Abstract
Visual perception and eye movements are considered to be tightly linked. Diverse fields, ranging from developmental psychology to computer science, utilize eye tracking to measure visual perception. However, this prevailing view has been challenged by recent behavioral studies. We review converging evidence revealing dissociations between the contents of perceptual awareness and different types of eye movements. Such dissociations reveal situations in which eye movements are sensitive to particular visual features that fail to modulate perceptual reports. We also discuss neurophysiological, neuroimaging and clinical studies supporting the role of subcortical pathways for visual processing without awareness. Our review links awareness to perceptual-eye movement dissociations and furthers our understanding of the brain pathways underlying vision and movement with and without awareness.
Keywords: awareness, eye movements, perception-action dissociation, visual pathways, visual perception
Using our eyes to actively explore the world and to gather information is a central part of human visual experience. The link between eye movements and visual perception is so tight that perception is facilitated even during the preparation of eye movements [1–5]. Recently, however, behavioral studies have revealed dissociations between perceptual reports, i.e., the contents of visual awareness, and different types of voluntary (e.g., saccades, smooth pursuit, vergence, see Glossary) and involuntary (e.g., microsaccades, ocular following, optokinetic nystagmus) eye movements. We review these perception-action dissociations, in which eye movements are sensitive to particular visual features, even though observers show no awareness of those features, as assessed by explicit perceptual reports. Some authors refer to “awareness” and “consciousness” interchangeably; we use the term “awareness” throughout and operationally define it as an explicit perceptual report. A further distinction can be made between situations in which visual processing could potentially lead to awareness, i.e., may or may not produce a perceptual report, and those in which visual processing is inaccessible to awareness, i.e., could not result in a perceptual report. Both situations may produce an eye movement in the absence of awareness, and our review focuses on these perception/action dissociations.
Dissociations provide important insights into the neural underpinnings of vision and movement with or without awareness; they may also further our understanding of diseases involving awareness deficits. We bring together these recent behavioral studies with neurophysiological, neuroimaging and clinical evidence supporting the role of the subcortical retino-collicular pathway [6,7] for visual processing without awareness. This fast-transmission pathway is associated with residual visual abilities in blindsight [8–11] and with the translation of unperceived visual signals into oculomotor outputs in these patients [12,13].
When eye movements reflect awareness
The visual content in our environment drives visually-guided eye movements, which in turn, serve perceptual judgments (reviewed in [14–20]). Accurate eye movements improve different aspects of vision, such as spatial acuity and the ability to discriminate motion direction or color [17,18]. Consistently, close links between perception and saccades/microsaccades have been demonstrated in visual illusions, fading paradigms, rivalry, and visual search [17,19–26].
Probably the tightest perception-action link is between the perception of visual motion and the control of smooth tracking movements–voluntary pursuit and reflexive ocular following responses (OFR). Motion perception and pursuit/OFR share anatomical substrates, namely the middle-temporal (MT) area and medial-superior temporal area (MST) [14]. Behavioral studies report similarities in perceptual and pursuit sensitivity in response to motion direction, speed, acceleration, biological motion and illusory motion [literature until 2011 reviewed in 16–18, and more recently 27,28].
Consistent with early studies [e.g., 29,30], recent studies of binocular rivalry [31–34], which is widely used to manipulate awareness [35], report similar perceptual alternations in rivalry with alternations in reflexive optokinetic nystagmus, OKN (both eyes track the perceived motion direction of the dominant percept). These studies advocate the use of eye movements and changes in pupil size as objective indicators of awareness, complementing subjective indicators [36,37].
When eye movements reflect processing of unaware information
Despite tight perception-action links, dissociations have also been reported. The prominent model of “vision-for-perception” and “vision-for-action” pathways [38,39] regards neuronal processing for perception and action to be largely separate in ventral and dorsal visual processing streams, respectively, although interactions between the two streams exist [38–40]. This model is based on decades of behavioral, neurophysiological, imaging and patient data comparing visual perception and goal-directed hand movements–reaching and grasping. Eye movements have classically been viewed as “information-seeking adjunct to visual perception” ([39] pp. 1567–1568). If perceptual reports and eye movements rely on the same processing mechanism and brain pathway, the two responses should be equally sensitive (same threshold) and highly correlated (same variability). However, recent research questions the tight coupling between perception and eye movements, with three main differences or dissociations emerging in studies simultaneously measuring perception (as explicit perceptual reports, indicating awareness) and eye movements (Table 1).
Table 1.
Behavioral studies revealing perception-eye movements differences, grouped by type of difference
| Study | Procedure (visibility manipulation) | Perceptual task | Eye movement | Evidence of perception-eye movements difference | |
|---|---|---|---|---|---|
| 1.1 VARIABILITY DIFFERENCES | |||||
| Gegenfurtner et al. [41] | subthreshold | 2AFC speed change discrimination; small dot | pursuit | perceptual and pursuit errors uncorrelated on a trial-by-trial basis; similar sensitivity in both responses | |
| Blum & Price [42] | subthreshold | continuous direction estimation; large random-dot pattern | OFR | perceptual and OFR errors uncorrelated on a trial-by-trial basis; similar sensitivity in both responses | |
| Price & Blum [43] | subthreshold | continuous direction estimation; OFR: large random-dot pattern; pursuit: small spot | OFR/pursuit | perceptual and OFR errors uncorrelated across observers, perceptual and pursuit errors correlated across observers | |
| 1.2 QUANTITATIVE DIFFERENCES | |||||
| Tavassoli & Ringach [44] | subthreshold | 2AFC speed discrimination of small spots | pursuit | pursuit more sensitive than perception; perceptual and pursuit errors uncorrelated | |
| Boström & Warzecha [45] | subthreshold | 2IFC speed discrimination; whole-screen random-dot pattern | OFR | perception more sensitive than OFR; perceptual and OFR errors uncorrelated | |
| Naber et al. [32] | binocular rivalry | 2AFC dominance judgment; two colored sinusoidal gratings with opposite direction and orientation | OKN | faster direction change in OKN than in perception | |
| Masson et al. [48] | brief dichoptic presentation, anticorrelated stimulus | 2AFC depth discrimination of large, dense random-dot patterns | vergence | patterns cannot be perceptually discriminated (humans) but elicit short-latency reflexive vergence eye movements (humans, monkeys) | |
| Van Zoest & Donk [49] | saccade-contingent masking of search display | 2AFC judgment of saccade correctness | saccades | no perceptual awareness of saccade goal despite correct saccadic selection | |
| Eggert et al. [50] | briefly flashed distractor | 2AFC target localisation | saccades | saccades land in between target and distractor (global effect); distractor does not affect perception | |
| Van der Stigchel et al. [51] | onset masking of distractor | 4AFC location of distractor | saccades | vertical saccade trajectories deviate away from unperceived distractor | |
| Zhaoping [52] | saccade-contingent masking of search display | 2AFC detection of orientation singleton in visual search | saccades | saccades to orientation singleton even though it was unperceived | |
| Rothkirch et al. [53] | CFS | localization and 2AFC orientation discrimination of a Gabor patch | saccades and fixations (dwell time) | saccades to unperceived (suppressed) Gabor patches | |
| 1.3 QUALITATIVE DIFFERENCES | |||||
| Glasser & Tadin [46] | surround suppression | 2AFC direction discrimination; small and large sinusoidal gratings | OFR | opposite spatial tuning in perception and OFR; small stimuli: perception > OFR; large stimuli: OFR > perception; perceptual and OFR errors uncorrelated | |
| Simoncini et al. [57] | subthreshold | 2IFC speed discrimination of textured patches | OFR | opposite sensitivity in perception and OFR in response to increasing spatial frequency; OFR pool across a wider range of frequency channels | |
| Spering & Gegenfurtner [58] | motion illusion | 2AFC speed discrimination of small dot surrounded by dynamic sinusoidal context | pursuit | perception follows relative motion of target and context, pursuit follows vector average of target and context | |
| Spering et al. [59] | monocular adaptation/Binocular rivalry flash suppression (BRFS) | continuous motion direction estimation of two dichoptically presented sinusoidal gratings | pursuit | perception follows component motion (horizontal/vertical), pursuit follows pattern motion (diagonal) | |
| Spering & Carrasco [60] | adaptation/BRFS | continuous motion direction estimation of two dichoptically presented sinusoidal gratings | pursuit | perception follows component, pursuit follows pattern motion; both responses biased in attended direction | |
| Badler et al. [47] | ambiguity in causal motion direction | 2AFC causality judgments of two colliding small spots | pursuit | opposite causal judgments in perception and pursuit; errors uncorrelated across and within observers | |
| Van der Steen & Dits [61] | dichoptic presentation creating different depth planes | continuous motion direction estimation of dichoptically presented random dot patterns | vergence | perception follows pattern motion, vergence is disjunctive and follows component motion with one eye tracking horizontal, the other eye vertical motion direction | |
| Kuhn & Land [62] | vanishing ball illusion | qualitative verbal assessment of perceived illusion | saccades and fixations | perception follows illusion, eye movements do not track illusory ball trajectory but remain fixated | |
Differences in variability
This section includes studies revealing differences in response variability between perception and the reflexive OFR or pursuit, despite similarities in sensitivity [41–43]. Blum and Price [42] used a continuous motion estimation task in which observers align an arrow shown on the screen with the motion direction of a large pattern of coherently moving random dots. This type of stimulus is known to produce a perceptual bias away from the reference (e.g., horizontal motion direction). The study reveals a similar bias in OFR; however, biases in perception and OFR are uncorrelated on a trial-by-trial basis, indicating a variability difference. In contrast, biases in perception and voluntary pursuit are correlated [43]. These results [42,43] indicate differences in motion processing between voluntary pursuit and reflexive OFR.
