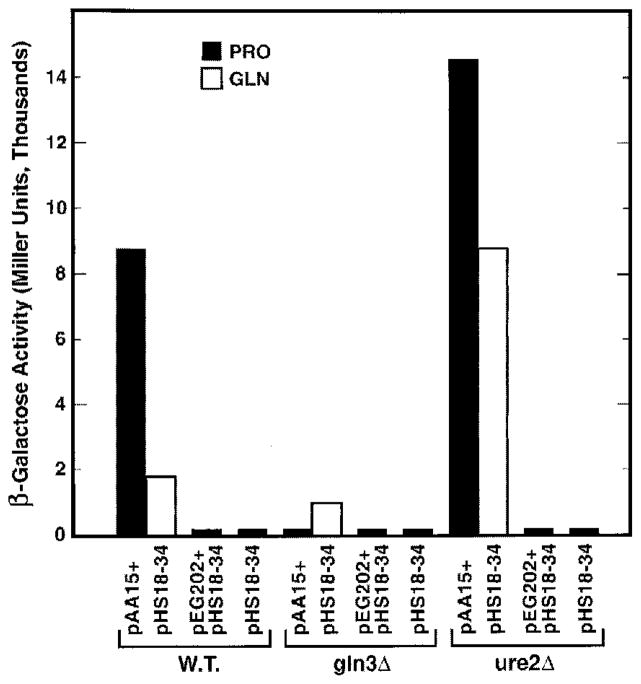
To identify the Ure2p residues required for interaction with Gln3p, we set-up a two-hybrid assay (Fig. 6A) with NLS-lexA-URE2 pAA15, lexAoperator-lacZ pHS18–34, and a GLN3-B42 plasmid (33, 44) as bait, reporter, and prey, respectively. To our initial surprise, transformants of wild type cells, containing only the bait and reporter plasmids (pAA15 plus pHS18–34) produce high level β-galactosidase (Fig. 7). This result is striking, because it occurred in the absence of a “prey” plasmid. In a two-hybrid assay, the prey plasmid insert normally encodes a protein, which activates transcription after being recruited to the promoter of a reporter gene via its interaction with the “bait” protein bound to multiple lexAoperator or Gal4p-binding sites upstream of that reporter gene; the reporter gene promoter is devoid of upstream activation sequence (UAS) elements (44) (Fig. 6A). pAA15 and pHS18–34 are both required for the β-galactosidase production (Fig. 7). Additional standard controls were performed to validate the surprising result, and the expected results were observed (data not shown). These data, and the fact that an SV40 NLS is fused to lexA sequences of bait vector pEG202, into which URE2 was cloned, argue that Ure2p is nuclear, binds to lexAoperator sites in the lacZ promoter, and successfully recruits a native, endogenous molecule capable of supporting transcriptional activation to the lacZ reporter gene promoter (Fig. 6B). This bait-transcriptional activator complex then mediates lacZ transcription. Upon further characterization, we found the LexA-Ure2p-dependent lacZ expression to be NCR-sensitive (Fig. 7). Note, however, that transcription with glutamine as nitrogen source is a bit higher than normally occurs if a typical NCR-sensitive gene, e.g. DAL5, had been assayed (11).
Fig. 6. Two-hybrid assay used to identify the regions of Ure2p required for its interaction with Gln3p.
A, normal two-hybrid assay components showing the assembled complex required for lacZ reporter gene expression. Here the putative Ure2p-interacting protein is fused to the transcriptional activation domain of B-42. The fusion protein is encoded by the insert of the prey plasmid. B, two-hybrid assay with native, endogenous Gln3p serving the role of the prey protein. In this instance, no prey plasmid is required because Gln3p is providing the trans-activation function, and no fusion to an activation domain is required because Gln3p itself possesses a strong activation domain (Gln3p-(126–138)). Full-length NLSSV40-lexA-URE2 as well as NLSSV40-lexA-ure2 deletion plasmids were used as baits for the experiment in Fig. 7.
