Abstract
The avian song control system provides an excellent model for studying transsynaptic trophic effects of steroid sex hormones. Seasonal changes in systemic testosterone (T) and its metabolites regulate plasticity of this system. Steroids interact with the neurotrophin brain-derived neurotrophic factor (BDNF) to influence cellular processes of plasticity in nucleus HVC of adult birds, including the addition of newborn neurons. This interaction may also occur transsynpatically; T increases the synthesis of BDNF in HVC, and BDNF protein is then released by HVC neurons on to postsynaptic cells in nucleus RA where it has trophic effects on activity and morphology. Androgen action on RA neurons increases their activity and this has a retrograde trophic effect on the addition of new neurons to HVC. The functional linkage of sex steroids to BDNF may be of adaptive value in regulating the trophic effects of the neurotrophin and coordinating circuit function in reproductively relevant contexts.
Keywords: Bird, song, songbird, plasticity, testosterone, estrogen, steroid, season, neurogenesis, neurotrophin, BDNF
Introduction
Seasonal changes of the environment that are critical to survival and reproduction have a pronounced effect on birds and essentially all other animals. It is therefore not surprising that seasonal plasticity of the adult brain has been observed in every vertebrate taxon [1]. The avian song control system provides the best model for studying the mechanisms and functional significance of seasonal plasticity in brain and behavior, with changes that are the most pronounced yet observed in any vertebrate model. Song is a learned stereotyped behavior that can be quantitatively analyzed, it is regulated by well-identified neural circuits, and testosterone (T) and its androgenic and estrogenic metabolites exert a strong influence on the morphology and physiology of these neural circuits.
Sex steroids released by the gonads or synthesized in the brain can serve various functions in the adult nervous system: 1) they activate neurons in sexually dimorphic brain regions to produce sex-typical behaviors; 2) hormones provide a means of restricting neural activity to appropriate environmental and physiological conditions, as seen in hormone-regulated growth of the song control system in birds early in the breeding season; and 3) secretion of steroids by the gonads and transport to the brain can coordinate neural and behavioral activation with reproductive physiology. An example of this latter role is the vernal enhancement of song production by T in male birds to attract and stimulate mates at the time of year when the reproductive axis is optimized for breeding in both sexes [2]. Steroids may act directly on neurons to have these effects. Neurons in limbic and other regions of the brain often have nuclear and/or membrane-bound receptors for different steroids. It has become clear recently that steroids may also have indirect actions on brain regions through transsynaptic mechanisms, even if neurons in the target regions lack steroid receptors. Transsynaptic trophic effects of steroids have been demonstrated most clearly in the context of seasonal plasticity of the adult avian song system, and that will be the focus of this review.
The avian song control system
Song is a learned behavior that is widely produced among the 4000 species of oscine songbirds and in many other avian taxa [3]. Songs have well-defined acoustic structures that are characteristic of each species. In most species that breed in temperate and high latitude regions, song is produced largely or only by males. In many tropical species, however, females also sing and may join with males in producing complex vocal duets. There is extensive taxonomic diversity in the age when song is learned [4; 5]. In many species, referred to as age-limited learners, song learning is completed by the onset of sexual maturity late in the first year of life. Other species, known as open-ended learners, may continue to learn new songs as adults. Song plays important roles in avian reproduction [3]. In many species, song is used to declare a territory from which other birds are aggressively excluded. Both males and females may use song in this context. In most songbird species males also use song to attract females. Females may select among potential mates on the basis of individual song characteristics. The male’s song may directly stimulate reproductive behavior in females. In addition to these two main functions, song may be used in other behavioral contexts. For example, song may be important in mediating dominance behavior among members of a social group.
Song behavior is regulated by a discrete network of interconnected nuclei that arises in the pallium, projects to brainstem nuclei that control vocalization and respiration, and receives input from pallial auditory regions. (Some authors regard the avian pallium as homologous to mammalian cortex [6; 7].) The song control system is organized into two functional pathways [for review see 8, Figure 1]. The main pathway for the motor production of song in adult birds includes projections from HVC to RA in the telencephalon, and from RA to nXIIts in the medulla, which innervates the muscles of the sound-producing organ, the syrinx, and brainstem respiratory pre-motor nuclei. The anterior forebrain pathway (AFP) includes HVC, area X in the striatum, DLM in the thalamus, LMAN in the pallium, and RA; it is analogous to the mammalian basal ganglia-thalamocortical circuit [9]. The AFP is essential for song learning, for the motor production of song in juveniles [10], and for adult song variability [reviewed in 11].
Figure 1.
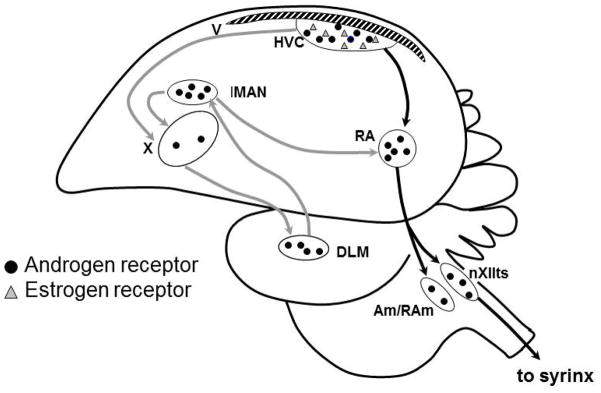
Simplified schematic sagittal view of the avian song control system showing the distribution of steroid receptors. Black arrows connect nuclei in the main descending motor circuit, and gray arrows connect nuclei in the anterior forebrain circuit. Abbreviations: AM- nucleus ambiguus; DLM, Dorsolateral nucleus of the medial thalamus; lMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; nXIIts, the tracheosyringeal portion of the hypoglossal nucleus; RA, the robust nucleus of the arcopallium; RAm – nucleus retroambigualis; syrinx, vocal production organ; V, lateral ventricle; X, area X of the medial striatum.
Steroid sex hormones affect song learning and production, and the juvenile development and adult plasticity of the song circuits [reviewed in 12]. In those species examined thus far, nuclear androgen receptors are present in the song nuclei HVC, RA, LMAN, area X, ICo (intercollicular nucleus), and nXIIts, as well as in the muscles of the syrinx. Classical nuclear estrogen receptors are found in HVC and ICo [Figure 1, reviewed in 12].
Seasonality of breeding and song behavior in birds
Photoperiod is the primary environmental factor that influences activation of the avian reproductive system. In arctic, temperate, and subtropical birds, breeding is usually restricted to spring and early summer. Reproduction may also be seasonal in tropical species in which there are seasonal changes in environmental factors such as rainfall that influence breeding. Song behavior occurs most often or only in the breeding season in most species.
Seasonal plasticity in the brain
Seasonal changes in brain structure were first reported in the song system of domestic canaries by Nottebohm [13]. The song control system provides the most pronounced example of seasonal plasticity in an adult brain, and is the leading model for study of this process.
Seasonal changes in song behavior are accompanied by changes in the morphology of song nuclei in essentially every seasonally breeding songbird species that has been examined, including Rufous-collared Sparrows (Zonotrichia capensis) that breed seasonally in the foothills of the Andes on the equator [14]. The volumes of HVC, RA, X, and nXIIts increase by up to 200% during the breeding season in both open-ended and closed-ended song learners. Cellular attributes of song regions also change [reviewed in 15]. These seasonal changes can be observed in vivo using fMRI [16]. The spontaneous neurophysiological activity of RA neurons is greater in breeding White-crowned Sparrows (Zonotrichia leucophrys) and Song Sparrows (Melospiza melodia) than non-breeding birds [17; 18; 19].
