Abstract
Malignant tumors are typically associated with altered rigidity relative to normal host tissue. Magnetic resonance elastography (MRE) enables the noninvasive quantitation of the mechanical properties of deep-seated tissue following application of an external vibrational mechanical stress to that tissue. In this preclinical study, we used MRE to quantify (kPa) the elasticity modulus Gd and viscosity modulus Gl of three intracranially implanted glioma and breast metastatic tumor models. In all these brain tumors, we found a notable softness characterized by lower elasticity and viscosity than normal brain parenchyma, enabling their detection on Gd and Gl parametric maps. The most circumscribed tumor (U-87 glioma) was the stiffest whereas the most infiltrative tumor (MDA-MB-231 metastatic breast carcinoma) was the softest. Tumor cell density and microvessel density correlated positively with elasticity and viscosity significantly, whereas there was no association with the extent of collagen deposition or myelin fiber entrapment. In conclusion, while malignant tumors tend to exhibit increased rigidity, intracranial tumors presented as remarkably softer than normal brain parenchyma. Our findings reinforce the case for MRE use in diagnosing and staging brain malignancies, based on the association of different tumor phenotypes with different mechanical properties.
Keywords: Magnetic Resonance Elastography, Brain tumors, Elasticity, Viscosity, Mechanical properties
Introduction
Altered tissue stiffness through loss of tensional homeostasis is a hallmark of cancer. The changes that occur at a cellular level during oncogenesis and tumor progression cause dramatic changes in the architecture and mechanical properties of both the tumor and surrounding host tissue. Malignant tumors have been typically associated with increased tissue rigidity relative to the normal host tissue, which may influence therapeutic response and promote metastasis (1-3) and invasiveness. There is also growing evidence that an increase in local stiffness may promote the malignant transformation of normal cells (4,5).
The morphology of both tumor cells and each component of their microenvironment (vasculature, fibroblast/immune cell infiltration, and extracellular matrix) manifestly shape the macroscopic mechanical properties of each tumor type, and may also define aggressive, invasive, metastatic and resistant phenotypes (6,7). Physical palpation exploits the differences in compliance between tumor and host tissue, and has provided an invaluable, yet limited, diagnostic tool for the detection of malignancies since before the origin of modern medicine.
Diagnostic imaging is an essential tool in the management of patients with brain cancer. MRI has become the methodology of choice due to its exquisite soft tissue image contrast, which enables the visualisation of detailed anatomical features with high resolution. Advanced functional MRI methodologies have improved radiological-based diagnosis of brain tumors, but still demonstrate low specificity. The final diagnosis still relies on pathological examination of the resected tumor or biopsy samples, both obtained at high risk of morbidity. Patients with grade IV malignant glioma only have a median survival of 12-15 months. While low-grade gliomas have a better prognosis, there is, as yet, no assured cure. Patients with brain metastases, which affect up to 40% of patients with metastatic cancer, have a similarly poor prognosis. With new promising targeted strategies for the treatment of patients with brain malignancies has come the unmet need for refined non-invasive imaging strategies that could provide more specific diagnostic, predictive (enabling the stratification of patients who would benefit from the treatment) and prognostic (to determine the potential to achieve favorable clinical outcome) biomarkers.
Elastography is the imaging of the mechanical properties of tissue, which may be accomplished with any conventional anatomical imaging modality such as MRI, ultrasound, X-ray and optical imaging. Magnetic resonance elastography (MRE) is an emerging MRI methodology, which enables the quantitative and non-invasive assessment of tissue mechanical properties in vivo. MRE relies on the ability of MRI to visualise the propagation of low-frequency shear waves, whose characteristics are directly determined by the local viscoelastic properties of the tissue of interest (8). Since low frequency waves can be mechanically applied through the skull, MRE is uniquely positioned to assess the viscoelastic properties of brain tissue in situ. MRE has already been successfully implemented in the clinic, in both healthy volunteers and patients with brain tumors (9-12).
In this study, we used MRE to investigate the viscoelastic properties of three intracranially implanted tumors with different infiltrative patterns of growth. Using histopathological correlates, we investigated the physiological and structural determinants of the different viscoelastic properties measured between brain parenchyma and tumors, as well as between different tumor types, and discuss the potential of MRE for the diagnosis and stratification of patients presenting with brain malignancies.
Materials and Methods
Cell Lines
U-87 MG human glioblastoma cells (American Type Culture Collection, LGC Standards, Teddington, UK) engineered to stably express pCDF1-MCS2-EFIPuro-luc, RG2 rat glioma cells (American Type Culture Collection) that stably express pGL4.50[luc2/CMV/hygro] (kind gift from Dr. D. Crichton, Cancer Research Technology, The Beatson Institute for Cancer Research, Glasgow) and luciferase-expressing MDA-MB-231 LM2-4 human triple negative breast carcinoma cells (13) (provided by Dr. R. Kerbel, University of Toronto, Canada) were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% (v/v) fetal bovine serum (Invitrogen, Life Technologies, Paisley, UK). All cells were tested for mycoplasma at the time of tumor propagation and were found to be negative.
Animals
Six week old female athymic (NCr nu/nu) mice were obtained from Charles River Ltd (Margate, UK). All experiments were performed in accordance with the local ethical review panel, the UK Home Office Animals (Scientific Procedures) Act 1986, the United Kingdom National Cancer Research Institute guidelines for the welfare of animals in cancer research (14) and the ARRIVE (animal research: reporting in vivo experiments) guidelines (15).
