Abstract
Pregnane X receptor (PXR) is a member of the nuclear receptor superfamily that differently expresses not only in human normal tissues but also in numerous types of human cancers. PXR can be activated by many endogenous substances and exogenous chemicals, and thus affects chemotherapeutic effects and intervenes drug–drug interactions by regulating its target genes involving drug metabolism and transportation, cell proliferation and apoptosis, and modulating endobiotic homeostasis. Tissue and context-specific regulation of PXR contributes to diverse effects in the treatment for numerous cancers. Genetic variants of PXR lead to intra- and inter-individual differences in the expression and inducibility of PXR, resulting in different responses to chemotherapy in PXR-positive cancers. The purpose of this review is to summarize and discuss the role of PXR in the metabolism and clearance of anticancer drugs. It is also expected that this review will provide insights into PXR-mediated enhancement for chemotherapeutic treatment, prediction of drug–drug interactions and personalized medicine.
Keywords: PXR, Pharmacogenomics, Chemotherapeutic drugs, Metabolism
PXR and its pharmacogenomics
Introduction
Belonging to the NR1I subfamily of nuclear receptors (NRs), pregnane X receptor (PXR), also referred as the steroid and xenobiotic receptors (SXR) or pregnane-activated receptor (PAR), was first cloned in 1998 [1–3]. Recent studies have revealed that PXR expresses not only in human normal tissues such as liver, small intestine, colon, kidney, ovary, breast, prostate, mononuclear blood cells, placenta, bone marrow, spinal cord, stomach and heart, but also in carcinoma tissues such as breast cancer, ovary cancer, endometrial cancer, colon cancer, prostate cancer and osteosarcoma [4, 5]. The expression levels of PXR are significantly different between normal and neo-plastic tissues [4, 5]. The basic expression of PXR exhibits marked individual variations, even in the same kind of neo-plastic tissues [5].
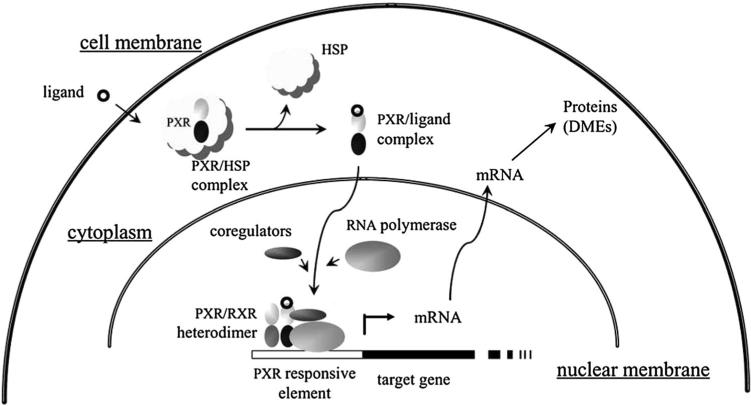
Originally recognized as a xenobiotic sensor, PXR holds an extremely large and flexible ligand-binding pocket, allowing the accommodation of numerous structurally diverse ligands that include many endobiotics, prescription drugs, environmental pollutants, herbal medicines and dietary supplements such as bile acid, cholesterol, estrogen, progesterone, 17-hydroxypregnenolone, 5-α-pregnan-3, 20-dione, rifampicin, clotrimazole, ritonavir, cyclosporin, tamoxifen, paclitaxel, troglitazone, bisphenol A, organochlorine pesticides, multijoint bromobenzene, flame retardants, St John's Wort (SJW), gugulipid, kava kava, Sweet Wormwood Herb, Schisandra, liquorice and so on [6, 7]. Inside the nucleus, PXR forms a heterodimer with the retinoid X receptor (RXR) and binds to xenobiotic responsive elements located in the promoter of its target genes [8, 9]. Upon ligand binding, the LBD (ligand-binding domain) of PXR went through a conformational change that facilitates the recruitment of co-activators or co-repressors, depending on the agonistic or antagonistic nature of the ligand, and alters the transcription of its target genes, thereafter [10–12] (Fig. 1). To coordinate the adaptive response against xenobiotic challenges, PXR regulates the transcriptional expression of numerous phase I and II drug-metabolizing enzymes and drug transporters such as CYP (Cytochrome P450 enzyme) 3A, CYP2B, CYP2C, aldehyde dehydrogenase, alcohol dehydrogenase, carboxylesterase, UGT (UDP-glucuronosyltransferase) 1A1, sulfotransferase, MDR (multidrug resistance gene) 1, ABCC (ATP-binding cassette transporter C) 2 and OATP (anion-transporting polypeptide) 2 [13–20]. In addition to the prototypical xenobiotic sensing effects, recent evidence has evolved PXR into an endobiotic sensor that impacts energy homeostasis and the pathogenesis of metabolic disorders [7], proinflammatory cytokines and inflammatory bowel disease [21], as well as cell proliferation, apoptosis and tumor development [22–24].
Fig. 1.
PXR regulation of expression of genes for DMEs, transporters and cell process [11]. Upon binding to ligands, PXR dissociates from heat shock protein (HSP), trans-locates into nucleus, forms PXR/RXR heterodimer and stimulates the transcription of target genes, which lead to altered cell functions such as resistance to chemotherapy
Genetic variants of PXR and their functional consequences
The PXR gene, located in chromosome 13q12-13.3, consists of nine exons and stretches approximately 37 kilo bases. To date, many PXR allelic variants have been identified in different ethnic populations [7, 25]. Among the reported 70 single nucleotide polymorphisms (SNPs) of PXR, 15 are nonsynonymous mutation in the coding regions, including four SNPs between the N terminal and the DNA-binding domain [A12T, E18K (PXR*9), P27S (PXR*2) and G36R (PXR*3)], three variants located in or near the DNA-binding domain [R98C (PXR*5), R122Q (PXR*4) and K109N] and eight variants in the LBD district of PXR [V140 M (PXR*10), R148Q (PXR*6), Q158K, D163G (PXR*11), A370T, C379G, R381 W and I403 V] [14]. Although the functional consequences are not fully illustrated, a number of SNPs are reported in the intron and promoter region of PXR gene, respectively [7].
Notably, altered expressions and activities of the PXR target genes might be associated with mutated PXR compared with the wild-type PXR. A PXR SNP at −566A > C is significantly correlated with higher expressions of both MDR and CYP3A two prototypical PXR target genes; nevertheless, this SNP was not associated with PXR expression [26]. Lower mRNA level of CYP3A was reported to be correlated with the variant 1359T > C, a SNP in the promoter region of PXR [27]. In primary human hepatocytes, the C10620T and G10799A variants in the 3′-UTR of PXR are correlated with lower CYP3A activity, and variants 11156C and 11193C could decrease the mRNA level of MDR [25]. The variants G10330A and C10483T of PXR are associated with higher activity of CYP3A [28].