Neuron number in HVC also changes seasonally. In wild-caught Song Sparrows, for example, neuron number in HVC increases from about 150,000 in the fall to 250,000 during the breeding season, a 67% increase [20]. This change in neuron number results from seasonal patterns of cell death and ongoing neurogenesis. At the end of the breeding season, circulating T levels drop and there is an increase in the death of mature HVC neurons [21; 22]. There is a subsequent increase in the addition of new neurons to HVC in nonbreeding birds [23; 24]. The death of mature HVC neurons increases the addition of new neurons to HVC [25; 26], and does so by increasing neural stem cell proliferation in the adjacent ventricular zone [27].
Seasonal changes in song behavior
Seasonal changes in various aspects of song behavior accompany plasticity of the song circuits. In some species of birds, such as the Spotted Towhee (Pipilo maculatus) and Sedge Warbler (Acrocephalus schoenobaenus), song is produced only during the breeding season and is absent at other times of year. Other species, such as Song Sparrows, White-crowned Sparrows, and Canaries, sing throughout most of the year. Even in these year-round singers, however, song is produced at much higher rates during the breeding season.
Song structure also changes seasonally in year-round singers. Songs typically become more variable after the breeding season. The morphology of individual song syllables becomes less stereotyped in species including Canaries, Song Sparrows, and White-crowned Sparrows [20; 28; 29; 30]. Song Sparrows sing a greater number of variations of specific song types outside the breeding season [20]. Songs also are shorter in nonbreeding Song Sparrows, White-Crowned Sparrows, and wild Island Canaries [20; 29; 31]. The stereotypy of song duration and of the “fee” note of Black-capped Chickadees (Poecile atricapillus) is greater during the breeding season [32]. Stable song produced during the breeding season, however, does not change in structure from year to year in closed-ended learners like Song Sparrows and White-crowned Sparrows [33; 34].
Adaptive value of seasonal plasticity
What is the adaptive value of the extensive seasonal changes observed in the song circuits? Regrowing the song system each spring must impose an energetic cost. Is the cost of such yearly growth outweighed by some advantage that is gained? Other hormone sensitive regions of the avian brain, such as the hippocampus, do not undergo the seasonal regression and growth characteristic of the song system [35].
One hypothesis of the benefit of seasonal plasticity was presented by Nottebohm [13] in the original study of this phenomenon in Canaries. Male Canaries develop new song patterns as adults, and do so in a seasonal manner. Nottebohm proposed that the plasticity associated with the seasonal changes in the song nuclei provides a neural basis for this adult song learning. He predicted that seasonal plasticity would be restricted to open-ended song learning species. Comparable patterns of seasonal changes in the song system, however, have been observed in essentially every species of seasonally breeding songbird examined thus far, regardless of whether they are open-ended or age-limited song learners. Thus, while seasonal plasticity may be necessary for adult song learning, it is not sufficient for such learning to occur and is therefore unlikely to have evolved explicitly as a mechanism for learning.
An alternative hypothes is that seasonal plasticity may be an adaptation to reduce the energetic costs imposed by the song system in the fall and winter. Oustide the breeding season, there is no need to produce frequent, stereotyped song for mate attraction and territorial defense. At the same time, birds may experience the energetic stress of feather molting (i.e., replacement), migration, increased thermoregulatory demands, and decreased food availability. Songbirds are relatively small animals with large surface area to volume ratios, and are therefore particularly subject to energetic constraints [36]. Given that the brain requires large amounts of energy to maintain signaling activities ([see 37]), regression of the song system outside the breeding season reduces the energetic costs imposed by the song nuclei and this is probably advantageous for birds. On balance, the reduced energy required by a regressed song system throughout the fall and winter may outweigh the energy required to regrow it the following spring.
Seasonal plasticity of the song control circuits, which appears to be widespread among the songbirds and is therefore perhaps an ancestral trait, may have served as a preadaptation that enabled the evolution of adult song learning in some species. In this view, the neural and behavioral plasticity that occurs with seasonal changes of the song system is necessary but not sufficient for adults to develop new songs. In ecological contexts where the development of new songs in adults may be adaptive, as in indicating a male’s relative age to potential mates, or in allowing birds to match the songs of new territorial neighbors, the plasticity associated with seasonal changes may be exploited to support the learning of new songs [see 15 for more extensive discussion].
Sex steroid influences on seasonal plasticity
T and its metabolites influence the development of song behavior and the song control circuits in juvenile birds [38]. These hormones also regulate seasonal plasticity of the song system. The secretion and metabolism of sex steroids vary with season; plasma T levels in male birds are high during breeding, and low after breeding. These seasonal changes in circulating hormone levels modulate song production (most birds sing frequently in the breeding season, and less or not at all outside of breeding), and are correlated with the morphological changes in the song control regions (which are fully grown when T levels are high, and regressed when T is low) [reviewed in 15; 39].
The seasonal changes in the song systems of the sparrow species that we study are primarily regulated by changes in gonadal T and its metabolites [15]. Smith et al. [40] showed that T induced volumetric and neuronal growth in HVC, RA, X, and nXIIts in castrated Gambels’ White-crowned Sparrows housed on either long days (LD) or short days (SD) [see also 41]. Other factors, such as social cues and photoperiod, have been reported to contribute to seasonal plasticity of the song system [39; 42; 43; 44; 45; 46]. The relative contributions of these different factors may vary among species. Caution should be used in invoking species differences, however, given the complex nature of interactions between sex steroids, neural and muscular function, gene expression, and behavior [see 15, pages 342–344 for discussion].
An LD photoperiod in the absence of a T implant induced only small increases in neuron size and spacing in RA. Other studies are consistent with the conclusion that growth of the song nuclei is due primarily to increased levels of T and its metabolites in breeding birds [e.g., 41; 47].
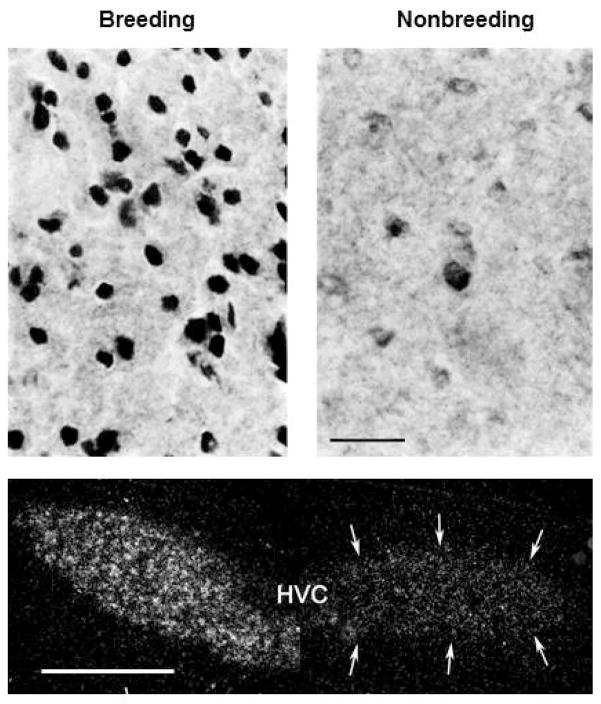
The sensitivity of neurons in song nuclei to sex steroids varies seasonally. Androgen receptor mRNA and protein in HVC are expressed at higher levels in breeding-condition birds [Figure 2 48; 49; 50]. The expression of ER mRNA by cells in HVC is greater in breeding canaries [49].
