Abstract
Executive function deficits and reward dysregulation, which mainly manifests as anhedonia, are well documented in drug abusers. We investigated specific aspects of executive function (inhibitory control and cognitive control), as well as anhedonia, in a cohort of current cocaine abusers in order to ascertain to what extent these factors are associated with more severe drug dependence. Participants filled out questionnaires relating to anhedonia and their addiction history. Participants also performed a response inhibition task while high-density event-related potentials (ERPs) were recorded. Electrophysiological responses to successful inhibitions (N2/P3 components) and to commission errors (ERN/Pe components) were compared between 23 current users of cocaine and 27 non-using controls. A regression model was performed to determine the association of our measures of reward dysregulation and executive function with addiction severity. As expected, cocaine users performed more poorly than controls on the inhibitory control task and showed significant electrophysiological differences. They were also generally more anhedonic than controls. Higher levels of anhedonia were associated with more severe substance use, whereas the level of executive dysfunction was not associated with more severe substance use. However, N2 amplitude was associated with duration of drug use. Further, inhibitory control and anhedonia were correlated, but only in controls. These data suggest that while executive dysfunction characterizes drug abuse, it is anhedonia, independent of executive dysfunction, that is most strongly associated with more severe use.
1. INTRODUCTION
Substance dependence is a multi-faceted problem. Substance abusers not only grapple with the inability to control and inhibit drug seeking behavior, but also with reward dysregulation. Reward dysregulation is usually manifested as anhedonia, the inability to experience pleasure from activities usually found enjoyable. In this study, we sought to gain a better understanding of the relationship between higher-order cognitive control and anhedonia in drug addiction, with a focus on users of cocaine. The study had two goals. The first was to assess the ability of cocaine users to successfully inhibit a prepotent response tendency and to see to what extent deficits in this ability is associated with addiction severity. The second was to examine the role of affective dysregulation in drug abuse and how this affective dysregulation may be associated with inhibitory capabilities in cocaine users.
The ability to withhold inappropriate responses and to monitor one’s actions fall under the umbrella of executive function. A well established paradigm to probe inhibition and monitoring is the Go/No-Go response inhibition task, which requires subjects to overcome a prepotent response tendency established by frequent Go stimuli to successfully inhibit response execution to No-Go stimuli. Inhibitory capability is measured by the number of correct withholds to No-Go stimuli, and performance monitoring can be measured by examining reaction time adjustments following incorrect executions to No-Go stimuli. Those who abuse drugs, including cocaine, have consistently demonstrated difficulties in their abilities to inhibit responses 1–6. Other work has revealed inhibitory difficulties in cocaine addiction that correlate to amount of cocaine used 7. We and others have shown that intact inhibitory processes 8–10 have been observed in those who are in recovery from drug dependence. Comparatively less is known about impairments in performance monitoring in cocaine abuse, though both behavioral and electrophysiological work has indicated deficits 11–13. Hester et al. (2007) assessed performance monitoring with post-error adjustments in response time and also the participants’ awareness of their errors as indicated by an additional button press. Cocaine using participants showed comparable post error slowing to controls when they were aware of their errors, but demonstrated awareness of fewer of their errors. These findings suggested that it is lack of awareness of errors that drives performance monitoring difficulties in cocaine abusers. Combined, the work in inhibitory control and error monitoring has suggested a strong role for executive dysfunction in cocaine addiction, and a need to determine to what extent inhibitory control and performance-monitoring deficits contribute to addiction severity, or vice versa. Understanding to what degree these specific components of executive functioning are associated with more severe cocaine addiction will enhance the development of more targeted interventions.
It is also important to examine the role of affective dysregulation. It is well established that cocaine’s subjective effects arise due to its impact on the re-uptake of the neurotransmitter dopamine. This neurotransmitter plays a strong role in reward and reward motivation 14. Dopamine D2 receptors are down-regulated in response to the high levels of DA that circulate as a result of cocaine use, resulting in poorer dopamine transmission when the drug is not being used 15–20. This poor transmission contributes to reward dysregulation. In this study, we focused specifically on drug user’s inability to derive adequate subjective reward from everyday stimulation. This reduction in reward response is typically referred to as anhedonia. Drug abusers have demonstrated higher levels of anhedonia than controls 21–23, and anhedonia is a key feature of withdrawal from many substances, including cocaine and methamphetamine 24, 25. The Reward Deficiency Syndrome theory of addiction proposes that reward deficiency associated with anhedonia may contribute to an increased desire for sources of high reward, such as drugs of abuse 26.
Both reward dysfunction and executive dysfunction may interact to worsen severity of substance abuse. This relationship has been suggested in gambling addicts, who showed increased self reported impulsivity that was correlated to sensitivity to reward during a gambling task 27. Previous work has suggested the presence of a relationship between direct measures of anhedonia and executive capabilities, notably in schizophrenia 28, 29. It has also been suggested that the presence of anhedonia may worsen executive capabilities as resources are put toward managing the affective dysregulation 30. Indeed, affective dysregulation in depressed individuals is known to affect performance monitoring capabilities 31, 32. The current study sought to determine the extent of anhedonia and deficits in inhibitory control and monitoring in healthy controls and in current cocaine abusers.
To study the behavioral and cortical underpinnings of inhibition and performance monitoring, control and cocaine-dependent participants performed a Go/No-Go task while high density event related potentials (ERPs) were recorded. The ERP components associated with successful inhibition are well characterized 33–37. The No-Go N2 is a fronto-centrally generated negativity arising between 200–400 ms, and the No-Go P3 is the later positive potential arising between 400–600 ms 38. The No-Go N2 is thought to reflect conflict monitoring mechanisms, while the No-Go P3 is a more direct reflection of motor inhibition37, 39, 40,41, 42, 38, 43, 44.
ERP measures associated with performance monitoring are also well defined. When participants fail to withhold a response, a negativity occurs approximately 50–100 ms after the error is made. This negativity is referred to as the Error-Related Negativity (ERN) 45. A subsequent slow wave that follows the ERN at approximately 120–400 ms is referred to as the error-related positivity, or the Pe. The ERN is thought to reflect a conflict monitoring signal, denoting cortical registration of an incorrect response execution 45. The Pe has been shown to reflect subsequent error awareness 46. Additionally, in order to explore the effect of anhedonia and determine whether it is associated with more severe substance abuse outcomes, we collected self-report information about trait and state anhedonia, as well as information about addiction severity 47, 48, 22.
We hypothesized that current cocaine abusers would demonstrate reduction in task accuracy, reduced post error slowing, and attenuation of ERP components related to inhibition, performance monitoring and error awareness. Furthermore, impairments would be correlated with addiction severity. We also hypothesized that the degree of executive impairment would be correlated with trait anhedonia in both cocaine users and controls. These findings may inform a more comprehensive model of the phenotype of substance dependence that incorporates information about both executive dysfunction and affective dysregulation.