Fig. 7. Effect of gln3 and ure2 deletion on LexA-Ure2p-mediated β-galactosidase production.
Growth and assay procedures are described under “Experimental Procedures.”
A priori, the most likely candidate of an activator to mediate NCR-sensitive transcription with LexA-Ure2p as bait is Gln3p, which predicts LexA-Ure2p-dependent β-galactosidase production should be Gln3p-dependent and Ure2p-regulated. As shown in Fig. 7, the predicted results are observed experimentally. It should be emphasized that native Ure2p encoded on the chromosome is regulating LexA-Ure2p-mediated transcription (Fig. 6B). This conclusion derives from the facts that (i) high levels of LexA-Ure2p are present in both wild type and ure2Δ transformants (LexA-URE2 expression is driven by the ADH promoter and the gene is carried on a 2-μ-based plasmid), and (ii) deletion of the resident, native URE2 gene exhibits a strong phenotype that is not complemented by LexA-Ure2p. Therefore, two reactions occur in which Gln3p complexes with Ure2p as follows: one in the cytoplasm between endogenous, native Ure2p and Gln3p, which imparts NCR-sensitive regulation; and the second in the nucleus between Gln3p and heterologous NLS-LexA-Ure2p, which accounts for transcriptional activation. The values observed with proline as nitrogen source reflect binding between LexA-Ure2p and Gln3p, since Gln3p is minimally influenced by Ure2p and has full access to the nucleus under this condition.
To identify Ure2p residues required for β-galactosidase production mediated by LexA-Ure2p, C- and N-terminal Ure2p truncations were constructed. C-terminal truncation of only eight amino acids diminishes lacZ expression by half (Fig. 8, pAA15 and pAA107). Loss of another 16 amino acids completely eliminates β-galactosidase production (pAA113). The N-terminal 101 Ure2p residues are not required for β-galactosidase production with proline as nitrogen source (Fig. 8, pAA110 and pAA114). Further N-terminal truncation, however, eliminates β-galactosidase production (pAA115), placing the N terminus between residues 101 and 151.
Fig. 8. β-Galactosidase production supported by truncated Ure2 proteins fused to LexA.
Experiments were performed in wild type STYC32. YNB glucose medium was used with 0.1% proline (PRO) or glutamine (GLN) as nitrogen source.
When the experiment was repeated with glutamine as nitrogen source, several differences were observed. (i) Truncation of the C-terminal eight amino acids of Ure2p didnt severely decrease reporter gene expression with proline (Fig. 8, pAA107). (ii) N-terminal truncation of 66 and 101 amino acids resulted in 6.5- and 10.2-fold increases in expression compared with 1.7-and 1.3-fold observed with proline. In other words, truncating LexA-Ure2p resulted in measurable loss of NCR sensitivity (Fig. 8, pAA15, pAA110, and pAA114). It is pertinent that lacZ expression in proline medium is influenced only by complex formation between Gln3p and LexA-Ure2p, whereas in gluta-mine medium, it is also influenced by complex formation between Gln3p and native Ure2p. By this reasoning, the loss of LexA-Ure2p residues 1–65 does not measurably affect its ability to bind Gln3p and mediate transcription, but it does affect Ure2p-Gln3p complex formation occurring in the cytoplasm and hence the ability of native Ure2p to function properly when cells are provided with glutamine as nitrogen source.
Finally, we constructed an internal deletion, which removed Ure2p amino acids 224–229. Although this truncation is situated within the LexA-Ure2p region required for lacZ expression, no deleterious effects are observed with proline as nitrogen source (Fig. 8). With glutamine, this truncation, like those encoded by pAA110 and pAA114, mediates reporter gene expression that is partially resistant to NCR (Fig. 8).