Variability differences also emerge when comparing perception and pursuit in response to brief speed changes [41] as well as in studies discussed below reporting quantitative differences [44,45] (Table 1.2) and qualitative differences [46,47] (Table 1.3). Variability differences are seemingly common in studies comparing perception and eye movements on a short timescale, either by examining reflexive OFR or by studying responses to brief speed perturbations in pursuit. These differences may rely on different sources of sensory and motor noise originating at different points along the sensorimotor processing hierarchy (see final section).
Quantitative differences
This section contains studies in which smooth tracking eye movements (smooth pursuit [44], OFR [45], OKN [32]), vergence [48] and saccades [49–53] are either more or less sensitive (including no response) than perceptual reports. Comparisons are mostly based on the analysis of detection or discrimination thresholds derived from psychometric and oculometric functions.
Pursuit
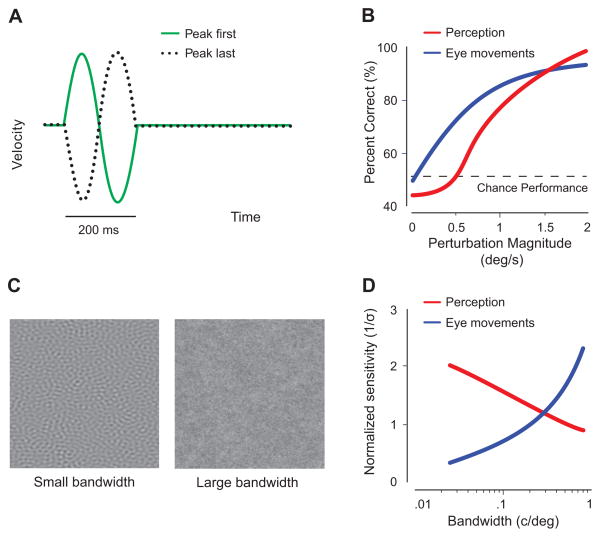
Consistent with an early report of superior sensitivity in pursuit [54], a recent study revealed more sensitive pursuit than perception when observers simultaneously track moving targets and discriminate changes in stimulus speed in a two-alternative forced choice (2AFC) task [44] (Fig. 1a). Eye velocity reflects fluctuations in stimulus speed, even when observers are unaware of the perturbation (Fig. 1b).
Figure 1. When eye movements and perception yield different results.
(A) Observers discriminated speed changes (perturbation magnitude between 0–2.5 deg/s). Stimulus speed either increased first and then decreased (peak first), or it decreased first and then increased (peak last); five speed perturbation magnitudes (in degrees per second) were tested. (B) Schematic of results from one observer. Pursuit eye movements were more sensitive to speed changes and discriminated between peak-first and peak-last changes reliably even when perception was at chance; data points below chance performance for perception, but above chance performance for pursuit. (C) OFR were elicited by large motion texture stimuli with varying spread of the spatial frequency distribution (bandwidth in cycles per degree) across trials. Eye movement velocity was compared to perceptual sensitivity in a speed discrimination task, in which mean spatial frequency and bandwidth were constant in a given trial but varied across trials. (D) Mean sensitivity results from three observers showing an opposite trend in OFR and perception. Eye movement sensitivity (inverse standard deviation of OFR velocity) increased, whereas perceptual sensitivity (inverse discrimination threshold) decreased with increasing bandwidth. [A and B adapted with the authors’ permission from Tavassoli and Ringach[44], Table 1.2; (C) and (D) adapted with the authors’ permission from Simoncini et al.[57], Table 1.3]
OKN
Many binocular rivalry studies have used reflexive OKN as a sensitive indicator of perceptual awareness, based on the assumption that eye movements reflect the dominant percept. However, two recent studies support quantitative differences between perception and action in rivalry by using OKN [32] and pupil dilation [32,33]. Whereas perceptual responses are typically “all-or-none” (observers see one stimulus or the other, except for occasional trials with “piecemeal” rivalry, where parts of both images are perceived), eye movement responses can reflect gradual differences in space and time. These studies found that transitions between perceptual states are reflected faster in eye movement responses than in perception. Pupil dilation and direction changes in slow-phase OKN velocity precede perceptual reports (awareness) by about half a second [33] and a second [32], respectively. Control experiments ruled out that this temporal lead is due to a faster response time of the eye vs. manual response. In consonance, frontal brain activity underlying binocular rivalry correlates better with eye movements than with perception [55].
Vergence
A study comparing humans and monkeys reports a quantitative difference between perception and vergence [48]. Random-dot patterns were shown separately to each eye through orthogonal polarizing filters. The patterns were anti-correlated (each black dot in one eye corresponded to a white dot in the other eye) and do not produce a consistent depth percept in humans. However, when small disparity steps (binocular misalignments) are induced these stimuli reliably elicit reflex-like vergence at ultra-short latencies even though observers are unaware of these changes.
Saccades
Notwithstanding similarities between perceptual and oculomotor target selection in paradigms such as visual search or under natural viewing conditions [17,21–24], some studies have revealed saccadic target selection in the absence of visual awareness [49–53]. For instance, in a visual search task [49] in which observers have to saccade to a salient object and ignore a distractor, correct saccades are made in about two-thirds of the trials and saccadic latencies are shorter in response to the target than to the distractor. However, observers are not perceptually aware of their saccadic choice, suggesting that low-level visual information can guide saccade target selection without awareness. These findings were confirmed by a subsequent study using continuous flash suppression, in which gaze is directed at a suppressed stimulus even though observers are not able to correctly locate it or discriminate its orientation, i.e., they are not aware of it [53]. Furthermore, saccades land in between a target and distractor (global effect), even when the distractor does not affect localization judgments [50], and they deviate away from unperceived distractors presented in the periphery [51]. Similar deviations in saccade trajectories were observed in blindsight patients when distractors are shown in the blind hemifield and thus do not reach awareness [12].