2. METHODS
2.1. Participants
For this study, twenty-seven (7 female) control participants with no drug use history were recruited using advertisements on Craigslist and through word of mouth. Twenty-three (7 female) current cocaine abusers were recruited using Craigslist (N = 14) and from the Next STEPs programs at Waters Place and Port Morris (N = 9), which are outpatient treatment programs located in Wellness Centers in the Bronx and affiliated with the Albert Einstein College of Medicine. The Next STEPs programs are dedicated treatment centers that focus on helping patients achieve abstinence from cocaine and provide outpatient treatment and counseling options. All potential participants were administered the Structured Clinical Interview for the DSM-IV and were also administered screening questionnaires related to their overall physical and mental health. Exclusion criteria for cocaine abusers and controls were as follows: 1) Any DSM IV, Axis 1 diagnosis (excluding dependence or a past diagnosis of depression or dysthymic disorder caused by drug use for the cocaine users); 2) Head trauma resulting in loss of consciousness for longer than 30 minutes; 3) Presence of any past or current brain pathology; 4) A diagnosis of HIV; 5) Age above 55 years and below 18 years. Because of the high rates of comorbidity of alcohol and drug abuse among the cocaine using population, cocaine abusers were not excluded if they abused other drugs or alcohol. However, cocaine abusers were excluded if cocaine was not their primary drug of choice. Years of drug use were recorded during the screening questionnaires and the addiction severity index (ASI) interviews. Controls were also excluded if they had any major Axis 1 disorder or alcohol/drug dependence diagnosis, including nicotine dependence, or if any first degree family members had an alcohol/drug dependence diagnosis. A urine screen was performed on all participants to test for the presence of metabolites related to cocaine, THC, or opiates. Participants were paid for their participation in the form of one $12 gift card to local department stores per hour of experiment time. All participants signed an informed consent document administered by HIPAA-certified staff. All procedures were approved by the Institutional Review Board of the Albert Einstein College of Medicine and the City College of the City University of New York. The study conformed to the principles outlined in the Declaration of Helsinki.
Demographic information for the users and controls are as follows: The average age of the control participants was 41 (SD = 8.5), and 44 for the cocaine users (SD = 6.6). Average duration of education for controls was high school (12.2 years; SD = 1.4), and this was also the case for the cocaine users (12.5 years; SD = 2.3). 3 substance abusers were left-handed, and 4 controls were left-handed. The groups did not significantly differ in age, gender or years of education.
Cocaine abusing participants were asked to abstain from cocaine for 24 hours before entering the laboratory. All cocaine abusing participants reported cocaine as their drug of choice and all self-reported as current users and reported having used within the past week. The average duration since last use of cocaine was 3.9 days, with a range between 1 day and 1 week. The average intensity for consumption of cocaine was 3× per week. This is consistent with typical “binge” patterns of cocaine use 49, and allowed us to investigate individuals without requiring them to change their typical usage pattern. Abstinence from alcohol was determined using a breathalyzer, Alcohawk Slim. No participants were under the influence of alcohol upon entering the laboratory. All but three cocaine users tested positive for metabolites of cocaine. Three cocaine-using participants also tested positive for THC and one cocaine-using participant also tested positive for opiates. It should be mentioned that the duration of effect for cocaine is approximately 1 hour after administration 50, 51, so it is very unlikely that participants entered the laboratory directly after ingesting cocaine and experiencing its effects. Even if they had, consent, interview procedures and electrode cap application took at least 2 hours, so acute cocaine intoxication during testing would be virtually impossible.
Eight of the cocaine using participants had never entered treatment for their substance use and expressed no interest in treatment. Twenty-one of the cocaine using participants reported nicotine use, and eight of these cocaine-using participants reported being heavy smokers who smoked multiple cigarettes a day.
2.2. Clinical Measurements
Upon arriving at the laboratory, participants were seated in a comfortable, private room at the Albert Einstein College of Medicine, where they were informed about the study and signed consent forms. Afterwards, a trained researcher administered the urine screen.
All participants were then requested to fill out three questionnaires related to anhedonia. The first, the Snaith Hamilton Pleasure Scale (SHPS), addressed the current experience of anhedonia in each participant, serving as a measure of “state” anhedonia 22. The other scale consisted of two sections, the Chapman Physical and Chapman Social Anhedonia Scales, which addressed lifetime prevalence of anhedonia, or “trait” anhedonia, and also addressed physical and social aspects of this characteristic separately 47.
Cocaine abusing participants were then administered two questionnaires in order to obtain a more comprehensive understanding of their addiction history and severity level. The first questionnaire was the Addiction Severity Index (ASI), which is a structured interview that addressed seven major aspects of the interviewee’s life: medical history, legal history, psychiatric history, their family history and social life, and their alcohol and drug use 48. The second was the Cocaine Selective Severity Index (CSSA), which is a questionnaire that addressed withdrawal symptoms from cocaine in the previous 24 hours, including irritability and anhedonia 52.
2.3. Go/No-Go Task
After the urine test and the subsequent questionnaires pertaining to anhedonia, participants performed a Go/No-Go task while EEG was recorded, and were asked to respond quickly and accurately to every stimulus presentation, while withholding responses to the second instance of any stimulus repeated twice in a row. The probability of Go and No-Go trials was 0.85 and 0.15 respectively. We used neutrally valenced pictures from the International Affective Picture System IAPS; 53, a set of normative photographs that includes content across a wide range of semantic categories (http://csea.phhp.ufl.edu/Media.html#topmedia). In this task, emotionally neutral stimuli were presented in a pseudorandom sequence depicting people, landscapes, abstract patterns and objects (valence: 5.2, which falls into the neutral range in a 1–9 point scale that ranges from pleasant to unpleasant; arousal: 3.5, which falls into the neutral range in a 1–9 point scale that ranges from calm to exciting). For details about images from the IAPS, see 54. Images were presented centrally every 1000ms for 800ms with an inter-stimulus-interval of 200ms. Images subtended 8.6° horizontally by 6.5° vertically. Seven blocks of the response inhibition task were run, and participants were allowed to take a break between blocks whenever they liked. Each block lasted 3.5 minutes and consisted of 180 trials, for a total of 1260 trials per participant, 189 of which were inhibition trials. Participant inclusion required at least 70% of trials be accepted after artifact rejection. All participants, both control and cocaine using, committed more than ten errors of commission over the 7 blocks of trials, and no participants were thus excluded for not having enough trials to achieve acceptable signal-to-noise ratios.
Previously, we had examined abstinent cocaine abusers using this same paradigm, and also with a paradigm that employed positive and negatively valenced stimuli from the IAPS 10. For this follow-up study investigating current users, we focused only on neutral stimuli.
2.4. Electrophysiology Procedures
Participants were seated in a dimly lit, sound-attenuated, electrically shielded room, 80 cm from a LCD monitor (Viewsonic VP2655WP, 55 × 65 cm). To ensure consistency of electrode placement across participants, measures were made between the inion and nasion and between the left and right pre-auricular notches, using a flexible tape-measure, to identify the vertex of the scalp. This was then designated as the Cz electrode site and the cap was adjusted accordingly. Central fixation was required throughout each block (180 trials). Participants completed one mandatory practice block before the main experiment began. If needed, additional practice blocks were allowed. Participants took 30 second breaks between blocks.
