Abstract
Alzheimer’s disease (AD) is the most common senile dementia in the world. Although important progress has been made in understanding the pathogenesis of AD, current therapeutic approaches provide only modest symptomatic relief. In this study, we evaluated the neuroprotective effect of quercetin (25 mg/kg) administration via i.p. injection every 48 hours for 3 months on aged (21–24 months old) triple transgenic AD model (3xTg-AD) mice. Our data show that quercetin decreases extracellular β-amyloidosis, tauopathy, astrogliosis and microgliosis in the hippocampus and the amygdala. These results were supported by a significant reduction in the paired helical filament (PHF), β-amyloid (βA) 1–40 and βA 1–42 levels and a decrease in BACE1-mediated cleavage of APP (into CTFβ). Additionally, quercetin induced improved performance on learning and spatial memory tasks and greater risk assessment behavior based on the elevated plus maze test. Together, these findings suggest that quercetin reverses histological hallmarks of AD and protects cognitive and emotional function in aged 3xTg-AD mice.
Keywords: Alzheimer’s disease, Quercetin, Neuroprotection, Tauopathy, β-amyloidosis, cognitive function
1. Introduction
The worldwide prevalence of dementia is estimated to be as high as 36 million and is predicted to reach 66 million by 2030 and 115 million by 2050, with approximately two-thirds of those patients living in developing countries (Prince et al., 2011). Alzheimer’s disease (AD) is the most common cause of dementia in elderly patients and is characterized by a progressive decline in cognitive function, which typically begins with deterioration of memory (Reitz et al., 2011). AD is characterized by the accumulation of extracellular β-amyloid (βA) plaques, the progressive appearance of intracellular tau pathology, the loss of synaptic connections from specific brain regions, and extensive oxidative stress. The deleterious activities of oxidized metabolites and free radicals include protein oxidation, lipid peroxidation, DNA oxidation and, ultimately, neuronal death (Duyckaerts et al., 2009; Iqbal and Grundke-Iqbal, 2008; Ittner and Gotz, 2011; Querfurth and LaFerla, 2010).
Although important progress has been made in understanding the pathogenesis of AD, current therapeutic approaches provide only modest relief of cognitive symptoms, including disturbances in memory and perception (Bassil and Grossberg, 2009; Neugroschl and Sano, 2009). Few medications have been approved by the US FDA for the treatment of AD; these drugs ameliorate symptoms but do not alter the course of disease progression and have even shown some undesired effects (Bassil and Grossberg, 2009; Mimica and Presecki, 2009; Soreq and Seidman, 2001). In this scenario, the use of natural substances for the treatment of neurodegenerative diseases such as AD is increasing (Mancuso et al., 2012; Russo et al., 2012).
Dietary flavonoids exhibit neuroprotective properties that involve several effects on the brain, including the protection of neurons against injury and promotion of memory, learning and cognitive function (Devi and Ohno, 2012; Spencer, 2009). Therefore, in principle, flavonoids could serve as a key class of molecules for the development of therapeutics for AD.
Quercetin (3,5,7,3′,4′-pentahydroxyflavone), a flavonoid generally found in fruits and vegetables, such as onions and apples, and red wine, has potential nutraceutical and pharmaceutical uses (Priprem et al., 2008). These potential uses may be due to its high oxygen radical scavenging activity or its ability to inhibit xanthine oxidase and lipid peroxidation in vitro (Fiorani et al., 2010). Furthermore, quercetin reliably exerts neuroprotective effects against agent-induced toxicity (Kanter et al., 2013) and increases the resistance of neurons to oxidative stress and excitotoxicity by modulating the mechanisms of cell death (Choi et al., 2014; Liu et al., 2013a). Other studies of quercetin have shown that it produces an anti-inflammatory effect (Garcia-Mediavilla et al., 2007) by inhibiting iNOS (Martinez-Florez et al., 2005) and regulating the expression of COX-2 (Banerjee et al., 2002; de Pascual-Teresa et al., 2004), as well as an anti-proliferative effect on some types of cancer (Park et al., 2005; Russo et al., 2014), via mechanisms that activate cell senescence, apoptosis (Russo et al., 2012) and autophagy (Psahoulia et al., 2007). Furthermore, its ability to penetrate the blood brain barrier (Fiorani et al., 2010; Youdim et al., 2004) and its protective effect against ischemia (Yao et al., 2012) and atherosclerosis (Lara-Guzman et al., 2012) have been reported.