To conclude, quantitative differences between perception and eye movements reflect that, with one exception [45], voluntary and reflexive eye movements are more sensitive than perception. Eye movements may even respond to visual stimuli that are rendered perceptually invisible (i.e., observers are not aware of them) through rivalry or flash suppression, akin to blindsight [8,9]. These results are complemented by a clinical study of one-eyed (enucleated) observers showing that eye movements can be more resilient than perception: Whereas motion perception was severely impaired in these observers, the accuracy of their pursuit did not differ from that of two-eyed observers tested either monocularly or binocularly [56]. The studies reported here favor a model incorporating the idea that visual information for perception and eye movements is processed in different populations of neurons within the same area(s) or in different pathways (see final section).
Qualitative differences (dissociations)
Cases in which eye movements and perception follow different response directions, rather than just a weaker or absent response, have been reported for OFR [46,57], pursuit [47,58–60], vergence [61] and saccades [62].
OFR/pursuit
Reflexive OFR and perception seem to integrate motion information across different spatial frequency ranges. Simoncini et al. [57] elicited reflexive OFR using large motion texture patches of constant speed but variable spatial frequency distribution (Fig. 1c). Larger bandwidth (‘richer’ stimuli, similar to natural scenes) results in stronger, less variable OFR and higher OFR sensitivity (Fig. 1d). In contrast, when discriminating the speed of a test stimulus that moves either slower or faster than a reference, observers’ perceptual sensitivity decreases with increasing spatial-frequency bandwidth (Fig. 1d). Eye movement responses integrate motion information across a wider range of spatiotemporal frequencies than do perceptual responses.
The oculomotor system also seems to integrate motion information across a larger spatial range than the perceptual system. Glasser and Tadin [46] asked observers to discriminate the motion direction of small (1° radius) and large (8° radius) sinusoidal luminance gratings. For small stimuli, perceptual discrimination is more reliable than discrimination by the elicited OFR. In contrast, for large stimuli, perceptual performance deteriorates, due to spatial suppression, while oculomotor performance improves, indicating spatial summation.
A qualitative difference between perception and pursuit was first reported when observers were asked to track a small, central moving target and ignore the motion of a peripheral context. Spering and Gegenfurtner [58] showed that context motion in the periphery affected perception and pursuit differently: While perception followed the relative motion of target and context, eye movements followed the vector average.
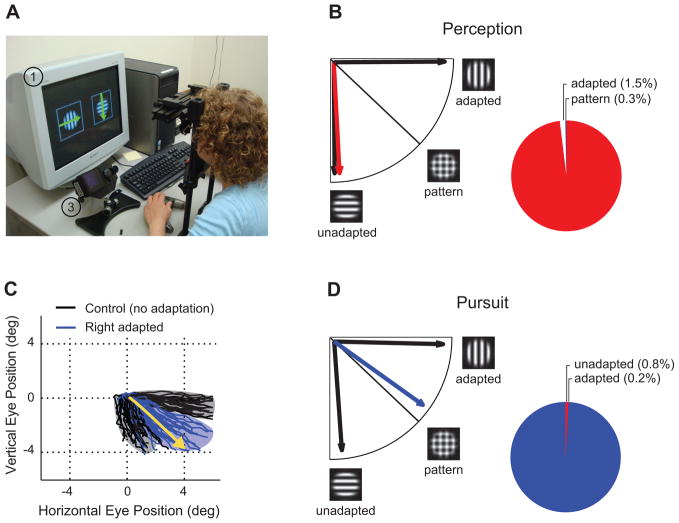
In a novel procedure developed by Spering and Carrasco [59,60], observers viewed a pattern that consisted of two superimposed gratings moving in two different directions, one horizontal and one vertical. The two gratings were shown separately to each eye, creating a dichoptic plaid (Fig. 2a, see also cover image). The strength of each grating was manipulated through monocular adaptation, similar to binocular rivalry flash suppression. Perceptual reports of the plaid’s motion direction followed the unadapted grating exclusively (e.g., vertical downward motion in Fig. 2b, c), indicating that observers were aware of such stimulus. The eyes, however, did not follow the perceived direction but tracked the vector average of both gratings (pattern motion), disregarding the perceived strength of each individual component (Fig. 2c, d).
Figure 2. When eye movements track unperceived motion.
(A) Set-up (1) Display monitor; left stimulus shows adapted motion direction, right stimulus shows unadapted motion direction; green arrows for illustration purposes only. (2) A 4-mirror stereoscope to present separate images to the two eyes. (3) Eye tracker recording binocular eye movements. (4) Track-ball mouse used to indicate perceived motion direction [right hand partially blocked by stereoscope]. (B) Perceived motion direction when rightward motion is adapted and downward motion is unadapted (red) or when both stimuli move rightward or downward as shown in control conditions (black). Pie chart indicates percent of total responses (pattern response and adapted response only happened in <1% of trials each). (C) Individual eye position traces for one representative observer; blue traces are for rightward motion adapted and downward motion unadapted, black traces are for control conditions (both stimuli to right or down, respectively). (D) Pursuit direction when rightward motion is adapted and downward motion is unadapted (blue) or in control conditions (black). Pie chart indicates percent of total responses (adapted responses and unadapted responses only happened in <1% of trials each). Eye movements were recorded binocularly and both eyes showed the same vector-averaging response. [Adapted from Spering et al. [59], Table 1.3]
The dissociation between perception and pursuit, which indicates that pursuit can track motion signals that do not reach awareness, was replicated under feature-based attention [60]. Observers were asked to attend to the motion direction of either the adapted (unaware) or the unadapted (aware) grating. Perception and pursuit were both shifted towards the attended motion direction regardless of whether the observers had adapted or not to the attended stimulus. Hence, feature-based attention affected perception and pursuit similarly, even though the former reflects awareness whereas the latter does not.
Perceptual judgments of causality and predictive pursuit responses [47] illustrate another qualitative difference. When observers judge a target’s motion direction (the ‘reaction’ target) as either caused or not caused by a colliding first stimulus (the ‘launcher’ target), perception and predictive pursuit show a similar preference for the “causal” motion direction, i.e. the motion angle that follows the physical laws of a collision event. When a time delay between collision and target motion is introduced, experienced observers base their judgments on direction information whereas naïve observers classify trials based on time information (as “non-causal”). Critically, whereas perception was strongly influenced by individual differences and seemed to rely on heuristic shortcuts, predictive pursuit consistently tracked causal motion, illustrating that pursuit can respond to aspects of stimulus motion that do not reach awareness.
Vergence
An opposite dissociation to those reported in Spering and Carrasco’s studies [59,60] (Fig. 2) occurs between vergence and motion perception when stimulus components are shown dichoptically in different depth planes [61]. When presenting two orthogonally oscillating random-dot patterns (horizontal and vertical motion directions) separately to the two eyes using red and green filters, observers perceived diagonal motion direction, i.e., the vector sum of the two images, as indicated in a continuous motion estimation task. However, each eye followed the direction of the stimulus motion presented to that eye; one eye tracked horizontal motion, the other eye tracked vertical motion. The finding of independent left and right eye movements violates the assumption that movements between the two eyes should be coupled and aimed at the same point in space, creating binocular retinal correspondence. These results may emerge because dichoptic presentation with red and green filters yields a stimulus with different depth planes, in which both components are equal in perceptual strength. Similar stimuli reliably elicit vergence even in the absence of a corresponding depth percept [48].