Event-related potentials (ERPs) were acquired from a 168-channel montage at a digitization rate of 512Hz with a pass-band of 0.05–100Hz using the BioSemi Amplifier System. BioSemi uses two electrodes—the Common Mode Sense (CMS), which is actively recorded, and the Driven Right Leg (DRL), a passive electrode—that together form a feedback loop that represent the reference. The acquisition of the data occurs referenced to the CMS-DRL ground which drives the average potential of the participant (i.e. the common mode voltage) as close as possible to the AC reference voltage of the Analog-to-Digital box (for a description of the BioSemi active electrode system referencing and grounding conventions, visit http://www.biosemi.com/faq/cms&drl.htm). Data were later referenced off-line to the nasion for purposes of illustration. Epochs of 800 ms, including a 400ms pre-stimulus baseline, were analyzed for commission errors and were locked to the erroneous response, and epochs of 900 ms, including a 100 ms pre-stimulus baseline, were analyzed for correct withholds and were locked to the onset of the stimulus. Trials with eye movements and blinks were rejected offline based on vertical and horizontal EOG recordings. An automatic artifact rejection criterion of +/− 70μV was used at all other scalp sites. All analyses were conducted on individual subject averages that were not digitally filtered but group data were subsequently low-pass filtered at 45Hz with a 48 db/octave slope, purely for purposes of illustration.
To ascertain times and regions of interest, we collapsed the grand mean ERP across groups (control and abstinent abusers) separately for each condition (successful withhold and commission error). Visual inspection of the successful withhold condition showed maximal N2 amplitude at 250 ms over fronto-central scalp locations (FCz) and was thus defined as the average amplitude in the time window between 230 and 270 ms at this location, matching the observed peak latency of this waveform. Maximal P3 amplitude was observed to begin at 350 ms and peak at 450 ms over central-parietal scalp sites (CPz) and was sustained until 600 ms. It was thus defined as the average amplitude in the time window between 350 and 600 ms at this location, matching the observed peak latency. Visual inspection of the commission error conditions showed maximal ERN amplitude at 50 ms over fronto-central scalp locations (FCz) and was thus defined as the average amplitude in the time window between 30 and 70 ms at this location, matching the observed peak latency of this waveform. Maximal Pe amplitude was observed to begin at 100 ms and peak at 150 over central-parietal scalp sites (CPz) and was sustained until 300 ms. It was thus defined as the average amplitude in the time window between 100 and 300 ms at this location, matching the observed peak latency. The epochs and scalp projections of these well-characterized ERP components were fully consistent with findings from a large body of literature that has examined these processes in the past. There is a plethora of ERP research on cognitive control that has examined cognitive control and inhibitory processes, which has focused upon activity projecting to fronto-central scalp sites 45, 12, 55, and has focused on similar time windows for the individual ERN (30–70 ms), Pe (100–150 ms), N2 (230–270 ms) and P3 (350–600 ms) components that we have defined here, including our previous work in inhibitory control in abstinent cocaine abusers 10.
2.5. Statistical Analyses
The Addiction Severity Index (ASI) was scored using the Composite Scores Manual, which provides rigorous, objective measures of severity (for the manual, see http://triweb.tresearch.org/wp-content/uploads/2012/09/CompositeManual.pdf). We also collected information about previous treatment entry and relapse rates using the ASI. For the anhedonia data, t-tests were employed to test for between group differences. To probe the relationship between executive function and reward dysfunction, correlation coefficients were computed separately for each ERP component with measures of anhedonia in both cocaine users and controls. A linear regression model was then developed separately for the addiction severity scores and for the reported instances of relapse in cocaine users, with predictors of ERP amplitudes (the N2, P3, ERN and Pe) and reported measures of state, social and physical anhedonia.
T-tests were employed to test for group differences in task accuracy (hit rates for Go trials and commission errors for No-Go trials) and reaction time on correct Go trials. A repeated measure ANOVA with Response type (Pre versus Post-Error RT) as within-subject factor and Group (cocaine user versus controls) as between-subject factor was used to test for group differences in post-error slowing. For the ERP data, repeated measures ANOVAs were employed for each component of interest. For the response-locked ERN and Pe, a 2×2 ANOVA was run for each with factors of Group (cocaine user versus controls) and Response Type (correct Go-response versus incorrect No-Go-response). For the stimulus-locked N2 and P3, a 2×2 ANOVA was run for each with factors of Group (cocaine user versus controls) and Response Type (correct Go-response versus correct No-Go response).
3. RESULTS
3.1. Clinical Data
Cocaine users scored significantly higher than controls on both physical (t = 5.4, p <.03) and social (t = 10.2, p <.002) trait measures of anhedonia. The mean physical anhedonia score was 14.6 (SD =8.7) for cocaine users and 9.0 (SD = 5.1) for controls. For social anhedonia, the mean score for cocaine users was 15.3 (SD = 9.3) and 7.5 (SD = 6.0) for controls. Cocaine users also scored higher on the measure of state anhedonia (t = 2.6, p <.01). The mean of the state anhedonia score was 2.6 (SD = 4.1) for the cocaine users and .13 (SD = .43) for the controls. The mean score of the cocaine users on the Cocaine Selective Severity Assessment was 26.30 (SD = 18.6). The ASI composite scores were as follows: medical = .27 (SD =.33); employment = .71 (SD =.26); legal = .09 (SD =.12); alcohol = .14 (SD =.20); drug = .22 (SD =.09); family history = .24 (SD =.21); and psychiatric = .16 (SD =.19).
3.2. Performance Data
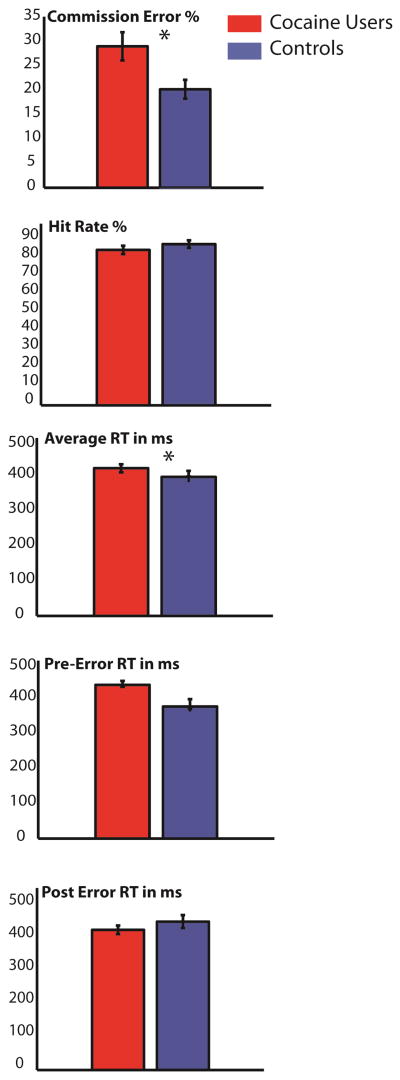
Figure 2 shows commission error rates, hit rates, reaction times and pre/post-error reaction times in both cocaine abusers and controls for the Go/No-Go task. Cocaine users made significantly more errors of commission than controls (t = 5.1 p <.03), and cocaine users were generally slower than controls, (t=6.3, p = .02). The repeated measure ANOVA assessing post-error slowing revealed an interaction between Pre/Post-Error RT and group (F1,49 =9.1, p = .01). Follow-up t-tests revealed post-error RT slowing in controls (p >.02), while cocaine users did not demonstrate such slowing (p > .3).
