Abstract
Caspase 3 activation has been linked to the acute neurotoxic effects of central nervous system damage, as in traumatic brain injury or cerebral ischaemia, and also to the early events leading to long-term neurodegeneration, as in Alzheimer’s disease. However, the precise mechanisms activating caspase 3 in neuronal injury are unclear. RhoB is a member of the Rho GTPase family that is dramatically induced by cerebral ischaemia or neurotrauma, both in preclinical models and clinically. In the current study, we tested the hypothesis that RhoB might directly modulate caspase 3 activity and apoptotic or necrotic responses in neurons. Over-expression of RhoB in the NG108-15 neuronal cell line or in cultured corticohippocampal neurons elevated caspase 3 activity without inducing overt toxicity. Cultured corticohippocampal neurons from RhoB knockout mice did not show any differences in sensitivity to a necrotic stimulus – acute calcium ionophore exposure – compared with neurons from wild-type mice. However, corticohippocampal neurons lacking RhoB exhibited a reduction in the degree of DNA fragmentation and caspase 3 activation induced by the apoptotic agent staurosporine, in parallel with increased neuronal survival. Staurosporine induction of caspase 9 activity was also suppressed. RhoB knockout mice showed reduced basal levels of caspase 3 activity in the adult brain. These data directly implicate neuronal RhoB in caspase 3 activation and the initial stages of programmed cell death, and suggest that RhoB may represent an attractive target for therapeutic intervention in conditions involving elevated caspase 3 activity in the central nervous system.
Keywords: cortex, hippocampus, mice, neurodegeneration, Rho GTPase, TUNEL
Introduction
Our understanding of the characteristics of neuronal cell death following acute central nervous system (CNS) injury is expanding, but therapeutic options remain limited and in general poorly effective. Traumatic injury to the brain or spinal cord, or ischaemic damage following stroke, initiates a complex sequence of events including inflammation and excitotoxicity, leading to a combination of necrotic and apoptotic neuronal death (Crowe et al., 1997; Keane et al., 2001; Lucas et al., 2006; Moskowitz et al., 2010). The apoptotic component of acute CNS injury is thought to involve caspase activation, in particular the ‘executioner’ caspase 3 – a key effector of apoptosis (Springer et al., 1999; Eldadah & Faden, 2000; Yakovlev & Faden, 2001; Taylor et al., 2008). Indeed, selective caspase 3 inhibition may improve recovery after trauma or stroke (Chen et al., 1998; Clark et al., 2000; Zhan et al., 2001; Citron et al., 2008). There is an evolving concept that there may be commonalities between the processes underlying neuronal death after an acute insult and the chronic neuronal loss associated with neurodegenerative disease. Indeed, traumatic brain injury is a known risk factor for Alzheimer’s disease (Nicoll et al., 1995; Magnoni & Brody, 2010). Strong evidence is currently emerging to link cortical and hippocampal caspase 3 activation with the early as well as the late stages of Alzheimer’s disease (Gervais et al., 1999; D’Amelio et al., 2010), suggesting a fundamental role in the disease process. Hence the mechanisms that mediate neuronal caspase 3 activation are currently of intense interest.
The low-molecular-weight GTPase RhoB is a possible mediator of pathological responses in the CNS. RhoB is rapidly and dramatically induced following neurotrauma, in both preclinical models and clinical samples (Brabeck et al., 2004; Conrad et al., 2005). A similar dramatic induction of RhoB expression has been observed after CNS ischaemic damage (Trapp et al., 2001; Brabeck et al., 2003; Erdö et al., 2004), where the degree of RhoB induction is predictive of the severity of the neurodegeneration (Trapp et al., 2001). RhoB is also one of the few genes identified in genome-wide scans for genes involved in ageing and senescence (Lee et al., 1999; Schwarze et al., 2002), suggesting that it contributes in some as yet unknown way to the cellular ageing process.
RhoB is a member of the Rho GTPase family of molecular switches: active, GTP-bound, molecules interact with downstream target proteins to induce a diversity of cellular responses, including modulation of the actin cytoskeleton and regulation of gene ranscription (reviewed by Hall, 1998). The best-characterized Rho family members – RhoA, Rac1 and cdc42 – have attracted a great deal of attention as regulators of cell morphology. RhoB appears to be important in regulating endosomal trafficking (Gampel et al., 1999), and is of particular importance in oncogenesis, where it has been implicated in both proliferative and apoptotic responses in tumour cells (Prendergast, 2000, 2001a,b; DeWard et al., 2009; Li et al., 2011).
Interestingly, RhoB gene expression in a number of cell types is greatly induced by DNA-damaging stimuli, and the levels of RhoB produced are inversely correlated with long-term survival (Fritz et al., 1995), suggesting that RhoB may participate at some level downstream of the DNA-damage-signalling machinery, as a death effector or death signal modifier (Liu et al., 2001a; Prendergast, 2001a). In contrast, a prominent role for RhoB in cell survival is supported by evidence that RhoB depletion or inhibition leads to reduced cell survival and increased apoptosis (Ader et al., 2002; Adini et al., 2003; Canguilhem et al., 2005; Hutchison et al., 2009). Thus the role of RhoB in adjusting the balance between cell survival and apoptosis appears to be complex, and may depend on the cell type. Hence induction of neuronal RhoB may be a key component of the neurodegenerative response to injury, or equally, based on evidence from peripheral cells, part of a survival response.
We have previously shown that RhoB is localized at excitatory synapses (O’Kane et al., 2003a,b), where it can be selectively activated by N-methyl-d-aspartate (NMDA) receptor stimulation (O’Kane et al., 2003a). Indeed, hippocampal or cortical neurons lacking RhoB show altered dendritic morphology, reduced spine density and impaired plasticity responses to NMDA receptor stimulation (McNair et al., 2010). Here we test the hypothesis that RhoB is a mediator of pathological as well as physiological plasticity in the CNS. To investigate the role of RhoB in neuronal necrosis, we exposed cultured corticohippocampal neurons to high concentrations of the calcium ionophore A23187, which induces a rapid necrotic cell death (Gwag et al., 1999). To investigate the role of RhoB in neuronal apoptosis, we employed staurospaurine (STS), a prototypical inducer of apoptosis, uncontaminated by necrosis, in a wide variety of cell types including neurons (Moore et al., 2002; Taylor et al., 2008; Leveille et al., 2010). STS induces robust apoptosis in cortical neurons in parallel with an induction of RhoB gene expression (Föcking et al., 2004).
Materials and methods
RhoB knockout mice
The production of mice with a targeted deletion of the RhoB gene (RhoB KO mice) has been described previously (Liu et al., 2001b). Corresponding wild-type (WT) controls were derived from littermates and maintained as a separate breeders with frequent intercrossing. The genotype of mice was determined by polymerase chain reaction and confirmed by Western blot analysis (Liu et al., 2001b; McNair et al., 2010). All procedures were performed in accordance with Home Office legislation.
Neuronal culture and transfection
The constitutively active RhoB (caRhoB) vector has been described elsewhere (Mellor et al., 1998; Conway et al., 2004; Wherlock et al., 2004): it expresses an active mutant of RhoB fused with green fluorescent protein (GFP). The corresponding empty vector lacking the RhoB insert, but expressing GFP, was used as a control (Conway et al, 2004). Corticohippocampal cultures were prepared as described previously (Morris, 1995), maintained in neurobasal medium with B27 supplement (Invitrogen, Paisley, UK), and used at 10–12 days in vitro. Transfection was conducted using either the calcium phosphate method or Lipofectamine 2000 (Invitrogen) as described (Clark et al., 2007; Greenwood et al, 2007) using 300 ng DNA per well. NG108-15 neuroblastoma-glioma cells were seeded into six-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with glutamine and penicillin/streptomycin, and 10% foetal calf serum (all from Invitrogen). Each well of NG108-15 cells was transfected with 250 ng vector, complexed with 2 µL Lipofectamine 2000 in 100 µL DMEM. After 6 h, cells were differentiated by switching the medium to DMEM with low (0.5%) foetal calf serum and 0.5% dimethyl sulphoxide (Johansson et al, 2007).
Assessment of neuronal viability
The reduction of XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) to assess mitochondrial activity was monitored as previously described (McDermott et al, 2003). After the specified time period, cells were incubated with XTT (1 mg/mL) plus phenazine methosulphate (5 µm) (dissolved in fresh warmed neurobasal medium) for 3 h. An aliquot of medium was then collected and the absorbance measured in triplicate at 450 nm.