Figure 2.
Seasonal changes in androgen receptor expression in HVC of White-crowned Sparrows. Bottom panel: AR mRNA is expressed at higher levels in HVC of breeding birds [adapted from 50]. Scale-bar = 0.5 mm. Top panel: The density and immunostaining intensity of cells positive for AR-like protein are greater in HVC of breeding sparrows. Note that nonbreeding birds have fainter staining within cell nuclei [adapted from 48]. Scale bar = 20 μm.
Both androgenic and estrogenic metabolites of T contribute to seasonal growth of the song circuits and song production. To measure the relative contributions of androgens and estrogens to seasonal growth, castrated Gambel’s White-crowned Sparrows with regressed song nuclei were implanted with either T, the androgenic metabolite 5-α dihyrotestosterone (DHT), 17 -β estradiol (E2), or a combination of DHT+E2 [47]. All three steroid treatments stimulated growth of HVC, RA, and X, showing that androgen- and estrogen receptor binding are each sufficient to trigger seasonal growth of the song circuits, and that T’s effects may depend, in part, upon enzymatic conversion of T to active metabolites. Song production was highly variable within these treatment groups. Interestingly, birds treated with E2 alone did not sing, whereas most birds implanted with T or DHT sang. Despite the lack of song in E2-treated birds, however, this hormone induced substantial growth of the song nuclei. This observation suggests that growth of the song nuclei and song behavior are under separate hormonal control.
Additional evidence in support of an estrogenic contribution to seasonal growth of the song circuits comes from a study of wild male Song Sparrows [51]. Territorial males in both the breeding and non-breeding seasons were treated with fadrozole, an inhibitor of the aromatase enzyme which catalyzes the conversion of T to E2. In breeding males, aromatase inhibition caused the volume of HVC to decrease, and this effect was partially rescued by concurrent estrogen replacement. It is noteworthy that fadrozole decreased HVC volume even though plasma T increased in breeding males due to disrupted neuroendocrine feedback. Fadrozole-treated males sang less than controls in response to song playback and a live decoy [52]. In nonbreeding males, E2 treatment caused HVC and X to grow to maximal breeding size within two weeks. Fadrozole decreased singing behavior and E2-treatment rescued song in the nonbreeding birds [52]. Taken together the results of Soma et al. [51] and Tramontin et al. [47] strongly suggest that both estrogenic and androgenic metabolites of gonadal T contribute to seasonal growth of the song circuits and song production.
Seasonal growth and regression of the song control circuits occur rapidly and sequentially
Field studies of wild birds show that growth of the song system occurs rapidly once plasma T levels first start to rise as day length increases in late winter, and precedes full seasonal reproductive development. [20; 53]. In a laboratory study of captive White-crowned Sparrows implanted with a systemic T pellet and exposed to a long day photoperiod to mimic breeding conditions, HVC grew to its full breeding size and neuron number increased from 90,000 to 150,000, a 67% change, within 7 days. This addition of 60,000 neurons to an adult brain region within such a short time period is unprecedented. HVC’s efferent targets, RA and area X, grew more slowly and were not significantly larger until 20 days after T implant [30]. These studies indicate that the song control circuitry grows rapidly and sequentially in response to seasonal hormonal cues.
Regression of the song system at the end of the breeding season also occurs rapidly and sequentially. HVC and RA in the wild Song Sparrows studied by Smith et al. [20] were already fully regressed in the early fall, just after the prebasic feather molt. We investigated the time course of seasonal regression of the song circuits in Gambel’s White-crowned Sparrows in the laboratory [22]. HVC regressed fully to a nonbreeding size by two days after withdrawing T. HVC lost over 40% of its neurons by two days as a result of caspase-mediated apoptosis [22; 54]. RA and X did not fully regress until day 20. These efferent targets therefore regress more slowly than does HVC.
Site(s) of hormone action
We can ask which nuclei within the song circuits are directly targeted to initiate growth; steroid receptors or their mRNA are present in all of the major song nuclei (Figure 1). Some insight is provided by the observation that HVC grows rapidly and its efferent targets RA and X change more slowly [22; 30]. This sequential pattern of growth raised the hypothesis that T initially acts directly on HVC, which subsequently stimulates growth of RA and X transsynaptically. Several observations support this hypothesis. In one study we lesioned HVC on one side of the brain in nonbreeding-condition White-crowned Sparrows and exposed them to breeding T and photoperiod conditions [55]. The unilateral HVC lesions completely blocked the growth of the ipsilateral RA and X, but the intact contralateral HVC, RA and X showed normal growth. In a second study we placed small T implants unilaterally in the brain near HVC or RA in male sparrows in nonbreeding condition [56]. The T implant near HVC produced significant growth of the ipsilateral (but not contralateral) HVC, RA, and X, and increased neuronal number in the ipsilateral HVC. The T implant near RA, however, did not produce growth of the ipsilateral RA, HVC, or X. The failure of this latter implant to stimulate growth of RA is noteworthy given that this nucleus has abundant AR [50]. In a third study unilateral intracerebral infusion of DHT + E2 adjacent to HVC produced significant growth of neurons, and increased spontaneous activity, in ipsilateral but not contralateral RA. Conversely, infusion of antagonists of the androgen receptor (flutamide) and estrogen receptor (faslodex) near HVC in breeding condition birds prevented neuronal growth and blocked the increase in spontaneous activity in RA [17]. These results together indicate that T and its metabolites induce seasonal growth by acting directly on HVC, which in turn provides trophic support of growth to its efferent targets RA and X. In this case, what function do the abundant ARs in RA serve? Infusion of the AR antagonist flutamide near RA in breeding condition sparrows with a systemic T implant blocked the increased size and firing rate of RA neurons [17]. Together with the results of steroid infusions near HVC described above, this observation suggests a scenario in which T and its metabolites act on HVC neurons to stimulate them to produce a trophic signal that is released on to RA neurons, and activation of the AR in RA neurons is permissive for them to respond the trophic signal coming from HVC.
Interactions between steroid hormones and neurotrophins
Androgens and estrogens may have similar trophic effects on the developing and adult brain as do neurotrophins such as brain-derived neurotrophic factor (BDNF), and act by common cellular and molecular mechanisms [57] [58; 59]. These commonalities suggest that steroids can play an important role in regulating the expression of the genes for BDNF and its cognate tropomyosin receptor kinase B (trkB), and/or that steroids and BDNF may act by convergent pathways [57]. Steroids can regulate the expression of BDNF via the hormone response element on the promotor region of the BDNF gene [60]. It is unclear whether there is a hormone response element in the promotor region of the avian gene for BDNF (Luche and Brenowitz, unpublished observations). The neurotrophic effects of steroids and BDNF are both mediated by the PI3-kinase/AKT signaling pathway [61; 62].