Figure 2.
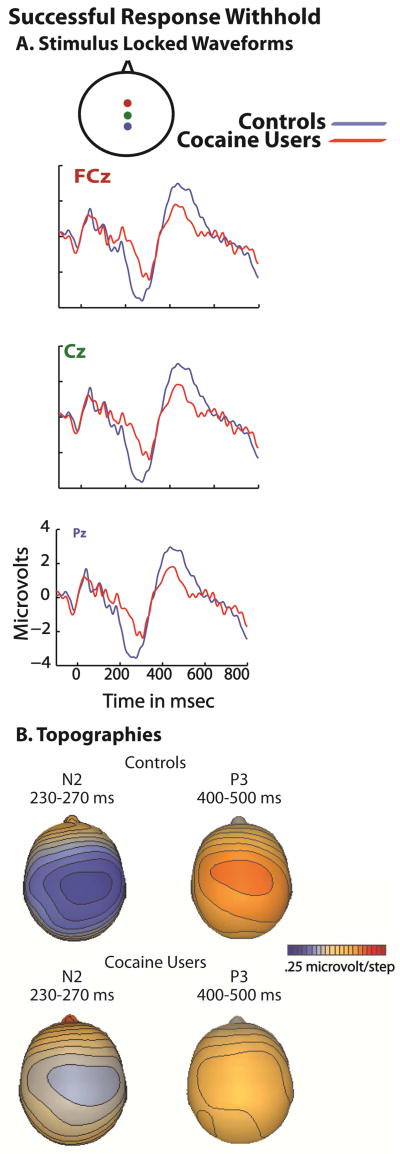
The No-Go N2 and No-Go P3 response waveforms associated with successful inhibitions for drug abusers and non-abusing control participants in the response inhibition task are displayed in Figure 2a. Topographic maps of activity across the scalp are displayed in 2b.
3.3. Electrophysiological data
3.3.1. Inhibition
Figure 3A shows the N2/P3 waveforms associated with successfully withholding a response at three midline electrodes over frontal, central and parietal scalp sites for controls (black trace) and cocaine users (red trace). Topographic maps of activity across the scalp for the N2 and for the P3 can also be seen in Figure 3B.
Figure 3.
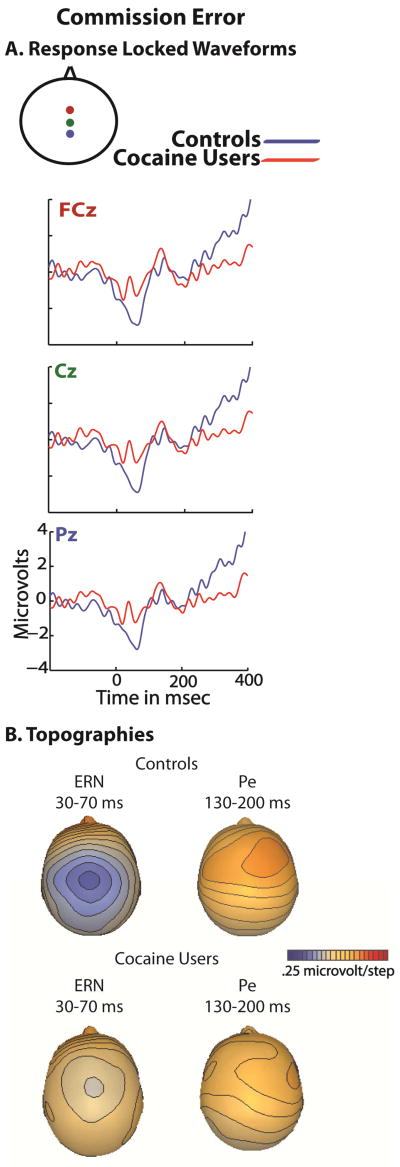
The ERN and Pe waveforms associated with commission errors for drug abusers and non-abusing control participants in the response inhibition task are displayed in Figure 3a. Topographic maps of activity across the scalp are displayed in 3b.
The ANOVA for the N2 revealed a main effect of response type (F49 = 8.1, p <.01) and a main effect of group (F49 = 7.1, p <.02), with an interaction of response type and group (F49 = 7.0, p <.02). Pairwise comparisons revealed the N2 amplitude to be smaller in the addicts than in the controls (t49 = 5.8, p <.01). Effect size for the interaction was .12.
Similarly, the ANOVA for the P3 revealed a main effect of response type (F49 = 8.4, p <.01) and a main effect of group (F49 = 4.1, p <.05), with an interaction of response type and group (F49 = 5.0, p <.03) . Pairwise comparisons revealed the P3 amplitude to be smaller in the addicts than in the controls (t49 = 6.2, p < .01). Effect size for the interaction was .095.
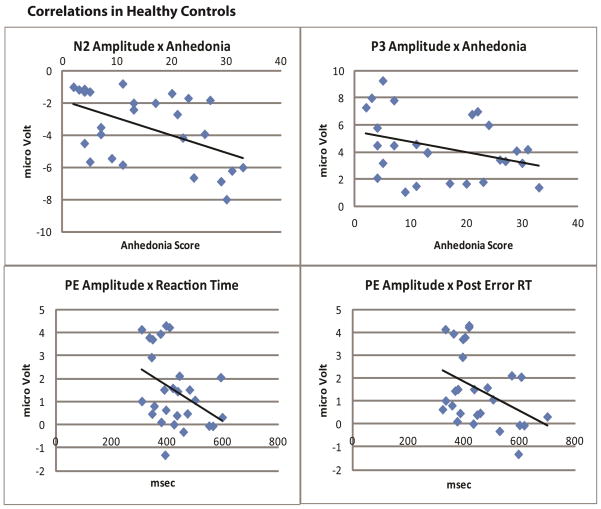
Correlations were performed between the mean amplitudes of the electrophysiological measures of interest in this task and the total anhedonia scores on the Chapman Physical and Social anhedonia scales, for both the cocaine abusers and the controls. In controls, a significant relationship was found between the mean amplitude of the N2 and anhedonia (r = −.513, p < .01) and between the mean amplitude of the P3 and anhedonia (r = −.429, p < .03). However, in cocaine abusers, no relationships were detected between total anhedonia score and amplitude of the N2 or P3 (p-values > .06).
3.3.2. Performance Monitoring
Figure 4A shows the response-locked waveforms at three midline electrodes over fronto-central and parietal scalp sites for controls (black trace) and cocaine users (red trace) for the instances in which participants committed a commission error. Topographic maps of the activity across the scalp for the ERN and the Pe can also be seen in Figure 4B.
Figure 4.