Saccades
Kuhn and Land [62] discovered an impressive qualitative difference between perception and saccades by showing observers a magician’s trick–the vanishing ball illusion: The magician pretends to throw a ball up in the air and uses social cues–following the imagined ball trajectory with his head and eyes–while the ball remains in his hand. Observers’ perception is driven by social cues–they report seeing the ball flying through the air–but their fixations are mostly on the magician’s face. Interestingly, observers report that their eyes are always on the ball. In contrast, when the ball is actually thrown observers’ fixations are on the ball at the peak of its trajectory. These results indicate that cognitive expectations alter perception, whereas eye movements are not deceived; instead, they more accurately reflect low-level visual information.
In sum, in studies reporting dissociations, perception and eye movements follow opposite motion directions or response patterns. These studies support the hypothesis of partly separate pathways for conscious perceptual reports and eye movements to visual stimuli of which the observers are not aware (see last section).
When do differences and dissociations emerge?
To conclude, the three groups of perception-eye movement differences discussed (Table 1) are not uniquely driven by the type of eye movement, task or stimulus used. However, certain configurations enable the emergence of differences and dissociations:
Eye movements: Reflexive OFR, predictive or short-scale pursuit eye movements are more prone to variability differences [41–47] than longer-scale pursuit in direction discrimination tasks [43,63].
Task: Most studies reporting similarities in sensitivity between perception and pursuit used 2AFC methods for perceptual judgments [27,41,63]. Interestingly, all studies reporting quantitative differences [32,44,45,48–53] also used alternative forced-choice methods (2AFC, 4AFC or 2IFC). In contrast, several studies with dissociations used continuous estimation tasks in perception [59–62].
Stimulus: Ambiguity in visual information, either through the use of dichoptically presented plaids, random-dot patterns [59–61] or illusory motion [47,58,62], as well as large stimulus size [46] and bandwidth [57] can give rise to qualitative dissociations.
Structural and physiological bases underlying perception-eye movement differences
What brain machinery could underlie both similarities and differences between perception and eye movements? Whereas variability differences between perception and eye movements can be reconciled with a model of similar processing mechanisms along shared brain pathways, quantitative and qualitative differences indicate separate pathways, neuronal populations, or mechanisms.
Same brain pathway
There is strong evidence for shared brain pathways for pursuit and motion perception [14,16]. The classical assumption is that the separation of pathways for perception and pursuit occurs downstream from visual areas, closer to the motor output stage. Studies reporting differences in variability (Table 1.1) are consistent with the idea of processing along the same pathway and postulate the existence of different sources of sensory and motor noise within this pathway, motion processing in different subpopulations of neurons, or different thresholds or decision criteria in perception and eye movements.
Different sources of noise
Variability similarities between the precision of sensory coding and the precision of pursuit/OFR have been taken to indicate that both systems rely on similar visual signals and are limited by the same neuronal noise. There is, however, a debate over whether perception and eye movements rely on shared or separate sources of noise. Some authors suggest that perception and the earliest, visually-driven phase of pursuit depend almost exclusively on noise in the sensory representation of motion signals in area MT [14,64]. Others affirm that motor noise can account for up to 50% of pursuit variability, even during pursuit initiation [65].
Studies reporting differences in variability [41–47] (Table 1) indicate that perception and eye movements likely rely on at least partly separate sources of noise. Because these studies focus on later time intervals of the eye movement response and/or transient changes in ongoing pursuit velocity, they are not necessarily in conflict with the idea that visually-driven eye movements may be guided by some amount of shared sensory noise. Common sensory noise may affect both perception and pursuit, but additional motor noise may be added to oculomotor areas downstream from visual areas (e.g., frontal eye field, cerebellum [14]). This explanation could account for results in studies finding differences in variability despite similar sensitivity [41–43].
Different subpopulations of neurons
Alternatively, different subpopulations of MT neurons may carry motion signals with different signal-to-noise ratios. Neurophysiological studies in monkeys have described MT neurons with different receptive field properties [66,67]. One type of neurons responds to wide-field motion stimuli that extend beyond the area of the classical receptive field, indicating an excitatory surround. Another type does not respond to these stimuli, indicating an inhibitory surround. Excitatory and inhibitory center-surround interactions may be related to the processing of global and local motion, respectively [68]; surround-suppressed MT neurons also integrate motion signals faster and respond better to briefly shown visual stimuli than non-surround suppressed neurons [69].
Such differences in receptive field properties in MT neurons could also underlie some of the quantitative and qualitative differences between perception and pursuit/OFR (Table 1.2–1.3). For instance, studies reporting opposite spatial or spatial frequency tuning in perception and OFR find superior sensitivity (lower threshold) in eye movements in response to larger stimuli [46] and stimuli with larger spatial frequency bandwidth [57]. Different subpopulations of MT neurons–surround-suppressed for perception and non-surround suppressed for OFR [58,70]–could explain these effects. According to this model, motion perception and eye movements rely on the same source of visual information but may integrate it differently through an adjustable, task-dependent tuning [57] in different subpopulations of neurons, potentially with different signal-to-noise ratios [44]. Such a model would reconcile similarities in perception and eye movements in behavior and neurophysiology with the differences and dissociations discussed here.
Different response thresholds or decision signals
Accumulate-to-threshold models have provided explanations based on differences in decision signal or response threshold (lower for eye movements than for perception [16]); these models could potentially explain quantitative differences in response sensitivity. However, this view is too simplistic and cannot explain the diverse differences discussed here, particularly those qualitative differences in which effects go in different directions. Underlying mechanisms likely include differences in neuronal decoding efficiency, firing rate, and/or in the way information is integrated or normalized [57].
Different brain pathways
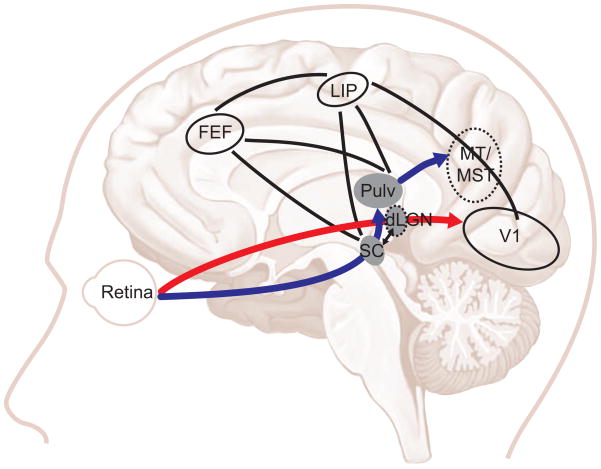
The main brain pathway for visual information processing, the retino-geniculate pathway (red arrow, Fig. 3), has been well characterized: the dorsal lateral geniculate nucleus (dLGN) of the thalamus receives information from retinal ganglion cells and projects mostly to primary visual cortex (V1). V1 sends feedback projections to the thalamus [6,7] and feedforward projections to higher-level visual areas, such as MT/MST, feeding into the dorsal or posterior parietal processing streams and into key fronto-parietal areas for oculomotor control [71]. Visual information for perception and eye movements could be processed largely in parallel, with separation into perceptual awareness and motor output control happening downstream from visual areas. However, quantitative and qualitative differences (Table 1.2–1.3) suggest that visual information is processed more readily for eye movements than for explicit perception, and that pathways may thus separate at an earlier stage of visual processing.
Figure 3. Brain pathways for eye movements to unaware visual information.
Two separate but interconnected pathways may mediate visual information for perception (retino-geniculate pathway, red) and eye movements in the absence of awareness (retino-collicular pathway, blue). In the retino-geniculate pathway, the dLGN transmits visual information from retina to V1; V1 then projects to higher-order visual and visual-motor areas in occipital, parietal and frontal cortex as well as to subcortical areas in midbrain and brainstem. The retino-collicular pathway directly connects the retina to the SC and pulvinar, thence to visual cortex, forming a subcortical route that can bypass the dLGN and V1. The dorsal pulvinar connects directly to cortical areas involved in eye movement control, such as frontal eye field (FEF) and lateral intraparietal sulcus (LIP). Structures that are not in the mid sagittal plane are indicated by dotted outlines; area illustration does not correspond to exact anatomical size and location; only feedforward connections are shown and not all areas implicated in oculomotor control are shown.