Assessment of caspase activity
Caspase 3 activity was determined using the fluorogenic caspase 3 substrate Ac-DEVD-AMC (Enzo Life Sciences, Exeter, UK). Cells were homogenized in cell lysis buffer (25 mm HEPES, and 5 mm MgCl2, pH 7.6), and the homogenates then centrifuged at 16,000g at 4 °C for 10 min. The cell lysate was incubated in assay buffer (25 mm HEPES and 0.5 mm EDTA, pH 7.6) with 10 µm Ac-DEVD-AMC. Caspase activity was measured by monitoring the accumulation of the fluorescent cleavage product 7-amino-4-methyl-coumarin (AMC) from the synthetic substrate (excitation wavelength 350 nm; emission wavelength 460 nm). To test the specificity of the reaction, the caspase 3 inhibitor Ac-DMQD-CHO (50 µm) was included in replicate samples. After determination of the protein concentration, based on the method of Bradford, activity was calculated using a standard curve for AMC (0–10 µm). Results were expressed as pmol AMC/min per mg protein. For the assessment of caspase 8 and caspase 9 activity, similar procedures were followed, except that the substrates used were Ac-IETD-AMC and Ac-LEHD-AMC, respectively (Enzo Life Sciences).
TUNEL staining
DNA fragmentation was assessed using the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay. This is based on the incorporation of brominated nucleotides onto the free 3’OH end of DNA fragments using terminal deoxynucleotidyl transferase enzyme (TdT) (basic in situ apoptosis detection kit, Trevigen, Gaithersburg, MD, USA). Cells were fixed with 3.7% formaldehyde and processed according to the manufacturer’s instructions. The streptavidin-peroxidase complex was detected with VIP solution (Vector Laboratories, Peterborough, UK). Subsequently, cells were counterstained with Eosin Y solution (Sigma, Poole, UK). Images were taken using Zeiss Axio Vision 2 software. The number of TUNEL-positive cells relative to counterstained cells within each image was determined using Image J (http://rsbweb.nih.gov/ij/) (W. Rasband, NIH). To determine nonspecific labelling, some sections were incubated with labelling reaction not containing TdT enzyme.
Immunocytochemical analysis of primary cultured neurons
Immunocytochemistry was performed as previously described (Simpson & Morris, 2000; McNair et al, 2010). Cultures were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min and incubated in 15% normal goat serum in PBS for 1 h. After incubation overnight with sheep anti-GFP (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-active caspase 3 (1 : 200; Promega, Southampton, UK) antibodies, cells were washed in PBS and incubated with Alexa 488 anti-sheep (1 : 1000; Santa Cruz Biotechnology) or Alexa 594 anti-rabbit (1 : 200; Molecular Probes, Paisley, UK) secondary antibody; cells were rinsed, coverslipped in Vectashield (Vector Laboratories, Peterborough, UK) and visualized by fluorescence microscopy.
Immunoblotting
Western blot studies were performed according to our standard procedures (Simpson & Morris, 2000; James et al, 2006; McNair et al., 2010). Proteins from corticohippocampal cultures were extracted with RIPA buffer with protease inhibitors added. Cellular extracts were then centrifuged at 12 000 r.p.m. for 10 min at 4 °C, supernatants were collected and protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad, Hemel Hempstead, UK), with concentrations normalized to the lowest-concentration sample. Samples were heated to 70 °C in Laemmli sample buffer [2% sodium dodecyl sulphate (SDS), 10% glycerol, 100 mm dithiothreitol, 60 mm Tris-HCl, pH 6.8, 0.01% bromophenol blue] before separation by SDS-PAGE in a 12% polyacrylamide gel and transfer to a polyvinylidene fluoride membrane. Blots were washed with TBST buffer (120 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.05% Tween 20), then placed in TBST buffer supplemented with 3% skimmed milk and blocked for 2 h at room temperature. After incubation with rabbit polyclonal anti-Bax antibody (1 : 500; Santa Cruz Biotechnology), overnight at 4 °C with constant agitation, and washing with TBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1 : 2000; Millipore, Oxford, UK) for 2 h. Membrane-bound secondary antibodies were detected using the ECL Plus procedure (GE Healthcare, Amersham, UK). Blots were reprobed using HRP-conjugated sheep anti-actin antibody (Santa Cruz Biotechnology). Integrative optical density readings were obtained using NIH Image (W. Rasband, NIH), and differences in specific density between WT and mutant animals were analysed for statistical significance as described previously (Rakhit et al, 2005; James et al, 2006).
Statistical analysis
Data were analysed using a general linear model analysis of variance with Tukey’s post-hoc analysis using Minitab software. Where data deviated from a normal distribution, analysis was instead performed by Mann-Whitney test. In all cases statistical significance was defined as P < 0.05.
Results
Effect of RhoB over-expression in neurons
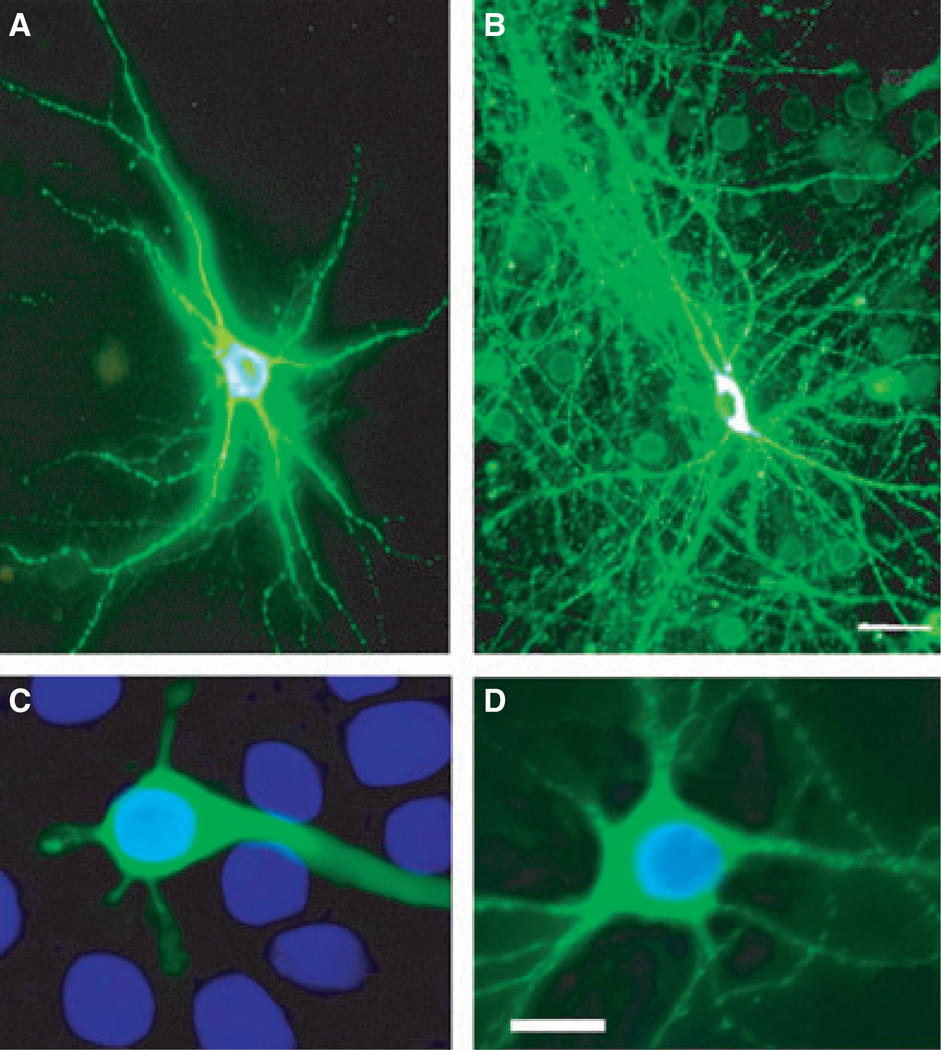
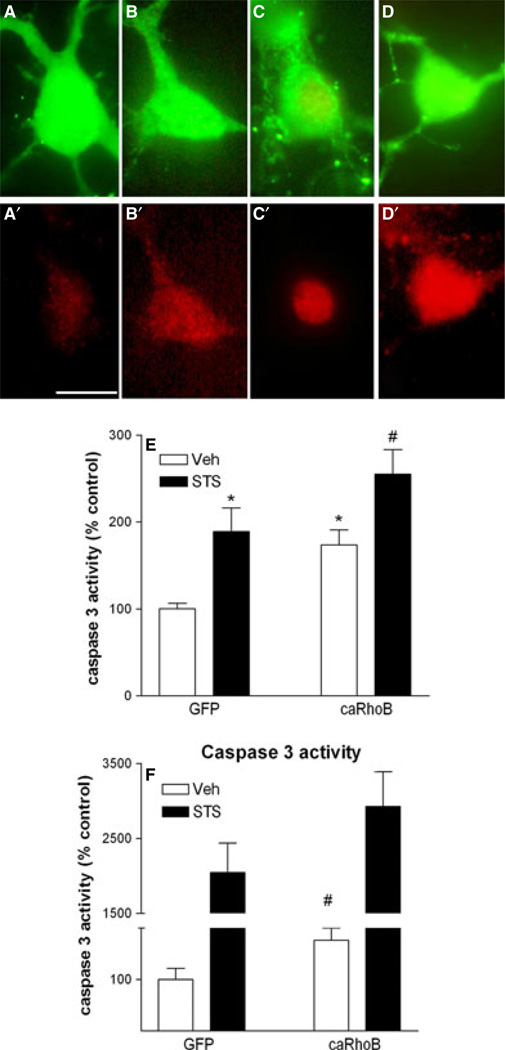
Initially we tested the hypothesis that over-expression of RhoB would be sufficient to induce neuronal degeneration. Cortico-hippocampal ultures from WT mice were transfected with the GFP-tagged caRhoB vector or control vector (GFP alone). While neurons expressing the control GFP vector had apparently normal morphology, neurons expressing caRhoB frequently displayed neuritic hypertrophy, with patterns of dramatically increased dendritic arborization (Fig. 1A and B). While cortico-hippocampal neurons expressing caRhoB appeared healthy, we tested whether an inceased level of RhoB expression led to the appearance of signs of apoptosis. Transfection of cortico-hippocampal neurons with caRhoB did not lead to any nuclear condensation at time points up to 72 h after transfection (Fig. 1C and D). As expected, STS treatment increased the levels of immunoreactive (ir-) caspase 3 (Fig. 2B′, D′ and E). However, neurons expressing caRhoB also showed elevated levels of active nuclear caspase 3 (Fig. 2A′, C′ and E), suggesting that an increase in neuronal RhoB activity is sufficient to trigger nuclear caspase 3 activation.