Moreover, PXR SNPs might affect the inducibility of PXR mediated by PXR agonists. It was found that variant R98C fails to transactivate CYP3A4 reporter by a luciferase reporter assay using the CYP3A4 promoter/ enhancer reporter in COS-7 or HepG2 cells when activated by human PXR agonists rifampicin, clotrimazole or paclitaxel [29]. The variants located in the PXR LBD (R98C, Q158K, V140 M, A370T, R381 W and I403v) significantly decreased the up-regulation of CYP3A expression by rifampin compared with wild-type PXR, while variant D136G increases the PXR inducibility activated by rifampin [25, 29–31]. It is reasonable to speculate that SNPs located in the LBD of PXR could have altered the secondary structure of PXR in this region, which resulted in varied binding affinities for antagonistic ligands such as rifampin. On the other hand, the variants A7635G, C8055T and T1359C located in the intron region of PXR have induced significantly higher expressions of CYP3A after rifampin treatment when compared with the wild-type [25]. A 6-bp deletion in intron 1a (−2,420) of PXR is associated with inactivity of the PXR promoter, which might reduce the inducible ability of PXR to induce its targeted genes [32].
Splice variants of PXR and their functional consequences
A number of alternative PXR splice variants have been identified in human tissues, including PXR.1, PXR.2 and PXR.3 [33, 34]. PXR.1 and PAR.2 are exclusive splice variants in extron 1 of PXR, and PXR.1 is the most common one [34]. PXR.1 encodes a 434-amino acid product and is expressed in both hepatic and extra-hepatic tissues. PXR.2 and PXR.3 come from the deletion of 111 and 123 nucleotides, resulting in the deletion of 37 and 41 amino acids in PXR LBD, respectively [33]. Notably, PXR.2 utilizes a downstream non-AUG codon in translation, which leads to a shorter iso-form and is largely nonresponsive to ligand stimulation [35].
Significant differences have been reported in the baseline level and inducible ability of PXR among different PXR splice variants [7]. The expression level of PXR splicing variants varied among liver samples from 15 Caucasian [36]. Mensah-Osman et al. [37] have observed that the wild-type PXR was not detectable in osteosarcoma cell lines OS187, WOL and COL, and the molecular size of the PXR protein expressed in these osteosarcoma cell lines was different from that of the wild-type PXR. Significant increases have been found for the mRNA levels of UGT1A1 after rifampin treatment when HepG2 cells or Caco-2 cells were transfected with PXR.1 or PAR.2, while no increase was observed for PXR.2 [35]. Compared with PXR.1, PXR.2 could significantly lower the basal level of CYP3A4 and decrease the inducible levels of CYP3A4, CYP2B6 and MDR1 by PXR agonists [29, 38]. By contrast, Tompkins et al. [39] has found that PXR.1 and PXR.2 demonstrated comparable activities in ligand-activated transcriptional up-regulation of CYP3A4. Further studies are needed to reveal the roles of PXR alternative splicing in PXR expression and function.
Role of PXR and its pharmacogenomics in chemotherapeutics metabolism and efficacy
In addition to the well-documented roles of PXR in xeno-biotic metabolism and clearance, as well as its emerging roles in modulating energy metabolism, steroid metabolism, bile acid and bilirubin metabolism, vitamin metabolism, retinoid acid metabolism, bone homeostasis and inflammation, PXR also plays a profound role in cell proliferation, apoptosis, carcinogenesis and cancer treatments [40–49]. Given that cancer patients are typically treated with multidrug regimes, PXR-mediated drug–drug interactions might be even more important in cancer chemo-therapy. Importantly, many drug-metabolizing enzymes and drug transporters participating in the metabolism and clearance of antineoplastic drugs are PXR down-stream target genes [7, 14, 43–49]. Moreover, PXR also regulates the expression of many genes associated with cell proliferation and apoptosis [22–24, 50]. For instance, PXR agonist rifampicin could significantly increase the mRNA level of fibroblast growth factor (FGF) 19 and stimulate cell proliferation and colon tumor invasiveness [51]. PXR activation could also increase the transcription and protein level of Bcl-2, BAG3, Bcl-XL, BIRC3 and MCL-1 and is negatively correlated with P53, an antitumor gene, leading to enhanced antiapoptosis effect in cancer cells [23, 52–54]. Since fat synthesis and energy metabolism are important in chemical carcinogenesis and chemotherapy of cancer [55], PXR might affect cancer development and chemotherapy by modulating cancer-specific lipid homeostasis [56, 57]. Recently, Xie and colleagues have revealed that PXR sensitized oxidative stress responses in cancerous cells, which might be implicated in increasing chemotherapy efficacy by sensitizing tumors to cellular oxidative damage [58]. In addition, PXR plays an important role in the chemotherapy of hormone-dependent tumor by modulating the steroid metabolic balance [44]. The following drugs are exemplified as chemotherapeutics that are associated with the role of PXR and its pharmacogenomics in cancer treatment (Table 1).
Table 1.