Dorsal vs. ventral visual processing streams
Some of the studies finding saccade/perception differences [49,53] refer to the dual-pathway model [38,39] as an explanation. This model proposes largely independent neuronal processing for perception and motor action in a ventral processing stream projecting to the inferotemporal cortex and a dorsal stream, projecting to the posterior parietal cortex, respectively. A separation between the two streams, where ‘vision-for-perception’ information is more accessible to awareness than is ‘vision-for-action’ information [72], could underlie perception-eye movement differences. However, there are known interconnections between some areas in the two streams [38–40]. Moreover, given the involvement of MT–located in the dorsal processing stream–for both perception and pursuit, these processing streams are unlikely to be the neural correlate for differences between perception and pursuit/OFR.
Subcortical pathways
Involvement of the retino-collicular pathway (blue arrows, Fig. 3) is a more likely explanation for why eye movements reveal a more sensitive processing of information, even when observers are unaware of it. This pathway directly connects the retina to superficial layers of the superior colliculus (SC); projections also go to the brainstem through the nucleus of the optic tract (NOT) and to the inferior (ventral) part of the pulvinar nucleus of the thalamus.
Because regions in this pathway (SC, NOT, pulvinar) have direct connections with area MT [7,71], providing fast transmission of retinal input to dorsal visual cortex [7,73] and mediating pursuit control and target selection [74], Spering and Carrasco [59,60] proposed that this pathway may also underlie pursuit eye movements’ responses to visual information of which the observers are not aware. Subsequent studies on perception-eye movement differences agree with this view [46,53]. Indeed, the fast transmission time from SC through pulvinar to MT via this pathway (~5 ms [73]) may mediate fast visual processing for the control of eye movements whereas readout for perception may take longer, or may occur at higher decision areas, enabling the differences and dissociations described in Table 1.2–1.3.
Psychophysical and neuroimaging studies in humans, neurophysiological studies in monkeys, and clinical studies in patients with awareness deficits have provided evidence for an involvement of this pathway in visual processing without awareness; however, there is an ongoing debate regarding exact role of areas such as the pulvinar and dLGN [75]. We cannot provide an in-depth review of this literature here; instead, we focus on representative studies illustrating recent progress in this topic.
The retino-collicular pathway is associated with residual visual abilities in blindsight [8–11] and with the translation of unperceived visual signals into oculomotor outputs in these patients. In these studies, blindsight patients can be prompted with a visual cue presented in an area of normal vision or by a sound to shift their gaze to unperceived but salient stimuli in the blind hemifield. (A recent study in monkeys has also revealed involuntary gaze shifts to objects in the blind hemifield during free viewing [76]). Blindsight patients with occipital cortex lesions and relative sparing of retino-collicular function exhibit saccade trajectories that deviate away from blind-field distractors [12]. Together with a parallel study in healthy observers [51], this suggests an involvement of the retino-collicular pathway in the processing of unperceived visual information for eye movements. This pathway is also implicated in blindsight studies assessing hand movements–reaching [77] and pointing [78].
The involvement of the SC in blindsight was confirmed by a study reporting that an unperceived stimulus, presented to the blind hemifield of a patient with unilateral blindsight, can quicken reaction times and enhance pupillary constriction when a perceived stimulus is presented simultaneously to the intact hemifield [13]. Critically, this effect was found for gray stimuli, which elicit SC activation, but not for purple stimuli, which are invisible to the SC due to its insensitivity to S-cone input (although the lack of S-cone input to the SC has been questioned, see [79]). The authors suggested that unperceived, gray stimuli may mediate perceptual and motor responses through direct projections via the SC.
Damage to the pulvinar has also been implicated in visual-spatial neglect [80], a frequent and disabling deficit of awareness. In visual-spatial neglect, objects and events in the contralesional visual hemifield are ignored or even unperceived and patients exhibit biased eye and head orientation towards the ipsilesional hemisphere [81]. There is debate over whether these patients show similar ‘blindsight’-like effects in perception or eye movements: On the one hand, distractors presented in the contralesional (neglected) hemifield do not affect saccade latencies to targets in the ipsilesional field [81–83], in contrast to what has been reported for blindsight [12]. On the other hand, saccades to targets in the neglected hemifield may be preserved even in the absence of awareness (as assessed in a verbal detection task) [83]. Selective inactivation of the pulvinar in monkeys also causes neglect symptoms [84]. Moreover, when monkeys are trained to report the visibility of a small, high-contrast target (by releasing a lever), changes in the spiking rate of pulvinar neurons reflect their perceptual awareness of the stimulus. In contrast, spiking activity of the LGN is solely driven by the physical presence or absence of the stimulus [85].
However, human neuroimaging during a binocular rivalry task suggests involvement of the dLGN in the processing of visual information of which observers are not aware [86,87]. Although these neuroimaging results could be caused by cortical feedback the idea that dLGN is a critical relay in visual processing without awareness is supported by a study in monkeys with chronic V1 lesions resulting in blindsight: Residual visual functions in their blind hemifield and neuronal activity in extrastriate areas, assessed by fMRI, are both eliminated by temporary dLGN inactivation in the V1-lesioned hemisphere [88]. These results agree with studies proposing that visual information is also transmitted to extrastriate areas directly from the dLGN, bypassing V1 [89].
In sum, retino-collicular and/or geniculo-extrastriate projections could provide rapid processing of visual information for motor actions in the absence of perceptual awareness. Furthermore, the retino-collicular pathway and its amygdala connection are associated with the rapid processing of emotional information and with primates’ heightened visual sensitivity to evolutionarily salient stimuli such as faces and snakes [90]. A subcortical route could thus provide a short-cut to drive motor actions, such as fast orienting eye movements to targets of interest, before visual information reaches awareness.
Conclusion
This review emphasizes that differences and dissociations between perception and eye movements are not the exception, especially when visual information is ambiguous, and when reflexive or short-scale eye movements are assessed with continuous estimation tasks. In these situations, observers’ eye movements demonstrate that they are processing visual information even when they are not aware of it.
Perception/eye movement dissociations may be adaptive responses to different task requirements and to diverse ecological demands. The motor system’s access to visual information that does not reach awareness may help manage limited bioenergetic resources [90]; it may also allow humans to act fast in fight or flight situations. On the one hand, eye movements rely mainly on estimation and are updated continuously. They provide a fast orienting response to information of which observers may be perceptually unaware; they may also respond faster than perception. They integrate information across space [46,50], a large range of spatial frequencies [57], and local motion directions (i.e., vector averaging [14,27,58–60]). On the other hand, the perceptual system needs to reliably discriminate or categorize visual information. Perceptual decisions are discrete; they may be based on visual signals at a specific moment in time or averaged across the whole presentation time and beyond. They are more prone to spatial suppression [46] and are constrained to a smaller spatial [50] and spatial-frequency range [57]. These differences in how information is processed across space, spatial frequencies, and time are crucial methodological factors to consider when directly comparing perception and eye movements [16,92].
Although there are still many unanswered questions about the origin, nature and functional significance of differences and dissociations between perception and eye movements (see Box 1), converging evidence suggests that the classical view of a tight link between perception and eye movements should be revised. Eye movements are often more sensitive than perception and may serve as an indicator of visual processing without awareness. Both reflexive and voluntary oculomotor responses can be earlier, faster and more accurate predictors of the way we locate and track events in the visual world than perceptual reports. Such differences can provide important insights into brain function and merit further investigation.
Box 1. Outstanding questions and future research directions.