Fig. 1.
Effect of elevated RhoB activity on morphology of corticohippocam-pal neurons. Neurons were transfected with vectors expressing GFP (A, C) or caRhoB-GFP (B, D). (A, B) caRhoB induces excessive dendritic arborisation. (C, D) Nuclear morphology, as assessed by DAPI staining (blue); note that elevated RhoB does not compromise nuclear morphology. Scale bars = 20 lm. For interpretation of color references in figure legend, please refer to the Web version of this article.
Fig. 2.
Effect of elevated RhoB activity on neuronal caspase 3 activity. (A–D) caRhoB and STS induce caspase 3 activation. Upper images in each pair show GFP-labelled neuronal soma (A–D), and lower images in each pair show the corresponding active caspase 3-immunoreactivity (red) (A′–D′). Scale bar = 10 µm. (E) Effect of RhoB expression and STS on active caspase 3-immunoreactivity in corticohippocampal neurons. Results are shown as percentage of signal in control (vehicle-treated, GFP-transfected) neurons, and are mean ± SEM, n = 8–16 per group. Effect of vector: F1,53 = 10.67, P < 0.01; effect of STS: F1,53 = 15.90, P < 0.001 (anova). *P < 0.05 vs. control (Veh-GFP) (Tukey’s post-hoc test); #P < 0.05 vs. Veh-RhoB. (F) Effect of elevated RhoB activity on caspase activity in response to STS exposure in NG108-15 cells. Results are shown as mean ± SEM, n = 6 per group. Effect of vector: F1,17 = 4.89, P < 0.05; effect of STS: F1,17 = 610.17, P < 0.001 (anova – transformed data). #P < 0.05 vs. control (Veh-GFP) (Mann–Whitney test). For interpretation of color references in figure legend, please refer to the Web version of this article.
To provide further verification of the causal relationship between RhoB and caspase 3 activity, we employed the NG108-15 neuronal cell line, where high transfection efficiencies can be achieved, allowing precise biochemical assessment of the effect of elevated RhoB on caspase 3 activity. We tested whether increased caRhoB expression would also be associated with elevated caspase 3 activity in differentiated NG108-15 cells. Indeed, exposure to STS dramatically enhanced the activity of caspase 3 in NG108-15 cells. Elevated expression of RhoB alone was sufficient to increase caspase 3 activity, and further potentiated the activation of caspase 3 by STS (Fig. 2F).
Targeted deletion of the RhoB gene does not affect necrosis
We next assessed whether neurons lacking RhoB showed altered sensitivity to an acute necrotic stimulus. Concentrations of A23187 above 1 µm induce a rapid necrosis in cultured cortical neurons (Gwag et al., 1999). We observed a concentration-dependent reduction in cell viability (as assessed by the reduction of XTT to assess mitochondrial activity) 2.5 h following exposure of cortico-hippocampal neurons from WT mice to A23187. An identical concentration-dependent effect was observed in cultures from RhoB KO mice [EC50 for A23187-induced reduction in neuronal viability: 18.1 ± 3.9 µm (WT) vs. 15.2 ± 3.4 µm (RhoB KO)].
Deletion of the RhoB gene enhances nuclear condensation during apoptosis
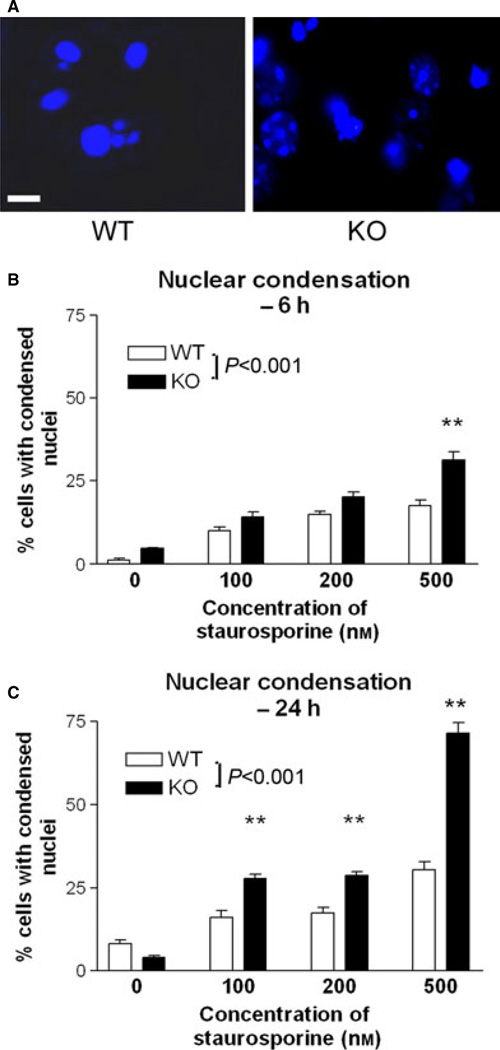
The above data indicate that elevated RhoB expression is not sufficient to induce nuclear condensation. However, RhoB may be necessary for nuclear condensation to occur during apoptosis. We monitored whether nuclear condensation during apoptosis is altered in neurons lacking RhoB. In fact, corticohippocampal neurons lacking RhoB were more sensitive to the induction of nuclear condensation after exposure to STS (Fig. 3). This was evident at both early and late time points (Fig. 3B and C).
Fig. 3.
Effect of RhoB deletion on nuclear morphology in corticohippocampal neurons following exposure to STS. (A) DAPI staining (blue) of corticohip-pocampal neurons from WT or RhoB KO mice 24 h following exposure to 500 nm STS. Scale bar = 10 µm. Note the higher proportion of nuclei showing condensation in the neurons lacking RhoB. (B,C) Nuclear morphology in corticohippocampal neurons from WT or RhoB KO mice 6 h (B) or 24 h (C) following exposure to STS. Results are shown as mean ± SEM, n = 3–5 per group. (B) Two-way anova: effect of STS concentration – F3,39 = 48.71, P < 0.001; effect of genotype – F3,39 = 39.71, P < 0.001. (C) Two-way anova: effect of STS dose – F3,39 = 254.38, P < 0.001; effect of genotype – F1,39 = 159.21, P < 0.001; **P < 0.01; post-hoc Tukey’s test vs. corresponding WT group. For interpretation of color references in figure legend, please refer to the Web version of this article.