In addition to neuroprotection, quercetin has been suggested to exert other beneficial effects on the central nervous system (CNS), such as anti-anxiety and cognitive enhancement, by stimulating or inhibiting enzyme activities/signal transduction pathways (Williams et al., 2004). However, whether quercetin reverses the primary histopathological hallmarks and the emotional and cognitive impairment of AD has yet to be determined. Therefore, we aimed to examine the neuroprotective effect of quercetin on a triple-transgenic AD model (3xTg-AD) mice.
2. Materials and Methods
2.1. Animals
Homozygous 3xTg-AD and non-transgenic (Non Tg) mice (Oddo et al., 2003b) from the in-house colony at the University of Antioquia maintained at the SIU (Sede de Investigación Universitaria) specific pathogen-free vivarium in Medellin, Colombia, were used at 18–21 months old to obtain homogeneus penetrance of tauopathy. The mice were maintained on a 12:12 hour dark:light cycle and received food and water ad libitum. The animals were handled according to Colombian standards (law 84/1989 and resolution 8430/1993) and guidelines. Special care was taken to minimize animal suffering and to reduce the number of animals used.
2.2. Administration of drugs
Quercetin (Cayman Chemical, Cat: 10005169) was dissolved in phosphate-buffered saline (PBS) containing 0.1% dimethyl sulfoxide (DMSO). The 3xTg-AD mice received intraperitoneal injections of 25 mg/kg quercetin or 0.1% DMSO (vehicle) every 48 hours for 3 consecutive months beginning at 18–21 months of age and sacrificed at 21–24 months old (Figure 1). The dose of quercetin (25 mg/kg) and the interval between the final drug treatment and the assays were selected based on previous in vivo studies (Cao et al., 2010; Carvalho et al., 2010; Patil et al., 2003). We carefully monitored the general health of the mice throughout the course of quercetin treatment and did not observe any adverse effects.
Figure 1. Scheme of experimental design.
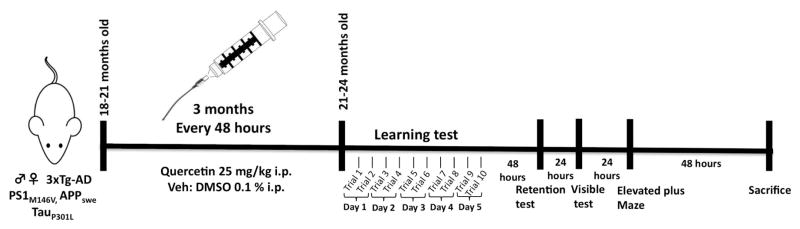
Quercetin (25 mg/Kg) and vehicle (DMSO 0.1 %) were i.p. administered at 18 – 21 months old no Tg and 3xTgAD mice during 3 months, every 48 hours. Learning (five days, ten trials) and memory tasks were evaluated by “Morris” water maze test at 21–24 months of age. After, elevated plus maze were realized (during two days). Later, mice were sacrificed for histological and biochemical analyses.
2.3. Histology
At 24 hours after the final behavioral test, the animals were anesthetized intraperitoneally using a mixture of ketamine (50 mg/kg) and xylazine (20 mg/kg) and were perfused with normal saline and 4% paraformaldehyde (in 0.1 M PBS, pH 7.4). The brains were carefully removed and post-fixed with 4% paraformaldehyde at 4°C for 48 hours, followed by cryopreservation using 30% sucrose and storage at −20°C. The brains were sectioned (50 μm) using a Leica VT1000S vibrating blade microtome (Leica Microsystems, Germany). Antero-posterior serial sections from each animal were evaluated via Nissl (toluidine blue) staining.