The function of PXR in the chemotherapy of cancer
| Drugs | PXR | PXR target genes | Chemotherapeutic effects | Models | References |
|---|---|---|---|---|---|
| Irinotecan | Expression ↑ by transfection | UGT1A1 ↑ UGT1A9 ↑ UGT1A10 ↑ CYP3A4 ↑ | Metabolism ↑ Sensitivity ↓ |
LS174T cells, HCT116 cells | [64, 65] |
| Expression ↓ by shRNA and siRNA | UGT1A1 ↓ | Metabolism ↓ Sensitivity ↑ |
LS174T cells, HCT116 cells | [64] | |
| Activation by rifampicin and SN-38 | UGT ↑ CYP3A4 ↑ CYP3A5 ↑ MRP2 ↑ P-gp ↑ | Metabohsm ↑ Efflux ↓ |
Mice, LS180 cells, HePG2 cells, HCT116 cells | [18, 24, 65, 66, 68] | |
| Tamoxifen | Expression ↑ by transfection | Ki-67 ↑ ABCC2 ↑ OATP1A2 ↑ | Drug resistance ↑ | MCF-7 cells, TAMR-MCF-7 cells | [19, 73] |
| Expression ↓ by shRNA | Sensitivity ↑ | MDA-MB-231 cells, MCF-7 cells | [74, 75] | ||
| Activation by TAM,4-OH-TAM, SR12813, rifampicin, anandamide and clotrimazole | CYP3A4 ↑ MDR1 ↑ MRP2 ↑ OATP1A2 ↑ | Metabolism ↑ Efflux ↑ Drug resistance ↑ |
MCF-7 cells, T-47D cells, ZR-75-1 cells, MCF-7 cells | [73-79] | |
| BAX ↑ P21 ↑ | Apoptosis ↑ | MCF-7 cells, ZR-75-1 cells | [50] | ||
| Inhibition by A-792611 | OATPlA2↓ | Sensitivity ↑ | T47-D cells | [75] | |
| Paclitaxel | Expression↓ by siRNA and shRNA | CYP3A4 ↓ MDR1 ↓ | Apoptosis ↑ Proliferation ↓ | HEC-1 cells, MDA-MB-231 cells | [22, 74, 86] |
| Activation by paclitaxel, SR12813, rifampicin | CYP3A ↑ CYP2B6 ↑ CYP2C8 ↑ MDR1 ↑ | Metabolism ↑ Efflux ↑ Apoptosis ↓ Proliferation ↑ |
HEC-1 cells, PC-3 cells, MDA-MB-231 cells, SKOV-3 cells | [24, 74, 86-89] | |
| Doxorubicin | Avtivation by rifampicin and VP-PXR | BAG3 ↓ BIRC2 ↓ MCL-1 ↓ BAK1 ↓ TP53/p53 ↓ P-gp ↑ | Apoptosis ↓ Sensitivity ↓ |
HCT116 cells, LS180 cells | [52, 77] |
| Inhibition by ecteinascidin-743 | P-gP↓ | Sensitivity ↑ | Osteosarcoma cells | [94] | |
| PXR polymorphisms | Patients harboring PXR*1B showed higher CYP3A4 levels compared with PXR*lA and PXR*lC | Patients harboring PXR*1B showed higher drug clearance compared with PXR*lA and PXR*lC | Patients | [95] | |
| Vinblastine | Activation by SR12813 and vinblastine | ABCC2 ↑ ABCC3 ↑ CYP3A4 ↑ | Sensitivity ↓ | PC-3 cells, LS174T cells, Patients | [86, 101, 102] |
| Expression↓ by siRNA | MDR1 ↑ CYP3A4 ↑ | Sensitivity ↑ | MCF-7 cells, MDA-MB-231 cells | [74] | |
| ATRA | Activation by PCN, Rifampin and dexamethasone | CYP3A ↑ CYP26 ↑ MDR1 ↑ ABCC3 ↑ OATP ↑ | AUC ↓ Sensitivity ↓ |
Mice | [109] |
| CPA&IFO | Activation by rifampin and dexamethasone | CYP3A4 ↑ CYP2C8 ↑ CYP2C9 ↑ | Metabolism ↑ Sensitivity ↑ |
Primary cultures hepatocytes | [114] |
Irinotecan
Irinotecan (7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin, CPT-11), a water-soluble camp-tothecin analog, is widely used in the treatment for small cell lung cancer, colorectal cancer and several other solid tumors. Being an inactive prodrug, therapeutic effects of irinotecan rely largely on its conversion to an active metabolite SN-38 (7-ethyl-10-hydroxycamptothecin) by carboxylesterase in vivo. After bond with DNA topoisomerase, SN-38 inhibits DNA replication, consequently leading to double-strand DNA break and cell apoptosis [59]. Irinotecan can also be metabolized into inactive by-products by CYP3A4 enzyme [60], whereas UGT1A1 represents the key enzyme inactivating the therapeutic SN-38 to yield its β-glucuronide (SN-38G) [61]. In addition, drugs transporters such as P-gp (P-glycoprotein, encoding MDR1 gene) and canalicular multispecific organic anion transporter (cMOAT) are associated with the efflux of irinotecan and SN-38 out of cancerous cells and decrease intracellular drug accumulation, resulting in drug resistance [62, 63].
An increasing body of evidence suggests that high PXR expression is correlated with decreased efficacy or increased chemo-resistance in irinotecan-based cancer treatment. Raynal et al. [64] have reported that PXR is strongly expressed in colon tumor tissues and displays a great variability of expression. Transfection of the PXR expression plasmid in the colon cancer cell line LS174T desensitized the cancer cells to SN-38; inversely reduced PXR expression by siRNA (small interfering RNA) restored drug sensitivity [64]. In another report, Basseville et al. [65] have found that SN-38 itself functions as a PXR activator and is associated with increased expression of CYP3A4, UGT1A1 and Pgp in both LS180 and HepG2 cells; and over-expression of PXR in LS180 and HCT 116 cells significantly increased their chemo-resistance to SN-38. As such, it is prompted that PXR expression may be regarded as a marker for predicting the chemotherapeutic efficacy of irinotecan and differential expression of PXR might partly account for inter-individual variations in the therapeutic efficacy of irinotecan. When mRNA expression of UGT1A1 elevated with higher PXR expression, intracellular SN-38G/SN-38 is increased and irinotecan efficacy is decreased [64].
Transgenic mice expressing a constitutively active form of human PXR (VP-hPXR) show significantly higher UGT1A1 activity than wild-type mice, which may lead to increased SN-38 glucuronidation, and decreased efficacy of irinotecan [18]. Gupta et al. [24] have found that rifampicin significantly increases chemoresistance to irinotecan in human ovarian cancerous cell line SKOV-3, and the intra-cellular ratio of SN-38G/SN-38 is higher than that of the control group. Clinical studies demonstrate that rifampicin can reduce the AUC (area under the curve) of irinotecan [66]. SJW (St. John's wort), an over-the-counter remedy for the treatment of depression, is a well-known and potent PXR agonist [67]. In cancer patients, when irinotecan was co-administrated with SJW, the plasma concentrations of SN-38 decreased by approximately 40 % in comparison to irinotecan treatment alone [68]. The expression of PXR downstream target CYP3A, UGT1A1 and P-gP was significantly increased by SJW, leading to accelerated metabolism and efflux of intracellular SN-38 [69]. Given that many drugs are activators of PXR and polypharmacy is often required for cancer treatment, attention should be paid to the drug–drug interactions which might lead to chemotherapeutics resistance by PXR activation. Activation of PXR is also one of the causes for irinotecan self-resistance since SN-38 can activate PXR and up-regulate PXR target genes such as CYP3A4, UGT1A1 and MDR1 [65].
Tamoxifen
Tamoxifen (TAM), a selective estrogen receptor modulator by virtue of competing estrogen receptor with endogenous estrogen, is widely used in the treatment and chemo-prevention of estrogen receptor-positive breast cancer. TAM is converted to a more active 4-hydroxytamoxifen predominantly catalyzed by CYP2D6 and can be further transformed into endoxifen (4-hydroxy-N-desmethyltamoxifen) which is equipotent to 4-hydroxytamoxifen [70]. TAM can also be metabolized into lowly reactive N-desmethyltamoxifen mainly by CYP3A4 [71]. P-gp could function as the efflux transporter facilitating the excretion of endoxifen from cancer cells and decrease the blood concentrations of endoxifen [72].