Deletion of the RhoB gene suppresses caspase 3 activation and DNA fragmentation during apoptosis
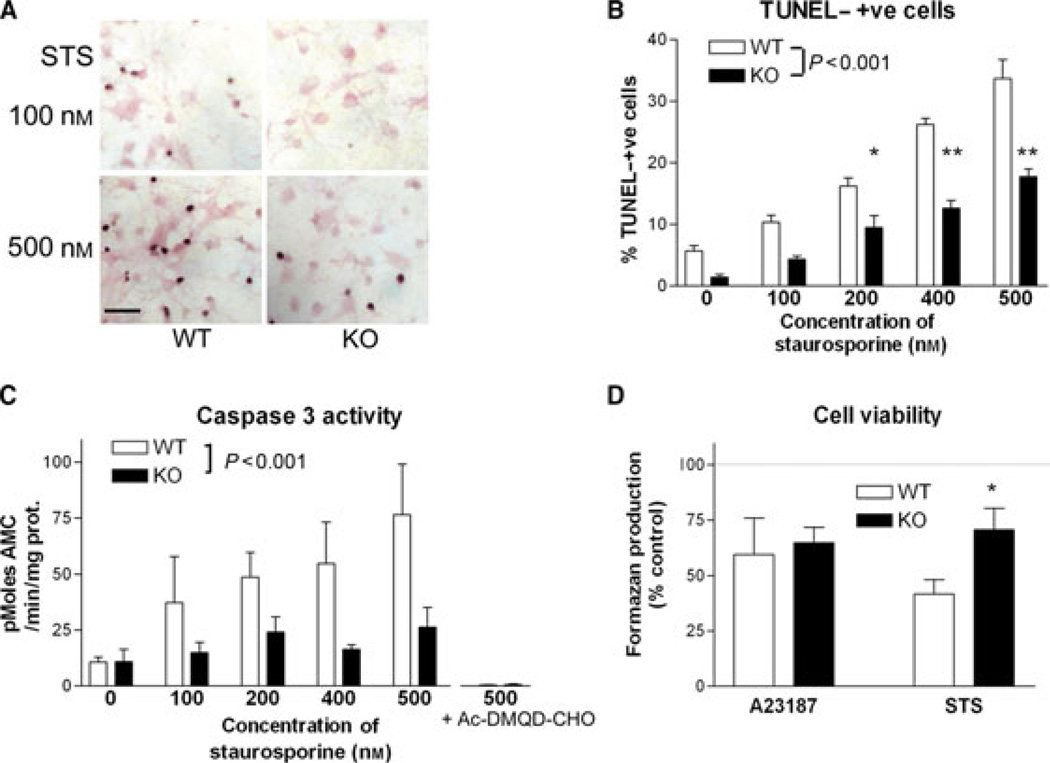
TUNEL staining was used to monitor DNA fragmentation after STS treatment in cortico-hippocampal neurons. STS treatment resulted in a dose-dependent increase in TUNEL staining in cultures from WT mice. While a dose-dependent effect of STS was also noted in cultures from RhoB KO mice, the degree of DNA fragmentation observed was dramatically reduced (Fig. 4A and B). A similar reduced sensitivity to STS was observed when caspase 3 activity was monitored in cultures from WT and RhoB KO mice. Cultures lacking RhoB showed reduced caspase 3 activation after exposure to STS (Fig. 4C), across a range of STS concentrations.
Fig. 4.
Altered apoptotic responses in corticohippocampal neurons lacking RhoB. (A) TUNEL staining in primary cortico-hippocampal cultures from WT (left panels) and RhoB KO (right panels) mice, treated for 24 h with either 100 nm (upper panels) or 500 nm (lower panels) STS. Note the reduced numbers of TUNEL-positive nuclei in the neurons lacking RhoB. Scale bar = 50 µm. (B) TUNEL staining in primary cortical cultures from WT and RhoB KO mice, treated for 24 h with STS at the doses shown. Results are shown as mean ± SEM, n = 5 per group. Two-way anova: effect of STS concentration – F4,49 = 74.98, P < 0.001; effect of genotype – F1,49 = 101.10, P < 0.001; *P < 0.05, **P < 0.01; post-hoc Tukey’s test. (C) Caspase 3 activity in primary cortico-hippocampal cultures from WT and RhoB KO mice, treated for 6 h with STS at the concentrations shown; n = 4–6 per group. Note that the selective caspase 3 inhibitor Ac-DMQD-CHO completely inhibits cleavage of the fluorogenic substrate. Two-way anova: effect of STS concentration – F4,52 = 4.2, P < 0.001; effect of genotype – F1,52 = 12.87, P < 0.001. (D) Neuronal survival, in neurons from WT or RhoB KO mice, as assessed by mitochondrial XTT reduction to formazan, following exposure to 10 µm A23187 for 2.5 h, or 500 nm STS for 24 h. Neurons lacking RhoB show increased survival after exposure to STS, *P < 0.05 vs. WT (t-test).
As the substrate used – Ac-DEVD-AMC – can be cleaved by caspase 7 as well as caspase 3, we additionally assessed whether any of the activity might be due to caspase 7 rather than caspase 3 using the selective caspase 3 inhibitor Ac-DMQD-CHO (Yoshimori et al., 2007). Cleavage activity was completely inhibited by this compound (Fig. 4C, right columns), indicating that the activity detected is solely due to caspase 3.
The elevated nuclear condensation, but reduced DNA fragmentation and caspase 3 activity, in neurons lacking RhoB after STS exposure left open the question as to whether lack of RhoB made neurons resistant or more sensitive to programmed cell death. However, when the level of neuronal survival was monitored 24 h after STS treatment, neurons lacking RhoB were found to be more resistant to the degenerative effects of STS as compared with WT neurons (Fig. 4D). This was in contrast to the lack of effect of RhoB deletion on neuronal survival after the necrotic stimulus – acute A23187 (Fig. 4E).
Effect of deletion of the RhoB gene on caspase 8 and caspase 9 activation and Bax induction during apoptosis
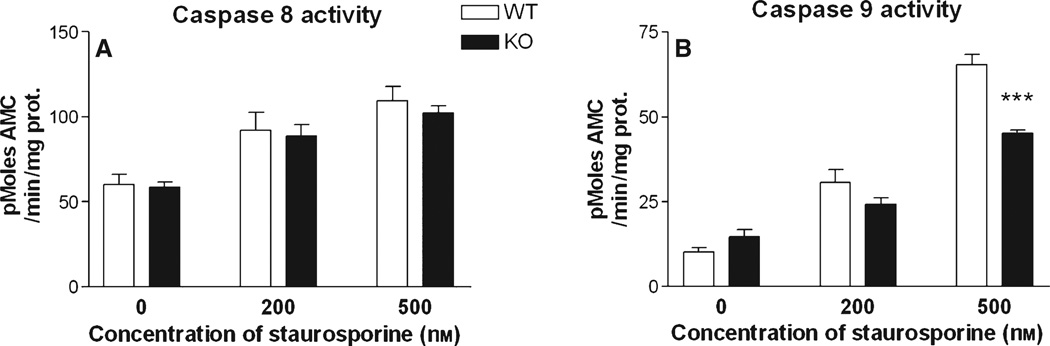
Caspase 8 and caspase 9 are regarded as ‘initiator’ caspases, activated at a relatively early stage in neurons exposed to apoptotic stimuli, which participate in the downstream activation of caspase 3 (Cao et al., 2002; Moore et al., 2002; Caballero-Benítez & Morán, 2003). To gain insight into the contribution of RhoB to early events in neuronal apoptosis, we monitored the levels of caspase 8 and caspase 9 activity in neurons exposed to STS for 4 h. STS treatment induced a concentration-dependent activation of both caspase 8 and caspase 9. While the degree of activation of caspase 8 was unaffected by the absence of RhoB, caspase 9 activation was decreased in corticohip-pocampal neurons lacking RhoB (Fig. 5).
Fig. 5.
Altered initiator caspase responses in corticohippocampal neurons lacking RhoB. (A) Caspase 8 activity and (B) caspase 9 activity in primary cortico-hippocampal cultures from WT and RhoB KO mice, treated for 4 h with vehicle, or either 200 or 500 nm STS. Results are shown as mean ± SEM, n = 8 per group. Two-way anova: caspase 8 activity, effect of STS concentration – F2,47 = 23.5, P < 0.001, effect of genotype – F2,47 = 0.51, P = 0.48; caspase 9 activity, effect of STS concentration – F2,47 = 162.9, P < 0.001, effect of genotype – F1,47 = 14.0, P < 0.001. ***P < 0.001 vs. corresponding WT value, post-hoc Tukey’s test.