Intracranial Injections and Bioluminescence Imaging
U-87 MG (5×104), RG2 or MDA-MB-231 cells (5×103) were implanted supratentorially in the brains of mice. Animals were anesthetized using 1-2% isoflurane in oxygen (1 l/min). A ~1cm incision was made in the skin on the top of the head, and a 1mm hole drilled 2mm posterior to the junction of sagittal and coronal sutures of the skull (bregma) and 1mm lateral to the midline using a surgical bone microdrill (Harvard Apparatus, Edenbridge, UK). Cell suspension (5μl) was then injected at a depth of 3mm from the dura, at a rate of 2μl/min, using a 10μl syringe (VWR International, Lutterworth, UK) and a nanomite syringe pump (Harvard Apparatus). The needle was removed 3 minutes after completion of the injection and the skin repaired with Vetbond™ Tissue Adhesive (3M Animal Care Products, St Paul, MN, USA). Tumor establishment and growth were monitored with bioluminescence imaging using a Xenogen IVIS® 200 system coupled with LivingImage software (Caliper Life Sciences, Runcorn, UK). Luciferin (150mg/kg, Caliper Life Sciences) was administered intraperitoneally 10 minutes before imaging. Total photon flux was established for automatically drawn ROIs at a constant threshold. MRE was performed when the photon flux reached a threshold value previously determined to represent a tumor of approximately 30-40mm3, a volume considered of sufficient size to acquire MRE data but not large enough to cause neurological effects in the mice. The average time from implantation to imaging was 18 days for the U-87 MG and MDA-MB-231 tumors and 22 days for the RG2 tumors.
Magnetic Resonance Elastography
All MRI studies were performed on a 7T Bruker horizontal bore MicroImaging system (Bruker Instruments, Ettlingen, Germany) using a 3cm birdcage volume coil. Anesthesia was induced by an intraperitoneal 10ml/kg injection of a combination of fentanyl citrate (0.315mg/ml) plus fluanisone (10mg/ml) (Hypnorm, Janssen Pharmaceutical, Oxford, UK) and midazolam (5mg/ml) (Roche, Welwyn Garden City, UK) and water (1:1:2). Core body temperature was maintained at ~37°C with warm air blown through the magnet bore.
MRE was performed as recently described (16). The mechanical vibrations were generated by an electromagnetic shaker (Brüel & Kjaer, Nærum, Denmark), and were transmitted through a flexible nylon rod to a square semi-curved piston positioned on the mouse head within the volume coil at the isocenter of the magnetic field (Supplementary Figure S1). Anatomical T2-weighted images (using a rapid acquisition with refocused echoes (RARE) sequence, with TE = 36ms, TR = 4.5s, RARE factor = 8, 40 contiguous 1mm thick transverse slices, 1 average, matrix size 128×128 over a 3×3cm2 field of view) covering the whole brain were used for determining tumor volumes, planning of the MRE acquisition, and optimisation of the local field homogeneity over the region of interest using the FASTMAP algorithm. MRE was performed using mechanical excitations at a vibration frequency of 1000 Hz, which generates mechanical waves inside the tumor with amplitude greater than 0.5μm. A 2D spin-echo sequence was modified with sinusoidal motion-sensitizing gradients synchronized to the mechanical excitation. Data were acquired in three orthogonal directions, from ten contiguous transverse slices (300μm thick), using 2 averages of 64 phase encoding steps over a 1.92×1.92cm2 FOV, with TE = 27ms, TR = 1001ms and 8 time sampling steps, giving an isotropic spatial sampling of 300×300×300μm3 of the mechanical wave propagation displacement inside the tumor. The total acquisition time was ~ 51min. High resolution anatomical T2-weighted images were subsequently acquired from the same ten contiguous transverse slices, (using a RARE sequence, with TE = 36ms, TR = 4.5s, RARE factor = 8, 300μm thick, 10 averages, matrix size 128×128 over a 1.92×1.92cm2 field of view)
Image Reconstruction and Analysis
Parametric maps of the absolute value of the complex shear modulus |G*|, elasticity Gd and viscosity Gl (where G*=Gd+iGl) were reconstructed using an in-house software from the three-dimensional displacement vector measured as described above, and using the following equation (17):
where is the complex-valued curl of the measured displacement field , ρ is the density of the material and ω is the angular frequency. For each slice, Gd and Gl (kPa) were determined pixelwise from a region of interest (ROI) covering the whole tumor identified from T2-weighted images.
