Abstract
Embolic insults account for a significant number of neurologic sequelae following many routine surgical procedures. Clearly, these post-intervention embolic events are a serious public health issue as they are potentially life altering. However, the pathway these emboli utilize to bypass the pulmonary microcirculatory sieve in patients without an intracardiac shunt such as an atrial septal defect or patent foramen ovale, remains unclear. In the absence of intracardiac routes and large diameter pulmonary arteriovenous malformations, inducible large diameter intrapulmonary arteriovenous anastomoses in otherwise healthy adult humans may prove to be the best explanation. Our group and others have demonstrated that inducible large diameter intrapulmonary arteriovenous anastomoses are closed at rest but can open during hyperdynamic conditions such as exercise in more than 90% of healthy humans. Furthermore, the patency of these intrapulmonary anastomoses can be modulated through the fraction of inspired oxygen and by body positioning. Of particular clinical interest, there appears to be a strong association between arterial hypoxemia and neurologic insults, suggesting a breach in the filtering ability of the pulmonary microvasculature under these conditions. In this review, we present evidence demonstrating the existence of inducible intrapulmonary arteriovenous anastomoses in healthy humans that are modulated by exercise, oxygen tension and body positioning. Additionally, we identify several clinical conditions associated with both arterial hypoxemia and an increased risk for embolic insults. Finally, we suggest some precautionary measures that should be taken during interventions to keep intrapulmonary arteriovenous anastomoses closed in order to prevent or reduce the incidence of paradoxical embolism.
Keywords: Intrapulmonary arteriovenous anastomoses, Shunt, Pulmonary gas exchange, Neurological sequelae, Paradoxical embolism, Fat embolism, Cryptogenic stroke, Transient ischemic attack, Migraine, Dementia
Introduction
Paradoxical embolization is increasingly mentioned in the literature as an explanation for numerous neurological insults including stroke,8,13,18,26,28,29,44,48,51,54,59,62,63,82 transient ischemic attack,18,57,82 migraines26,28,68,85,87,88 and several post-operative neurological se quelae including delirium,49 cognitive decline23 and cognitive dysfunction.55 Some of these neurological outcomes can be explained by the presence of an intracardiac shunt such as a patent foramen ovale (PFO), which allows for large diameter emboli to bypass the 8–10 mm diameter sieve created by the pulmonary capillaries and enter into the systemic circulation. However, there are a surprisingly large percentage of individuals without a PFO who suffer neurological insults. For example, up to 54% of patients who experience a cryptogenic stroke do not have a PFO.22,26,51 Furthermore, the overall incidence of PFO in the general population is ~30%,15 which cannot account for the prevalence of neurological insults being as high as 47 and 61% following cardiac and major orthopedic surgeries, respectively.49 Thus, in the absence of a large diameter intracardiac shunt, how can emboli of venous origin enter into the systemic arterial circulation? The answer to this question may be that these emboli bypass the pulmonary microcirculation via dynamic large diameter intrapulmonary arteriovenous anastomoses.
Existence of large diameter intrapulmonary arteriovenous anastomoses
Arteriovenous anastomoses were first reported by Sappey over 100 years ago58 and intrapulmonary arteriovenous anastomoses between 15 and 500 mm have been known to exist in adult human lungs for ~70 years.69,70,77,78 These landmark studies used gold standard, anatomic-based techniques such as microspheres and casting to definitively demonstrate the existence of these pathways in the isolated lung, albeit under various non-physiologic conditions. However, these understudied pathways have remained largely ignored by physicians and scientists because the conditions under which they are patent in healthy humans were unknown until very recently.24,65 Lovering and colleagues recently established that these dynamic pathways can be opened in isolated adult human and baboon lungs that are ventilated and perfused under physiologic conditions using 25 and 50 μm microspheres.35 Similarly, others have demonstrated the transpulmonary passage of large diameter microspheres in human infants84 and numerous animal models under varying conditions.4,38,47,50,52 Thus, these large diameter dynamic intrapulmonary arteriovenous anastomoses do exist and, as such, have the potential to play both physiological and pathophysiologic roles ranging from effecting pulmonary gas exchange efficiency33,67 to providing a pathway for emboli to bypass the pulmonary filter and cause cryptogenic stroke.26 Because these pathways may be involved in paradoxical embolism, which is associated with serious complications, it would be important to know when these important pathways are open and when they are closed.
Dynamic nature of large diameter intrapulmonary arteriovenous anastomoses
Saline contrast echocardiography is a non-invasive technique that uses ultrasound to visualize the delayed appearance of saline-contrast microbubbles in the left heart to detect the opening of large diameter intrapulmonary arteriovenous anastomoses. In the absence of intracardiac and intrapulmonary shunts such as arteriovenous malformations, pulmonary capillaries filter out intravenously injected saline contrast microbubbles and no bubbles appear in the left heart.3,40,42,56 According to Meltzer et al., the presence of a single microbubble in the left heart defines the presence of a pathologic shunt.41 As such, saline contrast echocardiography is widely regarded as the most sensitive technique in identifying any source of right-to-left shunt, even detecting subclinical microvascular pulmonary arteriovenous malformations which high resolution computed tomography is unable to visualize.21,46,73
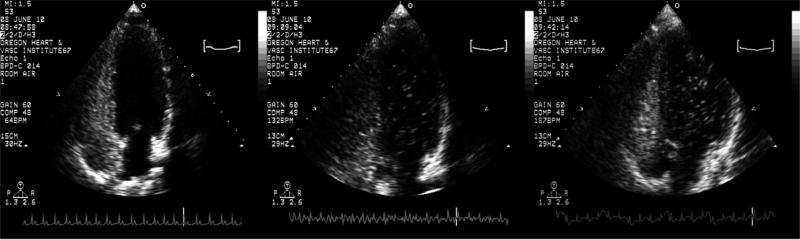
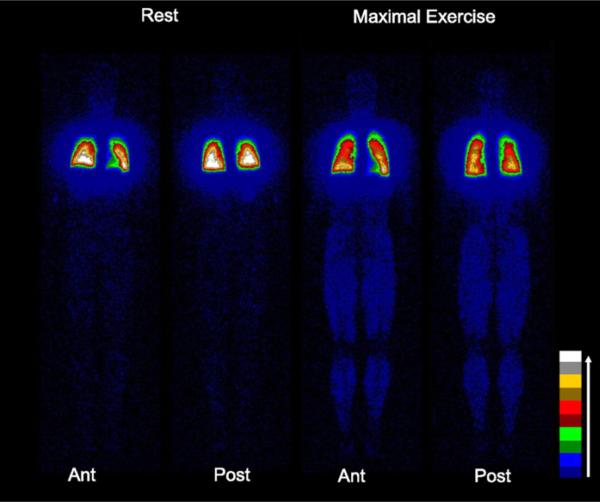
Using saline contrast echocardiography, our group and others have demonstrated that intrapulmonary arteriovenous anastomoses are closed at rest, but open during submaximal through maximal exercise in >90% of adult humans without an intracardiac shunt9,33,67 (Fig. 1). The detection of these open shunt pathways using saline contrast echocardiography was validated in exercising healthy adult humans using intravenously-injected, solid macroaggregates of albumin32 (Fig. 2) and microspheres of albumin83 and in exercising dogs using intravenously-injected 25 mm polymer microspheres.66 Thus, exercise is a condition in which these dynamic pathways have been clearly demonstrated to be open.