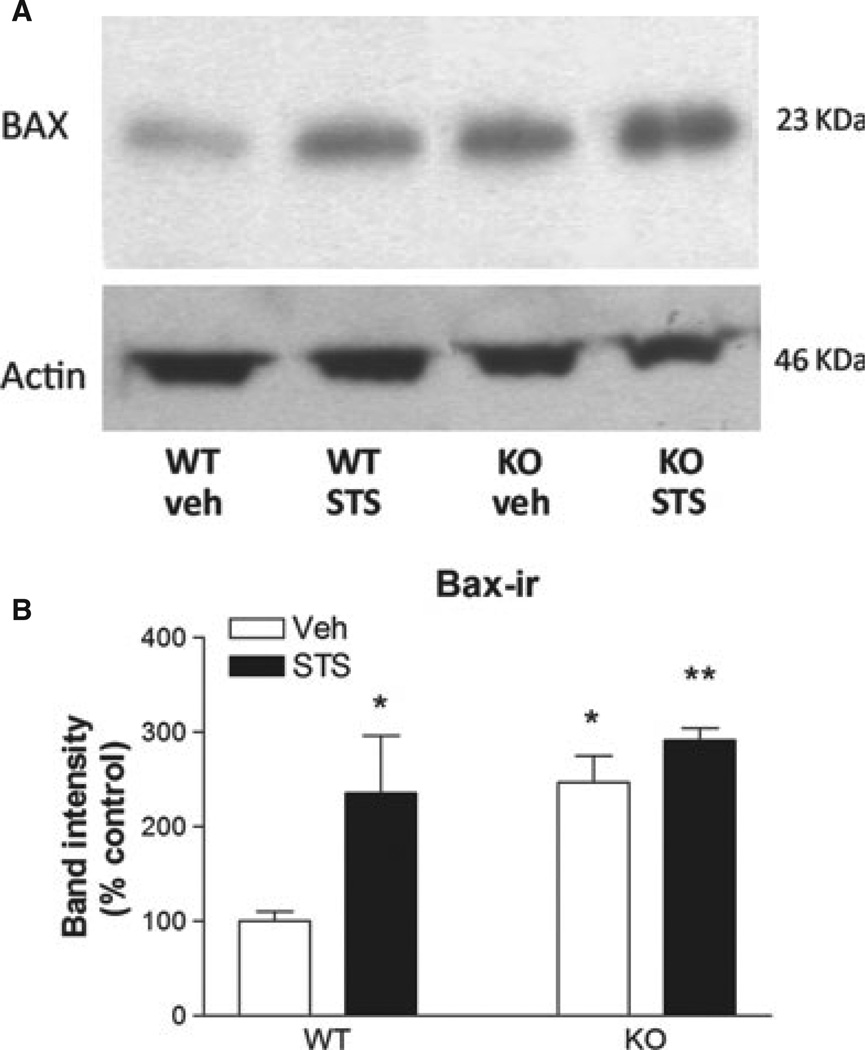
Bax is a pro-apoptotic member of the Bcl-2 family of proteins that plays a role in apoptosis in many cell types by releasing apoptogenic factors from mitochondria. Neurons undergoing apoptosis show rapid increases in Bax expression (Martin et al., 2009; Ma & Nowak, 2011), suggested to be mediated by Rho GTPases (Del Re et al., 2007). We therefore studied the levels of Bax in neuronal cultures from WT and RhoB KO mice exposed to STS. As expected, STS treatment for 2 h led to increased Bax levels in WT neurons. The basal levels of Bax expression were higher in neurons lacking RhoB compared with WT neurons, but the stimulated levels after STS treatment were equivalent to those after STS treatment in WT neurons (Fig. 6).
Fig. 6.
Altered Bax expression in corticohippocampal neurons lacking RhoB. (A) Western blot showing expression of Bax and actin (loading control) in extracts from cortico-hippocampal cultures treated for 2 h with either vehicle or 500 nm STS. (B) Quantification of Bax immunoblotting in primary cortico-hippocampal cultures from WT and RhoB KO mice, treated for 2 h with either vehicle or 500 nm STS. Results are shown as mean ± SEM, n = 3 per group. Two-way anova: effect of STS concentration – F1,10 = 10.4, P = 0.015; effect of genotype – F1,10 = 10.0, P = 0.016; no significant interaction. *P < 0.05; **P < 0.01 vs. corresponding WT vehicle.
Effect of deletion of the RhoB gene on constitutive caspase 3 activity in the brain
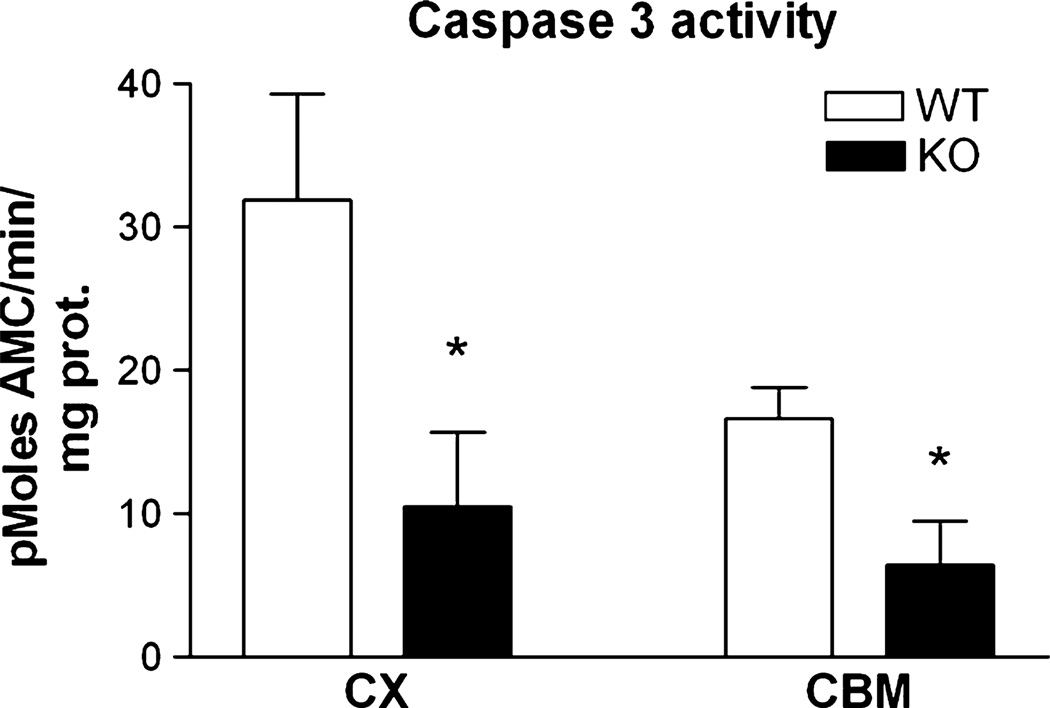
As caspase 3 has been linked to physiological plasticity downstream of NMDA receptor stimulation in the CNS (Sherstnev et al., 2006; Li et al., 2010), as well as the very early stages of Alzheimer’s disease (D’Amelio et al., 2010), we also investigated caspase 3 activity in adult brain tissue. A substantial level of caspase 3 activity was detected in both cortex and cerebellum from the brains of WT mice. However, the basal caspase 3 activity in both cortex and cerebellum was substantially lower in the RhoB KO mice (Fig. 7).
Fig. 7.
RhoB deletion modulates caspase 3 activity in adult brain. Caspase 3 activity in cortex and cerebellum samples from WT and RhoB KO (KO) mouse brain (aged 4 months). Results are shown as mean ± SEM, n = 4–5 per group. Activity in tissue from RhoB KO mice was significantly reduced relative to WT mice (two-way anova – factors: genotype and tissue; caspase 3 activity: effect of genotype F1,16 = 9.93, P = 0.008; *P < 0.05 relative to corresponding WT samples, post-hoc Tukey’s test.
Discussion
Rho GTPases have primarily been studied in peripheral tissues and cell lines and are well known for their potential role in anti-cancer therapies (Prendergast, 2000; Sebti & Hamilton, 2000). However, there is a growing awareness that they are also important signalling molecules in the CNS. Specifically, RhoB has been implicated in neuronal responses to glutamate receptor signalling (O’Kane et al., 2003a,b; McNair et al., 2010), in particular via the NMDA class of glutamate receptors. As NMDA receptor stimulation is implicated in pathological responses as well as physiological responses to glutamate, this makes RhoB of considerable interest as a potential mediator of CNS injury. RhoB expression is rapidly and dramatically elevated in neurons following toxic or traumatic stimuli, so it seems likely that these increases in RhoB levels play a major role in determining the fate of the neurons, but there is little evidence as to whether the RhoB induction is beneficial or detrimental to neuronal survival. We show here that the absence of RhoB does not affect cell survival following an acute necrotic stimulus. Neurons containing RhoB and lacking RhoB were equally sensitive to the toxic effects of Ca2+ influx, following A23187 treatment.
In contrast, the data clearly implicate RhoB in either direct mediation or modulation of apoptotic responses. STS is a prototypical inducer of apoptosis in neurons (Moore et al., 2002; Leveille et al., 2010), which induces apoptosis in cortical neurons in parallel with an induction of RhoB (Föcking et al., 2004). STS induced a dose-dependent increase in characteristic signs of apoptosis: nuclear condensation, caspase 3, 8 and 9 activation, and DNA fragmentation. Caspase 8 and caspase 9 are regarded as ‘initiator’ caspases, activated at a relatively early stage in apoptosis (Cao et al., 2002; Moore et al., 2002; Caballero-Benítez & Morán, 2003). Caspase 8 is typically associated with the ‘extrinsic’ or receptor-activated pathway of apoptosis, while caspase 9 mediates the mitochondrial apoptotic pathway following cytochrome C release. No activation of caspase 7 was detected in corticohippocampal neurons. This is consistent with previous reports that caspase 7 expression is very low in these cells (Li et al., 2010). Caspase 3 is a key effector caspase thought to be central to neurodegenerative processes in the CNS.