Histological Analysis
Tissue sections (5μm) were cut from tumor bearing brains that had been formalin-fixed and paraffin-embedded (FFPE). FFPE sections were stained with hematoxylin and eosin (H&E), visualized by light microscopy, then blind reviewed and scored by an experienced pathologist (S.P.) for in vasive growth, the presence of necrosis, edema and hemorrhage. Cellular density was also assessed on sections from two levels through each tumor, by counting the number of nuclei in four square ROIs from a total area of 0.01 mm2 per field (x200 magnification, three or more fields assessed per section) (18). Collagen I and III were detected on adjacent FFPE tissue sections using picrosirius red staining (Supplementary Methods and Materials). In addition, collagen IV expression was detected by immunohistochemistry (rabbit polyclonal antibodies, ab6586, Abcam, Cambridge, UK). Microvessel density was assessed on FFPE sections stained for the murine vascular endothelial marker CD31 (rabbit EP3095, Millipore, Watford, UK). The number of vessels in four or five random fields of a total area assessed of 0.015 mm2 each (x200 magnification). Luxol fast blue staining was also used to assess myelin fibers in adjacent sections (Supplementary Methods and Materials).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software Inc., La Jolla, USA). The mean of median values for all the quantitative MRE parameters, the mean values for tumor volume, cellular density and microvessel density were used for statistical analysis. Any significant differences in quantitative MRE parameters were identified using the non-parametric Mann-Whitney U test, with a 5% level of significance. Any significant differences in tumour volume and quantitative histopathological parameters were identified using Student’s 2-tailed unpaired t-test, with a 5% level of significance. Significant correlations between the mean values for all the quantitative MRE parameters and cellular and microvessel density were determined using linear regression analysis, confirmed by using the robust regression and outlier removal approach (19).
Results
Intracranially implanted U-87 MG, RG2 and MDA-MB-231 tumors are softer and less viscous than healthy brain parenchyma
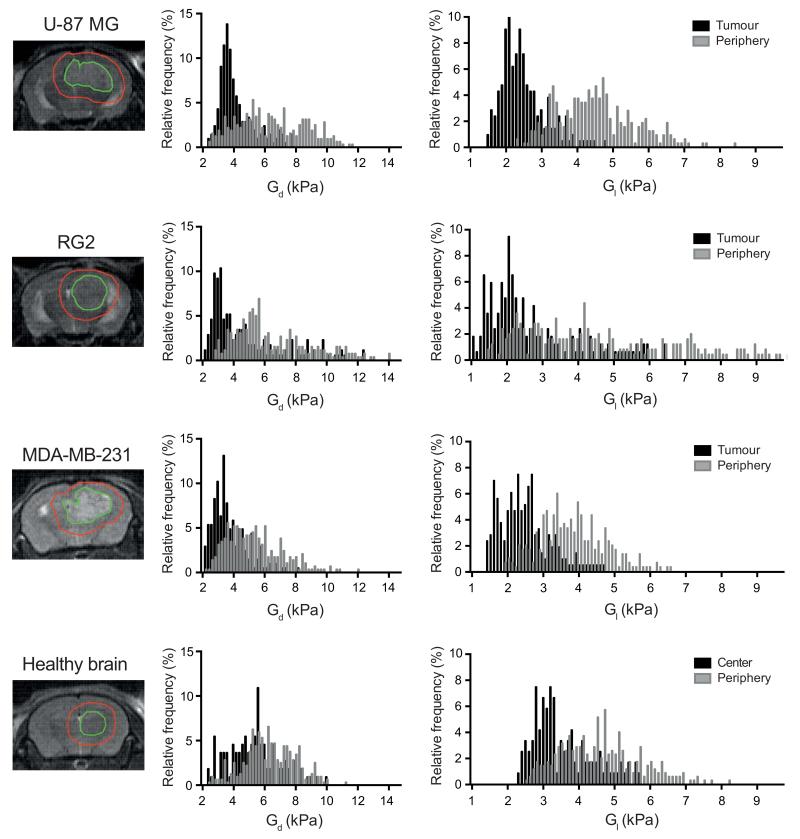
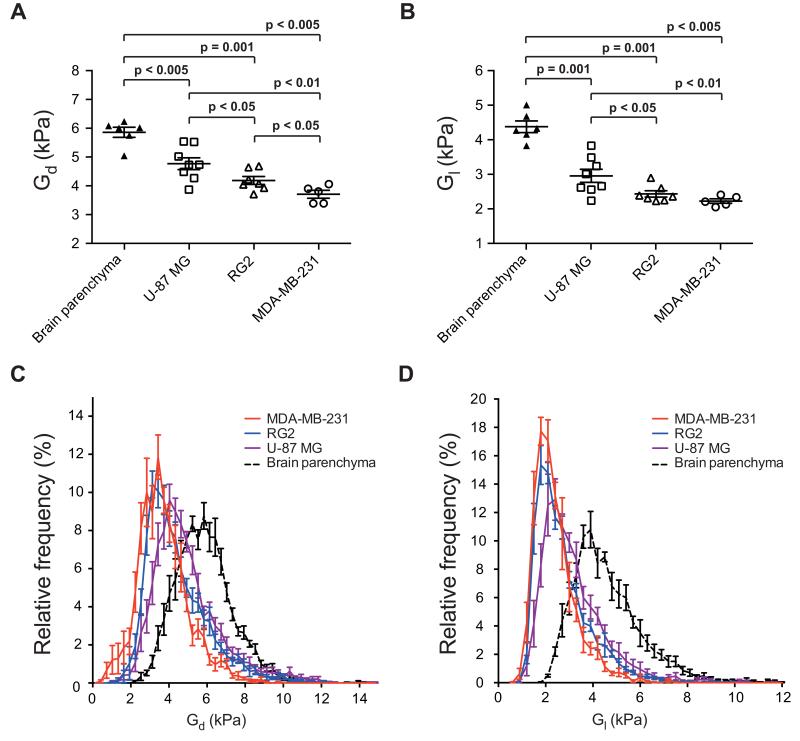
Parametric maps of elasticity (Gd) and viscosity (Gl) revealed the symmetrical and characteristic anatomical structures of the healthy mouse brain, including the relatively stiffer corpus callosum and the softer thalamus (Figure 1). In mouse brains bearing tumors derived from U-87 MG, RG2 or MDA-MB-231 cells, both elasticity and viscosity maps showed pronounced contrast between the established tumor (mean tumor volume 35±3 mm3) and the surrounding brain, based on viscoelastic image appearances and on analyses within regions of interest drawn using boundaries