Fig. 1.
Transpulmonary passage of saline contrast microbubbles with exercise. Representative saline contrast echocardiograms from a single subject pre-exercise, and during exercise at 50% and 90% of VO2max while breathing room air. Shunt scores were 0, 2, 3 for pre-exercise, 50% and 90% of VO2max respectively.
Fig. 2.
Transpulmonary passage of macroaggregates of albumin with exercise. Anterior (Ant) and posterior (Post) planar whole body images obtained following injections with technetium-99m macroaggregates of albumin at rest and during maximal treadmill exercise. The increased number of counts in the exercising muscles (legs) indicates intrapulmonary shunting of technetium-99m macroaggregates of albumin that have become trapped in systemic capillaries. The percent shunt in this individual at rest was 0.7%, which increased to 3.0% at maximal exercise. Color bar represents increasing count intensities with lighter colors. (From Lovering AT, Haverkamp HC, Romer LM, et al. Transpulmonary passage of 99mTc macroaggregates albumin in healthy humans at rest and during maximal exercise. J Appl Physiol 2009;106:1986–92; with permission.)
In addition to exercise, Stickland and colleagues have further demonstrated that simple changes in body positioning may alter the patency of these vessels. In a study of 8 healthy humans, these authors demonstrated that intrapulmonary arteriovenous anastomoses opened in 2 of 8 subjects going from upright to supine.67 These data suggest that minor changes in central blood volume or changes in regional pulmonary blood flow that result from changes in body position may be involved in determining the patency of these vessels. Thus, changes in body positioning, for instance head tilted up on the operating table, could prevent a breach in the pulmonary filter in as much as 25% of the general population.
Oxygen-modulation of inducible intrapulmonary arteriovenous anastomoses
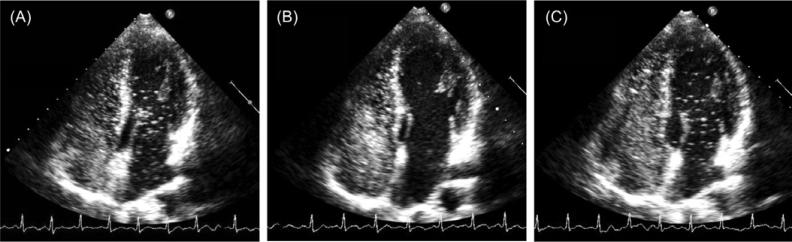
Von Euler and Liljestrand first showed that acute anoxia induces pulmonary vasoconstriction in the cat and suggested that the low oxygen was acting directly on the pulmonary vasculature in order to redistribute blood flow.76 Soon after, Motley et al. undertook this investigation in human subjects breathing 10% O2 and also demonstrated a rapid increase in pulmonary artery pressure which resolved upon return to ambient air breathing.43 Thus, it is now widely accepted that hypoxia induces pulmonary arterial vasoconstriction and it was under this premise that initial attempts to regulate intrapulmonary arteriovenous anastomoses were undertaken. Interestingly, while the fraction of inspired oxygen (FIO2) does in fact modulate intrapulmonary arteriovenous anastomoses during exercise, this regulation is opposite that of the normal pulmonary vasculature. For example, once these pathways have been opened up during exercise breathing room air, these pathways can be subsequently closed by breathing an FIO2=1.0 during submaximal through maximal exercise in the majority of healthy adult humans11,34 (Fig. 3). Importantly, a few bubbles still traverse the pulmonary circulation in some subjects breathing hyperoxia during exercise, just not as many bubbles.11,34 These data are supported by studies in dogs demonstrating that over-embolization of the lung results in the transpulmonary passage of microspheres, which is reduced in hyperoxic conditions.47 Furthermore, hyperoxia has been shown to redistribute pulmonary blood flow as measured with microspheres in intact sheep.39 Accordingly, these pathways can be opened up during hyperdynamic conditions such as exercise, but can be closed by increasing the FIO2.
Fig. 3.
Prevention of transpulmonary passage of saline contrast bubbles with exercise in hyperoxia. Saline contrast echocardiograms from a 36-year-old male subject during exercise at 180 W in normoxia and hyperoxia. (A) echocardiogram during exercise for 1 min at 180 W in normoxia. Note saline contrast bubbles in the left heart indicating arteriovenous shunting. Bubble score = 3. (B) Echocardiogram during exercise for 120 s at 180 W in hyperoxia (100% O2). Note absence of saline contrast bubbles in the left heart indicating no arteriovenous shunting. Bubble score = 0. (C) Echocardiogram upon returning to exercise for 60 s at 180 W in normoxia. Note appearance of saline contrast bubbles in the left heart recommenced indicating arteriovenous shunting. Bubble score = 3. (From Lovering AT, Stickland MK, Amann M, Murphy JC, O'Brien MJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008 Sep 15;586(Pt 18):4559–65; with permission.)