As noted above, RhoB has been implicated in both pro-apoptotic and anti-apoptotic processes depending on the cell type, although its role in relation to apoptosis in neurons has not previously been investigated. We found that corticohippocampal neurons lacking RhoB showed reduced activation of caspase 3, and suppressed DNA fragmentation (as assessed by the TUNEL assay), during STS-induced apoptosis. This is consistent with evidence from other cell types that RhoB can act as a death effector (Liu et al., 2001a; Prendergast, 2001a), and with evidence that suppression of neuronal apoptosis is associated with reduced RhoB induction (Trapp et al., 2003). The data suggest that elevated RhoB expression in neurons facilitates apoptosis.
STS exposure activated caspase 8 and caspase 9 as well as caspase 3. We observed that STS activation of caspase 9, but not caspase 8, was compromised by the lack of RhoB. In fact, there is evidence that caspase 9 rather than caspase 8 is linked to caspase 3 activation in neurons undergoing apoptosis (Hakem et al., 1998; Movsesyan et al., 2002; West et al., 2006), and following glutamate receptor stimulation (Budd et al., 2000; Zeron et al., 2004; Li et al., 2010). Furthermore, the abnormal CNS development observed in caspase 3-null mice is mirrored in caspase 9-null mice, suggesting a close functional relationship (Hakem et al., 1998), at least during development. The evidence that STS induction of caspase 9 and caspase 3 activity, but not caspase 8 activity, is compromised in neurons lacking RhoB implies that RhoB may act upstream of caspase 9 in this pathway, in injured neurons and downstream of glutamate receptor stimulation.
Our data suggest that RhoB can activate nuclear caspase 3 without leading to cell death. Other reports support the concept that caspase 3 activation in neurons does not necessarily induce apoptosis (Li et al., 2010). It may be that the caspase 3 activation we observe following RhoB over-expression is lower than a threshold required for mediating the nuclear events associated with apoptosis. Alternatively, other apoptotic mediators that are not activated by RhoB may be necessary for full apoptosis.
We observed that neurons lacking RhoB showed a reduced sensitivity to STS-induced caspase 3 activation and DNA fragmentation, along with an increased sensitivity to nuclear condensation. DNA fragmentation represents one of the key late-stage phenomena taking place in cells undergoing apoptosis, but while DNA fragmentation and nuclear condensation are both highly characteristic of apoptosis, a separation between these two phenomena has been noted previously (Andreau et al., 2004). In fact, there is evidence for distinct signalling pathways being involved in these apoptotic responses: the ERK pathway has been linked to nuclear condensation (Subramaniam et al., 2004), while caspase 3 activation has been implicated in DNA cleavage and fragmentation via ‘DNA Fragmentation Factor’ (Liu et al., 1997; Harada & Sugimoto, 1999; Mannherz et al., 2006). The data reported here are consistent with this evidence, and place RhoB unequivocally upstream of caspase 3 activation in the latter pathway in neurons. The elevated sensitivity to changes in nuclear morphology in neurons lacking RhoB may represent a compensatory response to the reduced caspase 3 activation, but clearly this is insufficient to impair survival, as the neurons lacking RhoB survive the STS exposure better than control neurons.
Basal levels of Bax expression were found to be elevated in neurons from RhoB KO mice. The reasons for this are not clear, but it is conceivable that this, too, represents a compensatory response to reduced caspase 3 activation, as Bax has been linked to caspase 3 activation under physiological conditions (Jiao & Li, 2011). However, there was no alteration in the degree of STS induction of Bax in neurons lacking RhoB, implying that RhoB does not lie upstream of Bax in this pathway. This may rather be a function of RhoA in apoptosis (Del Re et al., 2007).
There is mounting evidence that other molecules in the RhoB signalling pathway are also key mediators of apoptosis in the CNS. The guanine nucleotide exchange factor Lfc/GEF-H1/ARHGEF2 is an activator of RhoA and RhoB, and acts as an oncogene in many cell types, stimulating proliferation. However, when over-expressed in cortical neurones, Lfc induces a caspase-dependent apoptosis (Gauthier-Fisher et al., 2009). This could reflect actions via RhoA or RhoB. While the involvement of RhoA in neuronal survival has not been extensively investigated via genetic means, it has been linked on biochemical grounds primarily to pro-apoptotic actions in peripheral cells. A dominant-negative RhoA mutant (which may well suppress the function of RhoB as well as RhoA) decreases apoptosis of cortical neurons in vivo during development (Sanno et al., 2010). Similarly, pharmacological strategies inhibiting both RhoA and RhoB reduce caspase 3 activation and impair apoptosis in cultured cortical neurons (Zhang et al., 2007). Hence while available information is ambiguous concerning the relative contribution of RhoA and RhoB to neuronal survival, the data reported here link RhoB unequivocally to caspase 3 activation in neurons.
The precise link between RhoB and caspase 3 and 9 activation is not clear, but may involve Rho-kinase (ROCK), as in peripheral cells ROCK can activate effector caspases (Hebert et al., 2008). Alternatively, the apoptotic actions of RhoB may involve the mDia effector protein (DeWard et al., 2009), while another RhoB effector, PRK1/2, is also linked to apoptosis in peripheral cells (Takahashi et al., 1998; Prendergast, 2001a; Tomiyoshi et al., 2004; Zeng et al., 2010). We have recently shown that RhoB is the major regulator of cofilin phosphorylation in cortical and hippocampal neurons (McNair et al., 2010). RhoB regulates activity in the ROCK–Lim kinase–cofilin pathway downstream of glutamate receptor stimulation, and this pathway is involved in activity-dependent regulation of neuronal morphology. An absence of RhoB results in reduced Lim kinase activation and reduced activity-dependent cofilin phosphorylation (McNair et al., 2010). The neuritic hypertorophy we observed in neurons expressing active RhoB is probably related to elevated activity in this pathway. However, there is evidence that prolonged activation of Lim kinase contributes to apoptosis (Tomiyoshi et al., 2004).
As RhoB has been linked to the ageing process, we were also interested in the effect of RhoB on caspase 3 activity in the mature CNS. Basal caspase 3 activity was detected in both cortex and cerebellum from the brains of adult WT mice. This caspase 3 activity may conceivably relate to physiological processes, including synaptic plasticity and learning (Sherstnev et al., 2006; Li et al., 2010). Alternatively, there is thought to be a low level of ageing-associated apoptosis occurring in the adult brain, as other apoptotic mediators are found in association with caspase 3 activity (Higami & Shimokawa, 2000; Pollack et al., 2002). Interestingly, the caspase 3 activity in both cortex and cerebellum was dramatically reduced in the RhoB KO mice. This suggests that RhoB may be a critical regulator of caspase 3 activity, whether associated with physiological or pathological situations, in the mature CNS.
Evidence is currently emerging to link neuronal caspase 3 activation with the early stages of the degenerative response to traumatic CNS injury (Citron et al., 2008) and also Alzheimer’s disease (Harada & Sugimoto, 1999; D’Amelio et al., 2010). The fact that neurotrauma, which is associated with RhoB induction, is a known risk factor for Alzheimer’s disease (Magnoni & Brody, 2010) raises the possibility that the activation of caspase 3 is a key event in predisposition to the disease. Hence it can be speculated that RhoB, shown here to be an important regulator of CNS caspase 3 activity, may transpire to play an important role in long-term neurodegenerative diseases such as Alzheimer’s disease.
Conclusions
Neurons lacking RhoB were found to be more resistant to the degenerative effects of STS as compared with WT neurons. This is consistent with the reduced caspase 9 and caspase 3 activation and DNA fragmentation we observed after STS exposure in neurons from the RhoB KO mice, but is in contrast to the elevated nuclear condensation response to STS observed in the absence of RhoB. Increased nuclear condensation is apparently insufficient to lead to neuronal death. Similarly, our data suggest that over-expression of RhoB is not sufficient to cause apoptosis. This is consistent with the results showing that RhoB does not promote all aspects of apoptosis in neurons. These results suggest that the marked increase in RhoB levels that occur following CNS trauma or a neurotoxic stimulus are likely to make a substantial contribution to the neurodegenerative process. The increased RhoB expression promotes neurite outgrowth – this may be a response that is beneficial to neuronal survival by increasing the chances of making new synaptic contacts in an area of neuronal loss. However, the increased RhoB expression also promotes caspase 3 activation and DNA fragmentation, key contributors to programmed cell death. These results suggest that RhoB may be an attractive therapeutic target for intervention in such cases.