BDNF is an important factor in mediating the trophic effects of T and its metabolites on HVC and its efferent targets in songbirds, both in the contexts of development and adult plasticity.
The role of BDNF in development of the song system has been studied, though incompletely, in the Zebra Finch (Taenopygia guttata), a species with extreme neural and behavioral sexual dimorphisms. Steroid sex hormones influence the development of several attributes of the song system, whereas other traits appear to be under direct genetic regulation [63; 64; 65]. Estrogens, and to a lesser extent androgens, stimulate masculine growth of the song system when they are injected into very young (1–3 week posthatching) females (Gurney and Konishi, 1980, 1981; Gurney, 1982). The masculinizing effects of sex steroids have been confirmed repeatedly. Steroids induce growth of the overall volumes of HVC, RA, Area X and lMAN, stimulate increases in neuronal size and number, and alter biochemical properties and connectivity between neurons in these nuclei [66]. Estrogens may influence masculine growth of the song system by stimulating the synthesis, transport and secretion of BDNF. BDNF mRNA begins to be expressed in HVC of males but not females between days 30–35 [67]. Expression of BDNF mRNA could be induced within 24 hr in male HVC by systemic estrogen treatment at days 15 and 20–25 but not at day 10 (Dittrich et al., 1999). This suggests that E2 is naturally responsible for the upregulation of BDNF seen in HVC of males at this time. E2 appears to act even earlier to establish the estrogen-dependent increase in BDNF. BDNF in HVC is not upregulated by systemic E2 in juvenile females, unless they are also treated with E2 shortly after hatching (Dittrich et al., 1999), at ages when estrogens have their most potent and permanent masculinizing effects on song system growth (Adkins-Regan et al., 1994). These results show an important relationship between sex steroids and BDNF, one that may remain important in the adult brain in species that retain significant neural plasticity throughout life.
Interactions between steroid hormones and BDNF are also important in mediating seasonal plasticity of the adult song system. In adult male White-crowned Sparrows BDNF is significantly upregulated in HVC following a transition from nonbreeding to breeding conditions [68]. In situ hybridization confirms that BDNF mRNA is expressed widely throughout HVC of sparrows exposed to breeding conditions and that expression increased over 7 days following initial exposure to systemic T [Figure 3 69].
Figure 3.
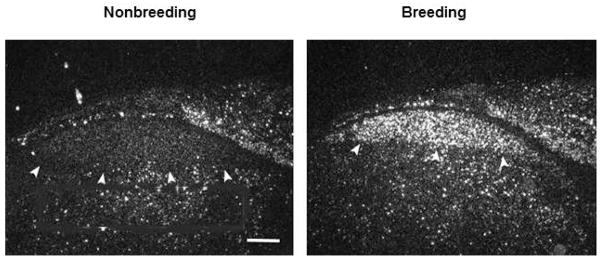
Dark-field photomicrographs of in situ hybridization showing seasonal change in BDNF mRNA expression in HVC of White-crowned Sparrows. BDNF is expressed at higher levels in HVC of breeding birds. Arrows delineate the ventral border of HVC. Scale bar, 300 μm.
BDNF supports the addition of newborn neurons to HVC in adult birds. Treatment of adult female canaries with exogenous T tripled the number of new neurons added to HVC [70; 71]. This effect of T on neuronal addition required BDNF [72]. T treatment increases both BDNF mRNA and protein in HVC cells [69; 72]. Intracerebral infusion of recombinant BDNF (rBDNF) into HVC increased the number of new neurons by the same amount as did T treatment. Infusion of a BDNF blocking antibody prevented the T-induced increase in new neurons in HVC [72]. In adult male canaries the number of new HVC neurons born during the spring decreased by 50% over the next four months [73]. Infusion of rBDNF into HVC 14–20 days after the birth of new neurons increased the number of neurons that survived for at least 8 months compared with birds that were infused with BDNF either 4–10 or 24–30 days after birth [74]
Steroid hormone interactions with BDNF occur widely in other neural systems [59, see special issue of Neuroscience (vol. 239, 2013) devoted to these interactions]. Levels of BDNF gene expression can vary with natural and experimentally induced changes in circulating steroid hormone levels, in a manner analogous to that seen seasonally in birds. In gonadally intact female rats, the expression of BDNF mRNA in CA1 and CA 3/4 of the hippocampus varies with different stages of the estrous cycle [75]. BDNF gene expression is highest on the morning of diestrus 2, when progesterone levels are highest, and lowest on the afternoon of pro-estrus, when progesterone levels are lowest. In ovariectomized rats, BDNF mRNA in the dentate granule cell layer, CA1, and CA 3/4 increases when the animals are treated with estrogen and progesterone [75].
Anterograde trophic effects
The “classical” model of neurotrophin action is for these proteins to be synthesized by peripheral targets, taken up by axon terminals, and retrogradely transported to the cell bodies where signaling cascades that support neuronal survival are activated [76]. This is the mode of action by which nerve-growth factor (NGF), the first growth factor to be discovered, stimulates neurite outgrowth from cultured chick sensory ganglia [77]. Recently, however, it has become clear that neurotrophins may also be synthesized by neurons in the central nervous system, transported anterogradely, and released by axons to influence the size and number of their post-synaptic targets [78]. This anterograde mode of action is important in seasonal growth of the avian song control circuit.
The synthesis and axonal transport of BDNF by HVC neurons likely stimulates seasonal growth and increased electrical activity of postsynaptic neurons in RA in adults birds. BDNF mRNA is expressed by cells in HVC but not RA in breeding condition sparrows [69]. Expression of the gene for the cognate tropomyosin receptor kinase B (TrkB) receptor for BDNF is expressed in RA, but does not vary seasonally [69]. Infusion of rBDNF protein into RA of nonbreeding condition sparrows stimulates growth of RA neurons [69] and increases spontaneous activity to breeding-typical levels (Wood et al., unpublished observation).
T-induced trophic support provided by HVC to its efferent targets requires the release of BDNF, and the release of and/or response of efferent neurons to BDNF is dependent on the electrical activity of neurons in these nuclei. It is not yet known whether the release of BDNF from HVC neurons occurs at axonal terminals following anterograde transport, or by exocytosis from extrasynaptic sites. Both mechanisms of BDNF release have been observed in other neural systems [79; 80; 81].
The transsynaptic action of BDNF on RA is activity dependent. Infusion of tetrodotoxin (TTX) near RA in a breeding-condition White-crowned Sparrow prevents the growth of RA neurons (Brenowitz and Lent, unpublished observation). TTX blocks voltage-gated Na+ channels and thus inhibits the electrical activity of HVC axon terminals and RA neurons. Co-infusing TTX with BDNF alone does not rescue RA neuronal growth from the effects of TTX. Co-infusing TTX, BDNF, and cAMP, however, does rescue growth of RA neurons (Brenowitz and Lent, unpublished observations). cAMP facilitates translocation of the TrkB receptor from the intracellular compartment to the plasma membrane in depolarized neurons in both retinal ganglion cells and spinal motor neurons in culture [82]. If a similar process of activity-dependent receptor translocation occurs in postsynaptic RA neurons, then cAMP may make these neurons responsive to BDNF released from HVC neurons.