Our understanding of perception/action will be further advanced by the integration of different levels of analysis and methodologies in both healthy and clinical populations. Ideally, future studies will shed light on these issues combining knowledge gathered from psychophysics, single-unit neurophysiology, neuroimaging, and computational techniques. Here we present some possible future directions in and approaches to the investigation of eye movements as an objective indicator of unperceived information that does not reach awareness.
Systematic investigations of which tasks, procedures and stimulus properties yield dissociations between perception and eye movements. Patterns may be identified by holding one property constant (e.g., same task) while systematically varying the other (e.g., different types of eye movements).
Preliminary evidence indicates that quantitative differences between perception and eye movements may also extend to non-visually triggered, reflexive eye movements, such as the vestibulo-ocular reflex (VOR) [93]. Conclusive interpretation of these findings awaits studies comparing perception and VOR in the same experiment and the same species.
Neurophysiological studies have identified MT neurons with different center-surround properties [66–69]. Several paradigms presented in our review lend themselves to the examination of perception-action difference using motion stimuli that are known to modulate MT neurons with excitatory and inhibitory receptive field properties (akin to studies identifying MT pattern vs. component cells [31,94]). For instance, Glasser and Tadin [46] and Spering and Gegenfurtner [58] used stimuli that could differentially activate either type of neuron.
There are still many unanswered questions regarding our understanding of the neuronal mechanisms underlying blindsight [75]. A novel combination of blindsight with suppression procedures (rivalry or adaptation), in which either the dominant/unadapted or suppressed/adapted stimulus is presented in the blind field, could provide insight into the processing mechanisms in blindsight. Effects on perception and eye movements could be compared.
Assessments of behavioral effects on eye movements and perception while characterizing single-unit activity. Basic electrophysiology studies are needed that link mechanistic properties of neurons along the retino-collicular pathway (i.e., to identify activity in different subpopulations of neurons) to perceptual and oculomotor processing. This research could be complemented by neuroimaging studies in humans to reveal activity in these pathways [95].
The pulvinar, which lies along the retino-collicular pathway associated with the processing of unaware visual information, also seems to play a critical role in visual attention [96,97]. Thalamic nuclei such as pulvinar and dLGN could be areas of interest in future behavioral and neuroimaging studies in healthy populations, to assess the interplay between attention and awareness [60]. Targeting the pulvinar and dLGN through a combination of psychophysics, neuroimaging and electrophysiology may also shed light on their connection to cortex–bottom-up projections and top-down inputs–and their role in blindsight.
A recent study has shown that feature-based attention affects both perceptual reports and eye movements similarly, even when qualitatively dissociated [60]. Further investigating whether and how spatial and feature-based attention [98–100] affect perceptual reports, assessed by different tasks, and different eye movements, would help probe situations in which visual processing could potentially lead to awareness.
Highlights.
We review differences and dissociations between visual perception and eye movements
These differences can be in response variability, magnitude (quantitative) or direction (qualitative dissociations)
Eye movements can be sensitive to visual features that fail to reach awareness
We discuss the possible role of subcortical pathways for visual processing without awareness
Acknowledgments
Both authors were supported by an International Visiting Research Award from the Peter Wall Institute for Advanced Studies (UBC) for MC to visit UBC. MS was supported by an NSERC Discovery Grant and by the Canada Foundation for Innovation John R. Evans Leadership Fund. MC was supported by NIH R01 EY016200. The authors thank Davis Glasser, Krystel Huxlin, Anna Montagnini, Antoni Valero-Cabré, and members of the Carrasco lab –Anasuya Das, Rachel Denison, Bonnie Lawrence, Hsin-Hung Li, Sarit Szpiro– for comments on an earlier version of this manuscript and Guillaume Masson for his contribution to Figure 1.
Glossary
Procedures to manipulate stimulus visibility
- Adaptation
Prolonged viewing of an image resulting in decreased sensitivity to the adapted stimulus during subsequent viewing.
- Binocular rivalry
When two different images are projected to corresponding retinal areas of the two eyes, observers report that the images alternate at a random rate, with one image dominant and the other suppressed, rather than fused into a coherent percept. Some of the physical visual information does not reach awareness, while the rest does, dissociating physical stimulation and awareness.
- Binocular rivalry flash suppression
Variation of binocular rivalry; one image is shown to one eye for a prolonged period of time (monocular adaptation), followed by a test period, during which the adapted eye sees the same stimulus as during adaptation and the unadapted eye sees a novel stimulus. The timing of awareness periods are under the experimenter’s control.
- Continuous flash suppression (CFS)
Procedure in which one eye is presented with a static stimulus, while the other eye sees a series of distinct images flashing successively at ~10 Hz. The dynamic stimuli suppress the perception of the static stimulus longer and deeper than binocular rivalry.
- Spatial suppression
Reduction in the activity of a neuron in response to a stimulus outside its classical receptive field.
Eye movements
- Microsaccades
Largest (<1°) and fastest fixational eye movement, occurring a couple of times per second. They are involuntary, we are generally unaware of their existence, but they are thought to play a functional role in visual perception/cognition.
- Ocular following response (OFR)
Reflexive, smooth tracking movement in response to sudden-onset, large-field, rapid stimulus motion; characteristic short latency (humans:~85ms).
- Optokinetic nystagmus (OKN)
Involuntary tracking movement evoked by large-field visual motion. Smooth tracking in the direction of stimulus motion (slow phase) alternates with fast backward saccades (quick phases) to reset the eye.
- Saccades
Discrete, ballistic movements that direct the eyes quickly toward a visual target. Smooth pursuit eye movements: Continuous, slow movements that keep the eyes close to a moving visual target.
- Vergence
Movements that rotate the eyes simultaneously in opposite directions to direct the fovea of both eyes at objects of interest located at different distances relative to the observer.
- Vestibuloocular reflex (VOR)
Compensates for head motion and is evoked by signals arising in the semicircular canals in the inner ear.
Brain structures for unaware processing of visual information
- Dorsal lateral geniculate nucleus (dLGN)
Thalamic nucleus that transmits visual signals from the retina to V1 along the retino-geniculate pathway, as well as directly to extrastriate areas, bypassing V1 through SC and pulvinar. Feedback connections from V1 and brainstem modulate information processing in the dLGN.
- Pulvinar nucleus
Largest nucleus of the human thalamus, processes visual information and lies posterior, medial and dorsal to the LGN; strongly connected to visual cortex.