Acknowledgements
This work was supported by the BBSRC. We thank Dr H. Mellor (University of Bristol) for the gift of the constitutively active RhoB vector.
Glossary
- AMC
7-amino-4-methyl-coumarin
- CNS
central nervous system
- DMEM
Dulbecco’s modified Eagle’s medium
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- KO
knockout
- NMDA
N-methyl-d-aspartate
- PBS
phosphate-buffered saline
- ROCK
Rho-kinase
- SDS
sodium dodecyl sulphate
- STS
staurospaurine
- TdT
terminal deoxynucleotidyl transferase enzyme
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labelling
- WT
wild-type
- XTT
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
References
- Ader I, Toulas C, Dalenc F, Delmas C, Bonnet J, Cohen-Jonathan E, Favre G. RhoB controls the 24 kDa FGF-2-induced radioresistance in HeLa cells by preventing post-mitotic cell death. Oncogene. 2002;21:5998–6006. doi: 10.1038/sj.onc.1205746. [DOI] [PubMed] [Google Scholar]
- Adini I, Rabinovitz I, Sun JF, Prendergast GC, Benjamin LE. RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev. 2003;17:2721–2732. doi: 10.1101/gad.1134603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreau K, Castedo M, Perfettini J-L, Roumier T, Pichart E, Souquere S, Vivet S, Larochette N, Kroemer G. Preapoptotic chromatin condensation upstream of the mitochondrial checkpoint. J. Biol. Chem. 2004;279:55937–55945. doi: 10.1074/jbc.M406411200. [DOI] [PubMed] [Google Scholar]
- Brabeck C, Mittelbronn M, Bekure K, Meyermann R, Schluesener HJ, Schwab JM. Effect of focal cerebral infarctions on lesional RhoA and RhoB Expression. Arch. Neurol. 2003;60:1245–1249. doi: 10.1001/archneur.60.9.1245. [DOI] [PubMed] [Google Scholar]
- Brabeck C, Beschorner R, Mittelbronn M, Bekure K, Meyermann R, Schluesener HJ, Schwab JM. Lesional expression of RhoA and RhoB following traumatic brain injury in humans. J. Neurotrauma. 2004;21:697–706. doi: 10.1089/0897715041269597. [DOI] [PubMed] [Google Scholar]
- Budd SL, Tenneti L, Lishnak T, Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc. Natl. Acad. Sci. USA. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Benítez A, Mora´n J. Caspase activation pathways induced by staurosporine and low potassium: role of caspase-2. J. Neurosci. Res. 2003;71:383–396. doi: 10.1002/jnr.10493. [DOI] [PubMed] [Google Scholar]
- Canguilhem B, Pradines A, Baudouin C, Boby C, Lajoie-Mazenc I, Charveron M, Favre G. RhoB protects human keratinocytes from UVB-induced apoptosis through epidermal growth factor receptor signaling. J. Biol. Chem. 2005;280:43257–43263. doi: 10.1074/jbc.M508650200. [DOI] [PubMed] [Google Scholar]
- Cao G, Luo Y, Nagayama T, Pei W, Stetler RA, Graham SH, Chen J. Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J. Cereb. Blood Flow Metab. 2002;22:534–546. doi: 10.1097/00004647-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J. Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Arnold PM, Haynes NG, Ameenuddin S, Farooque M, SantaCruz K, Festoff BW. Neuroprotective effects of caspase-3 inhibition on functional recovery and tissue sparing after acute spinal cord injury. Spine. 2008;33:2269–2277. doi: 10.1097/BRS.0b013e3181831f7e. [DOI] [PubMed] [Google Scholar]
- Clark RSB, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem. 2000;74:740–753. doi: 10.1046/j.1471-4159.2000.740740.x. [DOI] [PubMed] [Google Scholar]
- Clark CJ, McDade DM, O’Shaughnessy CT, Morris BJ. Contrasting roles of neuronal Msk1 and Rsk2 in Bad phosphorylation and feedback regulation of Erk signalling. J. Neurochem. 2007;102:1024–1034. doi: 10.1111/j.1471-4159.2007.04601.x. [DOI] [PubMed] [Google Scholar]
- Conrad S, Schluesener HJ, Trautmann K, Joannin N, Meyermann R, Schwab JM. Prolonged lesional expression of RhoA and RhoB following spinal cord injury. J. Comp. Neurol. 2005;487:166–175. doi: 10.1002/cne.20561. [DOI] [PubMed] [Google Scholar]
- Conway A-M, James AB, O’Kane EM, Rakhit S, Morris BJ. Regulation of myosin light chain phosphorylation by RhoB in neuronal cells. Exp. Cell Res. 2004;300:35–42. doi: 10.1016/j.yexcr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Crowe MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. RhoA/Rho Kinase Up-regulate Bax to Activate a Mitochondrial Death Pathway and Induce Cardiomyocyte Apoptosis. J. Biol. Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- DeWard AD, Leali K, West RA, Prendergast GC, Alberts AS. Loss of RhoB expression enhances the myelodysplastic phenotype of mammalian diaphanous-related formin mDia1 knockout mice. PLoS ONE. 2009;4:e7102. doi: 10.1371/journal.pone.0007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah BA, Faden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J. Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- Erdö F, Trapp T, Mies G, Hossmann K-A. Immunohistochemical analysis of protein expression after middle cerebral artery occlusion in mice. Acta Neuropathol. 2004;107:127–136. doi: 10.1007/s00401-003-0789-8. [DOI] [PubMed] [Google Scholar]
- Föcking M, Besselmann M, Trapp T. Statins potentiate caspase-3 activity in immortalized murine neurons. Neurosci. Lett. 2004;355:41–44. doi: 10.1016/j.neulet.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Fritz G, Kaina B, Aktories K. The Ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J. Biol. Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr. Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- Gauthier-Fisher A, Lin DC, Greeve M, Kaplan DR, Rottapel R, Miller FD. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nat. Neurosci. 2009;12:735–744. doi: 10.1038/nn.2339. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LHT, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-[beta] precursor protein and amyloidogenic A[beta] peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J. Biol. Chem. 2007;282:26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- Gwag BJ, Canzoniero LMT, Sensi SL, DeMaro JA, Koh JY, Goldberg MP, Jacquin M, Choi DW. Calcium ionophores can induce either apoptosis or necrosis in cultured cortical neurons. Neurosci-ence. 1999;90:1339–1348. doi: 10.1016/s0306-4522(98)00508-9. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for Caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Harada J, Sugimoto M. Activation of caspase-3 in b-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res. 1999;842:311–323. doi: 10.1016/s0006-8993(99)01808-9. [DOI] [PubMed] [Google Scholar]
- Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in jurkat cells. J. Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–132. doi: 10.1007/s004419900156. [DOI] [PubMed] [Google Scholar]
- Hutchison N, Hendry BM, Sharpe CC. Rho isoforms have distinct and specific functions in the process of epithelial to mesenchymal transition in renal proximal tubular cells. Cell. Signal. 2009;21:1522–1531. doi: 10.1016/j.cellsig.2009.05.012. [DOI] [PubMed] [Google Scholar]
- James AB, Conway A-M, Morris BJ. Regulation of the neuronal proteasome by Zif268 (Egr1) J. Neurosci. 2006;26:1624–1634. doi: 10.1523/JNEUROSCI.4199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Li Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron. 2011;70:758–772. doi: 10.1016/j.neuron.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson T, Elfverson M, Birgner C, Frändberg P-A, Nyberg F, Le Grevès P. Neurosteroids alter glutamate-induced changes in neurite morphology of NG108-15 cells. J. Steroid Biochem. Mol. Biol. 2007;104:215–219. doi: 10.1016/j.jsbmb.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Keane RW, Kraydieh S, Lotocki G, Alonso OF, Aldana P, Dietrich WD. Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J. Cereb Blood Flow Metab. 2001;21:1189–1198. doi: 10.1097/00004647-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Leveille F, Papadia S, Fricker M, Bell KFS, Soriano FX, Martel M-A, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, Tolkovsky AM, Hardingham GE. Suppression of the intrinsic apoptosis pathway by synaptic activity. J. Neurosci. 2010;30:2623–2635. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YD, Liu YP, Cao DM, Yan YM, Hou YN, Zhao JY, Yang R, Xia ZF, Lu J. Induction of small G protein RhoB by non-genotoxic stress inhibits apoptosis and activates NF-κB. J. Cell. Physiol. 2011;226:729–738. doi: 10.1002/jcp.22394. [DOI] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia J-M, Lo S-C, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Liu Ax, Cerniglia GJ, Bernhard EJ, Prendergast GC. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc. Natl. Acad. Sci. USA. 2001a;98:6192–6197. doi: 10.1073/pnas.111137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AX, Rane N, Liu J-P, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 2001b;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S-M, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Nowak FV. The RhoGAP domain-containing protein, Porf-2, inhibits proliferation and enhances apoptosis in neural stem cells. Mol. Cell. Neurosci. 2011;46:573–582. doi: 10.1016/j.mcn.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Magnoni S, Brody DL. New perspectives on amyloid-b dynamics after acute brain injury: moving between experimental approaches and studies in the human brain. Arch. Neurol. 2010;67:1068–1073. doi: 10.1001/archneurol.2010.214. [DOI] [PubMed] [Google Scholar]