These observations suggest the following scenario of interactions between T and BDNF in the regulation of seasonal plasticity. T acts on HVC to increase the synthesis of BDNF by neurons [69] as well as by endothelial cells [83]. BDNF acts locally and rapidly to increase the survival of new neurons added to HVC during seasonal growth of the nucleus. In addition, BDNF synthesized by HVC neurons is released on to postsynaptic RA neurons where it binds to TrkB receptors to increase activity and induce neuronal growth. The release of BDNF from HVC and/or response of RA neurons are activity dependent. Given the constitutive expression of TrkB mRNA in RA cells, the seasonal growth and increased activity of RA neurons reflects an increase in synthesis of the BDNF ligand by HVC cells, rather than increased sensitivity to the protein in RA neurons. A novel component of this model is that while T increases BDNF synthesis locally in HVC, BDNF exerts its effect on RA transsynaptically following axonal transport and synaptic release, or by extrasynaptic release, and binding to receptors on efferent neurons. In this system, therefore, neurons in the target nucleus RA respond only indirectly to T through the downstream effector BDNF.
[68][69, Woods et al., unpublished observations][84][78] It seems likely that there are also anterograde trophic effects of steroid hormones during post-natal development of the song system. Depriving RA and Area X of afferent input from HVC in juvenile Zebra Finches prevents these nuclei from growing to their normal size in males [85; 86], and blocks the masculinization of these regions in females caused by early treatment with E2 [87]. As discussed above, E2 induces growth of HVC, and expression of BDNF mRNA in male HVC neurons. The transsynaptic release of BDNF from HVC axons may be important for the growth of RA and Area X in juvenile male finches, though this remains to be tested.
Transsynaptic trophic effects of steroid hormones on neural circuits have been observed in other systems. Estrogen modulates BDNF mRNA and protein in the adult hippocampus and cortex of mammals, but there is only sparse co-localization of ERα and BDNF protein, and no co-localization of ERβ and BDNF, in the hypothalamus, amygdala, prelimbic cortex, and ventral hippocampus [88]. Axons from cortical ERβ-expressing inhibitory neurons, however, terminate on BDNF-immunoreactive pyramidal cells. These observations are consistent with a transsynaptic model of estrogen regulation of BDNF expression; by modulating GABAergic interneurons, estrogen may indirectly influence the activity and expression of BDNF-producing cortical neurons [88].
Anterograde transport and trophic effects of neurotrophins have been reported in other models [78; 89; 90]. Olfactory sensory neurons in mice express and anterogradely transport the neurotrophin NT-3 to the olfactory bulb [89]. BDNF and NT-3 are expressed by retinal ganglion cells in embryonic chicks and transported anterogradely to the optic tectum [79], where they increase the number of synapses, vesicle density, and the number and density of docked vesicles [90]. In mice BDNF and NT-3 are synthesized in cells located in the cerebral cortex, substantia nigra pars compacta, amygdala, and thalamus and transported anterogradely to the striatum; medium spiny neurons (MSNs) lack mRNA but show high levels of protein for these neurotrophins [78]. Midbrain dopaminergic neurons are the main source of BDNF in the developing striatum. BDNF-TrkB signaling is necessary for the survival of developing MSNs of the indirect striatal pathway, and NT-3-TrkC signaling is crucial for the development of MSNs of the direct strial pathway; delection of either BDNF or NT-3 in dopaminergic neurons leads to selective loss of MSNs in the indirect or direct pathways, respectively [78].
Retrograde Trophic Effects
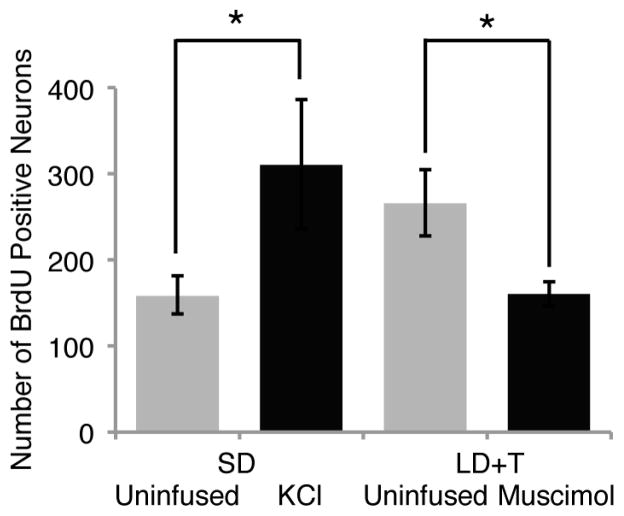
T and its metabolites also have retrograde trophic effects in the song control circuit. The addition of new RA-projecting neurons to HVC of adult White-crowned Sparrows is influenced by the electrical activity of target neurons in RA. The spontaneous activity of RA neurons is high in breeding sparrows and low in non-breeding birds [17; 18; 19]. As discussed above, androgen action on RA neurons is permissive for them to increase their activity in response to neurotrophins released by HVC neurons. Inhibiting activity in breeding-condition birds by infusing RA with the GABAA receptor agonist muscimol decreased the number of new neurons in HVC of adult white-crowned sparrows by 56% [91]. Increasing RA activity in non-breeding condition birds by infusing KCl increased the number of new HVC neurons by 93% (Figure 4; Larson, Wang, and Brenowitz, unpublished observations).
Figure 4.
Postsynaptic activity influences the addition of new RA-projecting neurons to HVC. Spontaneous activity of neurons in RA was decreased by unilateral infusions of muscimol [2.8 mg/ml 27] or increased by infusion of KCl (100 mM, n = 5 birds; Larson, Wang, and Brenowitz, unpublished observation ). Inhibiting activity decreased neuronal addition and increasing activity increased neurogenesis in HVC.
The mechanism by which neuronal activity in RA influences neuronal addition to HVC is not yet known. It seems likely that activity-induced regulation of genes encoding molecules that promote survival of adult-born HVC neurons, axonal path finding, and/or synapse formation are important [92; 93]. The retrograde transport of activity-induced trophic factors produced by target neurons that influence the survival of new RA-projecting neurons in HVC neurons may be modulated by activity in RA. Microarray analysis of cDNA extracted from RA showed that the expression of proneurogenic genes, including insulin-like growth factor 1 and neuromodulin, is increased in RA of breeding-condition birds [68]. In HVC, the expression of mRNA for sex steroid receptors, which facilitate the retrograde transport toward the neuronal soma of trophic factors bound to their receptor, also increases during breeding conditions [50; 94; 95]. Once transported back to the soma, trophic factors likely activate the PI3K/AKT and other signaling cascades that promote the growth and survival of new neurons [Brenowitz et al., unpublished observation 96; 97]. Inhibition of neural activity in RA may result in a failure of new HVC neurons to form synapses on RA neurons and/or a decreased production of activity-induced trophic factors in RA. The consequence would be a lack of retrograde transport of the trophic signals and, thus, a decrease in addition of adult-born neurons to HVC.