Miki et al. [19] have reported that PXR expression in breast cancer cells is positively correlated with Ki-67, a marker of cell proliferation, indicating that PXR possibly plays a pro-proliferative role in breast cancer pathogenesis. The expression of PXR target gene MRP2 (ABCC2) was significantly higher in TAM-resistant MCF-7 cells than that in TAM-sensitive MCF-7 cells; further analysis of revealed that PXR activation was associated with MRP2 over-expression in TAM-resistant MCF-7 cells [73]. In another report, Chen et al. [74] demonstrated that a potent and selective agonist of PXR, SR12813, significantly increased the drug resistance of breast cancer cell lines MCF-7 and MDA-MB-231 to TAM by elevating the expression of CYP3A4 and MDR1, while knockdown of PXR by shRNA (small hairpin RNA) sensitized TAM treatment. OATP 1A2 is a transporter modulating the cellular uptake of estrogen and its metabolites. The expression of mRNA and protein of OATP 1A2 was found to be significantly correlated with PXR expression in human breast cancer tissues [19]. Importantly, PXR agonist rifampin induces OATP1A2 expression and increases the uptake of estrogen, leading to competitive TAM resistance in breast cancer cell line T47-D, which could be reversed by siRNA of PXR or a specific PXR antagonist A-792611 [75]. In addition, PXR activation might account for TAM self-resistance since TAM and 4-OH-TAM both can activate PXR and increase the expressions of CYP3A4 and MDR1 [76–79], which eventually led to the increased metabolism and efflux of TAM and its active metabolites. On the other hand, PXR activation was also reported to inhibit the proliferation of MCF-7 and ZR-75 breast cancer cells via the up-regulation gene associated with cell cycle arrest, apoptosis such as p21, PUMA and BAX, leaving the net effects of PXR activation in breast cancer treatment rather controversial [50].
Paclitaxel
Paclitaxel, a member of the taxes family, is an important anticancer drug broadly used in the treatment for various malignancies such as breast cancer, ovarian cancer, endometrial cancer and lung cancer. Its mechanism of antitumor is stabilizing microtubules, which leads to defects in mitotic spindle assembly, chromosome segregation, cell cycle arrest, apoptosis and cytotoxicity [80, 81]. Unlike aforementioned irinotecan and tamoxifen, metabolites of paclitaxel are virtually inactive. In humans, Paclitaxel is inactivated by CYP2C8 and CYP3A4 [82, 83]. Inhibition of P-gp in the intestine was associated with significantly lower bioavailability of paclitaxel [84, 85].
PXR activation has been revealed to cause sub-therapeutic responses by enhancing paclitaxel metabolism and promoting the proliferation of cancer cells. Chen et al. [74] have shown that pretreatment with PXR agonist SR12813 increased drug resistance of breast cancer MDA-MB-231 cells to paclitaxel. In human prostate cancer PC-3 cells, activation of PXR by SR12813 up-regulating the expression of CYP3A4 and MDR1, and accelerating paclitaxel metabolism, which resulted in decreased concentration of paclitaxel [86]. PXR expression was also detected in a number of ovarian carcinoma cell lines, in SKOV-3 cells, activation of PXR by rifampin was associated with increased expression of CYP3A4, UGT1A1 but not MDR1 and MRP2, suggesting induction of different PXR target genes is a cell-specific event that could be affected by many cellular factors other than PXR. Notably, activation of PXR induces cell proliferation and paclitaxel resistance in SKOV-3 cells [24]. Furthermore, rifampin accelerates the tumor proliferation in mice carrying SKOV-3 xenografts and promotes SKOV-3 cell proliferation which could be ablated by a PXR antagonist ketoconazole [24].
On the other hand, down-regulation of PXR expression increased paclitaxel efficacy in human prostate carcinoma cell line PC-3 and enhanced apoptosis in human adenocarcinoma cell line HEC-1 [22, 86]. In MDA-MB-231 cells, shRNA-mediated knockdown of PXR significantly compromised the expression of CYP3A4, while sensitized the cells’ response to paclitaxel [74].
Moreover, PXR activation also plays a role in paclitaxel self-resistance. As a substrate of CYP3A4 and MDR1, paclitaxel could activate PXR and induce the expressions of these genes. Such auto-induction often leads to drug resistance by increasing the metabolism and elimination of paclitaxel and decreasing the drug concentrations in the target tissues [87–89].
Doxorubicin
Doxorubicin is a nuclear nonselective class I anthracycline antibiotics widely used in the treatment for lymphomas, leukemia, breast cancer and ovarian cancer. Mechanistically, doxorubicin interacts with DNA by intercalation and impairs both DNA and RNA synthesis during tumor cell division [90, 91]. Metabolism of doxorubicin occurs majorly in the liver, where it is converted to doxorubicinol carried out by carbonyl reductases. Both doxorubicin and doxorubicinol could be further transformed as aglycone metabolites via CYP3A enzymes [92]. A number of studies both in vitro and in vivo have established P-gp as a major efflux transporter responsible for excretion of doxorubicin from cells [93].
Both rifampin and the transfection of a VP-PXR plasmid rival cell apoptosis caused by doxorubicin treatment in human colon cancer cell line HCT116 [52]. Harmsen et al. [77] have observed that PXR activation by rifampin increases P-gp activity and reduces the cytotoxic activity of doxorubicin in human colon adenocarcinoma-derived cell line LS180. In contrast, augmented doxorubicin sensitivity in osteosarcoma cells was associated with down-regulation of P-gp by its antagonist ecteinascidin-743, indicating that specific and potent PXR antagonists could be used to increase doxorubicin efficacy [94].
Significant inter-individual and inter-ethnic variations have been observed in the pharmacokinetics and pharmacodynamics of doxorubicin [95]. Such variations could not be explained exclusively by drug–drug interactions. The study by Sandanaraj et al. [95] have shown that doxorubicin clearance and the expression of PXR, CYP3A4 and MDR1 in liver tissues harboring the PXR*1B haplotype are significantly lower than those harboring non-PXR*1B (PXR*1A and PXR*1C) clusters in 62 Asian (Chinese, Malay and Indian) breast cancer patients, indicating that the PXR genotype might be a prognostic factor of patients taking doxorubicin.
Vinblastine
Vinblastine, a vinca alkaloid, is extracted from the Madagascar periwinkle plant Catharanthus roseus [96]. It inhibits cell proliferation during mitosis by binding to the microtubules, which leads to mitotic block and apoptosis. Clinically vinblastine is extensively used in cancer chemo-therapy including acute leukemia, prostate cancer, breast cancer, lymphomas, myeloma, choriocarcinoma and malignant histiocytosis [97, 98]. Vinblastine is mainly metabolized by CYP3A4 and CYP3A5 [99] and transported by ABC (ATP-binding cassette) transporters [100].
Chen et al. [74] have found that PXR knockdown by siRNA increases the therapeutic sensitivity of vinblastine in human breast cancer cell lines MCF-7 and MDA-MB-231. The pretreatment for human prostate cancer cell line PC-3 cell with PXR agonist SR12813 elevates the mRNA level of CYP3A4 and increases vinblastine resistance, which is reversed by PXR knockout, suggesting that PXR is the key regulator in vinblastine resistance [86]. PXR-CYP3A4 might be the potential pathway in vinblastine resistance since PXR activation leads to the up-regulation of CYP3A4 and accelerates vinblastine metabolism, leading to drug resistance.