GFP-Gln3p Occasionally Exhibits Intranuclear Localization Similar to That Seen with GFP-Dal82p
Cells transformed with a GFP-DAL80 plasmid exhibit fluorescent foci that co-localize with 4,6-diamidino-2-phenylindole-positive material and follow DNA movement through the cell cycle (45). High resolution confocal and deconvolution microscopy delineate up to 16 distinct foci, which correlates with the number of S. cerevisiae chromosomes (45). DAL80 encodes a repressor protein, which binds to some of the same GATA sequences as Gln3p (10, 32, 46–48). GFP-Dal82p generates similar fluorescent foci, although they are considerably less well defined than seen with GFP-Dal80p (33, 45, 49, 50). Since we were viewing hundreds of fields of cells transformed with GFP-GLN3 plasmids, we queried whether or not images similar to those mentioned above also occurred with GFP-Gln3p. Intra-nuclear distribution of GFP-Gln3p fluorescent material similar to that seen with GFP-Dal82p were occasionally seen (compare Fig. 5, bottom image, arrows, with Fig. 2, row 4, of Ref. 45).
DISCUSSION
Present and past data show most Gln3p component functions localize to the N-terminal half of the molecule as follows: transcriptional activation, a predicted α-helical structure at residues 126–138 (30); Ure2p-interacting region at residues 1–301 (right two columns, Figs. 3 and 4); GATA binding, a C-4 zinc finger at residues 306–330 (29, 32, 51); nuclear localization at residues 344–365 (left two columns, Figs. 3–5), potentially nuclear export signals at residues 64–73 and/or 336–345, and Tor1p-binding at residues 510–720 (13) (Fig. 9).
Fig. 9.

Functional regions of Gln3p.
Although Gln3p residues 344–365 are necessary and sufficient for nuclear localization of Gln3p, residues 542–680 also influence this process. This conclusion derives from consistently observing more fluorescent material in the cytoplasm of transformants containing GFP-Gln3p-(1–730, 1–710, and 1–680) than with GFP-Gln3p-(1–542) (Fig. 3). Eliminated from consideration as potential NLS sites are Gln3p basic regions 390–394, 477–482, and 264–270. This conclusion is based on the cytoplasmic localizations of Gln3p-(344–493) (pKA56), containing residues 390–394 and 477–482 (Fig. 5), and Gln3p-(1–301) (pKA10), containing residues 264–270.
Two related assays localized the Ure2p-interacting region of Gln3p to residues 1–301. The nuclear exclusion assay in cells overexpressing URE2 localized the interacting region to residues 1–365. Further resolution could not be achieved because the assay requires functional integrity of NLS residues 344–365. Using the second assay, i.e. GFP-Gln3p generated punctate fluorescence when Ure2p is overproduced, resolved the interacting region to Gln3p residues 1–301 (Fig. 3, pKA10, right two columns). Data from pKA26 (Fig. 4, right two columns) might be suggested to localize the N terminus of the Ure2p-interacting region to residues 1 and 102. This interpretation isn’t justified, however, because Gln3p contains two nuclear export signal (NES)-homologous sequences, residues 336–345 and 64–73. If residues 64–73 were to serve as the unique Gln3p NES, their removal might result in nuclear localization of GFP-Gln3p due to lack of a mechanism for the protein to exit from the nuclear compartment. If, alternatively, residues 336–345 uniquely serve this function, the N terminus of the Ure2p-interacting region is somewhere between amino acids 1 and 102.
The Gln3p topology diagrammed in Fig. 9 influences interpretation of data addressing how Ure2p regulates Gln3p. For example, one mechanism envisions Ure2p binds directly to the NLS thereby sterically preventing it from functioning. Although elements of this idea remain tenable, such steric hindrance would have to occur indirectly as a result of Ure2p-binding N-terminal to the NLS. In this regard, it is interesting to note that the Gln3p transcriptional activation and DNA-binding domains are situated between the proposed Ure2p interaction site and the NLS. Also, because of their locations, it is reasonable to suggest the Gln3p DNA binding and transcriptional activation regions may point away from those needed for Ure2p interaction. At least for the DNA transcriptional activation domain, this suggestion is supported by the observation that Gln3p is able to mediate transcriptional activation when bound to LexA-Ure2p (Fig. 7).