Scatter plots demonstrating the relationship in non-abusing controls between scores as summed from the Chapman Physical and Social Anhedonia scale and the amplitudes of the N2 and P3 waveforms, as well as scatter plots demonstrating the relationship between amplitude of the Pe waveform and reaction time. Higher levels of anhedonia were associated with more robust, negative N2 waveforms. Higher levels of anhedonia were also associated with lower amplitudes in the P3. Lower Pe amplitudes were associated with slower reaction times and more post error slowing.
The ANOVA for the ERN revealed a main effect of response type (F49 = 10.1, p <.01) and a main effect of group (F49 = 4.7, p <.03), with an interaction of response type and group (F49 = 4.7, p <.03). Follow up t-tests revealed the ERN amplitude to be less robust in the addicts than in the controls (t49 = 4.2, p <.03). The effect size of the interaction was .085.
Similarly, the ANOVA for the Pe revealed a main effect of response type (F49 = 9.8, p <.01) and a main effect of group (F49 = 5.4, p <.02), with an interaction of response type and group (F49 = 4.3, p <.04). Follow up t-tests once again revealed the Pe amplitude to be smaller in the addicts than in the controls (t49 = 4.1, p < .04). The effect size of the interaction was .089.
Correlations were performed between the mean amplitudes of the electrophysiological measures in this task and the total anhedonia score on the Chapman Physical and Social anhedonia scales, for both the cocaine abusers and the controls. In controls, no significant relationships were found between the mean amplitude of the ERN and anhedonia or between the mean amplitude of the Pe and anhedonia (p > .07). Similarly in cocaine abusers, no relationships were found between total anhedonia score and amplitude of the ERN or PE.
In addition, we performed correlations between behavioral indices of inhibition and the ERP waveforms. In controls the amplitude of the Pe was correlated with reaction time (r = −.391, p < .03) and post error reaction time (r = −.414, p < .03). This was not the case in cocaine users.
3.3.3. Predictors of Addiction Severity
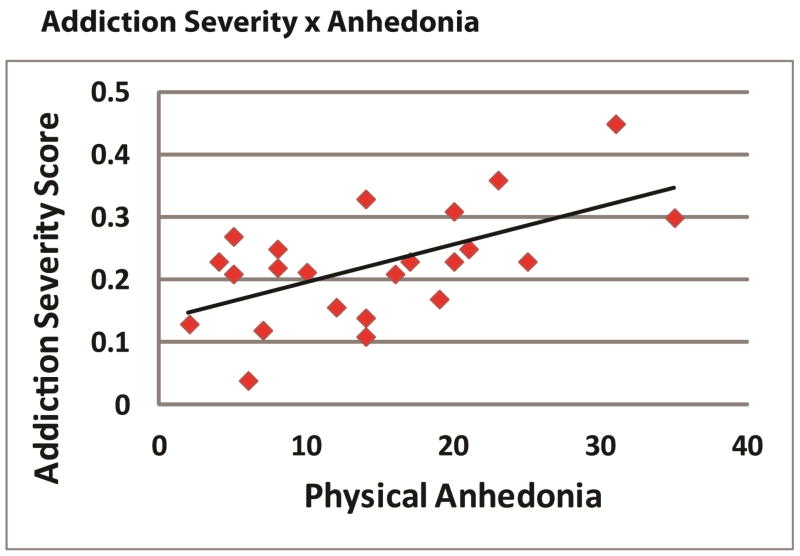
To assess predictors of addiction severity and relapse risk, two general linear models were developed for addiction severity and for reported instances of relapse with predictors of executive function (amplitudes of the N2, P3, ERN and Pe) and our measures of anhedonia (the SHPS and the Chapman physical and social trait anhedonia scales). The model was significant for addiction severity (F = 2.8, p <.05) but only trait physical anhedonia was a significant predictor (r = .58, p < .001). The model was not significant for the number of episodes of relapse (F = 2.1, p > .3). A scatter plot showing the significant relationship between physical anhedonia and addiction severity is illustrated in Figure 6.
Figure 6.
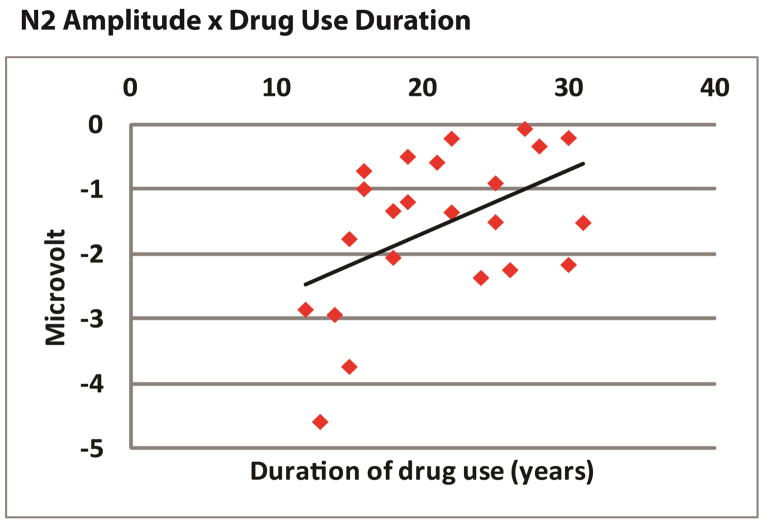
A graph demonstrating the relationship between drug use duration and the amplitude of the N2. Longer duration of drug use was associated with less robust N2 amplitude.
Correlations were also performed between the trait and state anhedonia and withdrawal scores as measured by the CSSA. CSSA scores were correlated with the state measure of anhedonia (r = .442, p < .03) but not with trait anhedonia measures. Correlations were also performed between our ERP and behavioral indices of interest and lifetime reported use of cocaine in years. Lifetime duration of cocaine use was found to be correlated with the amplitude of the N2 (r = .489, p < .02).
3.3.4 Post Hoc Analyses
A potential issue with our sample of cocaine abusers is that a sizable proportion of them were also alcohol users, raising the question of whether combined usage could impact the observations we report. As such, we conducted a series of exploratory follow-up analyses to assess this possibility. Five participants reported over 10 days of alcohol use to intoxication in the past month, and another 5 reported being bothered by their alcohol use in the past month1. These 10 participants were identified as being problematic alcohol users in comparison to 13 who did not report any problems with alcohol use and did not report any specific alcohol problems in the past month. The cocaine using group was thus split into high (Alcohol +) and low (Alcohol −) use cohorts, and ANOVAs were run for the dependent variables of interest between those two groups. ERP waveforms for both groups can be seen in supplementary figure 1.
The mean reaction time for alcohol users was 456.8 (SD =86.2) while the post-error reaction time was 492.6 (SD =91.2). The mean error rate for alcohol users was 29.9%, (SD =7.8) and the hit rate was 84.3% (SD =9.3). The mean reaction time for cocaine users with no alcohol problems was 448.3 (SD =86.2) while the post-error reaction time was 493.0 (SD =107.4). The mean error rate for cocaine users with no alcohol problems was 29.2%, (SD =11.7) and the hit rate was 85.7% (SD =12.5).
No significant between group differences were found for any behavioral measure (RT: F21 = 003, p > .9; mean Commission Error Rate: t21 = 1.6, p > .2; Hit Rate: t21 = .20, p > .6).