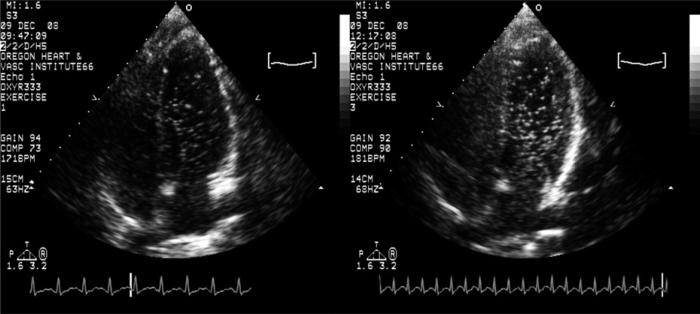
In addition to breathing high levels of oxygen, breathing low oxygen tensions also has an effect on these pathways. Due to hypoxic pulmonary vasoconstriction, it was initially hypothesized that intrapulmonary arteriovenous anastomoses could be kept closed during exercise if subjects breathed hypoxia.33 However, it was shown to be incorrect as breathing an FIO2 = 0.12 opened intrapulmonary arteriovenous anastomoses in 3/9 subjects at rest and caused an increase in the transpulmonary passage of saline contrast for any given workload during exercise (Fig. 4).33 Contrary to the original hypothesis, these dynamic intrapulmonary arteriovenous anastomoses respond to oxygen in a manner opposite that of the normal pulmonary vasculature and behave more like systemic vessels that vasodilate in response to hypoxia. However, this preliminary study used only one level of hypoxia and only 30% of the subjects demonstrated transpulmonary passage of saline contrast bubbles at rest. Thus, our most recent investigation set out to determine if we could induce shunting in all healthy human subjects breathing some level of hypoxia at rest.
Fig. 4.
Increased transpulmonary passage of saline contrast microbubbles with exercise in hypoxia. Representative saline contrast echocardiograms from a single subject during exercise in normoxia and hypoxia FIO2 = 0.14 at 75% of VO2max. Shunt score of 3 in normoxia and 4 during hypoxia. From Laurie SS, Yang X, Elliott JE, et al. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 2010;109(4):1072–9. © Am Physiol Soc, used with permission.
Using saline contrast echocardiography we have now demonstrated that breathing an FIO2 = 0.16 to 0.10 at rest opens inducible intrapulmonary arteriovenous anastomoses in a dose-dependent manner, with increased bubble density in the left ventricle as arterial hypoxemia worsens (Figs. 5, 6). In these studies we used a published bubble scoring system which assigns a 0 through 5 score based on the number and spatial distribution of bubbles in the left ventricle27,34 (Fig. 5). We found that bubble scores were significantly greater while breathing an FIO2 = 0.12 and 0.10 for 30 min compared to subjects breathing room air.27 While there was some variability in the duration of hypoxic exposure before the onset of shunting and in bubble scores between subjects for any given FIO2, all subjects demonstrated left sided contrast after 30 min breathing an FIO2 = 0.10 (Figs. 5, 6). Additionally, some subjects had significant left-sided contrast with saturations as high as 95% (Fig. 7). Because increased bubble density represents a greater breach in the pulmonary capillary filter,87 the variability between subjects in the opening of intrapulmonary arteriovenous anastomoses for any given arterial oxygen saturation may be linked to variability in susceptibility to embolic insults between individuals (Fig. 7).
Fig. 5.
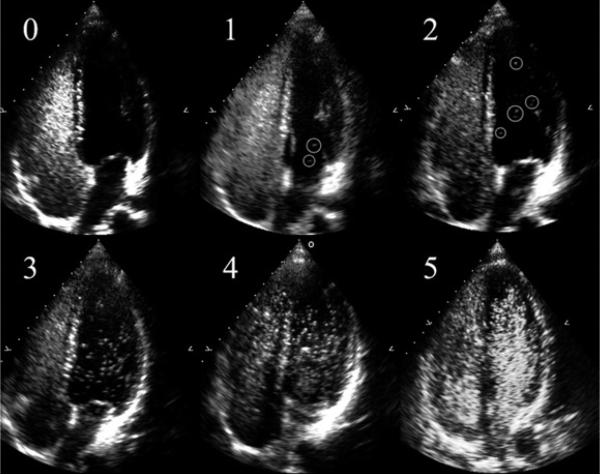
Transpulmonary passage of saline contrast microbubbles at rest in hypoxia. Representative echocardiograms of bubble scores 0–4 in a single subject at rest breathing (0) room air, (1) FIO2 = 0.16, (2) FIO2 = 0.14, (3) FIO2 = 0.12, (4) FIO2 = 0.10, and a second subject (5) with a bubble score of 5 breathing FIO2 = 0.10. Scores 1 and 2 have bubbles circled for clarity. From Laurie SS, Yang X, Elliott JE, et al. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 2010;109(4):1072–9. © Am Physiol Soc, used with permission.
Fig. 6.
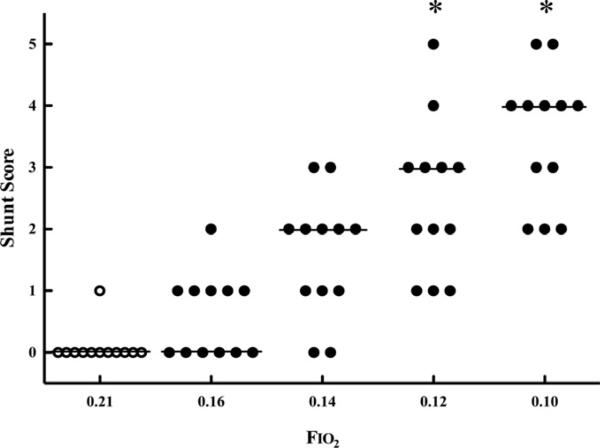
Bubble scores at rest in hypoxia. Individual subject bubble scores at rest in the left ventricle breathing FIO2 = 0.21 and after 30 min breathing FIO2 = 0.16, 0.14, 012, and 0.10. Horizontal lines indicate mode, *p < 0.01 vs. FIO2 = 0.21 (Friedman's test, Dunn's post-test). From Laurie SS, Yang X, Elliott JE, et al. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 2010;109(4):1072–9. © Am Physiol Soc, used with permission.
Fig. 7.
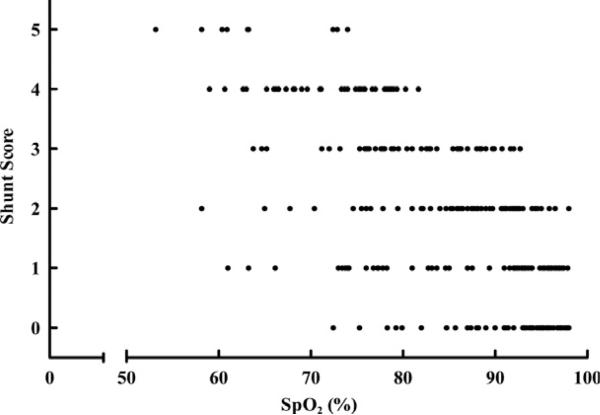
Bubble scores at rest in hypoxia as a function of arterial oxygen saturation. Bubble scores of the left ventricle at rest vs. corresponding SpO2 (n = 300). From Laurie SS, Yang X, Elliott JE, et al. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 2010;109(4):1072–9. © Am Physiol Soc, used with permission.