2.4. Immunohistochemistry
The sections were initially treated with methanol (50% v/v) and hydrogen peroxide (30% v/v) in 0.1 M PBS (pH 7.4) for 20 minutes to inhibit endogenous peroxidase activity. Then, three washes with 0.1 M PBS were performed, and the nonspecific binding sites were blocked for 1 hour using preincubation solution, consisting of 0.1 M PBS containing BSA (1%) and Triton X-100 (0.3% v/v). Next, the sections were incubated overnight at 4°C in a primary antibody that was diluted in incubation solution (0.3% BSA and Triton X-100 (0.3% v/v) in 0.1 M PBS). The anti-NeuN (mouse monoclonal, MAB377, Millipore, 1:500), anti-βA (monoclonal 1–16 (6E10), #SIG-39320, Covance, 1:500), anti-phospho-PHF-tau (pSer202/Thr205 (AT8), #MN1020, Thermo Scientific, 1:500), anti-glial fibrillary acidic protein (GFAP) (monoclonal, #G 3893, Sigma, 1:500), and anti-ionized calcium-binding adaptor molecule 1 (Iba1) (rabbit, #019-19741, Wako, 1:500) antibodies were used. The next day, the sections were washed three times (in 0.1 M PBS for 5 minutes each) and then incubated with the secondary antibody (1:250 dilution, biotin-conjugated goat anti-rabbit IgG (H+L), #31822, or biotin-conjugated goat anti-mouse IgG (H+L), #31800, Pierce, depending on the host species from which the primary antibody was prepared) for one hour at room temperature.
After three washes with 0.1 M PBS, the tissues were incubated in avidin biotin complex (1:250 reagents A and B, ABC Standard Peroxidase Staining Kit, #32020, Pierce) for 1 hour. Once the complex was removed, three additional washes were performed, and diaminobenzidine (DAB) was used to develop the reaction. Subsequently, the sections were dehydrated using alcohol series cleared with xylene and sealed using Consult-mount. Quantification of immunoreactivity in the areas examined was determined using a 10x or 40x objective and was analyzed using Fiji ImageJ 1.45 software (NIH, USA). The tissues incubated in the absence of primary antibody did not display immunoreactivity. The regions including the CA1 and subiculum (hippocampus), the entorhinal cortex (EC) and the amygdala were evaluated at Bregma −1.76 mm (Paxinos and Franklin, 2004).
2.5. Morris water maze test
At 48 hours after the final treatment, the animals were evaluated via the Morris water maze (MWM) test. A white plastic tank 1 min diameter and 30 cm in height was filled with water (22 ± 2°C) to a depth of 20 cm. The platform (7 cm diameter) was 1.5 cm below the surface of the water during spatial learning and 1.5 cm above the surface of the water during the visible session. Extramaze visual cues around the room remained in a fixed position throughout the experiment. Ten sessions or trials were performed, two complete sessions per day, during five days (Figure 1). Each session consisted of four successive subtrials (30 s inter-trial interval), and each subtrial began with the mouse placed pseudo-randomly in one of four starting locations. The animals had been trained to stay on the platform for 30 s prior to the initial trial. The latency to reach the platform was evaluated using a visible platform to control for any difference in visual-motor abilities or motivation between the experimental groups. If a mouse did not locate the platform after a maximum of 60 s, it was gently guided to the platform. Then, the animals were then provided with 48 h of retention time, followed by a probe trial of spatial reference memory, in which the animals were placed in the tank without the platform for 60 s (Figure 1). The latency to reach the exact former platform location and the number of crossings of the platform target quadrant were recorded during the probe trial. An automated system (Viewpoint, Lyon, France) recorded the behavior of the animals.