- Mannherz HG, Gonsior SM, Wu X, Polzar B, Pope BJ, Wartosch L, Weeds AG. Dual effects of staurosporine on A431 and NRK cells: microfilament disassembly and uncoordinated lamellipodial activity followed by cell death. Eur. J. Cell Biol. 2006;85:785–802. doi: 10.1016/j.ejcb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb. Cortex. 2009;19:1273–1293. doi: 10.1093/cercor/bhn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CJ, Bradley KN, McCarron JG, Palmer AM, Morris BJ. Striatal neurones show sustained recovery from severe hypoglycaemic insult. J. Neurochem. 2003;86:383–393. doi: 10.1046/j.1471-4159.2003.01853.x. [DOI] [PubMed] [Google Scholar]
- McNair K, Spike R, Guilding C, Prendergast GC, Stone TW, Cobb SR, Morris BJ. A role for RhoB in synaptic plasticity and the regulation of neuronal morphology. J. Neurosci. 2010;30:3508–3517. doi: 10.1523/JNEUROSCI.5386-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J. Biol. Chem. 1998;273:4811–4814. doi: 10.1074/jbc.273.9.4811. [DOI] [PubMed] [Google Scholar]
- Moore JD, Rothwell NJ, Gibson RM. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. Br. J. Pharmacol. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ. Stimulation of immediate-early gene expression in striatal neurones by nitric oxide. J. Biol. Chem. 1995;270:24740–24744. [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan VA, Yakovlev AG, Dabaghyan EA, Stoica BA, Faden AI. Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway. Biochem. Biophys. Res. Commun. 2002;299:201–207. doi: 10.1016/s0006-291x(02)02593-7. [DOI] [PubMed] [Google Scholar]
- Nicoll JAR, Roberts GW, Graham DI. Apolipoprotein E [epsi]4 allele is associated with deposition of amyloid [beta]-protein following head injury. Nat. Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- O’Kane EM, Stone TW, Morris BJ. Activation of Rho GTPases by synaptic transmission in the hippocampus. J. Neurochem. 2003a;87:1309–1312. doi: 10.1046/j.1471-4159.2003.02102.x. [DOI] [PubMed] [Google Scholar]
- O’Kane EM, Stone TW, Morris BJ. Distribution of Rho family GTPases in the adult rat hippocampus and cerebellum. Brain Res. Mol. Brain Res. 2003b;114:1–8. doi: 10.1016/s0169-328x(03)00121-9. [DOI] [PubMed] [Google Scholar]
- Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann. NY Acad. Sci. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Prendergast GC. Farnesyltransferase inhibitors: antineoplastic mechanism and clinical prospects. Curr. Opin. Cell Biol. 2000;12:166–173. doi: 10.1016/s0955-0674(99)00072-1. [DOI] [PubMed] [Google Scholar]
- Prendergast GC. Actin’ up: RhoB in cancer and apoptosis. Nat. Rev. Cancer. 2001a;1:162–168. doi: 10.1038/35101096. [DOI] [PubMed] [Google Scholar]
- Prendergast GC. Farnesyltransferase inhibitors define a role for RhoB in controlling neoplastic pathophysiology. Histol. Histopathol. 2001b;16:269–275. doi: 10.14670/HH-16.269. [DOI] [PubMed] [Google Scholar]
- Rakhit S, Clark CJ, O’Shaughnessy CT, Morris BJ. N-methyl-D-aspartate and brain-derived neurotrophic factor induce distinct profiles of extracellular signal-regulated kinase, mitogen- and stress-activated kinase, and ribosomal s6 kinase phosphorylation in cortical neurons. Mol. Pharmacol. 2005;67:1158–1165. doi: 10.1124/mol.104.005447. [DOI] [PubMed] [Google Scholar]
- Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HAR, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J. Neurosci. 2010;30:4221–4231. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, DePrimo SE, Grabert LM, Fu VX, Brooks JD, Jarrard DF. Novel pathways associated with bypassing cellular senescence in human prostate epithelial cells. J. Biol. Chem. 2002;277:14877–14883. doi: 10.1074/jbc.M200373200. [DOI] [PubMed] [Google Scholar]
- Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyl-transferase I inhibitors in cancer therapy: important mechanistic and bench to bedside issues. Expert Opin. Investig Drugs. 2000;9:2767–2782. doi: 10.1517/13543784.9.12.2767. [DOI] [PubMed] [Google Scholar]
- Sherstnev V, Yurasov V, Storozheva Z, Gruden’ M, Yakovleva N. Biochemical markers of apoptosis in different parts of the brain during learning. Neurosci. Behav. Physiol. 2006;36:915–919. doi: 10.1007/s11055-006-0107-8. [DOI] [PubMed] [Google Scholar]
- Simpson CS, Morris BJ. Regulation of neuronal cell adhesion molecule (NCAM) expression by NF-κB. J. Biol. Chem. 2000;275:16879–16884. doi: 10.1074/jbc.275.22.16879. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat. Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Zirrgiebel U, von Bohlen und Halbach O, Strelau J, Laliberteé C, Kaplan DR, Unsicker K. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J. Cell Biol. 2004;165:357–369. doi: 10.1083/jcb.200403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:11566–11571. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Tomiyoshi G, Horita Y, Nishita M, Ohashi K, Mizuno K. Caspase-mediated cleavage and activation of LIM-kinase 1 and its role in apoptotic membrane blebbing. Genes Cells. 2004;9:591–600. doi: 10.1111/j.1356-9597.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Trapp T, Olah L, Holker I, Besselmann M, Tiesler C, Maeda K, Hossmann KA. GTPase RhoB: an early predictor of neuronal death after transient focal ischemia in mice. Mol. Cell. Neurosci. 2001;17:883–894. doi: 10.1006/mcne.2001.0971. [DOI] [PubMed] [Google Scholar]
- Trapp T, Korhonen L, Besselmann M, Martinez R, Mercer EA, Lindholm D. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol. Cell. Neurosci. 2003;23:302–313. doi: 10.1016/s1044-7431(03)00013-7. [DOI] [PubMed] [Google Scholar]
- West T, Atzeva M, Holtzman DM. Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiol. Dis. 2006;22:523–537. doi: 10.1016/j.nbd.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J. Cell Sci. 2004;117:3221–3231. doi: 10.1242/jcs.01193. [DOI] [PubMed] [Google Scholar]
- Yakovlev A, Faden A. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol. 2001;24:131–144. doi: 10.1385/MN:24:1-3:131. [DOI] [PubMed] [Google Scholar]
- Yoshimori A, Sakai J, Sunaga S, Kobayashi T, Takahashi S, Okita N, Takasawa R, Tanuma S. Structural and functional definition of the specificity of a novel caspase-3 inhibitor, Ac-DNLD-CHO. BMC Pharmacol. 2007;7:8. doi: 10.1186/1471-2210-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P-Y, Rane N, Du W, Chintapalli J, Prendergast GC. Role for RhoB and PRK in the suppression of epithelial cell transformation by farnesyltransferase inhibitors. Oncogene. 2010;22:1124–1134. doi: 10.1038/sj.onc.1206181. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Fernandes HB, Krebs C, Shehadeh J, Wellington CL, Leavitt BR, Baimbridge KG, Hayden MR, Raymond LA. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington’s disease. Mol. Cell. Neurosci. 2004;25:469–479. doi: 10.1016/j.mcn.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zhan R-Z, Wu C, Fujihara H, Taga K, Qi S, Naito M, Shimoji K. Both caspase-dependent and caspase-independent pathways may be involved in hippocampal CA1 neuronal death because of loss of cytochrome c from mitochondria in a rat forebrain ischemia model. J. Cereb. Blood Flow Metab. 2001;21:529–540. doi: 10.1097/00004647-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu X, Yuan X. Phenylalanine activates the mitochondria-mediated apoptosis through the RhoA/Rho-associated kinase pathway in cortical neurons. Eur. J. Neurosci. 2007;25:1341–1348. doi: 10.1111/j.1460-9568.2007.05404.x. [DOI] [PubMed] [Google Scholar]