Of particular interest, within 15 min of returning these subjects to breathing room air, the intrapulmonary arteriovenous anastomoses closed and bubble scores returned to zero (no bubbles) regardless of the previous FIO2. Also, the degree of saline contrast bubbles able to traverse the pulmonary microcirculation was positively correlated with arterial oxygen desaturation and increased pulmonary artery systolic pressure. This suggests two things: (1) either the opening of these pathways results in a true shunt that worsens arterial hypoxemia or hypoxemia opens these pathways and (2) elevated pulmonary pressures are associated with the opening of these vessels.27 In addition to the human work outlined above, work in dogs has demonstrated that overembolization of the lung in hypoxic conditions results in a greater transpulmonary passage of microspheres than in normoxia.47 In combination, these studies reveal a clear link between arterial hypoxemia and a breach in the filtering ability of the pulmonary microcirculation.
Although the exact mechanisms controlling the patency of these inducible intrapulmonary arteriovenous anastomoses remain unknown, it is clear that oxygen is playing an important role in their regulation. It is possible that these vessels are regulated via a mechanism similar to that of the ductus arteriosus, a fetal vessel in the pulmonary circulation which is patent in the hypoxic in utero environment (PvO2 ~ 17)61 and closes after birth when the PvO2 in the pulmonary vasculature increases to ~40 Torr with the onset of room air breathing. Interestingly, breathing hypoxia at rest reduces the PvO2 from ~40 Torr to less than 30 Torr,79 a level similar to that which occurs during submaximal exercise. Thus, reductions in the PvO2 represent a commonality between exercise and hypoxia that could be responsible for controlling the patency of intrapulmonary arteriovenous anastomoses. Accordingly, an oxygen sensing location that responds to changes in PvO2, caused by exercise and/or changes in FIO2, may exist in the pulmonary arterial circulation and may regulate the patency of intrapulmonary arteriovenous anastomoses.
Validity of saline contrast echocardiography in subjects submitted to hypoxia and hyperoxia
Much of the work described above regarding intrapulmonary arteriovenous anastomoses and their regulation is based on evidence from saline contrast echocardiography. Critics of our work using saline contrast echocardiography to detect the patency of intrapulmonary arteriovenous anastomoses are skeptical that changes in the FIO2 alone are responsible for the opening and closing of these pathways, despite the fact that our work is supported by animal studies utilizing gold standard techniques such as microspheres.10,39,47 For example, microspheres(25mm) traverse the pulmonary circulation during exercise but not at rest in dogs.66 Likewise, the percentage of albumin microspheres and macroaggregates that traverse the pulmonary circulation increases during exercise in healthy humans32,83 and this is consistent with human bubble method data.9,11,25,33,67 Furthermore, the transpulmonary passage of microspheres (60–420 mm) increases under hypoxic conditions and decreases under hyperoxic conditions in dogs.47 Again, this is all consistent with human bubble method data.11,27,33,34
Despite these studies using microspheres which strongly support the saline contrast echocardiography data, they argue that our results can be explained by altered in vivo bubble dynamics caused by changing the FIO2.30 Their rationale is that if saline contrast bubbles are created from room air, the internal partial pressure environment of the bubbles is held constant, but changing the inspired oxygen tension results in an alteration to the external partial pressure environment, subsequently altering bubble stability. Therefore, depending on the FIO2, room air bubbles could either grow in size to become larger than patent intrapulmonary arteriovenous pathways, and thus get filtered out, or shrink in size and dissolve prior to reaching the left heart. In either case, no left-sided contrast would be detected whether intrapulmonary arteriovenous pathways are opened or closed.2,17,74,75
The theoretical basis behind this critique is best explained by examining the partial pressure changes that occur in the blood of subjects breathing FIO2 = 1.0. In this scenario, the PN2 within the venous and arterial blood would be very low, while the PO2 within the mixed venous blood would be ~48 Torr. Saline contrast bubbles created from room air have an internal PN2 and PO2 of ~590 Torr and ~150 Torr, respectively. Despite the slowly permeating characteristics of nitrogen, there exists a very large “sink” for the diffusion of nitrogen out of the bubbles and into the blood. Thus, when breathing 100% O2, the nitrogen within intravenously injected bubbles created from room air should very quickly diffuse out of the bubbles and cause them to dissolve down to ~20% of their original size.16,17,74,89–91 Subsequently, other influences (e.g. surface tension, pressure, and flow), should further promote their rapid dissolution before the bubbles ever reach the left heart, regardless of the patency of intrapulmonary arteriovenous anastomoses.40,71,81
Recently, we directly tested these theoretical critiques through a series of experiments designed to determine if they were valid. Using saline contrast echocardiography and our previously published scoring system27,34 these studies evaluated the degree of intrapulmonary arteriovenous shunt during exercise at 60% VO2peak while altering the internal and external partial pressures of the saline contrast bubbles. Subjects performed exercise on a cycle ergometer while breathing either room air, an FIO2 = 0.14 or an FIO2 = 1.0, to alter the partial pressures of oxygen in the blood. Additionally, five saline contrast injections were done during each exercise bout and each injection was made with a different gas (either room air, 100% O2, 100% N2, 100% CO2 or 100% He) to alter the internal partial pressures of the bubbles. The resulting bubble scores within each FIO2 produced identical results, regardless of the initial bubble gas composition.11 These data demonstrate that intrapulmonary arteriovenous anastomoses are in fact opening and closing in response to changes in the FIO2 and that our results are not simply due to changes in bubble dynamics.11,20 Thus, the data collected by our group support the use of room air bubbles in subjects breathing any FIO2 and validates our previous work demonstrating a link between arterial hypoxemia and a breach in the filtering ability of the pulmonary microcirculation via patent hypoxia-induced intrapulmonary arteriovenous anastomoses.27
Proposed physiological and pathophysiologic roles of these vessels
The exact role of intrapulmonary arteriovenous anastomoses remains controversial.19,20,30,31 With respect to physiological roles, we have previously hypothesized that they may be remnant fetal vessels as they would be beneficial for life in utero because their ability to shunt blood away from the pulmonary capillaries when the lung is not being used for gas exchange.33–35 Support for this idea comes from studies done in fetal lambs demonstrating the existence of intrapulmonary arteriovenous anastomoses that close as the lamb matures.38 Furthermore, the existence of these pathways in baboon lungs suggests that they must have some evolutionary advantage since humans, gorillas and chimpanzees diverged from the old world monkeys (baboons, macaques, etc.) approximately 25 to 30 million years ago.14,53,64 Despite the apparent evolutionary advantage of these vessels, we have suggested a negative physiological role for these pathways as well. Specifically, as a shunt vessel that bypasses the pulmonary capillaries preventing gas exchange from occurring.9,27,33,67 Stickland and colleagues demonstrated a correlation between shunt and pulmonary gas exchange efficiency during exercise,65,67 implicating these vessels as major contributors to the reduction in pulmonary gas exchange efficiency that occurs during exercise in all healthy humans.7 However, none of these proposed roles have been directly demonstrated.