2.6. Elevated plus maze
To evaluate anxiolytic activity at 24 hours after the visible test in the Morris water maze, the animals were exposed to the elevated plus maze (EPM) according to previously published data (Lister, 1987; Vissiennon et al., 2012). The EPM was composed of white Plexiglas and was illuminated at approximately 30–40 lux. The apparatus consisted of two open arms (30 × 5 × 0.25 cm) and two closed arms (30 × 5 × 15 cm) extending from a common central platform (5 × 5 cm), and the entire apparatus was elevated on a single central support to a height of 60 cm above the floor. Each mouse was placed in the middle section facing an open arm and was allowed to explore the maze for a single 5 min session, during which the experimenter was out of view. After each trial, the floor was wiped clean with 10% alcohol.
The following parameters were recorded: frequency of open and closed arm entries (arm entry was defined as all four paws in the arm), total arm entries, and the amount of time spent by the animals in the open and closed sections of the maze. These data were used to calculate the % open or closed arm entries (e.g., open entries/total entries x 100) and the % time spent in the open or closed arms (e.g., open time/300 × 100). The following measurements were added to these standard parameters: rearing frequency and duration (all rearing occurred against the walls of the enclosed arms), the frequency of discrete behaviors, such as head dipping or deeping (exploratory movement of the head/shoulders over the side of the maze), stretched attentive postures (stretching, an exploratory posture in which the mouse stretches forward and retracts to the original position without locomoting forward), closed arm returns (exiting a closed arm with only the forepaws and returning/doubling back to the same arm), and the duration of grooming (a species-typical sequence beginning with the snout, progressing to the ears and ending by grooming the entire body). Each experiment was videotaped using a high-resolution video camera. These data were collected using X-Plo-Rat 2005 software (Taverna-Chaim, 2008).
2.7. Western blot
After behavioral testing, the animals were sacrificed via decapitation, and the hippocampus and the amygdala were dissected, immediately frozen in liquid nitrogen and stored at −80°C until analysis. The tissues were dissected and homogenized in lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% NP40, 1 nM orthovanadate, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (1:1000) (Sigma-Aldrich) (Cardona-Gomez et al., 2004). Then, 10–12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed using a Mini-Protean system (Bio-Rad, Hercules, CA) and wide range molecular weight standards (Bio-Rad). Each lane was loaded with 50 mg of protein in buffer containing 0.375 M Tris (pH 6.8), 50% glycerol, 10% SDS, 0.5 M DTT and 0.002% bromophenol blue. The samples were heated at 95°C for 5 minutes before loading on the gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes (Amersham) using an electrophoretic transfer system (Mini Trans-Blot Electrophoretic Transfer Cell, Bio-Rad, USA) at 300 mA for 2 hours. The membranes were washed with Tris-buffered saline (pH 7.4) (20 mM Tris-HCl (pH 7.5) and 500 mM NaCl) containing 0.05% Tween-20 (TTBS) and were blocked with 5% skim milk for 1 hour. TTBS was used for all subsequent washes and incubations. The membranes were incubated overnight in the following primary antibodies: monoclonal anti-PHF-1, which recognizes Tau pSer-396/404 and was donated by P. Davies (Feinstein Institute for Medical Research, Manhasset, NY), anti-phospho-PHF-tau (pSer202/Thr205 (AT8), #MN1020, Thermo Scientific, 1:500), and anti-tau 5 (mouse monoclonal, #MAB361, Chemicon International, 1:1000). Tubulin (mouse monoclonal anti-βIII tubulin antibody, #G712A, Promega, 1:10,000) or actin (mouse monoclonal anti actin AC-40, #A3853, Sigma, 1:1000) was used as a loading control. To detect the C-terminal fragments, we used 10–20% gradient gels (Mini-PROTEAN Cat#:456-3114) and the anti-amyloid precursor protein C-terminal antibody (rabbit polyclonal, #A8717, Sigma, 1:1000). IRDye 800CW goat anti-mouse or anti-rabbit (LI-COR; 1:10,000) was used as the secondary antibody. The membranes were developed using the Odyssey Infrared Imaging System (LI-COR, USA).