In addition, vinblastine could activate PXR and elevated the mRNA levels and activities of ABCC2, ABCC3 and CYP3A4 [101, 102], which leads to increased metabolism and efflux of vinblastine, resulted in drug self-resistance.
Other antitumor drugs
ATRA (all-trans retinoic acid) is broadly used in the treatment for APL (acute promyelocytic leukemia), breast cancer, Kaposi's sarcoma and glioma [103]. The antineoplastic role of ATRA is implemented by binding to RARs (retinoic acid receptors) and RXRs to modulate the transcription of a set of genes correlated with cellular differentiation, growth and apoptosis [104–106]. The clinical application of ARTA is restricted by drug resistance [107, 108]. PXR agonist PCN (pregnenolone-16α-carbonitrile), rifampin and dexamethasone are revealed to significantly increase the metabolism and decrease the AUC of ATRA in wild-type mice, but not in PXR-null mice. Further study has found that compared with PXR-null mice, mPXR agonist significantly increases the mRNA levels of mPXR, CYP3A,CYP26, MDR1, ABCC3 and OATP in wild-type mice [109], which might contribute to reduced blood concentrations of ATRA, indicating that PXR antagonists might be hopeful in reversing ARTA resistance.
Cyclophosphamide (CPA) and ifosfamide (IFO), two commonly used DNA alkylating agents in cancer chemo-therapy, are widely used in the treatment for various cancers [110, 111]. They are prodrugs activated via 4-hydroxylation by CYP2B6 and CYP3A4 to generate alkylating nitrogen mustards which could alkylate DNA to form DNA–DNA cross-links, leading to inhibition of DNA synthesis and cell apoptosis [112, 113]. Chang et al. [114] have observed that PXR agonists rifampin and dexamethasone could accelerate the metabolism of CPA and IFO and increase the 4-hydroxylation products in human primary cultures hepatocytes. Meanwhile, the protein expressions of CYP3A4, CYP2C8 and CYP2C9 are induced by the PXR agonists [114], which might accelerate the metabolism of CPA and IFO and be accounted for the enhanced drug efficacy.
Conclusions
To date, chemotherapy continues to be one of the major strategies battling various solid as well as systematic malignances in humans [115, 116]. Although great success has been achieved in this area, inter-individual variability of chemotherapeutic responses has often resulted in drug resistance or unwanted toxicity in clinics [115]. As a promiscuous xenobiotic receptor, PXR plays an important role in chemotherapeutic resistance, drug– drug and gene-drug interactions in cancer treatment [11, 15, 43, 117, 118]. A large number of chemotherapeutic agents are either inducer/inhibitor or substrate of drug-metabolizing enzymes or transporters that can be affected by PXR, leading to inter-individual variability of chemotherapeutic responses in cancer treatment. Therefore, the pharmacogenomics and regulation of PXR hold the promise to be a predict biomarker dictating the prognosis of chemotherapy [19, 22, 64, 65, 73, 74, 86, 109]. Targeted PXR antagonists may potentially improve chemotherapeutics efficacy by inhibiting the metabolism and efflux of anticancer drugs and suppressing the antiapoptosis genes [119]. Nevertheless, comparing to the well-established role of PXR in xenobiotic detoxification, its effects on cancer treatment and tumor development are yet controversial, and to find out tissue-context aspect of PXR should not be ignored [120]. Direct extrapolation findings from animal studies to human beings are inaccurate and sometimes risky. Thus, clinically targeting PXR as a therapeutic molecule warrants further investigations.
Contributor Information
Wei Zhuo, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha 410008, People's Republic of China; Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, Changsha 410078, Hunan, People's Republic of China; Hunan Key Laboratory of Pharmacogenetics, Changsha 410078, People's Republic of China.
Lei Hu, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha 410008, People's Republic of China; Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, Changsha 410078, Hunan, People's Republic of China; Hunan Key Laboratory of Pharmacogenetics, Changsha 410078, People's Republic of China.
Jinfeng Lv, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha 410008, People's Republic of China; Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, Changsha 410078, Hunan, People's Republic of China; Hunan Key Laboratory of Pharmacogenetics, Changsha 410078, People's Republic of China.
Hongbing Wang, Department of Pharmaceutical Sciences, The University of Maryland School of Pharmacy, 20 Penn Street, Baltimore, MD 21201, USA.
Honghao Zhou, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha 410008, People's Republic of China; Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, Changsha 410078, Hunan, People's Republic of China; Hunan Key Laboratory of Pharmacogenetics, Changsha 410078, People's Republic of China.
Lan Fan, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha 410008, People's Republic of China; Pharmacogenetics Research Institute, Institute of Clinical Pharmacology, Central South University, Changsha 410078, Hunan, People's Republic of China; Hunan Key Laboratory of Pharmacogenetics, Changsha 410078, People's Republic of China.