With respect to pathophysiologic roles, we have also proposed that these dynamic intrapulmonary arteriovenous anastomoses may provide a pathway for emboli to circumvent the pulmonary microcirculation. However, if these pathways are open during exercise and when breathing a low FIO2, why are there not more people who exercise and who travel to high altitude who also suffer neurological insults? We believe there are several lines of evidence that can explain this apparent discrepancy. First, many healthy and physically fit people, like the ones expected to exercise and travel to high altitudes, have healthy clotting profiles compared to less physically active individuals.86 Thus, they are less likely to clot. Also, any prothrombotic responses augmented by exercise are counteracted by concomitant release of soluble nucleotide-inactivating enzymes,92 preventing any clotting from occurring. Second, we have measured the percentage of cardiac output traveling through these pathways to be ~2% in healthy humans at maximal exercise.32 Thus, despite being potentially open in healthy people on a regular basis, the fact that healthy people are less likely to clot and <3% of their cardiac output travels through these pathways prevents the majority of people from experiencing serious neurological consequences. Nevertheless, exercise is known to induce stroke and migraines in healthy young adults37,45,62 with an increased incidence at altitude in otherwise healthy individuals.1 Therefore, these vessels may play a pathophysiologic role in some healthy humans, but clearly more research in this area is needed.
Conditions associated with arterial hypoxemia & embolic insults
Our data strongly support the idea that hypoxemia opens intrapulmonary arteriovenous anastomoses which may lead to a breach in the pulmonary filter in healthy humans. However, there are also several diseases associated with arterial hypoxemia and neurologic insults of embolic origin. One of these diseases is hereditary hemorrhagic telangiectasia (HHT). Patients with HHT are more likely to have large diameter arteriovenous malformations (AVMs), including grossly distended capillaries22,29,36,68 that often result in low arterial oxygen saturations. Thus, even without a PFO in these individuals, there is a breach in the pulmonary capillary filter caused by AVMs and possibly hypoxia-inducible intrapulmonary arteriovenous anastomoses that would allow for emboli to enter into the systemic circulation. Not surprisingly, patients with HHT also are at an increased risk for neurological insults such as stroke and migraines.22,68
Patients with chronic obstructive pulmonary disease (COPD) and arterial hypoxemia, with and without a PFO, have an increased risk of developing neurological insults of embolic origin such as stroke and transient ischemic attacks, as well as ischemic heart disease.6,51,60,72 Individuals with COPD are also prothrombotic, potentially increasing the number of blood clots in their circulation.5,12,80 However, the reason for the elevated risk of embolic neurological insults in this patient population remains unknown, as it is generally accepted that the lung filters out blood clots. Our previous work has clearly established that intrapulmonary arteriovenous anastomoses are opened with arterial hypoxemia in healthy adult humans.27,33 Thus, it is tempting to speculate that patients with COPD or other lung diseases and concomitant arterial hypoxemia may have patent intrapulmonary arteriovenous pathways at rest, as a result of their underlying arterial hypoxemia.27,33 Based on our previous research demonstrating that these pathways are closed with normal resting arterial oxygen saturations, judicious supplemental oxygen therapy could significantly reduce the risk of neurological insults in patients with COPD and other diseases associated with arterial hypoxemia.
To summarize, conditions favoring paradoxical embolization such as open intrapulmonary arteriovenous pathways, arterial hypoxemia, clotting problems, or surgically-induced emboli and/or large percentages of blood flow traveling through these pathways may put individuals at an increased risk of neurological sequelae. Thus, care should be taken to avert these conditions, if possible, in order to prevent embolic injury.
Future directions & prevention of neurological insults following surgery
The previous sections of this review provided evidence for the existence of intrapulmonary arteriovenous anastomoses and focused on identifying the conditions under which these vessels are open or closed. The sections below are intended to inform surgeons, anesthesiologists and other clinical specialists what could be done to minimize the possibility that these pathways may be open during an interventional procedure.
Arterial oxygen saturation
We have established that maintaining normal, or better than normal arterial oxygen tension keeps these large diameter pathways closed. Our previous work has shown that in some individuals significant amounts of bubbles get through the pulmonary circulation when saturations are as high as 95%. Thus, one very simple method of keeping these pathways closed may be to keep patients on supplemental oxygen to keep saturations as high as possible. Our lab group continues to investigate the mechanisms involved in the oxygen mediation of these dynamic pathways.
Body positioning
Work by Stickland and colleagues demonstrated that body positioning can play a role in opening these pathways, specifically in the supine position.67 These data suggest that perfusion of the apices of the lung may open shunts and this is supported by the work of Tobin & Zariquiey who demonstrated that shunts 20 to 500 mm are located in the apex of the lung.70 Therefore, simply changing patient orientation to the reverse-Trendelenburg position may reduce the chances that these pathways would be open during an interventional procedure. Future work investigating the role of body positioning during interventional procedures on the long-term neurological outcome of patients may have a significant impact on the quality of life for patients undergoing major surgical procedures.
Identification of pre-existing conditions favorable for embolization
Conditions favorable for paradoxical embolization include: compromising the pulmonary capillary filter, arterial hypoxemia, excessive formation or introduction of emboli, increased pulmonary pressures and large shunt fractions. Extensive precautionary measures should be taken in patients with these characteristics, such as individuals with HHT who have pulmonary arteriovenous malformations and thus lungs with reduced filtering capabilities. Indeed, these individuals are at risk for developing neurological insults even without surgical intervention. Our work has also demonstrated a correlation between pulmonary artery systolic pressure and the patency of these pathways. Thus, conditions associated with pulmonary hypertension may also favor a breach in the pulmonary filter.