The LexA-Ure2p experiment generated further insight into the functioning of Ure2p, Gln3p, and their interaction. LexA-Ure2p-mediated transcription argues that Ure2p is cytoplasmic, not because it is tethered but because it simply lacks a functional NLS or, alternatively, that NLSSV40LexA fused to Ure2p inactivates the tethering mechanism. We favor the first explanation and feel that the inability of LexA-Ure2p to complement ure2Δ mutations could be one outcome of it.
NLSSV40 fused to Ure2p can mediate nuclear uptake, whereas the NLS provided by Gln3p, when it forms the cytoplasmic Ure2pnative-Gln3pnative complex, is insufficient to permit nuclear entry. One explanation of the data would posit that single molecules, but not complexes consisting of multiple molecules, can be transported into the nucleus. Alternatively, this experimental result may support the question of whether NLSGln3p phosphorylation or Ure2p binding is primarily responsible for Gln3p exclusion from the nucleus. Gln3pS344A,S347A,S355A (pKA52) demonstrates that serine residues per se at these positions are not required for NLS function. The Gln3p-(344–493)S344D,S347D,S355D variant most closely mimics the effects of phosphorylating these serines, because they confer a negative charge to this region of Gln3p. In addition, these residues encompass a potential PKA modification site, KRIS355. Although these mutant data are consistent with the contention that NLS phosphorylation plays a regulatory role, they certainly do not prove it. On the other hand, the in vivo demonstration that URE2 overexpression excludes Gln3p and Gat1p from the nucleus and represses NCR-sensitive transcription in proline-grown cells indicates that high level NCR can be exerted in the absence of the cellular signal generated by excess nitrogen, because proline-grown cells do not contain it. Therefore, by the current models in the literature, this NCR would be occurring in under conditions of hypo-phosphorylation (9, 15) and hence argues in favor of Gln3p-Ure2p complex formation being the more important regulatory determinant. That conclusion, however, does not exclude a role for phosphorylation outside of its influence on Gln3p-Ure2p complex formation. It is quite conceivable that NCR-sensitive GAT1 and GAP1 expression that occurs in a gln3Δure2Δ mutant derives from phosphorylating Gat1p in response to nitrogen excess (52). Similarly by this reasoning, NCR-sensitive DAL5 expression that occurs in a gat1Δure2Δ mutant can be argued to derive from phosphorylating Gln3p when glutamine is supplied as sole nitrogen source (52).
The LexA-Ure2p truncation experiments offer additional insights into Ure2p itself. Genetic studies led Wickners laboratory to conclude the Ure2p prion-forming domain was N-terminal (amino acids 1–65), with the C terminus being sufficient for NCR-sensitive transcriptional regulation imposed upon Gln3p (18, 19). Our LexA-Ure2p data with proline as nitrogen source (Fig. 8) confirm their view, demonstrating the N and C termini of the Ure2p region needed for Gln3p binding to be between residues 101–151 and 330–346, respectively. Higher resolution experiments demonstrated that although prion formation was predominantly N-terminal, C-terminal portions of Ure2p also participated (24). Data presented here similarly argue that whereas NCR-sensitive transcriptional regulation is predominantly a Ure2p C-terminal function, the first 65 amino acids participate in this control as well (Fig. 8).