defined by the T2-weighted MR images (Figure 2, Supplementary Figure S2). The presence and location of the tumors were confirmed by H&E staining of FFPE tissue sections. Quantitative analysis of the elastic modulus Gd and the viscosity modulus Gl revealed that the three tumor types were significantly less elastic (Gd, U-87 MG: 4.80 ± 0.21 kPa > RG2: 4.22 ± 0.14 kPa > MDA-MB-231: 3.74 ± 0.14 kPa) and viscous (Gl, U-87 MG: 2.94 ±0.19 > RG2: 2.41 ± 0.09 kPa > MDA-MB-231: 2.21 ± 0.07 kPa) than healthy brain parenchyma Gd, 5.89 ± 0.17 kPa and Gl, 4.36 ± 0.17 kPa) (Figure 3A and B). Histogram analysis demonstrated a clear shift in the distribution of Gd and Gl values in the tumor compared with healthy brain tissue (Figure 3C and D). In the majority of mice with tumors, the lateral ventricles were markedly dilated and demonstrated low elasticity and viscosity (Figure 1).
Figure 1.
Non-invasive imaging of the viscoelastic properties of intracranial tumors assessed by magnetic resonance elastography (MRE). Anatomical T2-weighted MR images, and parametric maps of elasticity (Gd) and viscosity (Gl), acquired from a non-tumor bearing mouse, and mice bearing tumors derived from human adult U-87 MG glioblastoma, N-ethyl-N-nitrosourea-induced RG2 rat glioma, or human triple negative MDA-MB-231 breast carcinoma cells are shown. (---) indicates the tumor boundaries defined on T2-weighted images. Images of whole hematoxylin and eosin stained sections obtained from the same mice are also shown, with the tumor location arrowed.
Figure 2.
Frequency histograms showing the distribution of elasticity (Gd) and viscosity (Gl) and in tumours and their periphery, in representative mice bearing intracranially implanted U-87 MG, RG2, or MDA-MB-231 tumors. Tumor regions of interest were defined on T2-weighted images. Histogram analysis was also performed in the brain of a control healthy mouse.
Figure 3.
Quantitative assessment of A) elasticity (Gd) and B) viscosity (Gl), determined in vivo from healthy brain parenchyma in control mice (n=6), and intracranially implanted U-87 MG (n=8), RG2 (n=7) and MDA-MB-231 (n=5) tumors, using magnetic resonance elastography. Data are presented as the median values for each tumor, and as the mean ± 1 s.e.m. across each cohort. Significance testing employed Mann-Whitney U test with a 5% level of significance. The associated frequency histograms showing the distribution of C) elasticity (Gd) and D) viscosity (Gl) for brain parenchyma and the three tumor types are also shown.
Intracranially implanted U-87 MG, RG2 and MDA-MB-231 tumors have different viscoelastic properties
Significantly different values of Gd were determined across the three tumor models, with tumors derived from U-87 MG cells being the stiffest and tumors derived from MDA-MB-231 the softest (Figures 3A & C). A similarly significant trend was also found for Gl, with tumors derived from U-87 MG cells significantly more viscous than tumors derived from either RG2 or MDA-MB-231 cells (Figures 3B & D).