References
- 1.Blumberg B, Kang H, Bolado J, Jr, Chen H, Craig AG, Moreno TA, Umesono K, Perlmann T, De Robertis EM, Evans RM. BXR, an embryonic orphan nuclear receptor activated by a novel class of endogenous benzoate metabolites. Genes Dev. 1998;12:1269–1277. doi: 10.1101/gad.12.9.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M, Naito S, Yokoi T. Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokinet. 2004;19:135–149. doi: 10.2133/dmpk.19.135. [DOI] [PubMed] [Google Scholar]
- 5.Pondugula SR, Mani S. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer Lett. 2013;328:1–9. doi: 10.1016/j.canlet.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu M, Fan L, Zhou HH, Tomlinson B. Theranostics meets traditional Chinese medicine: rational prediction of drug-herb interactions. Expert Rev Mol Diagn. 2012;12:815–830. doi: 10.1586/erm.12.126. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Xie W, Krasowski MD. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics. 2008;9:1695–1709. doi: 10.2217/14622416.9.11.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orans J, Teotico DG, Redinbo MR. The nuclear xenobiotic receptor pregnane X receptor: recent insights and new challenges. Mol Endocrinol. 2005;19:2891–2900. doi: 10.1210/me.2005-0156. [DOI] [PubMed] [Google Scholar]
- 9.Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279:49307–49314. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Nie D. Pregnane X receptor and its potential role in drug resistance in cancer treatment. Recent Pat Anticancer Drug Discov. 2009;4:19–27. doi: 10.2174/157489209787002498. [DOI] [PubMed] [Google Scholar]
- 12.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7:e001. doi: 10.1621/nrs.07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai X, Zeng S, Xie W. Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol. 2013;9:253–266. doi: 10.1517/17425255.2013.754010. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem Pharmacol. 2012;83:1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- 17.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 18.Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, Evans RM. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA. 2003;100:4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 20.Guzelian J, Barwick JL, Hunter L, Phang TL, Quattrochi LC, Guzelian PS. Identification of genes controlled by the pregnane X receptor by microarray analysis of mRNAs from pregnenolone 16alpha-carbonitrile-treated rats. Toxicol Sci. 2006;94:379–387. doi: 10.1093/toxsci/kfl116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J, Shah YM, Gonzalez FJ. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci. 2012;33:323–330. doi: 10.1016/j.tips.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells. Mol Pharmacol. 2007;72:1045–1053. doi: 10.1124/mol.107.037937. [DOI] [PubMed] [Google Scholar]
- 23.Zucchini N, de Sousa G, Bailly-Maitre B, Gugenheim J, Bars R, Lemaire G, Rahmani R. Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim Biophys Acta. 2005;1745:48–58. doi: 10.1016/j.bbamcr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Gupta D, Venkatesh M, Wang H, Kim S, Sinz M, Goldberg GL, Whitney K, Longley C, Mani S. Expanding the roles for pregnane X receptor in cancer: proliferation and drug resistance in ovarian cancer. Clin Cancer Res. 2008;14:5332–5340. doi: 10.1158/1078-0432.CCR-08-1033. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Kuehl P, Green ED, Touchman JW, Watkins PB, Daly A, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Wrighton SA, Hancock M, Kim RB, Strom S, Thummel K, Russell CG, Hudson JR, Jr, Schuetz EG, Boguski MS. The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics. 2001;11:555–572. doi: 10.1097/00008571-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 26.King CR, Xiao M, Yu J, Minton MR, Addleman NJ, Van Booven DJ, Kwok PY, McLeod HL, Marsh S. Identification of NR1I2 genetic variation using resequencing. Eur J Clin Pharmacol. 2007;63:547–554. doi: 10.1007/s00228-007-0295-3. [DOI] [PubMed] [Google Scholar]
- 27.Lamba J, Lamba V, Strom S, Venkataramanan R, Schuetz E. Novel single nucleotide polymorphisms in the promoter and intron 1 of human pregnane X receptor/NR1I2 and their association with CYP3A4 expression. Drug Metab Dispos. 2008;36:169–181. doi: 10.1124/dmd.107.016600. [DOI] [PubMed] [Google Scholar]
- 28.Koyano S, Kurose K, Ozawa S, Saeki M, Nakajima Y, Hasegawa R, Komamura K, Ueno K, Kamakura S, Nakajima T, Saito H, Kimura H, Goto Y, Saitoh O, Katoh M, Ohnuma T, Kawai M, Sugai K, Ohtsuki T, Suzuki C, Minami N, Saito Y, Sawada J. Eleven novel single nucleotide polymorphisms in the NR1I2 (PXR) gene, four of which induce non-synonymous amino acid alterations. Drug Metab Pharmacokinet. 2002;17:561–565. doi: 10.2133/dmpk.17.561. [DOI] [PubMed] [Google Scholar]
- 29.Koyano S, Kurose K, Saito Y, Ozawa S, Hasegawa R, Komamura K, Ueno K, Kamakura S, Kitakaze M, Nakajima T, Matsumoto K, Akasawa A, Saito H, Sawada J. Functional characterization of four naturally occurring variants of human pregnane X receptor (PXR): one variant causes dramatic loss of both DNA binding activity and the transactivation of the CYP3A4 promoter/enhancer region. Drug Metab Dispos. 2004;32:149–154. doi: 10.1124/dmd.32.1.149. [DOI] [PubMed] [Google Scholar]
- 30.Lim YP, Liu CH, Shyu LJ, Huang JD. Functional characterization of a novel polymorphism of pregnane X receptor, Q158 K, in Chinese subjects. Pharmacogenet Genomics. 2005;15:337–341. doi: 10.1097/01213011-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Hustert E, Zibat A, Presecan-Siedel E, Eiselt R, Mueller R, Fuss C, Brehm I, Brinkmann U, Eichelbaum M, Wojnowski L, Burk O. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos. 2001;29:1454–1459. [PubMed] [Google Scholar]
- 32.Uno Y, Sakamoto Y, Yoshida K, Hasegawa T, Hasegawa Y, Koshino T, Inoue I. Characterization of six base pair deletion in the putative HNF1-binding site of human PXR promoter. J Hum Genet. 2003;48:594–597. doi: 10.1007/s10038-003-0076-5. [DOI] [PubMed] [Google Scholar]
- 33.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 34.Keightley MC. Steroid receptor isoforms: exception or rule? Mol Cell Endocrinol. 1998;137:1–5. doi: 10.1016/s0303-7207(97)00236-0. [DOI] [PubMed] [Google Scholar]
- 35.Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab Dispos. 2004;32:340–347. doi: 10.1124/dmd.32.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuen S, Fukuda T, Matsuda H, Sumida A, Yamamoto I, Inaba T, Azuma J. Identification of the novel splicing variants for the hPXR in human livers. Biochem Biophys Res Commun. 2002;298:433–438. doi: 10.1016/s0006-291x(02)02469-5. [DOI] [PubMed] [Google Scholar]
- 37.Mensah-Osman EJ, Thomas DG, Tabb MM, Larios JM, Hughes DP, Giordano TJ, Lizyness ML, Rae JM, Blumberg B, Hollenberg PF, Baker LH. Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer. 2007;109:957–965. doi: 10.1002/cncr.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YS, Yasuda K, Assem M, Cline C, Barber J, Li CW, Kholodovych V, Ai N, Chen JD, Welsh WJ, Ekins S, Schuetz EG. The major human pregnane X receptor (PXR) splice variant, PXR.2, exhibits significantly diminished ligand-activated transcriptional regulation. Drug Metab Dispos. 2009;37:1295–1304. doi: 10.1124/dmd.108.025213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tompkins LM, Sit TL, Wallace AD. Unique transcription start sites and distinct promoter regions differentiate the pregnane X receptor (PXR) isoforms PXR 1 and PXR 2. Drug Metab Dispos. 2008;36:923–929. doi: 10.1124/dmd.107.018317. [DOI] [PubMed] [Google Scholar]