The LexA-Ure2p truncation experiments leave an apparent paradox. Full-length LexA-Ure2p mediates NCR-sensitive lacZ, arguing the fusion protein plays no role in the cytoplasm. However, removing either the first 65 Ure2p residues or those between 223 and 230 diminishes NCR-sensitive regulation. How could this be? The explanation that makes most sense to us posits the occurrence of negative complementation. Although Ure2p is a dimer in solution (26, 27), whether this is required for its regulatory role isn’t known. Our data are most easily explained if it is. Both regions of Ure2p, the truncation of which are deleterious to NCR-sensitive regulation, are associated with Ure2p-Ure2p interaction, the prion-forming domain (residues 1–65) (18), and the α5 helix which forms the Ure2p dimerization interface (26, 27). Therefore, Ure2p heterodimers consisting of Ure2pnative and LexA-Ure2p-(66–354) or LexA-Ure2p-(224–229Δ) would be expected to form less stably than the Ure2pnative-Ure2pnative homodimer, which contains both interacting regions. Note that pAA111 in which the dimerization interface is directly damaged also possesses the stronger phenotype, i.e. one-third of the NCR sensitivity is lost. But why wasn’t an even stronger phenotype in glutamine medium observed since there is far more lexA-ure2 than URE2 expression in the cell? Although this is true, the SV40 NLS fused to LexA-Ure2 mutant protein would result in its nuclear concentration, resulting in less mutant LexA-Ure2p being available in the cytoplasm to dimerize with Ure2pnative molecules. Analogously, it is appropriate to question that if LexA-Ure2p-(66–354)-Ure2pnative forms a less stable complex with Gln3p than Ure2pnative-Ure2pnative, why isn’t this reflected in similarly weaker LexA-Ure2p-(66–354)-LexA-Ure2p-(66–354)-Gln3p complex being formed at the site of transcription, hence lowering lacZ expression supported by pAA15 compared with pAA110 in proline medium. Two possibilities come to mind. (i) The interaction of the LexA-Ure2p-LexA-Ure2p-Gln3p complex with components of the core transcription complex contribute to stabilization such as is seen when the presence of Dal82p suppresses mutations in uasNTR. (ii) Ure2p in its dimer form is not required for binding to Gln3p but is required for Ure2p to prevent Gln3p from gaining entry into the nucleus. Crystallographic data for Ure2p complexed with Gln3p will, no doubt, greatly contribute to a more complete understanding of these in vivo data.
The LexA-Ure2p is not the first protein shown to support Gln3p-dependent reporter gene transcription and/or complex with Gln3p. (i) LexA-Dal82p mediates Gln3p-dependent lacZ expression under experimental conditions similar to those reported here (33, 44). Furthermore, there is independent genetic evidence supporting the contention that Dal82p and Gln3p interact with one another, i.e. mutations in the UASNTR, Gln3p-binding site are suppressed by placing a functioning Dal82p-binding site adjacent to the mutated uasNTR (50). This suppression is Dal82p-dependent. (ii) The coiled-coil domain of Dal82p interacts with Dal81p (44). (iii) The Gln3p, Dal80p, and Deh1p/Gzf3p zinc finger motifs all interact with one another in a two-hybrid reaction but not with the zinc finger motif of Put3p (47). From this perspective, we propose the following interpretation of the recent report that a Tor1p bait plasmid, fused to a Galp-DNA-binding domain, yields positive interactions with prey plasmids in which the Gal4p activation domain was fused to Gln3p-(510–720), Ure2p, Dal82p, Dal81p, Dal80p, Gat1p, and Deh1p/Gzf3p (13). In our view, these data derive from Tor1p interacting with Gln3p and/or Gat1p and that the remaining interactions occur through the intermediacy of these transcriptional activators. This interpretation also points to the possibility that Gln3p, Gat1p, Dal81p, Dal82p, and perhaps the transcriptional repressors, Dal80p and Deh1p/Gzf3p, are situated in a larger multitranscription factor complex as suggested earlier (44, 50).
Table II.