Cellular and microvessel density contribute to the relative stiffness of intracranially implanted brain tumors
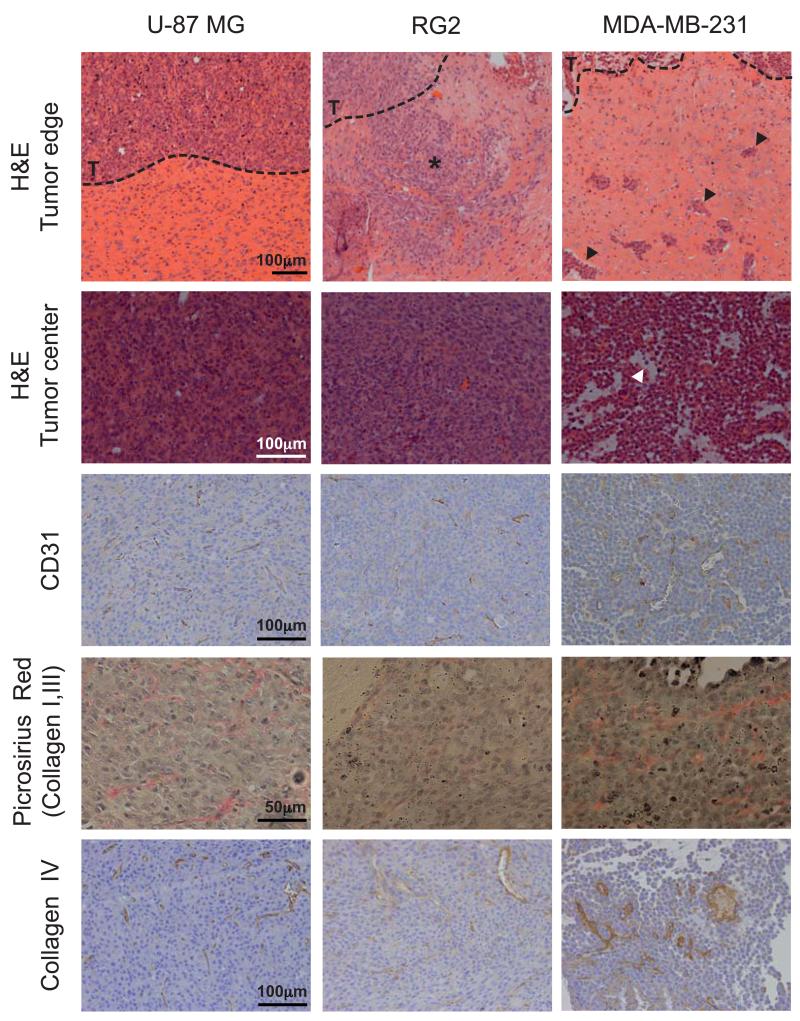
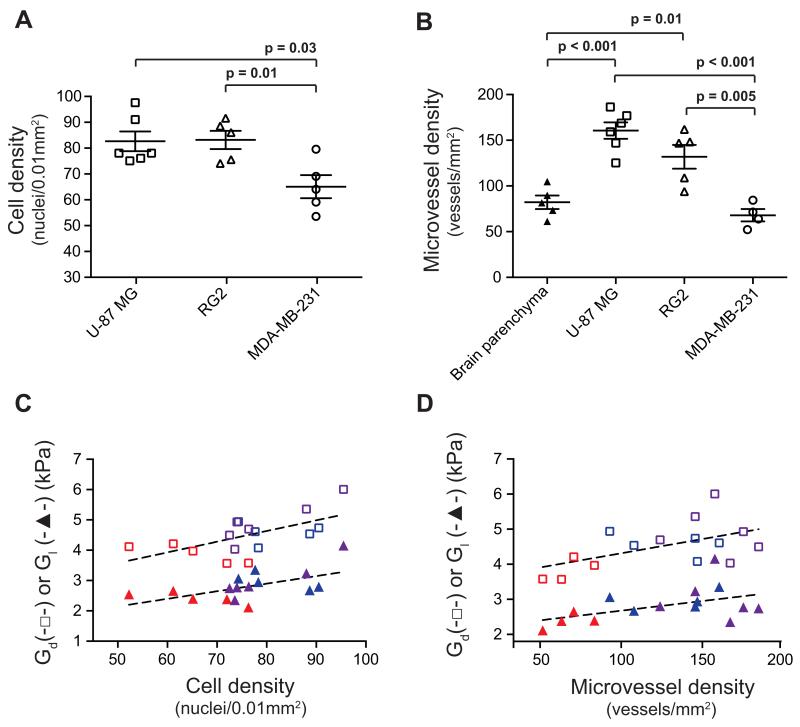
Table 1 summarizes the histopathological features of each tumor model. Markedly different growth patterns were apparent, with tumors derived from U-87 MG cells being well-circumscribed with sparse foci of infiltrative cells, whilst tumors derived from MDA-MB-231 cells were substantially more infiltrative (Figure 4). Tumors derived from U-87 MG and RG2 cells had a homogeneous dense cellularity, whereas tumors derived from MDA-MB-231 cells presented with regions of sparse cell density and edema. Quantitative analysis confirmed a significantly lower cellular density in tumors derived from MDA-MB-231 cells compared with tumors derived from U-87 MG or RG2 cells (Figure 5A). At the time of imaging or excision, no areas of gross necrosis were detected in any of the tumor models.
Table 1.
Summary of the histopathological features of tumors derived from adult human U-87 MG glioblastoma cells, N-ethyl-N-nitrosourea-induced rat RG2 glioma cells, or human triple negative MDA-MB-231 breast carcinoma cells implanted intracranially in athymic mice (n=3 per tumor type).
| M | Growth pattern | Cellularity | Necrosis | Edema | Hemorrhage | Myelin fibres | |
|---|---|---|---|---|---|---|---|
| U-87 MG | 1 | Well circumscribed Presence of infiltrative foci |
Dense | - | - | Small foci | Foci of very fine fibers in the centre |
|
| |||||||
| 2 | Well circumscribed Presence of infiltrative foci |
Dense | - | - | - | Foci of cells infiltrating highly myelinated part of the brain | |
|
| |||||||
| 3 | Well circumscribed Presence of infiltrative foci |
Dense | - | - | - | Few, very fine fibres within the tumor | |
|
| |||||||
| RG2 | 1 | Moderately infiltrative | Dense | - | - | + (center) | Fine fiber are seen between tumor islands at the periphery |
|
| |||||||
| 2 | Moderately infiltrative | Dense | - | - | - | - | |
|
| |||||||
| 3 | Moderately infiltrative | Dense | - | - | - | Infiltrative part of the tumor contains entrapped highly myelinated structures | |
|
| |||||||
| MDA-MB-231 | 1 | Highly infiltrative | Dense at the periphery Loose texture in centre |
- | + (center) | + (center) | Infiltrative margin is intermixed with moderately myelinated structures of normal brain |
|
| |||||||
| 2 | Highly infiltrative | Dense at the periphery Loose texture in centre |
- | + (center) | - | Infiltrative margin is intermixed with moderately and highly myelinated structures of normal brain | |
|
| |||||||
| 3 | Highly infiltrative | Heterogeneous | - | + | - | Infiltrative margin is intermixed with well-myelinated structures of normal brain | |
Figure 4.