2.8. ELISA for βA
The brain levels of soluble βA 1–40 and 1–42 were determined via ELISA according to the manufacturer’s protocol. Then, the levels of soluble βA were measured via sandwich ELISA using Colorimetric BetaMark βA x-40 (SIG-38954- Covance Laboratories) and x-42 ELISA Kits (SIG-38956, Covance Laboratories).
2.9. Statistics
At least 3 mice were used for each histological study; 4–6 mice were used for each biochemical study; and 10–16 mice were used for each behavioral assay. The parametric data were evaluated via analysis of variance (ANOVA) to compare 4 groups followed Tukey’s test for post hoc multiple comparison between-group analyses. The nonparametric data were evaluated using the Kruskal-Wallis test. The escape latency during the training and transfer test was determined via two-way ANOVA followed by a Dunnett’s post hoc test for multiple comparisons. The statistical analysis was performed using GraphPad Prism software (version 6.0), and the results were considered to be significant when p≤0.05. The values are expressed as the means ± SEM.
3. Results
3.1. Quercetin protects the neuronal population in the subiculum of 3xTg-AD mice
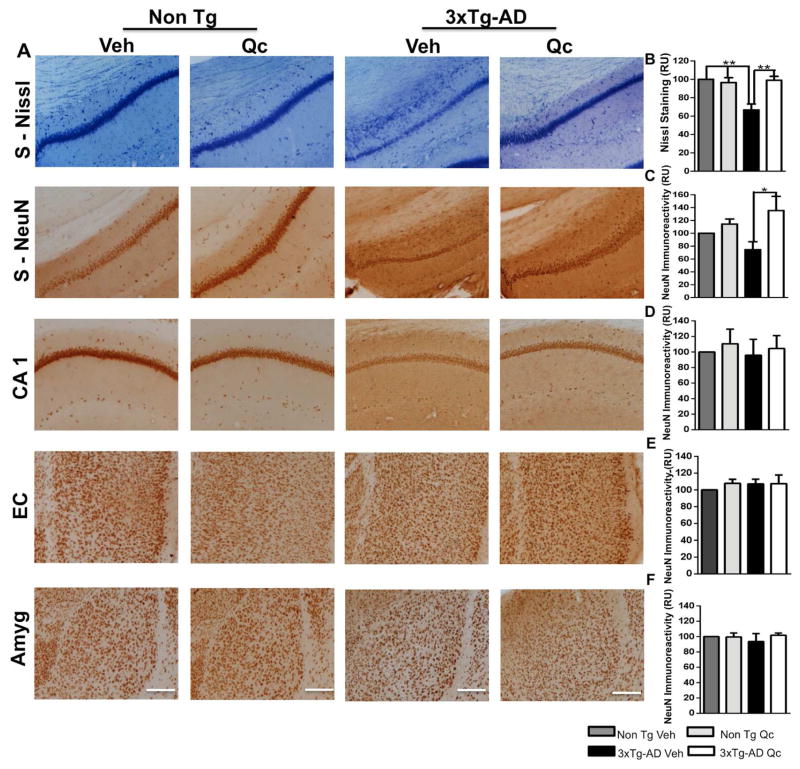
After sacrificing the animals, the brains were removed and processed for Nissl staining to identify the changes in the cytoarchitecture and cellular morphology in 3xTg-AD animals treated with vehicle or quercetin. The subiculum displayed a loss of cell density in the vehicle-treated 3xTg-AD mice, and quercetin treatment increased the cell density in the subiculum to a level similar to that in Non Tg mice treated with vehicle or quercetin (Fig. 2A–B). No changes in cell density were detected in the other structures evaluated (data not shown). These findings were supported by a significant neuronal loss of NeuN immunoreactivity in the subiculum of 3xTg-AD mice treated with vehicle, which was prevented by quercetin treatment (Fig. 2A–C). NeuN immunoreactivity in the subiculum of the quercetin-treated 3xTg-AD mice was similar to that in the Non Tg mice. The CA1 area, the EC and the amygdala did not display any alteration in NeuN immunoreactivity (Fig. 2D–F).