- Superior colliculus (SC)
Multilayered brainstem structure on the roof of the midbrain; plays a major role in the control of eye movements. It receives direct projections from retinal ganglion cells and conveys information to V1 through dLGN and to extrastriate visual areas through pulvinar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Miriam Spering, University of British Columbia, Dept. Ophthalmology & Visual Sciences and Centre for Brain Health, 818 W10th Avenue, Vancouver, BC V5Z 1M9, Canada
Dr. Marisa Carrasco, New York University, Dept. Psychology and Center for Neural Science, 6 Washington Pl, New York, NY 10003, USA
References
- 1.Kowler E, et al. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- 2.Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 3.Rolfs M, et al. Predictive remapping of attention across eye movements. Nat Neurosci. 2011;14:252–256. doi: 10.1038/nn.2711. [DOI] [PubMed] [Google Scholar]
- 4.Rolfs M, Carrasco M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. J Neurosci. 2012;32:13744–13752. doi: 10.1523/JNEUROSCI.2676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White A, et al. Adaptive deployment of spatial and feature-based attention before saccades. Vision Res. 2013;85:26–35. doi: 10.1016/j.visres.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurtz RH, et al. Thalamic pathways for active vision. Trends Cogn Sci. 2011;15:177–184. doi: 10.1016/j.tics.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoerig P. Varieties of vision: from blind responses to conscious recognition. Trends Neurosci. 1996;19:401–406. doi: 10.1016/S0166-2236(96)10051-5. [DOI] [PubMed] [Google Scholar]
- 9.Kentridge RW. Blindsight. In: Nadel L, editor. The Encyclopaedia of Cognitive Science. Nature Publ. Group; 2012. pp. 390–397. [Google Scholar]
- 10.Huxlin KR, et al. Perceptual relearning of complex visual motion after V1 damage in humans. J Neurosci. 2009;29:3981–3991. doi: 10.1523/JNEUROSCI.4882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, et al. Different properties of visual relearning after damage to early versus higher-level visual cortical areas. J Neurosci. 2012;32:5414–5425. doi: 10.1523/JNEUROSCI.0316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Stigchel S, et al. The influence of ‘blind’ distractors on eye movement trajectories in visual hemifield defects. J Cogn Neurosci. 2008;20:2025–2036. doi: 10.1162/jocn.2008.20145. [DOI] [PubMed] [Google Scholar]
- 13.Tamietto M, et al. Collicular vision guides nonconscious behavior. J Cogn Neurosci. 2010;22:888–902. doi: 10.1162/jocn.2009.21225. [DOI] [PubMed] [Google Scholar]
- 14.Lisberger SG. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron. 2010;66:477–491. doi: 10.1016/j.neuron.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowler E. Eye movements: the past 25 years. Vision Res. 2011;51:1457–1483. doi: 10.1016/j.visres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spering M, Montagnini A. Do we track what we see? Evidence for common and independent processing of motion information for perception and smooth pursuit eye movements. Vision Res. 2011;51:836–852. doi: 10.1016/j.visres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Schütz AC, et al. Eye movements and perception: a selective review. J Vision. 2011;11(5):9, 1–30. doi: 10.1167/11.5.9. [DOI] [PubMed] [Google Scholar]
- 18.Spering M, Gegenfurtner KR. The analysis of visual motion and smooth pursuit eye movements. In: Chubb C, editor. Human information processing: vision, memory, and attention. Ch 3. APA Press; 2013. [Google Scholar]
- 19.Rolfs M. Microsaccades: small steps on a long way. Vision Res. 2009;49:2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Conde S, et al. The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat Rev Neurosci. 2013;14:83–96. doi: 10.1038/nrn3405. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein MP, et al. Similar neural representations of the target for saccades and perception during search. J Neurosci. 2007;27:1266–1270. doi: 10.1523/JNEUROSCI.3975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomplun M, et al. The effect of task difficulty on visual search strategy in virtual 3D displays. J Vision. 2013;13(3):24, 1–22. doi: 10.1167/13.3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruno N, et al. A metaanalysis of the effect of the Müller-Lyer illusion on saccadic eye movements: no general support for a dissociation of perception and oculomotor action. Vision Res. 2010;50:2671–2682. doi: 10.1016/j.visres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Fracasso A, et al. Fooling the eyes: the influence of a sound-induced visual motion illusion on eye movements. PLoS One. 2013;8(4):e62131. doi: 10.1371/journal.pone.0062131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, et al. Visibility states modulate microsaccade rate and direction. Vision Res. 2009;49:228–236. doi: 10.1016/j.visres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonneh YS, et al. Motion-induced blindness and microsaccades: cause and effect. J Vision. 2010;10(14):22, 1–15. doi: 10.1167/10.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu YQ, Lisberger SG. Sensory versus motor loci for integration of multiple motion signals in smooth pursuit eye movements and human motion perception. J Neurophysiol. 2011;106:741–753. doi: 10.1152/jn.01025.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szpiro SFA, et al. Perceptual learning modifies untrained pursuit eye movements. J Vision. 2014;14(8):8, 1–13. doi: 10.1167/14.8.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logothetis NK, Schall JD. Binocular motion rivalry in macaque monkeys: Eye dominance and tracking eye movements. Vision Res. 1990;30:1409–1419. doi: 10.1016/0042-6989(90)90022-d. [DOI] [PubMed] [Google Scholar]
- 30.Sun F, et al. Multidirectional shifts of optokinetic responses to binocular-rivalrous motion stimuli. Brain Res. 2002;944:56–64. doi: 10.1016/s0006-8993(02)02706-3. [DOI] [PubMed] [Google Scholar]
- 31.Tailby C, et al. Binocular integration of pattern motion signals by MT neurons and by human observers. J Neurosci. 2010;30:7344–7349. doi: 10.1523/JNEUROSCI.4552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naber M, et al. Perceptual rivalry: reflexes reveal the gradual nature of visual awareness. PLoS One. 2011;6:e20910. doi: 10.1371/journal.pone.0020910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahle MW, et al. How much of the “unconscious” is just pre-threshold? Front Hum Neurosci. 2011;5:120. doi: 10.3389/fnhum.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi R, Tanifuji M. Which image is in awareness during binocular rivalry? Reading perceptual status from eye movements. J Vision. 2012;12:5, 1–11. doi: 10.1167/12.3.5. [DOI] [PubMed] [Google Scholar]
- 35.Blake R, et al. Can binocular rivalry reveal neural correlates of consciousness? Phil Trans R Soc B. 2014;369:20130211. doi: 10.1098/rstb.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandberg K, et al. Measuring consciousness: is one measure better than the other? Conscious Cogn. 2010;19:1069–1078. doi: 10.1016/j.concog.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Yang E, et al. On the use of continuous flash suppression for the study of visual processing outside of awareness. Front Psychol. 2014 doi: 10.3389/fpsyg.2014.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Op Neurobiol. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Goodale MA. Transforming vision into action. Vision Res. 2011;51:1567–1587. doi: 10.1016/j.visres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Yeatman JD, et al. The vertical occipital fasciculus: A century of controversy resolved by in vivo measurements. Proc Nat Acad Sci. 2014;111:5214–5223. doi: 10.1073/pnas.1418503111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gegenfurtner KR, et al. A comparison of pursuit eye movement and perceptual performance in speed discrimination. J Vision. 2003;3:865–876. doi: 10.1167/3.11.19. [DOI] [PubMed] [Google Scholar]
- 42.Blum J, Price NS. Reflexive tracking eye movements and motion perception: one or two neural populations? J Vision. 2014;14(3):23, 1–12. doi: 10.1167/14.3.23. [DOI] [PubMed] [Google Scholar]
- 43.Price NS, Blum J. Motion perception correlates with volitional but not reflexive eye movements. J Neurosci. 2014;277:435–445. doi: 10.1016/j.neuroscience.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 44.Tavassoli A, Ringach D. When your eyes see more than you do. Curr Biol. 2010;20:R83–R94. doi: 10.1016/j.cub.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boström KJ, Warzecha AK. Open-loop speed discrimination performance of ocular following response and perception. Vision Res. 2010;50:870–882. doi: 10.1016/j.visres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Glasser DM, Tadin D. Modularity in the motion system: independent oculomotor and perceptual processing of brief motion stimuli. J Vision. 2014;14(3):28, 1–13. doi: 10.1167/14.3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badler JB, et al. Divergence between oculomotor and perceptual causality. J Vision. 2012;12(5):3, 1–15. doi: 10.1167/12.5.3. [DOI] [PubMed] [Google Scholar]