Histopathology of intracranially implanted U-87 MG, RG2 and MDA-MB-231 tumors. Note the different infiltrative patterns at the tumor boundary (--- T) between the RG2 (*) and MDA-MB-231 (arrowed) tumors. Also note the presence of edema evident on H&E stainined sections (arrowed, white), and large distended vessels as shown by immunohistochemical staining for the murine vascular endothelial marker CD31, in the MDA-MB-231 tumor.
Figure 5.
Quantitative histological assessment of A) cell density and B) microvessel density, determined from intracranially implanted U-87 MG (n=6), RG2 (n=5) and MDA-MB-231 (n=5) tumors. Microvessel density was also quantified in healthy brain parenchyma in control mice (n=6). Data are presented as the median values for each tumor, and as the mean ± 1 s.e.m. across each cohort. Significance testing employed Student’s two-tailed unpaired t-tests with a 5% level of significance. The relationship of MR elastography-derived elasticity (Gd) and viscosity (Gl) with C) cell density (r=0.61, p=0.01 and r=0.57, p=0.02 respectively), and D) microvessel density (r=0.54, p=0.03 and r=0.48, p=0.07 respectively) across all the intracranially implanted tumors is shown ( U-87 MG,
U-87 MG,  RG2,
RG2,  MDA-MB-231).
MDA-MB-231).
Myelin was only detected as sparse and very fine fibers within tumors derived from U-87 MG cells. Myelin fibers were also detected at the invasive margin of all tumors in the myelinated structures of the brain that were being invaded (Supplementary Figure S3). Type I, III and IV collagen were more abundant in tumor compared with brain parenchyma, but were mainly localized to the basement membrane of the vasculature in all tumor types. There was no difference in collagen content between the tumor types. The three models demonstrated significantly different microvessel density (MVD) as measured on CD31-stained FFPE tissue sections of the MRE-imaged mice, with the highest vessel density in U-87 MG tumors and the lowest score in MDA-MB-231 tumors (Figures 4 & 5B). Microvessel density was significantly lower in normal brain parenchyma compared with U-87 MG and RG2 tumors (*p<0.05).
The functional vascular phenotype of the three tumor models was also investigated in separate cohorts using the perfusion marker Hoechst 33342 (see Supplementary Materials and Methods). Fluorescence microscopy of Hoechst 33342 uptake revealed no significant difference in the degree of functionally perfused vasculature (Supplementary Figures S4A & B). However, subsequent processing and quantitative analysis of CD31 immunofluoresence on the same frozen tissue sections corroborated the CD31 MVD data acquired on FFPE sections of the imaged tumors (Supplementary Figures S4 A & C).
Linear regression analysis revealed significant positive correlations between tumor cellular density and MVD with both elasticity Gd (r=0.61, p=0.01 and r=0.54, p=0.03 respectively), and viscosity Gl (r=0.57, p=0.02 and r=0.48, p=0.07 respectively) (Figures 5C & D).
Discussion
It is well established that increased tissue rigidity in extracranial tumors is associated with an invasive phenotype, and can influence therapeutic response (3). Reciprocally, therapeutic prevention of tissue stiffening is predicted to impede cancer progression and metastasis, and drugs targeting the mechanical properties of the extracellular matrix are being developed (4,20-22). In this setting, the ability of MRE to non-invasively quantify tumor mechanical properties is being actively exploited and should prove beneficial for cancer diagnosis, prognosis and for monitoring response to these novel therapeutics (23,24).
Using MRE, we have shown that three intracranially implanted tumors derived from U-87 MG human glioma, RG2 rat glioma or MDA-MB-231 metastatic human breast carcinoma cells, which present with different growth patterns were all significantly softer than the surrounding brain tissue in vivo. Given the well-described association of increased stiffness with malignancy in, for example, breast or liver cancer (23), the relative softness of these intracranially implanted tumor models compared with normal brain was initially unexpected. However, this observation is consistent with the reported soft consistency of brain malignancies, based on pathological examination or intra-operative assessment (25-27). In a recent clinical prospective study, preliminary evidence of the relative softness of malignant primary brain tumors and metastases with a range of differentiation status and grade was provided by MRE (12).
In interrogating the pathological determinants underpinning our MRE data we have demonstrated that both cellular density and MVD contribute to the relative stiffness of these soft brain tumor models. More generally, cellular architecture (cellular arrangement, size, shape and density), the vascular network and its collagen-supported scaffold are major structural components of the tumor microenvironment, and as such represent important determinants of the intrinsic stiffness of tumors. This is corroborated by recent MRE studies showing a decrease in the magnitude of the shear modulus (G*) upon therapy-induced necrosis or a decrease in MVD, and the correlation between increased G* and MVD upon progression in in vivo models of colon cancer and lymphoma (28-30).