Oligonucleotides used in this work
| GLN-3Top1 | 5′-GGATTCCATATGCAAGACGACCCCGAAAATTC-GAAGCT-3′ |
| AJ301 | 5′-TCCCAAGCTTTCATTTTTTATTTTGTCCTGATGG-3′ |
| AJ365 | 5′-TCCCAAGCTTTCAGTTTGGGTCCGTTTGTTTGGC-3′ |
| AJ487 | 5′-TCCCAAGCTTTCACGATGAAGTACTACTTCGTC-3′ |
| AJ542 | 5′-TCCCAAGCTTTCAAATTCTTGGTGAGGATGCGAC-3′ |
| AJ680 | 5′-TCCCAAGCTTTCAGTTAAATTTGTGATTTGAGAT-3′ |
| AJ710 | 5′-TCCCAAGCTTTCATTTTGTGGAATTATCCTCACT-3′ |
| AJ102 | 5′-ACCAATCATATGGATGATGATATTGCG-3′ |
| AJ187 | 5′-GGATTCCATATGTCCAGCACGTCCAACAGCAACAT-CAAC-3′ |
| AJ256 | 5′-GGATTCCATATGTCATCCAACACAACAAATTC-TGTA-3′ |
| AJ297 | 5′-GGATTCCATATGGGACAAAATAAAAAACCTCTG-3′ |
| AJ344 | 5′-GGATTCCATATGTCCTTAAAATCGGACGTTATC-3′ |
| AJ384 | 5′-GGATTCCATATGACAAATGCTAAACCCATACGA-3′ |
| AJ344M1 | 5′-GGATTCCATATGgCCTTAAAAgCGGACGTTATCAA-AAAGAGGATTgCAAAGAAGAGAGCCA-3′ |
| AJ344M2 | 5′-GGATTCCATATGgaCTTAAAAgatGACGTTATCAAA-AAGAGGATTgatAAGAAGAGAGCCA-3′ |
| AJNLSMUT | 5′-GGATTCCATATGTCCTTAAAATCGGACGTTATCA-AAgAGgaGATTTCAAAGgAGgaAGCCAAACAAACG-3′ |
| AG3LAST | 5′-TCCCAAGCTTTCAACTGGAACTTGAGGTGTTCGA-3′ |
| AAUurER | 5′-GTAGAATTCATGATGAATAACAAC-3′ |
| AAUrLHN | 5′-ATGTAAAGCTTGTCCAATCATTGG-3′ |
| UreLD65 | 5′-CGCGTCGACTCAGCGGCCGCTGTTATT-3′ |
| UreL100 | 5′-CGCGTCGACTCAGGAATACTCCACGTG-3′ |
| UreLD150 | 5′-CGCGTAGACTCATTCGCCAAGATTGAA-3′ |
| UreLD200 | 5′-CGCGTCGACTCATAATGGATTACCAGT-3′ |
| UreLD250 | 5′-CGCGTCGACTCAATCCGTATATCTTTC-3′ |
| UreLD275 | 5′-CGCGTCGACTCAGTCTAATTCCATCAC-3′ |
| UreLD300 | 5′-CGCGTCGACTCACCATACGGGATAATC-3′ |
| UreLD346 | 5′-CGCGTCGACTCACGCGGGTCTTCTCAT-3′ |
| UreDSphH | 5′-ATGTAAAGCTTGCCCTGACGT-3′ |
| UreUD65 | 5′-GGCCGCGAATTCAATGGTAGCCAAAAT-3′ |
| UreSaL | 5′-AAATTTTGGTCGACTGTGGTTGGG-3′ |
| UreDN100 | 5′-CGCGAATTCAGAATTACAAAATTT-3′ |
| UreDN150 | 5′-CGCGAATTCCATAGGGCCCCCGAA-3′ |
| UreDN200 | 5′-CGCGAATTCCTCTGGTCCGATGAT-3′ |
| UreDN250 | 5′-CGCGAATTCGAGGTTAGAAGAGTT-3′ |
| UreDN275 | 5′-CGCGAATTCACGGAAAATGCGGCT-3′ |
Acknowledgments
We thank Tim Higgins for preparing the artwork and the University of Tennessee Yeast Group for suggested improvements to the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant GM-35642.