Retrograde trophic effects of neurotrophins have been demonstrated in other steroid-sensitive neural systems. Blocking TrkB receptors in target muscles of the spinal nucleus of the bulbocavernosus (SNB) in rats reduces the number of motoneurons that survive during a sexually dimorphic perinatal period of cell death [reviewed in 98]. Both the SNB motoneurons and their target muscles contain AR. Application of BDNF (but not CNTF, GDNF, or NT-4) to axotomized SNB motoneurons prevented a decrease in AR expression, and T treatment in the absence of exogenous BDNF is insufficient to maintain ARs [99]. In cultured hippocampal neurons, BDNF signaling at axon terminals induces rapid endocytosis and retrograde transport of the BDNF-TrkB receptor complex to the cell body of the presynaptic neuron [100; 101]. Hippocampal neurons express receptors for various steroids including androgens, estrogens, glucocorticoids, and mineralocorticoids. Steroids may influence axonal transport mechanisms [102]. It is thus possible that retrograde trophic effects of steroid hormones may be mediated by tranpsort of target-derived neurotrophins.
Summary and Conclusions
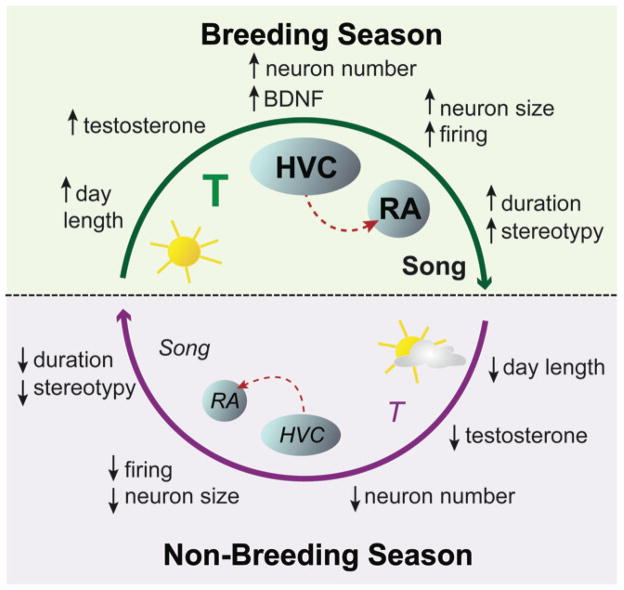
A summary of some of the interactions between photoperiod, hormones, neurotrophins, neural circuits, and song behavior that characterize seasonal plasticity of the song control system is presented in Figure 5. As day length increases beyond a threshold level in late winter, the hypothalamic-pituitary-gonadal axis is stimulated. The testes begin to recrudesce and secrete increased levels of testosterone into the blood. Testosterone is transported to the brain, where it acts on cells in HVC. Steroid hormones act on HVC neurons to increase the synthesis of BDNF. BDNF acts locally within HVC to increase neuron number by promoting the survival of adult-born neurons. BDNF is also transported anterogradely and released from HVC neurons to induce the growth and increased electrical activity of postsynaptic neurons in RA. Increased electrical activity of RA neurons has a retrograde transsynaptic trophic effect to increase the number of new neurons added to HVC. As the song circuits grow and the syringeal muscles hypertrophy, songs become longer and more stereotyped in structure. There is also an increase in the rate of song production, perhaps due to the action of sex steroids on areas of the brain outside the song system that are associated with sexual and aggressive motivation [103]. The neural activity associated with increased singing may reinforce or consolidate the growth of the song circuits by increased expression of the gene for BDNF in HVC neurons.
Figure 5.
A summary of the main interactions between photoperiod, hormones, neurotrophins, brain, and behavior that characterize seasonal plasticity of the song system. See text for explanation. (Figure prepared by T. Larson.)
The magnitude of seasonal changes in HVC neuron number is quite dramatic. In both White-crowned Sparrows and Song Sparrows, HVC has ca. 67% more neurons in breeding than in non-breeding birds [20] [30]. Neuron number in HVC of Eastern Towhees (Pipilo erythrophthalmus) increases by ca. 62% in breeding compared with non-breeding birds [104]. These increases in neuron number coincide with the time when song stereotypy increases. This relationship between neuronal addition to HVC and song quality is consistent with observations in adult male Zebra Finches that the maintenance of song after deafening and recovery of song after botox-induced paralysis of the syringeal muscles are positively correlated with the number of new HVC neurons [105; 106]. Together these observations suggest that new HVC neurons are essential for the recovery and maintenance of previously learned song.
Testosterone and its metabolites play an important role in regulating seasonal plasticity of the adult avian song control system. Both androgenic and estrogenic action are necessary to produce the full range of changes in different attributes of the song circuits, including volume of the song nuclei, cell structure and activity, the addition of new neurons, patterns of gene expression, and circuit connectivity. Steroids may act directly on cells that contain classical nuclear receptors to influence expression of genes that encode trophic factors, neurite extension, vascularization, apoptosis, and electrical activity. Hormones may have indirect, anterograde effects via the expression and transsynaptic release of neurotrophins from HVC axons that bind to receptors on the membranes of postsynaptic neurons in RA. Indirect effects of hormones may also be retrograde, whereby the activity of target neurons in RA influences the addition of new afferent neurons to HVC.
Transsynaptic trophic effects of steroid hormones and neurotrophins are likely to be common in neural circuits in other model systems. Good candidates for analysis include circuits in which steroid-sensitive brain regions project to efferent nuclei that are either sexually dimorphic in structure or function, or that show plasticity in response to changes in circulating steroid levels related to reproductive or seasonal cycles. Regions in which neurotrophin protein levels are high but mRNA is low or absent are also likely to be of interest [78]. Intracerebral manipulations of steroid action in selected brain regions similar to those discussed above can be used to probe for transsynaptic effects.
Interactions between steroid hormones and neurotrophins observed in the song system occur commonly among vertebrates. One can ask why these different classes of molecules came to be functionally linked with each other through evolution. Steroids serve various functions in the adult brain: 1) they activate neurons in sexually dimorphic brain regions to produce sex-typical behaviors; 2) hormones provide a means of restricting neural activity to appropriate environmental and physiological conditions, as seen in the T-induced growth of the song system in birds early in the breeding season; 3) secretion of steroids by the gonads and transport to the brain can coordinate neural and behavioral activation with reproductive physiology, as with the increase in song production in breeding birds. BDNF has a wide of range of effects on target cells in the brain, including proliferation, differentiation, survival, growth of processes, neuronal activation, neurotransmission, and synaptic plasticity. In neural systems where it would be maladaptive to have constitutive trophic activity, such as in developmental, sexual, or seasonal contexts, it is important to regulate BDNF expression and to do so in coordination with other molelcular and physiological processes. Sex steroids such as the androgens and estrogens are well suited to providing the timing cue to initiate the trophic activity of BDNF in these contexts. At a circuit level, the production of effective behavior requires connectivity and coordinated activity of neurons at different levels. Transsynaptic steroid interactions with neurotrophins provide a mechanism for functional coordination within distributed neural circuits.