Other interventions
Currently, understanding the regulation of these pathways remains in its infancy. To our knowledge, there are no published studies demonstrating the ability to open or close these pathways with any pharmaceutical intervention. Thus, the best ways to keep these pathways closed are listed above. However, in subjects with severe lung disease, keeping arterial oxygen saturations high may not be possible. Also, some surgical procedures may demand that patients are in the Trendelenburg position or in the supine position. Thus, the need for future research aimed at better understanding the mechanisms mediating the patency of these intrapulmonary arteriovenous pathways remains vitally important. Ultimately, if these investigations fail to produce the required knowledge, then it will be necessary to develop other surgical tools and techniques to reduce emboli formation and/or to filter out potential emboli so that the risk of paradoxical embolization is reduced.
Acknowledgements
We thank Dr. Michael K. Stickland for helpful, critical review of this work.
Support: The work presented in this review that was performed by the authors has been generously supported by the following agencies:
National Institutes of Health (NIH) HL15469, NIH T32 HL07654, Oregon Health & Science University Foundation Medical Research Foundation Grant #0820, American Heart Association (AHA) Grant-in-Aid 0550176Z, AHA Scientist Development Grant 2280238, University of Oregon/Peace Health Oregon Region Translational Research Award Program and the American Physiological Society's Giles F. Filley Memorial Award for Excellence in Respiratory Physiology & Medicine.
Funding
Financial support for the authors came from Federal and National sources only. The authors received no financial support from industry or private sources. As such, only the authors had a role in making decisions about study designs, data collection, analysis, interpretation of the data, in the writing of the review and this review was an invited submission.
Footnotes
Competing interests
The authors have no conflict of interest to disclose.
References
- 1.Basnyat B, Wu T, Gertsch JH. Neurological conditions at altitude that fall outside the usual definition of altitude sickness. High Alt Med Biol. 2004;5:171–9. doi: 10.1089/1527029041352126. [DOI] [PubMed] [Google Scholar]
- 2.Burkard ME, Van Liew HD. Simulation of exchanges of multiple gases in bubbles in the body. Respir Physiol. 1994;95:131–45. doi: 10.1016/0034-5687(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 3.Butler BD, Hills BA. The lung as a filter for microbubbles. J Appl Physiol. 1979;47:537–43. doi: 10.1152/jappl.1979.47.3.537. [DOI] [PubMed] [Google Scholar]
- 4.Cheney FW, Pavlin J, Ferens J, Allen D. Effect of pulmonary microembolism on arteriovenous shunt flow. J ThoracCardiovascSurg. 1978;76:473–78. [PubMed] [Google Scholar]
- 5.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 6.De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol. 2007;14:989–92. doi: 10.1111/j.1468-1331.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87:1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- 8.Di Tullio M, Sacco RL, Gopal A, et al. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–5. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 9.Eldridge MW, Dempsey JA, Haverkamp HC, et al. Exercise-induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97:797–805. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- 10.Elliott JE, Choi Y, Lauire SS, et al. Reply to: Sonic echocardiography: what does it mean when there are no bubbles in the left ventricle? J Appl Physiol. 2010 doi: 10.1152/japplphysiol.01241.2010. doi: 10.1152/japplphysiol.01229.2010. [DOI] [PubMed] [Google Scholar]
- 11.Elliott JE, Choi Y, Laurie SS, et al. Effect of initial gas bubble composition on detection of inducible intrapulmonary arteriovenous shunt during exercise in normoxia, hypoxia or hyperoxia. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00145.2010. doi:10.1152/japplphysiol.00145.2010. [DOI] [PubMed] [Google Scholar]
- 12.Fisman EZ, Benderly M, Esper RJ, et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol. 2006;98:14–8. doi: 10.1016/j.amjcard.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Foulkes MA, Wolf PA, Price TR, et al. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–54. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 14.Goodman M, Porter CA, Czelusniak J, et al. Toward a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol. 1998;9:585–98. doi: 10.1006/mpev.1998.0495. [DOI] [PubMed] [Google Scholar]
- 15.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 16.Hlastala MP, Farhi LE. Absorption of gas bubbles in flowing blood. J Appl Physiol. 1973;35:311–6. doi: 10.1152/jappl.1973.35.3.311. [DOI] [PubMed] [Google Scholar]
- 17.Hlastala MP, Van Liew HD. Absorption of in vivo inert gas bubbles. Respir Physiol. 1975;24:147–58. doi: 10.1016/0034-5687(75)90109-7. [DOI] [PubMed] [Google Scholar]
- 18.Homma S, Di Tullio MR, Sacco RL, et al. Surgical closure of patent foramen ovale in cryptogenic stroke patients. Stroke. 1997;28:2376–81. doi: 10.1161/01.str.28.12.2376. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins SR, Olfert IM, Wagner PD. Last Word on Point:Counterpoint: exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol. 2009;107:1002. doi: 10.1152/japplphysiol.00652.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins SR, Olfert IM, Wagner PD. Point: Exercise-induced intrapulmonary shunting is imaginary. J Appl Physiol. 2009;107:993–4. doi: 10.1152/japplphysiol.91489.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjeldsen AD, Oxhoj H, Andersen PE, et al. Pulmonary arteriovenous malformations: screening procedures and pulmonary angiography in patients with hereditary hemorrhagic telangiectasia. Chest. 1999;116:432–9. doi: 10.1378/chest.116.2.432. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen AD, Oxhoj H, Andersen PE, et al. Prevalence of pulmonary arteriovenous malformations (PAVMs) and occurrence of neurological symptoms in patients with hereditary haemorrhagic telangiectasia (HHT). J Intern Med. 2000;248:255–62. doi: 10.1046/j.1365-2796.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 23.Koch S, Forteza A, Lavernia C, et al. Cerebral fat microembolism and cognitive decline after hip and knee replacement. Stroke. 2007;38:1079–81. doi: 10.1161/01.STR.0000258104.01627.50. [DOI] [PubMed] [Google Scholar]