- 48.Masson GS, et al. Vergence eye movements in response to binocular disparity without depth perception. Nature. 1997;389:283–286. doi: 10.1038/38496. [DOI] [PubMed] [Google Scholar]
- 49.Van Zoest W, Donk M. Awareness of the saccade goal in oculomotor selection: Your eyes go before you know. Conscious Cogn. 2010;19:861–871. doi: 10.1016/j.concog.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Eggert T, et al. Differential effect of a distractor on primary saccades and perceptual localization. Vision Res. 2002;42:2969–2984. doi: 10.1016/s0042-6989(02)00392-9. [DOI] [PubMed] [Google Scholar]
- 51.Van der Stigchel S, et al. Eye cannot see it: the interference of subliminal distractors on saccade metrics. Vision Res. 2009;49:2104–2109. doi: 10.1016/j.visres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Zhaoping L. Gaze capture by eye-of-origin singletons: Interdependence with awareness. J Vision. 2012;12(2):17, 1–22. doi: 10.1167/12.2.17. [DOI] [PubMed] [Google Scholar]
- 53.Rothkirch M, et al. A direct oculomotor correlate of unconscious visual processing. Curr Biol. 2012;22:R514–R515. doi: 10.1016/j.cub.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 54.Mack A, et al. Smooth pursuit eye movements: is perceived motion necessary? Science. 1979;2:1361–1363. doi: 10.1126/science.424761. [DOI] [PubMed] [Google Scholar]
- 55.Frässle S, et al. Binocular rivalry: frontal activity relates to introspection and action but not to perception. J Neurosci. 2014;34:1738–1747. doi: 10.1523/JNEUROSCI.4403-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.González EG, et al. Unaffected smooth pursuit but impaired motion perception in monocularly enucleated observers. Vision Res. 2014;101:151–157. doi: 10.1016/j.visres.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Simoncini C, et al. More is not always better: adaptive gain control explains dissociation between perception and action. Nat Neurosci. 2012;15:1596–1605. doi: 10.1038/nn.3229. [DOI] [PubMed] [Google Scholar]
- 58.Spering M, Gegenfurtner KR. Contrast and assimilation in motion perception and smooth pursuit eye movements. J Neurophysiol. 2007;98:1355–1363. doi: 10.1152/jn.00476.2007. [DOI] [PubMed] [Google Scholar]
- 59.Spering M, et al. Tracking without perceiving: a dissociation between eye movements and motion perception. Psych Sci. 2011;22:216–225. doi: 10.1177/0956797610394659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spering M, Carrasco M. Similar effects of feature-based attention on motion perception and pursuit eye movements at different levels of awareness. J Neurosci. 2012;32:7594–7601. doi: 10.1523/JNEUROSCI.0355-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Steen J, Dits J. Binocular eye movement control and motion perception: what is being tracked? Invest Ophthalmol Vis Sci. 2012;53:7268–7275. doi: 10.1167/iovs.12-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn G, Land MF. There’s more to magic than meets the eye. Curr Biol. 2006;16:R950–R951. doi: 10.1016/j.cub.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Stone LS, Krauzlis RJ. Shared motion signals for human perceptual decisions and oculomotor actions. J Vision. 2003;3:725–736. doi: 10.1167/3.11.7. [DOI] [PubMed] [Google Scholar]
- 64.Osborne LC, et al. A sensory source for motor variation. Nature. 2005;437:412–416. doi: 10.1038/nature03961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rasche C, Gegenfurtner KR. Precision of speed discrimination and smooth pursuit eye movements. Vision Res. 2009;49:514–52. doi: 10.1016/j.visres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Allman JM, et al. Direction- and velocity-specific responses from beyond the classical receptive field in the middle temporal visual area (MT) Perception. 1985;14:105–126. doi: 10.1068/p140105. [DOI] [PubMed] [Google Scholar]
- 67.Born RT, et al. Segregation of object and background motion in visual area MT: effects of microstimulation on eye movements. Neuron. 2000;2:725–734. doi: 10.1016/s0896-6273(00)81208-8. [DOI] [PubMed] [Google Scholar]
- 68.Born RT, Tootell RB. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357:497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- 69.Churan J, et al. Brief motion stimuli preferentially activate surround-suppressed neurons in macaque visual area MT. Curr Biol. 2008;18:R1051–R1052. doi: 10.1016/j.cub.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Barthélemy FV, et al. Behavioral receptive field for ocular following in humans: Dynamics of spatial summation and center-surround interactions. J Neurophysiol. 2006;95:3712–3726. doi: 10.1152/jn.00112.2006. [DOI] [PubMed] [Google Scholar]
- 71.Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- 72.Milner AD. Is visual processing in the dorsal stream accessible to consciousness? Proc Biol Sci. 2012;279:2289–2298. doi: 10.1098/rspb.2011.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berman RA, Wurtz RH. Functional identification of a pulvinar pathway from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91:591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- 75.Leopold DA. Primary visual cortex: awareness and blindsight. Annu Rev Neurosci. 2012;35:91–109. doi: 10.1146/annurev-neuro-062111-150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida M, et al. Residual attention guidance in blindsight monkeys watching complex natural scenes. Curr Biol. 2012;22:1429–1434. doi: 10.1016/j.cub.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 77.Striemer CL, et al. “Real-time” obstacle avoidance in the absence of primary visual cortex. Proc Nat Acad Sci. 2009;106:15996–16001. doi: 10.1073/pnas.0905549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buetti S, et al. Dissociation between goal-directed and discrete response localization in a patient with bilateral cortical blindness. J Cogn Neurosci. 2013;25:1769–1775. doi: 10.1162/jocn_a_00404. [DOI] [PubMed] [Google Scholar]
- 79.Hall N, Colby C. S-cone visual stimuli activate superior colliculus neurons in old world monkeys: implications for understanding blindsight. J Cogn Neurosci. 2014;26:1234–1256. doi: 10.1162/jocn_a_00555. [DOI] [PubMed] [Google Scholar]
- 80.Karnath HO, Rorden C. The anatomy of spatial neglect. Neuropsychologia. 2012;50:1010–1017. doi: 10.1016/j.neuropsychologia.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker R, Findlay JM. Saccadic eye movement programming in unilateral neglect. Neuropsychologia. 1996;34:493–508. doi: 10.1016/0028-3932(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 82.Van der Stigchel S, Nijboer TC. The imbalance of oculomotor capture in unilateral visual neglect. Conscious Cogn. 2010;19:186–197. doi: 10.1016/j.concog.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Benson V, et al. Eye movements and verbal report in a single case of visual neglect. PLoS One. 2012;7(8):e43743. doi: 10.1371/journal.pone.0043743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilke M, et al. Pulvinar inactivation disrupts selection of movement plans. J Neurosci. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilke M, et al. Neural activity in the visual thalamus reflects perceptual suppression. Proc Nat Acad Sci. 2009;106:9465–9470. doi: 10.1073/pnas.0900714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haynes JD, et al. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wunderlich K, et al. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmid MC, et al. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466:373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sincich LC, et al. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;7:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- 90.Tamietto M, De Gelder B. Neural basis of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2011;11:697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- 91.Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 92.Cardoso-Leite P, Gorea A. On the perceptual/motor dissociation: a review of concepts, theory, experimental paradigms and data interpretations. Seeing Perceiving. 2011;23:89–151. doi: 10.1163/187847510X503588. [DOI] [PubMed] [Google Scholar]
- 93.Haburcakova C, et al. Frequency dependence of vestibuloocular reflex thresholds. J Neurophysiol. 2012;107:973–983. doi: 10.1152/jn.00451.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rust How MT cells analyze the motion of visual patterns. Nat Neurosci. 2006;9:1421–1431. doi: 10.1038/nn1786. [DOI] [PubMed] [Google Scholar]
- 95.Kastner S, et al. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol. 2004;91:438–448. doi: 10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- 96.Fischer J, Whitney D. Attention gates visual coding in the human pulvinar. Nat Commun. 2012;3:1051. doi: 10.1038/ncomms2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saalmann YB, et al. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ling S, et al. How spatial and feature-based attention affect the gain and tuning of population responses. Vision Res. 2009;49:1194–1204. doi: 10.1016/j.visres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anton-Erxleben K, Carrasco M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci. 2013;14:188–200. doi: 10.1038/nrn3443. [DOI] [PMC free article] [PubMed] [Google Scholar]