Abstract
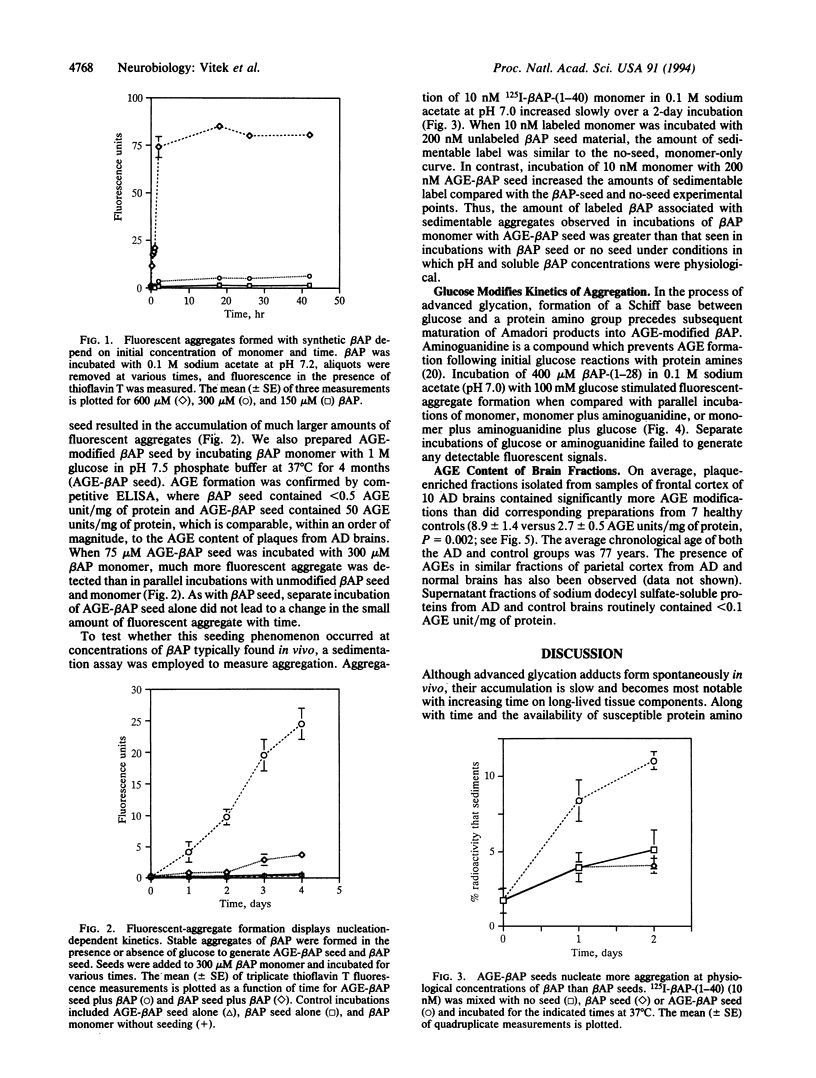
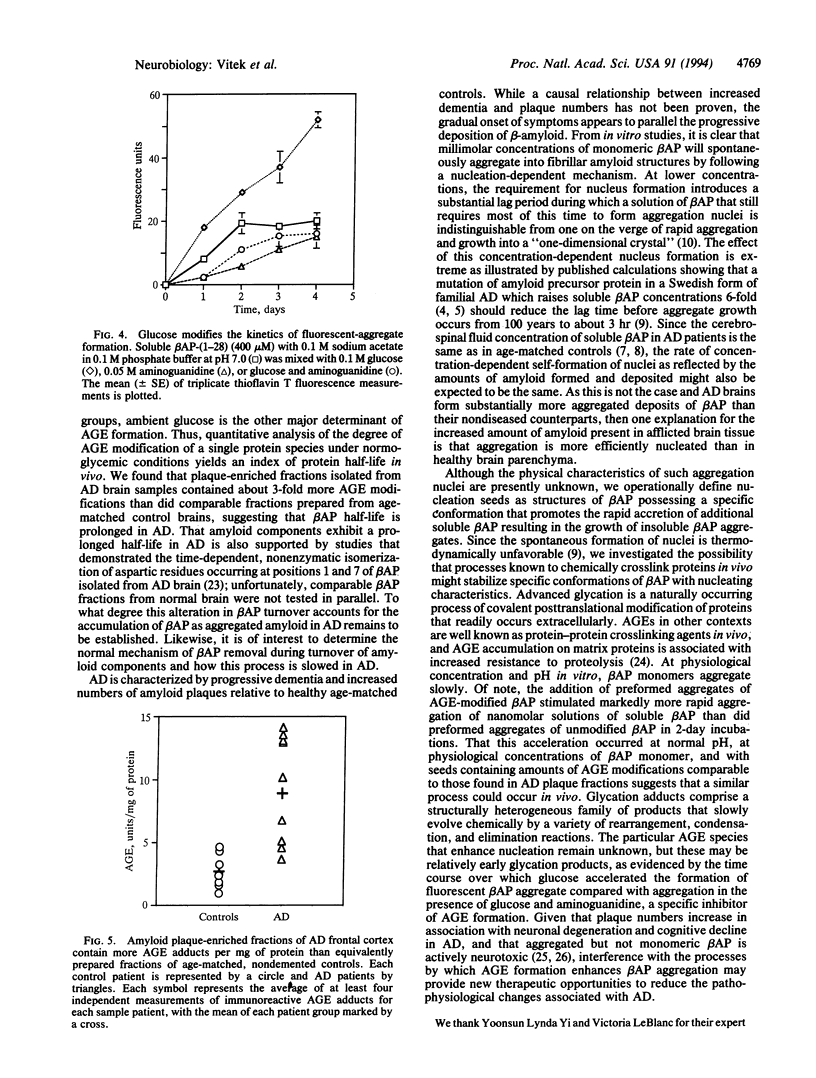
Alzheimer disease (AD) is characterized by deposits of an aggregated 42-amino-acid beta-amyloid peptide (beta AP) in the brain and cerebrovasculature. After a concentration-dependent lag period during in vitro incubations, soluble preparations of synthetic beta AP slowly form fibrillar aggregates that resemble natural amyloid and are measurable by sedimentation and thioflavin T-based fluorescence. Aggregation of soluble beta AP in these in vitro assays is enhanced by addition of small amounts of pre-aggregated beta-amyloid "seed" material. We also have prepared these seeds by using a naturally occurring reaction between glucose and protein amino groups resulting in the formation of advanced "glycosylation" end products (AGEs) which chemically crosslink proteins. AGE-modified beta AP-nucleation seeds further accelerated aggregation of soluble beta AP compared to non-modified "seed" material. Over time, nonenzymatic advanced glycation also results in the gradual accumulation of a set of posttranslational covalent adducts on long-lived proteins in vivo. In a standardized competitive ELISA, plaque fractions of AD brains were found to contain about 3-fold more AGE adducts per mg of protein than preparations from healthy, age-matched controls. These results suggest that the in vivo half-life of beta-amyloid is prolonged in AD, resulting in greater accumulation of AGE modifications which in turn may act to promote accumulation of additional amyloid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki N., Ueno N., Chakrabarti B., Morino Y., Horiuchi S. Immunochemical evidence for the presence of advanced glycation end products in human lens proteins and its positive correlation with aging. J Biol Chem. 1992 May 25;267(15):10211–10214. [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation products on collagen covalently trap low-density lipoprotein. Diabetes. 1985 Sep;34(9):938–941. doi: 10.2337/diab.34.9.938. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Come J. H., Fraser P. E., Lansbury P. T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R. J., Rasool C. G., Bartus R., Vitek S., Blume A. J., Vitek M. Multiple forms of beta-amyloid peptide precursor RNAs in a single cell type. Neurobiol Aging. 1988 Jul-Aug;9(4):333–338. doi: 10.1016/s0197-4580(88)80078-2. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993 Mar;2(3):404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992 Aug;1(5):345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. doi: 10.1016/0922-4106(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5190–5192. doi: 10.1073/pnas.78.8.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]