- 24.Krahl VE, Fenn WO, Rahn H. Handbook of Physiology, section III. American Physiological Society; Washington, DC: 1964. Anatomy of the mammalian lung. pp. 213–84. [Google Scholar]
- 25.La Gerche A, Macisaac AI, Burns AT, et al. Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol. 2010;109:1307–17. doi: 10.1152/japplphysiol.00457.2010. [DOI] [PubMed] [Google Scholar]
- 26.Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002;33:706–11. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 27.Laurie SS, Yang X, Elliott JE, et al. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol. 2010;109:1072–9. doi: 10.1152/japplphysiol.00150.2010. [DOI] [PubMed] [Google Scholar]
- 28.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–52. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 29.Lee WL, Graham AF, Pugash RA, et al. Contrast echocardiography remains positive after treatment of pulmonary arteriovenous malformations. Chest. 2003;123:351–8. doi: 10.1378/chest.123.2.351. [DOI] [PubMed] [Google Scholar]
- 30.Lovering AT, Eldridge MW, Stickland MK. Counterpoint: Exercise-induced intrapulmonary shunting is real. J Appl Physiol. 2009;107:994–7. doi: 10.1152/japplphysiol.91489.2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovering AT, Eldridge MW, Stickland MK. Last Word on Point:Counterpoint: Exercise-induced intrapulmonary shunting is imaginary vs. real. J Appl Physiol. 2009;107:1003. doi: 10.1152/japplphysiol.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovering AT, Haverkamp HC, Romer LM, et al. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J Appl Physiol. 2009;106:1986–92. doi: 10.1152/japplphysiol.01357.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovering AT, Romer LM, Haverkamp HC, et al. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104:1418–25. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- 34.Lovering AT, Stickland MK, Amann M, et al. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008;586:4559–65. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25-and 50-mm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart CircPhysiol. 2007;292:H1777–81. doi: 10.1152/ajpheart.01024.2006. [DOI] [PubMed] [Google Scholar]
- 36.Mager JJ, Zanen P, Verzijlbergen F, et al. Quantification of right-to-left shunt with (99m)Tc-labelled albumin macroaggregates and 100% oxygen in patients with hereditary haemorrhagic telangiectasia. Clin Sci (Lond) 2002;102:127–34. [PubMed] [Google Scholar]
- 37.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–75. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 38.McMullan DM, Hanley FL, Cohen GA, et al. Pulmonary arteriovenous shunting in the normal fetal lung. J Am Coll Cardiol. 2004;44:1497–500. doi: 10.1016/j.jacc.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 39.Melsom MN, Flatebo T, Nicolaysen G. Hypoxia and hyperoxia both transiently affect distribution of pulmonary perfusion but not ventilation in awake sheep. Acta Physiol Scand. 1999;166:151–8. doi: 10.1046/j.1365-201x.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer RS, Sartorius OE, Lancee CT, et al. Transmission of ultrasonic contrast through the lungs. Ultrasound Med Biol. 1981;7:377–84. doi: 10.1016/0301-5629(81)90048-x. [DOI] [PubMed] [Google Scholar]
- 41.Meltzer RS, Tickner EG, Popp RL. Why do the lungs clear ultrasonic contrast? Ultrasound Med Biol. 1980;6:263–9. doi: 10.1016/0301-5629(80)90022-8. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer RS, Tickner EG, Sahines TP, Popp RL. The source of ultrasound contrast effect. J Clin Ultrasound. 1980;8:121–7. doi: 10.1002/jcu.1870080205. [DOI] [PubMed] [Google Scholar]
- 43.Motley HL, Cournand A, et al. The influence of short periods of induced acute anoxia upon pulmonary artery pressures in man. Am J Physiol. 1947;150:315–20. doi: 10.1152/ajplegacy.1947.150.2.315. [DOI] [PubMed] [Google Scholar]
- 44.Movsowitz C, Podolsky LA, Meyerowitz CB, et al. Patent foramen ovale: a nonfunctional embryological remnant or a potential cause of significant pathology? J Am Soc Echocardiogr. 1992;5:259–70. doi: 10.1016/s0894-7317(14)80346-5. [DOI] [PubMed] [Google Scholar]
- 45.Nadelson C. Sport and exercise-induced migraines. Curr Sports Med Rep. 2006;5:29–33. doi: 10.1097/01.csmr.0000306516.25172.21. [DOI] [PubMed] [Google Scholar]
- 46.Nanthakumar K, Graham AT, Robinson TI, et al. Contrast echocardiography for detection of pulmonary arteriovenous malformations. Am Heart J. 2001;141:243–6. doi: 10.1067/mhj.2001.112682. [DOI] [PubMed] [Google Scholar]
- 47.Niden AH, Aviado DM., Jr Effects of pulmonary embolism on the pulmonary circulation with special reference to arteriovenous shunts in the lung. Circ Res. 1956;4:67–73. doi: 10.1161/01.res.4.1.67. [DOI] [PubMed] [Google Scholar]
- 48.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case–control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 49.Parikh SS, Chung F. Postoperative delirium in the elderly. Anesth Analg. 1995;80:1223–32. doi: 10.1097/00000539-199506000-00027. [DOI] [PubMed] [Google Scholar]
- 50.Pavlin DJ, Ferens J, Allen DR, Cheney FW. Pulmonary arteriovenous shunts during halothane anesthesia in dogs. Br J Anaesth. 1980;52:763–8. doi: 10.1093/bja/52.8.763. [DOI] [PubMed] [Google Scholar]
- 51.Petty GW, Khandheria BK, Chu CP, et al. Patent foramen ovale in patients with cerebral infarction. A transesophageal echocardiographic study. Arch Neurol. 1997;54:819–22. doi: 10.1001/archneur.1997.00550190013008. [DOI] [PubMed] [Google Scholar]
- 52.Prinzmetal M, Ornitz ME, Simkin B, Bergman HC. Arterio-venous anastomoses in liver, spleen and lungs. Am J Physiol. 1948;152:48–52. doi: 10.1152/ajplegacy.1947.152.1.48. [DOI] [PubMed] [Google Scholar]
- 53.Purvis A. A composite estimate of primate phylogeny. Philos Trans R Soc Lond B Biol Sci. 1995;348:405–21. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- 54.Ranoux D, Cohen A, Cabanes L, et al. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke. 1993;24:31–4. doi: 10.1161/01.str.24.1.31. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez RA, Tellier A, Grabowski J, et al. Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty. 2005;20:763–71. doi: 10.1016/j.arth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Roelandt J. Contrast echocardiography. Ultrasound Med Biol. 1982;8:471–92. doi: 10.1016/0301-5629(82)90079-5. [DOI] [PubMed] [Google Scholar]