Adult avian song control system shows pronounced seasonal plasticity
Plasticity is regulated by testosterone (T) and its androgenic and estrogenic metabolites
T can act directly on neurons or transsynaptically by steroid interactions with neurotrophins
Transsynaptic trophic effects can be anterograde or retrograde
Acknowledgments
Supported by NIH MH53032 and NS075331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–8. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 2.Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann N Y Acad Sci. 2003;1007:211–31. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- 3.Catchpole CK, Slater PJB. Bird song: Biological themes and variations. Cambridge University Press; Cambridge, U.K: 2008. [Google Scholar]
- 4.Beecher MD, Brenowitz EA. Functional aspects of song learning in the songbirds. Trends Ecol Evol. 2005;20:143–149. doi: 10.1016/j.tree.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Brenowitz EA, Beecher MD. Song learning in birds: diversity, plasticity, opportunities and challenges. Trends Neurosci. 2005;28:127–32. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis ED. Evolution of the Pallium in Birds and Reptiles. In: Marc NH, Binder D, Windhorst Uwe, editors. New Encyclopedia of Neuroscience. Springer-Verlag GmbH; Berlin: 2009. pp. 1390–1400. [Google Scholar]
- 7.Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proceedings of the National Academy of Sciences. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeigler HP, Marler P. Neuroscience of birdsong. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- 9.Luo M, Ding L, Perkel DJ. An avian basal ganglia pathway essential for vocal learning forms a closed topographic loop. J Neurosci. 2001;21:6836–45. doi: 10.1523/JNEUROSCI.21-17-06836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS biology. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brainard MS. The anterior forebrain pathway and vocal plasticity. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge: 2008. pp. 240–255. [Google Scholar]
- 12.Schlinger B, Brenowitz EA. Neural and Hormonal Control of Birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2009. pp. 897–941. [Google Scholar]
- 13.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–70. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 14.Moore IT, Wingfield JC, Brenowitz EA. Plasticity of the Avian Song Control System in Response to Localized Environmental Cues in an Equatorial Songbird. J Neurosci. 2004;24:10182–10185. doi: 10.1523/JNEUROSCI.3475-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenowitz EA. Plasticity of the song control system in adult birds. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge: 2008. pp. 332–349. [Google Scholar]
- 16.De Groof G, Verhoye M, Van Meir V, Balthazart J, Van der Linden A. Seasonal rewiring of the songbird brain: an in vivo MRI study. Eur J Neurosci. 2008;28:2475–2485. doi: 10.1111/j.1460-9568.2008.06545.x. [DOI] [PubMed] [Google Scholar]
- 17.Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–57. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meitzen J, Perkel DJ, Brenowitz EA. Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:677–83. doi: 10.1007/s00359-007-0222-1. [DOI] [PubMed] [Google Scholar]
- 19.Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ. Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci. 2009;29:6558–67. doi: 10.1523/JNEUROSCI.5571-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–10. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson CK. Cell death, the song control system: A model for how sex steroid hormones regulate naturally-occurring neurodegeneration. Development, Growth & Differentiation. 2011;53:213–224. doi: 10.1111/j.1440-169X.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci U S A. 2007;104:15520–5. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–26. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning [published erratum appears in Science 1990 Oct 19;250(4979):360] Science. 1990;249:1444–6. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- 25.Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CK, Brenowitz EA. Neurogenesis in an adult avian song nucleus is reduced by decreasing caspase-mediated apoptosis. J Neurosci. 2009;29:4586–91. doi: 10.1523/JNEUROSCI.5423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson TA, Thatra NM, Lee BH, Brenowitz EA. Reactive neurogenesis in response to naturally occurring apoptosis in an adult brain. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3316-13.2014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–71. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- 29.Brenowitz EA, Baptista LF, Lent K, Wingfield JC. Seasonal plasticity of the song control system in wild Nuttall’s white- crowned sparrows. J Neurobiol. 1998;34:69–82. doi: 10.1002/(sici)1097-4695(199801)34:1<69::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20:854–61. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leitner S, Voigt C, Gahr M. Seasonal changes in the song pattern of the non-domesticated island canary (Serinus canaria), a field study. Behaviour. 2001;138:885–904. [Google Scholar]
- 32.Smulders T, Lisi MD, Tricomi E, Otter KA, Chruszcz B, Ratcliffe LM, deVoogd T. Failure to detect seasonal changes in the song system nuclei of the black-capped chickadee. J Neurobiol. 2006;66 doi: 10.1002/neu.20281. [DOI] [PubMed] [Google Scholar]
- 33.Hough GE, II, Nelson DA, Volman SF. Re-expression of songs deleted during vocal development in white-crowned sparrows, Zonotrichia leucophrys. Anim Behav. 2000;60:279–287. doi: 10.1006/anbe.2000.1498. [DOI] [PubMed] [Google Scholar]
- 34.Nordby J, Campbell S, Beecher MD. Adult song sparrows do not alter their song repertoires. Ethology. 2002;108:39–50. [Google Scholar]
- 35.Lee DW, Smith GT, Tramontin AD, Soma KK, Brenowitz EA, Clayton NS. Hippocampal volume does not change seasonally in a non food-storing songbird. Neuroreport. 2001;12:1925–8. doi: 10.1097/00001756-200107030-00031. [DOI] [PubMed] [Google Scholar]
- 36.Calder WA, King JR. Thermal and caloric relations in birds. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Academic Press; New York: 1974. pp. 259–413. [Google Scholar]
- 37.Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 38.Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Academic Press; San Diego: 2002. pp. 105–135. [Google Scholar]
- 39.Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal Plasticity in the Song Control System: Multiple Brain Sites of Steroid Hormone Action and the Importance of Variation in Song Behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- 40.Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32:426–42. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Bernard DJ, Ball GF. Photoperiodic condition modulates the effects of testosterone on song control nuclei volumes in male European starlings. Gen Comp Endocrinol. 1997;105:276–83. doi: 10.1006/gcen.1996.6829. [DOI] [PubMed] [Google Scholar]
- 42.Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–83. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF. Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur J Neurosci. 2010;32:2116–2132. doi: 10.1111/j.1460-9568.2010.07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66:1044–60. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- 45.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 46.Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:233–44. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- 47.Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–40. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- 48.Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1998;409:224–236. [PubMed] [Google Scholar]
- 49.Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44:509–17. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 50.Fraley GS, Steiner RA, Lent K, Brenowitz EA. Seasonal Changes in Androgen Receptor mRNA in the Brain of the White-crowned Sparrow. Gen Comp Endocrinol. 2010;166:66–71. doi: 10.1016/j.ygcen.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–21. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- 52.Soma KK, Alday NA, Schlinger BA. 3 Beta-HSD, aromatase in songbird brain: DHEA metabolism, aggression, song. Soc Neurosci. 2002:Abstracts 28. [Google Scholar]