- 40.di Masi A, De Marinis E, Ascenzi P, Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol Aspects Med. 2009;30:297–343. doi: 10.1016/j.mam.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Koutsounas I, Patsouris E, Theocharis S. Pregnane X receptor and human malignancy. Histol Histopathol. 2013;28:405–420. doi: 10.14670/HH-28.405. [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen T. Overcoming drug resistance by regulating nuclear receptors. Adv Drug Deliv Rev. 2010;62:1257–1264. doi: 10.1016/j.addr.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koutsounas I, Theocharis S, Patsouris E, Giaginis C. Pregnane X receptor (PXR) at the crossroads of human metabolism and disease. Curr Drug Metab. 2013;14:341–350. doi: 10.2174/1389200211314030009. [DOI] [PubMed] [Google Scholar]
- 45.Qiao E, Ji M, Wu J, Ma R, Zhang X, He Y, Zha Q, Song X, Zhu LW, Tang J. Expression of the PXR gene in various types of cancer and drug resistance. Oncol Lett. 2013;5:1093–1100. doi: 10.3892/ol.2013.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 47.Gollamudi R, Gupta D, Goel S, Mani S. Novel orphan nuclear receptors–coregulator interactions controlling anti-cancer drug metabolism. Curr Drug Metab. 2008;9:611–613. doi: 10.2174/138920008785821701. [DOI] [PubMed] [Google Scholar]
- 48.Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6:369–383. doi: 10.2174/1389200054633880. [DOI] [PubMed] [Google Scholar]
- 49.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 50.Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC Cancer. 2009;9:3. doi: 10.1186/1471-2407-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, Zhu L, Kaubisch A, Wang L, Pullman J, Whitney K, Kuro-o M, Roig AI, Shay JW, Mohammadi M, Mani S. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J, Liu M, Zhai Y, Xie W. The antiapoptotic role of pregnane X receptor in human colon cancer cells. Mol Endocrinol. 2008;22:868–880. doi: 10.1210/me.2007-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, Laffins B, Yao H, Zhou B, Tian Y. Pregnane X receptor suppresses proliferation and tumorigenicity of colon cancer cells. Br J Cancer. 2010;102:1753–1761. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias A, Wu J, Chen T. Tumor suppressor protein p53 negatively regulates human pregnane X receptor activity. Mol Pharmacol. 2013;83:1229–1236. doi: 10.1124/mol.113.085092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 56.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem. 2006;281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 58.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, Ren S, Kagan VE, Day BW, Zimniak P, Xie W. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 59.Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem. 2003;10:41–49. doi: 10.2174/0929867033368619. [DOI] [PubMed] [Google Scholar]
- 60.Haaz MC, Rivory L, Riché C, Vernillet L, Robert J. Metabolism of irinotecan (CPT-11) by human hepatic microsomes: participation of cytochrome P-450 3A and drug interactions. Cancer Res. 1998;58:468–472. [PubMed] [Google Scholar]
- 61.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- 62.Chu XY, Kato Y, Sugiyama Y. Possible involvement of P-glycoprotein in biliary excretion of CPT-11 in rats. Drug Metab Dispos. 1999;27:440–441. [PubMed] [Google Scholar]
- 63.Chu XY, Suzuki H, Ueda K, Kato Y, Akiyama S, Sugiyama Y. Active efflux of CPT-11 and its metabolites in human KB-derived cell lines. J Pharmacol Exp Ther. 1999;288:735–741. [PubMed] [Google Scholar]
- 64.Raynal C, Pascussi JM, Leguelinel G, Breuker C, Kantar J, Lallemant B, Poujol S, Bonnans C, Joubert D, Hollande F, Lumbroso S, Brouillet JP, Evrard A. Pregnane X Receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation. Mol Cancer. 2010;9:46. doi: 10.1186/1476-4598-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basseville A, Preisser L, de Carné Trécesson S, Boisdron-Celle M, Gamelin E, Coqueret O, Morel A. Irinotecan induces steroid and xenobiotic receptor (SXR) signaling to detoxification pathway in colon cancer cells. Mol Cancer. 2011;10:80. doi: 10.1186/1476-4598-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yonemori K, Takeda Y, Toyota E, Kobayashi N, Kudo K. Potential interactions between irinotecan and rifampin in a patient with small-cell lung cancer. Int J Clin Oncol. 2004;9:206–209. doi: 10.1007/s10147-004-0394-4. [DOI] [PubMed] [Google Scholar]
- 67.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VK. St John's wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 68.Mathijssen RH, Verweij J, de Bruijn P, Loos WJ, Sparreboom A. Effects of St. John's wort on irinotecan metabolism. J Natl Cancer Inst. 2002;94:1247–1249. doi: 10.1093/jnci/94.16.1247. [DOI] [PubMed] [Google Scholar]
- 69.Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, Mulcahy F, Feely J. St Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997;57:3402–3406. [PubMed] [Google Scholar]
- 71.Jacolot F, Simon I, Dreano Y, Beaune P, Riche C, Berthou F. Identification of the cytochrome P450 IIIA family as the enzymes involved in the N-demethylation of tamoxifen in human liver microsomes. Biochem Pharmacol. 1991;41:1911–1919. doi: 10.1016/0006-2952(91)90131-n. [DOI] [PubMed] [Google Scholar]
- 72.Teft WA, Mansell SE, Kim RB. Endoxifen, the active metabolite of tamoxifen, is a substrate of the efflux transporter P-glycoprotein (multidrug resistance 1). Drug Metab Dispos. 2011;39:558–562. doi: 10.1124/dmd.110.036160. [DOI] [PubMed] [Google Scholar]
- 73.Choi HK, Yang JW, Roh SH, Han CY, Kang KW. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer. 2007;14:293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Tang Y, Chen S, Nie D. Regulation of drug resistance by human pregnane X receptor in breast cancer. Cancer Biol Ther. 2009;8:1265–1272. doi: 10.4161/cbt.8.13.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res. 2008;68:9338–9347. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos. 2002;30:608–612. doi: 10.1124/dmd.30.5.608. [DOI] [PubMed] [Google Scholar]
- 77.Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagaoka R, Iwasaki T, Rokutanda N, Takeshita A, Koibuchi Y, Horiguchi J, Shimokawa N, Iino Y, Morishita Y, Koibuchi N. Tamoxifen activates CYP3A4 and MDR1 genes through steroid and xenobiotic receptor in breast cancer cells. Endocrine. 2006;30:261–268. doi: 10.1007/s12020-006-0003-6. [DOI] [PubMed] [Google Scholar]
- 79.Sane RS, Buckley DJ, Buckley AR, Nallani SC, Desai PB. Role of human pregnane X receptor in tamoxifen- and 4-hydroxytamoxifen-mediated CYP3A4 induction in primary human hepatocytes and LS174T cells. Drug Metab Dispos. 2008;36:946–954. doi: 10.1124/dmd.107.018598. [DOI] [PubMed] [Google Scholar]