As with the ERP data between users and controls, for the response-locked ERN and Pe, a 2×2 ANOVA was run for each with factors of Group (Alcohol + and Alcohol −) and Response Type (correct Go-response versus incorrect No-Go-response). For the stimulus-locked N2 and P3, a 2×2 ANOVA was run for each with factors of Group (Alcohol + and Alcohol −) and Response Type (correct Go-response versus correct No-Go response). The ANOVA for the ERN revealed an effect of Response Type (F = 7.3, p < .04) but no effect of group (F21 = .9, p > .2) or any interaction effect (F21 = .62, p > .1). Similarly, an ANOVA for the Pe revealed an effect of Response Type (F21 = 8.1, p < .01) but no effect of group (F21 = .48, p > .5) or any interaction effect (F21 = .91, p > .2).
An ANOVA for the N2 revealed an effect of Response Type (F21 = 10.3, p < .01) but no effect of group (F21 = .3, p > .5) or any interaction effect (F21 = .01, p > .9). Similarly, ANOVAs for the P3 revealed an effect of Response Type (F21 = 9.1, p < .01) but no effect of group (F21 = .038 p >.8) or any interaction effect (F21 = .01, p > .9).
4. DISCUSSION
The purpose of this work was to investigate the relationship between executive dysfunction, anhedonia, and addiction severity in cocaine abusers. This was accomplished by collecting self report data on anhedonia and addiction severity, and by measuring the integrity of the cognitive control systems of both healthy and cocaine abusing participants using high-density electrophysiological methods.
4.1. Executive function in drug abuse
Executive function deficits are pronounced in all forms of substance abuse and addictive behaviors see review by 56. In line with these findings, and as predicted, cocaine abusing participants show a decrement in inhibition and performance monitoring. They performed more poorly on the Go/No-Go task, committed significantly more errors of commission and were generally slower than controls when responding. Unlike Hester et al., 2007, there were significant group differences in post error response adjustments, where cocaine users did not show the same post error slowing that controls did. The higher number of observed commission errors and evidence of poorer performance monitoring in cocaine users corresponds well to previous findings in cocaine addicted populations, where cocaine users have demonstrated poorer cognitive control and impaired inhibition circuitry 3, 57, 58.
Similarly, the ERP analyses revealed that the N2 and P3, as well as the ERN and Pe, were substantially reduced in current cocaine abusers. This suggests dysfunction in inhibitory control (indexed by the N2 and P3), conflict monitoring (indexed by the ERN), and error awareness (indexed by the Pe). Our findings correspond to previous ERP work that investigated cognitive control in cocaine users who had been abstinent for at least one month 12, in current cocaine users 59 and smokers 55. All of these studies found reduced inhibitory control in drug using populations indexed by reduced task performance and altered ERP amplitudes. However, our study differs from the Sokhadze study in one respect. In the Sokhadze study, an analysis on a subset of their participants who committed a high number of commission errors revealed that current cocaine abusers had larger, not smaller, ERN amplitudes, suggestive of improved cognitive control capabilities. Our data aligns with the Franken study, and the combination in our data of participants showing smaller ERN amplitudes while also committing more errors of commission suggests that these processes are generally impaired, not improved, in current cocaine abusers. It is likely that both functions, inhibition and performance monitoring, are impaired in cocaine abuse, and both may contribute to the difficulties that drug abusers encounter when attempting to withhold their responses.
One finding of note was the relationship between post-error reaction time and the amplitude of the Pe, which was observed in controls and not addicts. Previous research on the Pe has suggested that it is an indicator of awareness of an error 46, 60, 61. The finding that it is associated with adjustment of behavior after an error further supports that interpretation and the reduced amplitude of the Pe accompanied by the lack of such a relationship in cocaine addicts might suggest reduced awareness of errors in cocaine addiction.
4.2. Anhedonia in drug abuse and its relationship to executive function
Current cocaine users were more anhedonic than controls, as indexed by increased scores on both the state and trait anhedonia questionnaires. This extended to both physical and social trait anhedonia measures. This coincides well with previous research, which has revealed increased anhedonia in cocaine abuse 62, 23, 63.
There was a relationship between neural indices of inhibition and anhedonia in healthy controls. The N2 was more pronounced in more anhedonic controls, while the P3 was smaller. Depressed individuals, for whom elevated anhedonia is characteristic, have demonstrated a similar pattern of N2 and P3 alterations 64. However, contrary to our predictions and contrary to what has been observed in other clinical populations like those with schizophrenia 28, this pattern was not observed in drug users. Degree of anhedonia was not associated with any of the measures of inhibition or cognitive control in this population, suggesting that the executive function deficits measured in this task are entirely separate from the reward deficits reported by the participants via the anhedonia scales. However, it is apparent from the data here that both executive and reward deficits are present in the addicted phenotype. It is also somewhat surprising that the relationship between anhedonia and ERP measures of inhibitory control existed in controls and not in cocaine users. It is possible that impaired fronto-striatal circuits in cocaine addiction 65 can lead to a disruption of this normal relationship between reward systems and top-down control systems. Future imaging work should investigate this possibility.
4.3. Differential relationship of Executive Function and Anhedonia to addiction severity
Regression analysis revealed that trait physical anhedonia was associated with more severe addiction in cocaine abusers, while neural indices of inhibition and performance monitoring were not. While it is not possible to ascertain causality from a correlation analysis, there are two supplementary findings from our study that suggest a role of trait anhedonia in more severe drug addiction. The first is that our measure of state anhedonia, the Snaith Hamilton Pleasure Scale which measures anhedonia experience in the last 24 hours, was not correlated with addiction severity. This suggests that it was not recent intense drug use that was associated with an increased state of anhedonia. The other finding was the lack of a relationship between the withdrawal scale and trait anhedonia. Previous research has established that withdrawal contributes to anhedonia 25 and it was indeed correlated with our state measure of anhedonia. However, the lack of relationship between trait anhedonia and withdrawal suggests that our use of the Chapman scales captured trait anhedonia throughout the lifespan that was independent of any anhedonia caused by the short 24 hour period of abstinence participants underwent for this study.
Of course, it is always possible that lifetime drug use contributed to anhedonia rather than anhedonia contributing to lifetime drug use, and it is not possible to fully disentangle these two interpretations. The fact that the trait anhedonia measures correlated with addiction severity suggests a need for future investigations into the interplay of this trait in severe drug use. Other data have suggested the same. Those who have low baseline response to reward report greater responses to drugs than others 62, and smokers with increased anhedonia reported greater motivation to smoke 66. Further, research examining the idea of the “Reward deficiency syndrome,” which postulates that reward deficiency comes about due to low levels of dopamine receptors in the brain 26, found repeatedly that individuals with fewer dopamine D2 type receptors reported more pleasure from acutely administered methylphenidate 67, 68. Finally, it is well known that anhedonia is associated with craving during treatment 21, 69, and is considered a risk factor for relapse 63. These studies, along with our findings, suggest that anhedonia may be a trait that contributes to more severe dependence. Future work should investigate the development of drug use longitudinally with anhedonia as a predictor. It is possible that anhedonia contributes to more severe drug dependence via a “self-medication” mechanism. This is especially relevant considering the finding that physical, but not social, anhedonia was correlated with severity.