- 53.Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinology. 2001;122:1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- 54.Thompson CK, Brenowitz EA. Caspase Inhibitor Infusion Protects an Avian Song Control Circuit from Seasonal-Like Neurodegeneration. J Neurosci. 2008;28:7130–7136. doi: 10.1523/JNEUROSCI.0663-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenowitz EA, Lent K. Afferent input is necessary for seasonal growth and maintenance of adult avian song control circuits. J Neurosci. 2001;21:2320–9. doi: 10.1523/JNEUROSCI.21-07-02320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci U S A. 2002;99:12421–6. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Frontiers in Neuroendocrinology. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharfman HE, Maclusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends in Neurosciences. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Sohrabji F, Miranda RCG, Toran-Allerand CD. Identification of a Putative Estrogen Response Element in the Gene Encoding Brain-Derived Neurotrophic Factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo Trs. Implication of the Phosphatidylinositol-3 Kinase/Protein Kinase B Signaling Pathway in the Neuroprotective Effect of Estradiol in the Striatum of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Mice. Molecular Pharmacology. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- 62.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–43. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 63.Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Academic Press; New York: 2002. pp. 799–839. [Google Scholar]
- 64.Wade J, Arnold AP. Sexual Differentiation of the Zebra Finch Song System. Ann N Y Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- 65.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnold AP. Hormonally-induced alterations in synaptic organization in the adult nervous system. Exp Gerontol. 1992;27:99–110. doi: 10.1016/0531-5565(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 67.Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci USA. 1999;96:8241–6. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson CK, Meitzen J, Replogle K, Drnevich J, Lent KL, Wissman AM, Farin FM, Bammler TK, Beyer RP, Clayton DF, Perkel DJ, Brenowitz EA. Seasonal Changes in Patterns of Gene Expression in Avian Song Control Brain Regions. PLoS ONE. 2012;7:e35119. doi: 10.1371/journal.pone.0035119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci. 2009;29:6461–71. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries [see comments] Proc Natl Acad Sci U S A. 1994;91:7854–8. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balthazart J, Boseret G, Konkle AT, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur J Neurosci. 2008;27:801–17. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- 72.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 73.Nottebohm F, O’Loughlin B, Gould K, Yohay K, Alvarez-Buylla A. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of year when these cells are born [see comments] Proc Natl Acad Sci U S A. 1994;91:7849–53. doi: 10.1073/pnas.91.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci U S A. 2004;101:3957–61. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibbs RB. Levels of trkA, BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Research. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- 76.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–25. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 77.Levi-Montalcini R. The saga of the nerve growth factor. Neuroreport. 1998;9:R71–R83. [PubMed] [Google Scholar]
- 78.Baydyuk M, Xu B. BDNF signaling, survival of striatal neurons. Frontiers in Cellular Neuroscience. 2014;8 doi: 10.3389/fncel.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379:830–3. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- 80.Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–23. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 81.Trueta C, De-Miguel FF. Extrasynaptic exocytosis, its mechanisms: a source of molecules mediating volume transmission in the nervous system. Frontiers in Physiology. 2012;3 doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP Elevation Rapidly Recruit TrkB to the Plasma Membrane of CNS Neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z, Ye R, Goldman SA. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain. Neuroscience. 2013;239:139–148. doi: 10.1016/j.neuroscience.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. The Journal of Comparative Neurology. 1997;386:529–539. [PubMed] [Google Scholar]
- 85.Akutagawa E, Konishi M. Two separate areas of the brain differentially guide the development of a song control nucleus in the zebra finch. Proc Natl Acad Sci U S A. 1994;91:12413–7. doi: 10.1073/pnas.91.26.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson F, Bottjer SW. Afferent influences on cell death and birth during development of a cortical nucleus necessary for learned vocal behavior in zebra finches. Development. 1994;120:13–24. doi: 10.1242/dev.120.1.13. [DOI] [PubMed] [Google Scholar]
- 87.Herrmann K, Arnold AP. Lesions of HVc block the developmental masculinizing effects of estradiol in the female zebra finch song system. J Neurobiol. 1991;22:29–39. doi: 10.1002/neu.480220104. [DOI] [PubMed] [Google Scholar]
- 88.Blurton-Jones M, Kuan P, Tuszynski M. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. The Journal of Comparative Neurology. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- 89.Liu H, Lu M, Guthrie KM. Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo. Exp Neurol. 2013;241:125–137. doi: 10.1016/j.expneurol.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Butowt R, Von Bartheld CS. Presynaptic neurotrophin-3 increases the number of tectal synapses, vesicle density, and number of docked vesicles in chick embryos. The Journal of Comparative Neurology. 2003;458:62–77. doi: 10.1002/cne.10558. [DOI] [PubMed] [Google Scholar]
- 91.Larson TA, Wang T-W, Gale SD, Miller KE, Thatra NM, Caras ML, Perkel DJ, Brenowitz EA. Postsynaptic neural activity regulates neuronal addition in the adult avian song control system. Proceedings of the National Academy of Sciences. 2013;110:16640–16644. doi: 10.1073/pnas.1310237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Tounekti O, Akerman B, Goodyer CG, LeBlanc A. 17-beta-estradiol induces an inhibitor of active caspases. J Neurosci. 2001;21:RC176. doi: 10.1523/JNEUROSCI.21-20-j0007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kay L, Humphreys L, Eickholt BJ, Burrone J. Neuronal activity drives matching of pre- and postsynaptic function during synapse maturation. Nat Neurosci. 2011;14:688–690. doi: 10.1038/nn.2826. [DOI] [PubMed] [Google Scholar]
- 94.Fusani L, Van’t Hof T, Hutchison JB, Gahr M. Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. Journal of neurobiology. 2000;43:254–68. [PubMed] [Google Scholar]
- 95.Jezierski MK, Sohrabji F. Estrogen Enhances Retrograde Transport of Brain-Derived Neurotrophic Factor in the Rodent Forebrain. Endocrinology. 2003 doi: 10.1210/en.2003-0724. [DOI] [PubMed] [Google Scholar]
- 96.Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. Signaling Mechanisms Mediating BDNF Modulation of Synaptic Plasticity in the Hippocampus. Learning & Memory. 1999;6:243–256. [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 98.Ottem EN, Bailey DJ, Jordan CL, Marc Breedlove S. With a little help from my friends: Androgens tap BDNF signaling pathways to alter neural circuits. Neuroscience. 2013;239:124–138. doi: 10.1016/j.neuroscience.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 99.Al-Shamma HA, Arnold AP. Brain-derived neurotrophic factor regulates expression of androgen receptors in perineal motoneurons. Proc Natl Acad Sci U S A. 1997;94:1521–6. doi: 10.1073/pnas.94.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 101.Zheng J, Shen W-H, Lu T-J, Zhou Y, Chen Q, Wang Z, Xiang T, Zhu Y-C, Zhang C, Duan S, Xiong Z-Q. Clathrin-dependent Endocytosis Is Required for TrkB-dependent Akt-mediated Neuronal Protection and Dendritic Growth. Journal of Biological Chemistry. 2008;283:13280–13288. doi: 10.1074/jbc.M709930200. [DOI] [PubMed] [Google Scholar]
- 102.Dai J, Buijs R, Swaab D. Glucocorticoid hormone (cortisol) affects axonal transport in human cortex neurons but shows resistance in Alzheimer’s disease. British Journal of Pharmacology. 2004;143:606–610. doi: 10.1038/sj.bjp.0705995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brenowitz EA, Nalls B, Wingfield JC, Kroodsma DE. Seasonal changes in avian song nuclei without seasonal changes in song repertoire. J Neurosci. 1991;11:1367–74. doi: 10.1523/JNEUROSCI.11-05-01367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pytte C, Yu Y-L, Wildstein S, George S, Kirn JR. Adult Neuron Addition to the Zebra Finch Song Motor Pathway Correlates with the Rate and Extent of Recovery from Botox-Induced Paralysis of the Vocal Muscles. The Journal of Neuroscience. 2011;31:16958–16968. doi: 10.1523/JNEUROSCI.2971-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pytte CL, George S, Korman S, David E, Bogdan D, Kirn JR. Adult Neurogenesis Is Associated with the Maintenance of a Stereotyped, Learned Motor Behavior. The Journal of Neuroscience. 2012;32:7052–7057. doi: 10.1523/JNEUROSCI.5385-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]