These pathological correlates, however, do not explain the softness of brain tumors. The ECM is another major contributor to the viscoelastic properties of tumors. Breast cancer cells have been shown to be more compliant (softer) than their non-malignant counterparts (31), yet malignant breast tumors are stiff due to their ECM, being rich in cross-linked collagen fibers (32). In contrast, the ECM of brain tumors has been shown to share the unique composition of that of the healthy brain, characterized by high concentrations of hyaluronic acid and the absence of fibrillar networks such as collagen, fibronectin and vitronectin, or basement membrane proteins such as laminin, which are only found in the vascular or perivascular space (33). The unique composition of the ECM contributes to making brain the softest tissue in the human body (34). The lower viscoelastic properties of brain tumors relative to the brain parenchyma itself may be attributed to the absence of structural anisotropy due to the rapid and chaotic tumor cell growth, which contrast with the highly networked and organized microstructure of brain tissue. The heterogeneous and symmetrical distribution of mechanical properties in the healthy brain, observed in our study, correlate with the regional differences in the microstructure and organization of the brain parenchyma (35). For example, the elevated viscoelasticity associated with the corpus callosum, a compact bundle of myelin-sheathed axonal projections connecting the two brain hemispheres, is a prime example of the direct relationship between increased organization and increased stiffness in the brain. In contrast, the lower stiffness observed in the dilated ventricles in tumor-bearing mice is a consequence of the inability of cerebrospinal fluid to withstand a shear stress (36).
Our histopathologically-correlated MRE data provide further in vivo evidence of the sensitivity of MRE-measured viscoelastic properties for changes in tissue microstructure at scales that are far below the resolution of the MR images (37,38), demonstrated here for the first time in brain tumors. This is consistent with the predictions of models for the mechanical properties of networked microstructures which have been shown to scale into unique macroscopic mechanical signatures (39), making MRE a very promising methodology for the detection and differential diagnosis of malignancies (37), as illustrated herein by the discrimination of the three intracranial tumor models based on their differential mechanical phenotypes.
MRE is already in use clinically for the staging of liver fibrosis and the differential diagnosis of malignant nodules in breast, liver and prostate cancer (23,40-42). Having overcome the challenges of transmitting waves through the human cranium, MRE has also been successfully implemented for the routine examination of neurology patients including those with brain malignancies (9,11,12), and shown to detect changes in brain tissue integrity associated with several neurological disorders, including multiple sclerosis (MS) (43,44). The potential use of MRE in pre-operative planning in patients with meningioma, in which the complexity of surgical resection increases with tumor stiffness, has been recently highlighted (45).
The ability of MRE to assist in the delineation of brain tumors for preoperative management is also being actively investigated. The provision of strong conclusions on the accuracy of tumor delineation on our viscoelastic maps is challenging, as nonlinear deformations occurring during tissue processing preclude the use of H&E-stained sections as a gold standard to define tumor boundaries. Further in vivo studies co-registering MRE-acquired viscoelastic maps with images acquired with techniques such as stimulated Raman scattering (SRS) microscopy, shown to accurately delineate tumors in orthotopic models of human gliomas both in situ and ex vivo using fresh tissue slices (46), would provide definitive conclusions on the utility of MRE to delineate brain tumors.
In conclusion, our pre-clinical study provides definitive evidence for the relative softness of intracranial tumors in vivo. In addition to measuring stiffness, we demonstrate that MRE is sensitive to changes in intracranially implanted tumor microstructure, including those within the cellular and vascular networks, allowing the discrimination of three phenotypically different tumor models based on their different mechanical properties. Our study thus supports further evaluation of clinical MRE for the detection and differential diagnosis of brain tumors (or metastases) and reinforces MRE as a promising and attractive addition to the multi-parametric diagnostic neuroimaging methodologies, which already play a crucial role in the management of patients with brain malignancies (47).
Supplementary Material
Acknowledgments
Financial support: We acknowledge support from The Institute of Cancer Research Cancer Research UK and EPSRC Cancer Imaging Centre, in association with the MRC and Department of Health (England) grant C1060/A10334, NHS funding to the NIHR Biomedical Research Centre, Cancer Research UK funding to the Cancer Therapeutics Unit grant C309/A11566, the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust, The Wellcome Trust grant #091763Z/10/Z, EPSRC Platform Grant #EP/H046526/1, and a Paul O’Gorman Postdoctoral Fellowship funded by Children with Cancer UK (Y.J.), a Dorothy Hodgkin Postgraduate Award (DHPA) #EP/P505828/1 (J.L.), and AstraZeneca.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose. John Waterton has employment, stock and stock options in AstraZeneca, a for-profit company engaged in the discovery, development, manufacture and marketing of proprietary therapeutics. He does not consider that this creates any conflict of interest with the subject-matter of the manuscript.