- 80.Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH. Taxanes: a new class of antitumor agents. Cancer Invest. 1995;13:381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 81.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 82.Harris JW, Rahman A, Kim BR, Guengerich FP, Collins JM. Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res. 1994;54:4026–4035. [PubMed] [Google Scholar]
- 83.Rahman A, Korzekwa KR, Grogan J, Gonzalez FJ, Harris JW. Selective biotransformation of taxol to 6 alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res. 1994;54:5543–5546. [PubMed] [Google Scholar]
- 84.van Asperen J, van Tellingen O, Sparreboom A, Schinkel AH, Borst P, Nooijen WJ, Beijnen JH. Enhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833. Br J Cancer. 1997;76:1181–1183. doi: 10.1038/bjc.1997.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tell-ingen O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007;67:10361–10367. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- 87.Masuyama H, Suwaki N, Tateishi Y, Nakatsukasa H, Segawa T, Hiramatsu Y. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Mol Endocrinol. 2005;19:1170–1180. doi: 10.1210/me.2004-0434. [DOI] [PubMed] [Google Scholar]
- 88.Nallani SC, Goodwin B, Maglich JM, Buckley DJ, Buckley AR, Desai PB. Induction of cytochrome P450 3A by paclitaxel in mice: pivotal role of the nuclear xenobiotic receptor, pregnane X receptor. Drug Metab Dispos. 2003;31:681–684. doi: 10.1124/dmd.31.5.681. [DOI] [PubMed] [Google Scholar]
- 89.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 90.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 91.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 92.Lee HJ, Lee MG. Effects of dexamethasone on the pharmacokinetics of adriamycin after intravenous administration to rats. Res Commun Mol Pathol Pharmacol. 1999;105:87–96. [PubMed] [Google Scholar]
- 93.Lal S, Mahajan A, Chen WN, Chowbay B. Pharmacogenetics of target genes across doxorubicin disposition pathway: a review. Curr Drug Metab. 2010;11:115–128. doi: 10.2174/138920010791110890. [DOI] [PubMed] [Google Scholar]
- 94.Mensah-Osman E, Lin H-L, Reinke D, Hollenberg P, Baker L. Ecteinascidin-743 is a potent inhibitor of P450 3A4 enzyme and accumulates cytoplasmic PXR to inhibit transcription of P450 3A4 and MDR1: implications for the enhancement of cytotoxicity to chemotherapeutic agents in osteosarcoma. J Clin Oncol (Meeting Abstracts) 2005;23(16s):9026. [Google Scholar]
- 95.Sandanaraj E, Lal S, Selvarajan V, Ooi LL, Wong ZW, Wong NS, Ang PC, Lee EJ, Chowbay B. PXR pharmacogenetics: association of haplotypes with hepatic CYP3A4 and ABCB1 messenger RNA expression and doxorubicin clearance in Asian breast cancer patients. Clin Cancer Res. 2008;14:7116–7126. doi: 10.1158/1078-0432.CCR-08-0411. [DOI] [PubMed] [Google Scholar]
- 96.Noble RL. The discovery of the vinca alkaloids–chemotherapeutic agents against cancer. Biochem Cell Biol. 1990;68:1344–1351. [PubMed] [Google Scholar]
- 97.Jordan MA, Wilson L. Microtubules as a target for anti-cancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 98.Levêque D, Wihlm J, Jehl F. Pharmacology of Catharan-thus alkaloids. Bull Cancer. 1996;83:176–186. [PubMed] [Google Scholar]
- 99.Levêque D, Jehl F. Molecular pharmacokinetics of catharanthus (vinca) alkaloids. J Clin Pharmacol. 2007;47:579–588. doi: 10.1177/0091270007299430. [DOI] [PubMed] [Google Scholar]
- 100.Gruol DJ, King MN, Kuehne ME. Evidence for the locations of distinct steroid and Vinca alkaloid interaction domains within the murine mdr1b P-glycoprotein. Mol Pharmacol. 2002;62:1238–1248. doi: 10.1124/mol.62.5.1238. [DOI] [PubMed] [Google Scholar]
- 101.Huang R, Murry DJ, Kolwankar D, Hall SD, Foster DR. Vincristine transcriptional regulation of efflux drug transporters in carcinoma cell lines. Biochem Pharmacol. 2006;71:1695–1704. doi: 10.1016/j.bcp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 102.Smith NF, Mani S, Schuetz EG, Yasuda K, Sissung TM, Bates SE, Figg WD, Sparreboom A. Induction of CYP3A4 by vinblastine: role of the nuclear receptor NR1I2. Ann Pharmacother. 2010;44:1709–1717. doi: 10.1345/aph.1P354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong WK, Itri LM. Retinoids and human cancer. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids. Biology, chemistry and medicine. 2nd edn. Raven Press Ltd; New York: 1994. pp. 597–630. [Google Scholar]
- 104.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 105.Nagy L, Thomázy VA, Shipley GL, Fésüs L, Lamph W, Heyman RA, Chandraratna RA, Davies PJ. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995;15:3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elstner E, Müller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muindi JR, Frankel SR, Huselton C, DeGrazia F, Garland WA, Young CW, Warrell RP., Jr Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 1992;52:2138–2142. [PubMed] [Google Scholar]
- 108.Marill J, Cresteil T, Lanotte M, Chabot GG. Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol Pharmacol. 2000;58:1341–1348. doi: 10.1124/mol.58.6.1341. [DOI] [PubMed] [Google Scholar]
- 109.Wang T, Ma X, Krausz KW, Idle JR, Gonzalez FJ. Role of pregnane X receptor in control of all-trans retinoic acid (ATRA) metabolism and its potential contribution to ATRA resistance. J Pharmacol Exp Ther. 2008;324:674–684. doi: 10.1124/jpet.107.131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. doi: 10.2165/00003088-199120030-00002. [DOI] [PubMed] [Google Scholar]
- 111.Dechant KL, Brogden RN, Pilkington T, Faulds D. Ifosfamide/mesna. A review of its antineoplastic activity, pharmacokinetic properties and therapeutic efficacy in cancer. Drugs. 1991;42:428–467. doi: 10.2165/00003495-199142030-00006. [DOI] [PubMed] [Google Scholar]
- 112.Sladek NE. Metabolism of oxazaphosphorines. Pharmacol Ther. 1988;37:301–355. doi: 10.1016/0163-7258(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 113.Jing Zhang, Quan Tian, Shu-Feng Zhou. Clinical pharmacology of cyclophosphamide and ifosfamide. Curr Drug Ther. 2006;1:55–84. [Google Scholar]
- 114.Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- 115.Lee W, Lockhart AC, Kim RB, Rothenberg ML. Cancer pharmacogenomics: powerful tools in cancer chemotherapy and drug development. Oncologist. 2005;10:104–111. doi: 10.1634/theoncologist.10-2-104. [DOI] [PubMed] [Google Scholar]
- 116.Chabner BA, Roberts TG., Jr Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 117.Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug–drug interactions in oncology. Cancer Treat Rev. 2007;33:369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Meijerman I, Beijnen JH, Schellens JH. Herb–drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11:742–752. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- 119.Biswas A, Mani S, Redinbo MR, Krasowski MD, Li H, Ekins S. Elucidating the ‘Jekyll and Hyde’ nature of PXR: the case for discovering antagonists or allosteric antagonists. Pharm Res. 2009;26:1807–1815. doi: 10.1007/s11095-009-9901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robbins D, Chen T. Tissue-specific regulation of pregnane X receptor in cancer development and therapy. Cell Biosci. 2014;4:17. doi: 10.1186/2045-3701-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]