Figure 2. Quercetin protects the neuronal population in the subiculum of 3xTg-AD mice.
(A) Representative images of Nissl staining in the subiculum and NeuN immunohistochemistry in the evaluated areas of vehicle- and quercetin-treated Non Tg and 3xTg-AD mice at 21–24 months of age. Magnification: 10x; scale bar: 50 μm. The values in the bar graph are expressed in densitometric relative units (RU), and the statistical significance of Nissl staining in the subiculum (B) and NeuN immunoreactivity quantification in the subiculum (C), the CA1 area (D), the EC, (E), and the amygdala (F). Veh: vehicle (DMSO); Qc: quercetin; S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p: <0.05; **p: <0.01‚ ***p: <0.001.
3.2. Quercetin treatment reverses β-amyloidosis in 3xTg-AD mice
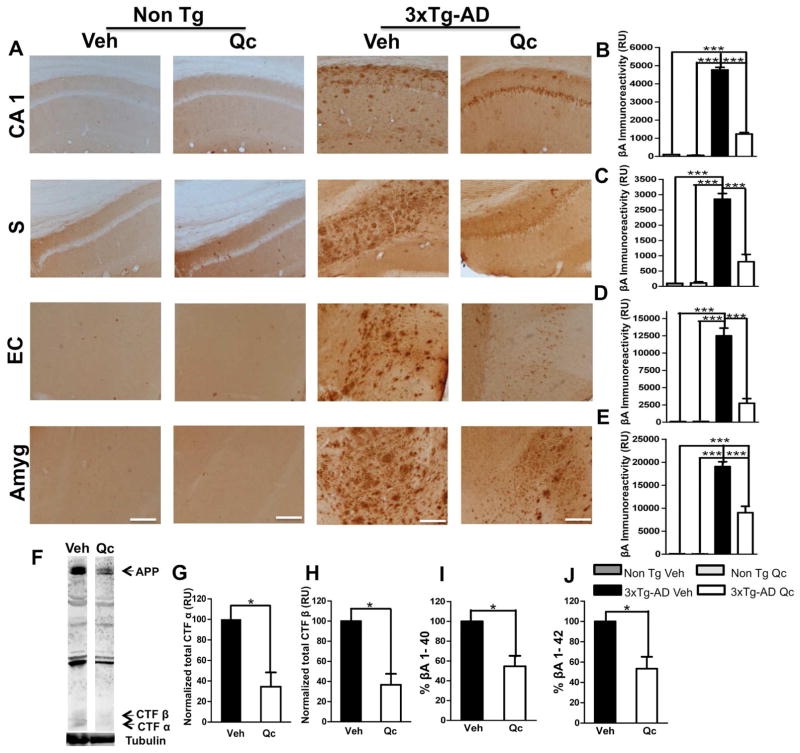
The 3xTg-AD mice displayed strong βA immunoreactivity, a neuropathological manifestation of AD, compared with the Non-Tg mice (21–24 months old). When we evaluated the effect of quercetin treatment on βA immunoreactivity, a significant reduction in the amount of extracellular βA deposition was detected in all of the examined cerebral regions of the brain compared to vehicle treatment, confirming the robustness of this result (Fig. 3A–E). Neither the vehicle- nor quercetin-treated Non Tg animals displayed βA immunoreactivity (Fig. 3A–E).
Figure 3. Quercetin ameliorates β-amyloidosis in the evaluated brain areas.