The abbreviations used are: NCR, nitrogen catabolite repression; GFP, green fluorescent protein; NLS, nuclear localization signal; PCR, polymerase chain reaction.
References
- 1.Weiss MJ, Orkin SH. Exp Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 2.Kelley C, Blumberg H, Zon LI, Evans T. Development. 1993;118:817–827. doi: 10.1242/dev.118.3.817. [DOI] [PubMed] [Google Scholar]
- 3.Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. J Biol Chem. 1994;269:23177–23184. [PubMed] [Google Scholar]
- 4.Cooper TG. In: The Mycota III. Marzluf G, Bambrl R, editors. Springer-Verlag; Berlin: 1996. pp. 139–169. [Google Scholar]
- 5.ter Schure EG, van Riel NA, Verrips CT. FEMS Microbiol Rev. 2000;24:67–83. doi: 10.1111/j.1574-6976.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman-Bang J. Mol Biotechnol. 1999;12:35–73. doi: 10.1385/MB:12:1:35. [DOI] [PubMed] [Google Scholar]
- 7.Cox KH, Pinchak AB, Cooper TG. Yeast. 1999;15:703–713. doi: 10.1002/(SICI)1097-0061(19990615)15:8<703::AID-YEA413>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Proc Natl Acad Sci U S A. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham TS, Andhare R, Cooper TG. J Biol Chem. 2000;275:14408–14414. doi: 10.1074/jbc.275.19.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham T, Dorrington RA, Cooper TG. J Bacteriol. 1994;176:4718–4725. doi: 10.1128/jb.176.15.4718-4725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugherty JR, Rai R, El Berry HM, Cooper TG. J Bacteriol. 1993;175:64–73. doi: 10.1128/jb.175.1.64-73.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck T, Hall MN. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 13.Bertram PG, Choi JH, Carvalho J, Ai W, Zeng C, Chan TF, Zheng XF. J Biol Chem. 2000;275:35727–35733. doi: 10.1074/jbc.M004235200. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox KH, Rai R, Distler M, Daugherty JR, Coffman JA, Cooper TG. J Biol Chem. 2000;275:17611–17618. doi: 10.1074/jbc.M001648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drillen R, Lacroute F. J Bacteriol. 1972;109:203–208. doi: 10.1128/jb.109.1.203-208.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drillen R, Aigle M, Lacroute F. Biochem Biophys Res Commun. 1973;53:367–372. doi: 10.1016/0006-291x(73)90671-2. [DOI] [PubMed] [Google Scholar]
- 18.Wickner RB. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 19.Masison DC, Wickner RB. Science. 1995;270:93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- 20.Masison DC, Maddelein ML, Wickner RB. Proc Natl Acad Sci U S A. 1997;94:12503–12508. doi: 10.1073/pnas.94.23.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deleted in proof
- 22.Edskes HK, Gray VT, Wickner RB. Proc Natl Acad Sci U S A. 1999;96:1498–1503. doi: 10.1073/pnas.96.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Bellot E, Guillemet E, Baudin-Baillieu A, Gaumer S, Komar AA, Cullin C. Biochem J. 1999;338:403–407. [PMC free article] [PubMed] [Google Scholar]
- 24.Maddelein ML, Wickner RB. Mol Cell Biol. 1999;19:4516–4524. doi: 10.1128/mcb.19.6.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickner RB, Taylor KL, Edskes HK, Maddelein ML, Moriyama H, Roberts BT. J Struct Biol. 2000;130:310–322. doi: 10.1006/jsbi.2000.4250. [DOI] [PubMed] [Google Scholar]
- 26.Umland TC, Taylor KL, Rhee S, Wickner RB, Davies DR. Proc Natl Acad Sci U S A. 2001;98:1459–1464. doi: 10.1073/pnas.041607898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thual C, Bousset L, Komar AA, Walter S, Buchner J, Cullin C, Melki R. Biochemistry. 2001;40:1764–1773. doi: 10.1021/bi001916l. [DOI] [PubMed] [Google Scholar]