- 57.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–90. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 58.Sappey PC. Traité d'anatomie descriptive. V.A. Delahaye; Paris: 1879. [Google Scholar]
- 59.Sardesai SH, Marshall RJ, Mourant AJ. Paradoxical systemic embolisation through a patent foramen ovale. Lancet. 1989;1:732–3. doi: 10.1016/s0140-6736(89)92253-8. [DOI] [PubMed] [Google Scholar]
- 60.Sidney S, Sorel M, Quesenberry CP, Jr, et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 61.Siggaard-Andersen O, Huch R. The oxygen status of fetal blood. Acta Anaesthesiol Scand Suppl. 1995;107:129–35. doi: 10.1111/j.1399-6576.1995.tb04347.x. [DOI] [PubMed] [Google Scholar]
- 62.Stern BJ, Kittner S, Sloan M, et al. Stroke in the young. Part I. Md Med J. 1991;40:453–62. [PubMed] [Google Scholar]
- 63.Stern BJ, Kittner S, Sloan M, et al. Stroke in the young. Part II. Md Med J. 1991;40:565–71. [PubMed] [Google Scholar]
- 64.Stewart CB, Disotell TR. Primate evolution – in and out of Africa. Curr Biol. 1998;8:R582–88. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- 65.Stickland MK, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34:99–106. doi: 10.1249/00003677-200607000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Stickland MK, Lovering AT, Eldridge MW. Exercise-induced arteriovenous intrapulmonary shunting in dogs. Am J Respir Crit Care Med. 2007;176:300–5. doi: 10.1164/rccm.200702-206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stickland MK, Welsh RC, Haykowsky MJ, et al. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561:321–9. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thenganatt J, Schneiderman J, Hyland RH, et al. Migraines linked to intrapulmonary right-to-left shunt. Headache. 2006;46:439–43. doi: 10.1111/j.1526-4610.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 69.Tobin CE. Arteriovenous shunts in the peripheral pulmonary circulation in the human lung. Thorax. 1966;21:197–204. doi: 10.1136/thx.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tobin CE, Zariquiey MO. Arteriovenous shunts in the human lung. Proc Soc Exp Biol Med. 1950;75:827–9. doi: 10.3181/00379727-75-18360. [DOI] [PubMed] [Google Scholar]
- 71.Tsujino T, Shima A. The behaviour of gas bubbles in blood subjected to an oscillating pressure. J Biomech. 1980;13:407–16. doi: 10.1016/0021-9290(80)90034-2. [DOI] [PubMed] [Google Scholar]
- 72.van Dijk EJ, Vermeer SE, de Groot JC, et al. Arterial oxygen saturation, COPD, and cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75:733–6. doi: 10.1136/jnnp.2003.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Gent MW, Post MC, Luermans JG, et al. Screening for pulmonary arteriovenous malformations using transthoracic contrast echocardiography: a prospective study. Eur Respir J. 2009;33:85–91. doi: 10.1183/09031936.00049008. [DOI] [PubMed] [Google Scholar]
- 74.Van Liew HD, Burkard ME. Bubbles in circulating blood: stabilization and simulations of cyclic changes of size and content. J Appl Physiol. 1995;79:1379–85. doi: 10.1152/jappl.1995.79.4.1379. [DOI] [PubMed] [Google Scholar]
- 75.Van Liew HD, Vann RD. Sonic echocardiography: what does it mean when there are no bubbles in the left ventricle? J Appl Physiol. 2010 doi: 10.1152/japplphysiol.01241.2010. doi: 10.1152/ japplphysiol.01241.2010. [DOI] [PubMed] [Google Scholar]
- 76.von Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12:301–20. [Google Scholar]
- 77.von Hayek H. Translated from Die Menschliche Lunge by V.E. Krahl. Hafner; New York and London: 1960. The Human Lung. [Google Scholar]
- 78.von Hayek H. Über einen Kurzschlusskreislauf (arterio-venöse Anastomosen) in der menschlichen Lunge. Z Anat EntwGesch. 1940;110:412–22. [Google Scholar]
- 79.Wagner PD, Gale GE, Moon RE, et al. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–70. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- 80.Wedzicha JA, Seemungal TA, MacCallum PK, et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84:210–5. [PubMed] [Google Scholar]
- 81.Weinberg M. Surface-tension effects in gas bubble dissolution and growth. Chem Eng Sci. 1981;36:407–16. [Google Scholar]
- 82.White RI, Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988;169:663–9. doi: 10.1148/radiology.169.3.3186989. [DOI] [PubMed] [Google Scholar]
- 83.Whyte MK, Peters AM, Hughes JM, et al. Quantification of right to left shunt at rest and during exercise in patients with pulmonary arteriovenous malformations. Thorax. 1992;47:790–6. doi: 10.1136/thx.47.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilkinson MJ, Fagan DG. Postmortem demonstration of intrapulmonary arteriovenous shunting. Arch Dis Child. 1990;65:435–7. doi: 10.1136/adc.65.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilmshurst P, Nightingale S. Relationship between migraine and cardiac and pulmonary right-to-left shunts. Clin Sci (Lond) 2001;100:215–20. [PubMed] [Google Scholar]
- 86.Womack CJ, Nagelkirk PR, Coughlin AM. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003;33:795–807. doi: 10.2165/00007256-200333110-00002. [DOI] [PubMed] [Google Scholar]
- 87.Woods TD, Harmann L, Purath T, et al. Small and moderate size right-to-left shunts identified by saline contrast echocardiography are normal and unrelated to migraine headache. Chest. 2010;138:264–9. doi: 10.1378/chest.09-2797. [DOI] [PubMed] [Google Scholar]
- 88.Woods TD, Patel A. A critical review of patent foramen ovale detection using saline contrast echocardiography: when bubbles lie. J Am SocEchocardiogr. 2006;19:215–22. doi: 10.1016/j.echo.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 89.Yang WJ. Dynamics of gas bubbles in whole blood and plasma. J Biomech. 1971;4:119–25. doi: 10.1016/0021-9290(71)90022-4. [DOI] [PubMed] [Google Scholar]
- 90.Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. I. Stationary bubbles. J Biomech. 1971;4:275–81. doi: 10.1016/0021-9290(71)90033-9. [DOI] [PubMed] [Google Scholar]
- 91.Yang WJ, Echigo R, Wotton DR, Hwang JB. Experimental studies of the dissolution of gas bubbles in whole blood and plasma. II. Moving bubbles or liquids. J Biomech. 1971;4:283–8. doi: 10.1016/0021-9290(71)90034-0. [DOI] [PubMed] [Google Scholar]
- 92.Yegutkin GG, Samburski SS, Mortensen SP, et al. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–64. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]