Of surprise to us was the finding that neither behavioral nor electrophysiological measures of inhibition or performance monitoring were associated with addiction severity in our regression model, especially considering the finding that duration of drug use was indeed correlated with measures of cognitive control. Previous research has established a relationship between self-reported amount of drug used and self reported impulsivity, as well as between self reported impulsivity and treatment outcome 70. Research by Verdejo and colleagues found that drug severity scores on the Addiction Severity Index and disinhibition subscale scores as measured by the self reported Frontal Systems Behavioral Scale were correlated 71. However, similar to the findings here, they found no correlation between the ASI score and actual performance on a Go/No-Go task. In a study investigating cocaine and heroin abusers, it was once again found that inhibition and cognitive control, as measured via a battery of neuropsychological tests, were not significantly correlated with severity 5. Another study that investigated correlations between severe cocaine use and executive function 7 identified a correlation between performance on a Stroop task and amount of cocaine used during peak use. However, users were at least 15 days abstinent in that study, so their result does not bear on acute effects. It is possible that while executive dysfunction contributes to drug dependency, and cumulative neurotoxic effects of long periods of drug use may contribute to worsened cognitive control, worsened executive dysfunction, at least as measured by commonly used laboratory tasks, is not necessarily associated with increased intensity of drug use.
The current findings also suggest that the toxic vaso-constrictive effects of cocaine 72 may not necessarily result in more impaired executive function as drug use grows more severe in the short term, but instead may operate over much longer periods of time, as evidenced by the correlation between duration of drug use and the amplitude of the N2 component. Of course, it is also possible that the associations observed here were due to earlier onset of drug use in those with increased drug use durations 73. Work explicitly investigating indices of executive control as a function of age of onset, and how age of onset and subsequent duration of drug use each relate to executive functions will be required to shed further light on this issue.
Finally, it is surprising to us that no variables in our regression analyses predicted relapse rates as reported on the ASI. However, considering that relapse rates were determined from self-reports of previous times in treatment, it is possible that the lack of specificity weakened any effects. In addition, not all participants were treatment seekers and a significant cohort had never entered treatment throughout their lifetime (N=8).
4.4. Limitations
Examination of drug abusing populations always raises the risk that the effects observed are due to acute effects of the drug or due to sudden abstinence from the drug (i.e. withdrawal effects). While participants were asked to refrain from drug use for 24 hours, and a goal of this work was to examine the neurocognitive profiles of cocaine users without requiring them to alter their normal usage patterns, it is always possible that the effects observed could be due to acute effects of cocaine or to effects resulting from abstinence, especially in those who did not show cocaine-positive urines. However, the 24-hour period of abstinence we asked participants to undergo was not onerous considering the “binge” use patterns seen in typical cocaine users 74, 49. In addition, our data is a snapshot of the neurocognitive profile of users who have not, or who have just entered, treatment, which is valuable information for treatment providers.
Another difficulty that arises when investigating drug use is the tendency of drug users to abuse more than one substance. It is difficult to generalize the findings here only to cocaine, despite this substance being the drug of choice for every participant, as most participants also used nicotine and many reported alcohol problems. However, a strength of this study is that this population more accurately reflects general drug using populations, making our findings very relevant to treatment providers who seek to treat individuals who may report drugs of choice but actually abuse many different substances.
4.5. Conclusions
Despite these limitations, our findings suggest that drug abuse is a result of a unique phenotype of affective dysregulation and executive dysfunction. Inhibition and cognitive control deficits are present in drug abusers, but these executive factors are not related to affective dysregulation in this population. The usual relationship between anhedonia and executive function observed in healthy controls was not detected in cocaine users, but duration of drug use was associated with alterations in a neural marker associated with cognitive control. Our findings also suggest that it is anhedonia, not executive dysfunction, that contributes most strongly to more severe recent cocaine use. Future work should examine abstinent drug abusers longitudinally and establish whether executive function and anhedonia can recover once cocaine abuse has ceased.
Supplementary Material
Figure 1.
Reaction times, accuracy rates and commission error rates for drug abusers and non-abusing control participants are displayed for the response inhibition task. Drug abusers committed significantly more errors of commission than non-abusing controls.
Figure 5.
A graph demonstrating the relationship in drug abusers between the Chapman Physical Anhedonia scale and the composite scores for drug use from the Addiction Severity Index. More anhedonia was associated with higher levels of severity.
Highlights.
Cocaine users show reward dysregulation in the form of anhedonia
Cocaine users have worse performance in tasks of inhibitory control and performance monitoring
Cocaine users show reduced amplitudes of ERP indices of inhibitory control and performance monitoring
ERP indices of inhibitory control are correlated with anhedonia in controls, but not in cocaine users
A regression model demonstrates that anhedonia, not executive functioning, is correlated to addiction severity in cocaine users
Acknowledgments
The Human Clinical Phenotyping Core, where the participants enrolled in this study were recruited and clinically evaluated, is a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (IDDRC) which is funded through a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593). Major support for the work of The Cognitive Neurophysiology Laboratory is provided through a grant from the Sheryl and Daniel R. Tishman Charitable Foundation. Dr. Foxe’s work is supported in part by a grant from the U.S. National Institute of Mental Health (NIMH) (RO1 - MH085322). We thank the Albert Einstein Division of Substance Abuse, Dr. Julia Arnsten, Dr. Sarah Church, Juan Martinez, Amy Greengrass, Marsha Dommel, and all of the staff at the Next Steps Addiction Treatment Centers in the Bronx for all of their help in recruitment efforts. The work would simply not have been possible without the dedication of these individuals. We would also like to express our sincere gratitude to the participants for giving their time to this effort. Additionally, we would like to thank Dr. Ryan Bell, Ms. Sarah Ruberman and Mr. Frantzy Acluche for their help during data collection. We would also like to thank three anonymous reviewers for their comments on the paper, which helped to greatly improve it.
Footnotes
Note that comparing composite scores from the ASI across measures, i.e. comparing the composite score for drug use to the composite score for alcohol use to ascertain which is the most problematic for the individual, is not considered a valid comparison. The sections are scored separately using different scoring systems, as the drug section collects information about every possible drug of abuse.
Author Contributions
KPM, JJF and HG were responsible for initial study concept and design. KPM was responsible for participant recruitment, phenotyping and coordinating data collection. KPM, PDS and JJF all contributed to data analysis and data interpretation. KPM wrote the first draft of the manuscript. JJF, PDS, and HG provided extensive editorial input throughout the process, and critical revisions of the manuscript for important intellectual content. All authors critically reviewed the content of the paper and approved the final version for publication. The senior author, JJF, attests that all authors had full access to the data and subsequent analyses throughout the editorial process.