- 28.Bousset L, Belrhali H, Janin J, Melki R, Morera S. Structure. 2001;9:39–46. doi: 10.1016/s0969-2126(00)00553-0. [DOI] [PubMed] [Google Scholar]
- 29.Omichinski JG, Clore GM, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl SJ, Gronenborn AM. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 30.Svetlov V, Cooper TG. J Bacteriol. 1997;179:7644–7652. doi: 10.1128/jb.179.24.7644-7652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor, Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 32.Cunningham TS, Svetlov VV, Rai R, Smart W, Cooper TG. J Bacteriol. 1996;178:3470–3479. doi: 10.1128/jb.178.12.3470-3479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott S, Dorrington R, Svetlov V, Beeser AE, Distler M, Cooper TG. J Biol Chem. 2000;275:7198–7204. doi: 10.1074/jbc.275.10.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smart WC, Coffman JA, Cooper TG. Mol Cell Biol. 1996;16:5876–5887. doi: 10.1128/mcb.16.10.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper TG, Ferguson D, Rai R, Bysani N. J Bacteriol. 1990;172:1014–1018. doi: 10.1128/jb.172.2.1014-1018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cokol M, Nair R, Rost B. EMBO Rep. 2000;1:411–415. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young MR, Suzuki K, Yan H, Gibson S, Tye BK. Genes Cells. 1997;2:631–643. doi: 10.1046/j.1365-2443.1997.1510349.x. [DOI] [PubMed] [Google Scholar]
- 38.Azuma Y, Takio K, Tabb MM, Vu L, Nomura M. Biochimie (Paris) 1997;79:247–259. doi: 10.1016/s0300-9084(97)83512-2. [DOI] [PubMed] [Google Scholar]
- 39.Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jans DA, Hubner S. Physiol Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- 41.Jans DA, Moll T, Nasmyth K, Jans P. J Biol Chem. 1995;270:17064–14067. doi: 10.1074/jbc.270.29.17064. [DOI] [PubMed] [Google Scholar]
- 42.Moll T, Tebb G, Surana U, Robitsch H, Nasmyth K. Cell. 1991;66:743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- 43.Blinder D, Coschigano PW, Magasanik B. J Bacteriol. 1996;178:4734–4736. doi: 10.1128/jb.178.15.4734-4736.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott S, Abul-Hamd AT, Cooper TG. J Biol Chem. 2000;275:30886–30893. doi: 10.1074/jbc.M005624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Distler M, Kulkarni A, Rai R, Cooper TG. J Bacteriol. 2001;183:4636–4642. doi: 10.1128/JB.183.15.4636-4642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham TS, Cooper TG. J Bacteriol. 1993;175:5851–5861. doi: 10.1128/jb.175.18.5851-5861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svetlov VV, Cooper TG. J Bacteriol. 1998;180:5682–5688. doi: 10.1128/jb.180.21.5682-5688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chisholm G, Cooper TG. Mol Cell Biol. 1982;2:1088–1095. doi: 10.1128/mcb.2.9.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai R, Genbauffe FS, Sumrada RA, Cooper TG. Mol Cell Biol. 1989;9:602–608. doi: 10.1128/mcb.9.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Vuuren HJJ, Daugherty JR, Rai R, Cooper TG. J Bacteriol. 1991;173:7186–7195. doi: 10.1128/jb.173.22.7186-7195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minehart PL, Magasanik B. Mol Cell Biol. 1991;11:6216–6228. doi: 10.1128/mcb.11.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coffman JA, Rai R, Cunningham T, Svetlov V, Cooper TG. Mol Cell Biol. 1996;16:847–858. doi: 10.1128/mcb.16.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coffman JA, El Berry HM, Cooper TG. J Bacteriol. 1994;176:7476–7483. doi: 10.1128/jb.176.24.7476-7483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]