References
- 1.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 2.Akiri G, Sabo E, Dafni H, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 3.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Mouw JK, Yui Y, Damiano L, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 9.Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21:755–764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 10.Kruse SA, Rose GH, Glaser KJ, et al. Magnetic resonance elastography of the brain. NeuroImage. 2008;39:231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sack I, Beierbach B, Hamhaber U, Klatt D, Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed. 2008;21:265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- 12.Simon M, Guo J, Papazoglou S, et al. Non-invasive characterization of intracranial tumors by magnetic resonance elastography. New J Physics. 2013;15:085024. [Google Scholar]
- 13.Munoz R, Man S, Shaked Y, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 14.Workman P, Aboagye E, Balkwill F, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Jamin Y, Boult JK, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Brit J Cancer. 2014 doi: 10.1038/bjc.2014.76. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinkus R, Siegmann K, Xydeas T, Tanter M, Claussen C, Fink M. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn Reson Med. 2007;58:1135–1144. doi: 10.1002/mrm.21404. [DOI] [PubMed] [Google Scholar]
- 18.Schnapauff D, Zeile M, Niederhagen MB, et al. Diffusion-weighted echo-planar magnetic resonance imaging for the assessment of tumor cellularity in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2009;29:1355–1359. doi: 10.1002/jmri.21755. [DOI] [PubMed] [Google Scholar]
- 19.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox TR, Bird D, Baker AM, et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 22.Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 23.Garteiser P, Doblas S, Daire JL, et al. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. Eur Radiol. 2012;22:2169–2177. doi: 10.1007/s00330-012-2474-6. [DOI] [PubMed] [Google Scholar]
- 24.Sinkus R, Tanter M, Xydeas T, Catheline S, Bercoff J, Fink M. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn Reson Imaging. 2005;23:159–165. doi: 10.1016/j.mri.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 25.Jones H, Steart PV, Weller RO. Spindle-cell glioblastoma or gliosarcoma? Neuropath Appl Neuro. 1991;17:177–187. doi: 10.1111/j.1365-2990.1991.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Sugimoto T, Shibuya M, Sugita K, Patel SJ. Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochirurgica. 1994;129:39–46. doi: 10.1007/BF01400871. [DOI] [PubMed] [Google Scholar]
- 27.Aas AT, Brun A, Blennow C, Stromblad S, Salford LG. The RG2 rat glioma model. J Neurooncol. 1995;23:175–183. doi: 10.1007/BF01059948. [DOI] [PubMed] [Google Scholar]
- 28.Juge L, Doan BT, Seguin J, et al. Colon tumor growth and antivascular treatment in mice: complementary assessment with MR elastography and diffusion-weighted MR imaging. Radiology. 2012;264:436–444. doi: 10.1148/radiol.12111548. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Jamin Y, Boult JK, et al. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br J Cancer. 2014;110:1727–1732. doi: 10.1038/bjc.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepin KM, Chen J, Glaser KJ, et al. MR elastography derived shear stiffness-a new imaging biomarker for the assessment of early tumor response to chemotherapy. Magn Reson Med. 2013;71:1834–1840. doi: 10.1002/mrm.24825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan V, Mythreye K, O’Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Bilston LE. Brain Tissue Mechanical Properties. In: Bilston LE, editor. Neural Tissue Biomechanics. Springer; Berlin Heidelberg: 2011. pp. 11–24. [Google Scholar]
- 35.Guo J, Hirsch S, Fehlner A, et al. Towards an elastographic atlas of brain anatomy. Plos One. 2013;8:e71807. doi: 10.1371/journal.pone.0071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pattison AJ, Lollis SS, Perrinez PR, et al. Time-harmonic magnetic resonance elastography of the normal feline brain. J Biomech. 2010;43:2747–2752. doi: 10.1016/j.jbiomech.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sack I, Johrens K, Wurfel J, Braun J. Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft matter. 2013;9:5672–5680. [Google Scholar]
- 38.Schregel K, Wuerfel Nee Tysiak E, Garteiser P, et al. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc Natl Acad Sci U S A. 2012;109:6650–6655. doi: 10.1073/pnas.1200151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, Posnansky O, Hirsch S, et al. Fractal network dimension and viscoelastic powerlaw behavior: II. An experimental study of structure-mimicking phantoms by magnetic resonance elastography. Phys Med Biol. 2012;57:4041. doi: 10.1088/0031-9155/57/12/4041. [DOI] [PubMed] [Google Scholar]
- 40.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 41.Xydeas T, Siegmann K, Sinkus R, Krainick-Strobel U, Miller S, Claussen CD. Magnetic resonance elastography of the breast: correlation of signal intensity data with viscoelastic properties. Invest Radiol. 2005;40:412–420. doi: 10.1097/01.rli.0000166940.72971.4a. [DOI] [PubMed] [Google Scholar]
- 42.Sahebjavaher RS, Frew S, Bylinskii A, et al. Prostate MR elastography with transperineal electromagnetic actuation and a fast fractionally encoded steady-state gradient echo sequence. NMR Biomed. 2014;27:784–794. doi: 10.1002/nbm.3118. [DOI] [PubMed] [Google Scholar]
- 43.Wuerfel J, Paul F, Beierbach B, et al. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. NeuroImage. 2010;49:2520–2525. doi: 10.1016/j.neuroimage.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Freimann FB, Streitberger KJ, Klatt D, et al. Alteration of brain viscoelasticity after shunt treatment in normal pressure hydrocephalus. Neuroradiol. 2012;54:189–196. doi: 10.1007/s00234-011-0871-1. [DOI] [PubMed] [Google Scholar]
- 45.Murphy MC, Huston J, 3rd, Glaser KJ, et al. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013;118:643–648. doi: 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji M, Orringer DA, Freudiger CW, et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Science Transl Med. 2013;5:201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Essig M, Anzalone N, Combs SE, et al. MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. Am J Neuroradiol. 2012;33:803–817. doi: 10.3174/ajnr.A2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.