All authors of this paper declare that they have no conflict-of-interest, financial or otherwise, that would bias their contributions to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fillmore MT, Rush CR, Hays L. Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug and alcohol dependence. 2002;67:157–167. doi: 10.1016/s0376-8716(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman JN, Ross, Thomas J, Stein, Elliot A, Garavan H. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. The Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 5.Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology. 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- 6.Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albein-Urios N, Martinez-Gonzalez JM, Lozano O, Clark L, Verdejo-Garcia A. Comparison of impulsivity and working memory in cocaine addiction and pathological gambling: Implications for cocaine-induced neurotoxicity. Drug and alcohol dependence. 2012;126:1–6. doi: 10.1016/j.drugalcdep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug and alcohol dependence. 2012 doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): A functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): A high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Li CS, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and alcohol dependence. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biological psychology. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- 14.Wise RA. Dopamine and Reward: The Anhedonia Hypothesis 30 Years on. Neurotoxicity Research. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyatt R, Karoum F, Suddath R, Fawcett R. Persistently decreased brain dopamine levels and cocaine. J Am Med Assoc. 1988;259:2996. [PubMed] [Google Scholar]
- 16.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 17.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-Induced Dopamine Release: Markedly blunted in cocaine dependence and predictive of the choice to selfadminister cocaine. The American journal of psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in Drug Abuse and Addiction: Results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 20.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of Low Striatal Dopamine D2 Receptor availability with nicotine dependence similar to that seen with other drugs of abuse. American Journal of Psychiatry. 2008;165:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 21.Janiri L, Martinotti G, Dario T, Reina D, Paparello F, Pozzi G, Addolorato G, Di Giannantonio M, De Risio S. Anhedonia and substance-related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- 22.Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of affective disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and clinical psychopharmacology. 2010;18:562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. Journal of psychiatry & neuroscience: JPN. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- 25.D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Current topics in behavioral neurosciences. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- 26.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJH, Comings DE. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000:32. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Moya EM, Ochoa C, Jimenez-Murcia S, Aymami MN, Gomez-Pena M, Fernandez-Aranda F, Santamaria J, Moragas L, Bove F, Menchon JM. Effect of executive functioning, decision-making and self-reported impulsivity on the treatment outcome of pathologic gambling. Journal of psychiatry & neuroscience: JPN. 2011;36:165–175. doi: 10.1503/jpn.090095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophr Res. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Tully LM, Lincoln SH, Hooker CI. Impaired executive control of emotional information in social anhedonia. Psychiatry Res. 2012 doi: 10.1016/j.psychres.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clinical psychology review. 2010;30:621–634. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: relationship to subclinical measures of depression. Emotion. 2007;7:68–76. doi: 10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of general psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalography Clinical Neurophsyiology. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- 34.Eimer M. Effects of attention and stimulus probability on ERPs in a Go/NoGo task. Biol Psychiatry. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- 35.Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M. The time course of brain activations during response inhibition: evidence from event-related potentials in a go/no go task. Neuroreport. 1998;9:765–770. doi: 10.1097/00001756-199803090-00037. [DOI] [PubMed] [Google Scholar]
- 36.Roche RA, Garavan H, Foxe JJ, O’Mara SM. Individual differences discriminate event-related potentials but not performance during response inhibition. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2005;160:60–70. doi: 10.1007/s00221-004-1985-z. [DOI] [PubMed] [Google Scholar]
- 37.Katz R, De Sanctis P, Mahoney JR, Sehatpour P, Murphy CF, Gomez-Ramirez M, Alexopoulos GS, Foxe JJ. cognitive control in late-life depression: response inhibition deficits and dysfunction of the anterior cingulate cortex. American journal of geriatric psychiatry. 2010;18:1017–1025. doi: 10.1097/JGP.0b013e3181d695f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 39.De Sanctis P, Butler JS, Green JM, Snyder AC, Foxe JJ. Mobile brain/body imaging (MoBI): High-density electrical mapping of inhibitory processes during walking. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2012;2012:1542–1545. doi: 10.1109/EMBC.2012.6346236. [DOI] [PubMed] [Google Scholar]
- 40.De Sanctis P, Foxe JJ, Czobor P, Wylie GR, Kamiel SM, Huening J, Nair-Collins M, Krakowski MI. Early sensory-perceptual processing deficits for affectively valenced inputs are more pronounced in schizophrenia patients with a history of violence than in their non-violent peers. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 43.Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage. 2010;51:877–887. doi: 10.1016/j.neuroimage.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 44.De Sanctis P, Butler JS, Malcolm BR, Foxe JJ. Recalibration of inhibitory control systems during walking-related dual-task interference: A Mobile Brain-Body Imaging (MOBI) Study. NeuroImage. 2014;94C:55–64. doi: 10.1016/j.neuroimage.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 46.O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. The European journal of neuroscience. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- 47.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 48.McLellan ATLL, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;3:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21:35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- 50.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 51.Bigelow GE, SLW . Evaluation of potential pharmacotherapies: response to cocaine challenge in the human laboratory. New York: Academic Press; 1998. [Google Scholar]
- 52.Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 53.Lang PB, Cuthbert BN. International Affective Picture System (IAPS): technical manual and affective ratings. Gainesville, FL: 1997. [Google Scholar]
- 54.Mikels JA, Frederickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the International affective picture system. Behavioral Research Methods. 2005;37:626–630. doi: 10.3758/bf03192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franken IH, van Strien JW, Kuijpers I. Evidence for a deficit in the salience attribution to errors in smokers. Drug and alcohol dependence. 2010;106:181–185. doi: 10.1016/j.drugalcdep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of psychiatry & neuroscience: JPN. 2013;38:130052. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. The American journal of drug and alcohol abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- 59.Sokhadze E, Stewart C, Hollifield M, Tasman A. Event-Related Potential Study of Executive Dysfunctions in a Speeded Reaction Task in Cocaine Addiction. Journal of neurotherapy. 2008;12:185–204. doi: 10.1080/10874200802502144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, Fitzgerald M, Foxe JJ, Robertson IH. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD) Neuropsychologia. 2009;47:1149–1159. doi: 10.1016/j.neuropsychologia.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Murphy PR, Robertson IH, Allen D, Hester R, O’Connell RG. An electrophysiological signal that precisely tracks the emergence of error awareness. Frontiers in human neuroscience. 2012;6:65. doi: 10.3389/fnhum.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2008;17:218–223. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Frontiers in psychiatry / Frontiers Research Foundation. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang BW, Zhao L, Xu J. Electrophysiological activity underlying inhibitory control processes in late-life depression: a Go/Nogo study. Neuroscience letters. 2007;419:225–230. doi: 10.1016/j.neulet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 66.Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. American Journal of Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 68.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 69.Martinotti G, Cloninger CR, Janiri L. Temperament and character inventory dimensions and anhedonia in detoxified substance-dependent subjects. The American journal of drug and alcohol abuse. 2008;34:177–183. doi: 10.1080/00952990701877078. [DOI] [PubMed] [Google Scholar]
- 70.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 71.Verdejo-Garcia A, Bechara A, Recknor, Emily C, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence an examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- 72.Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. The British Journal of Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 73.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. The British journal of psychiatry: the journal of mental science. 2013;203:35–43. doi: 10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 74.Gawin F. Cocaine Dependence. Annual Reviews Medicine. 1989;40:149–161. doi: 10.1146/annurev.me.40.020189.001053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.