(A) Representative images of βA (anti-βA 6E10) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of vehicle- and quercetin-treated Non Tg and 3xTg-AD mice at 21–24 months of age. Magnification: 10x; scale bar: 50 μm. (B) The values in the bar graph are expressed in densitometric relative units (RU) of βA immunoreactivity in the CA1 area, (C) the subiculum, (D) the EC and (E) the amygdala. (F) Representative bands of CTFα and CTFβ using an anti-C-terminal APP antibody. Tubulin was used as a loading control. (G) Densitometric quantification of CTFα and (H) CTFβ expression (I). The relative βA 1–40 and (J) βA 1–42 fragment levels were analyzed in the hippocampal lysates via ELISA. Veh: vehicle (DMSO); Qc: quercetin; S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4–5. *p: <0.05; **p: <0.01; ***p: <0.001.
These findings were clearly supported by a significant decrease in the levels of C-terminal APP fragments (β) in the quercetin-treated 3xTg-AD mice, which were significantly lower than those in the vehicle-treated 3xTg-AD mice (Fig. 3F–H). In addition, we detected a remarkable reduction in the βA 1–40 and βA 1–42 levels in the hippocampus of the quercetin-treated 3xTg-AD mice compared to the vehicle-treated 3xTg-AD mice (Fig. 3I–J).
3.3. Quercetin decreases tauopathy in 3xTg-AD mice
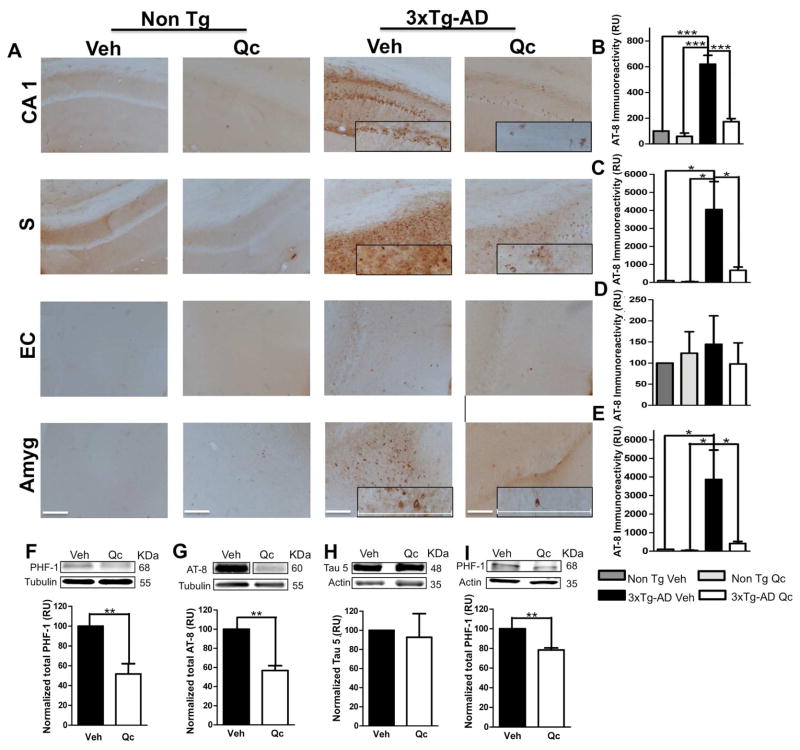
The 3xTg-AD mice treated with vehicle displayed abundant AT-8 immunoreactivity, whereas the 3xTg-AD mice treated with quercetin displayed a significant decrease in these neurofibrillary tangles in the CA1 area, the subiculum and the amygdala but not in the EC. These findings are similar to the results of Non Tg mice treated with quercetin or vehicle (Fig. 4A–E). These findings were confirmed by the significant reduction in the protein levels of PHF-1 (Fig. 4F) and AT-8 (Fig. 4G) in hippocampal and amygdalar lysates from quercetin-treated 3xTg-AD mice compared with those from vehicle-treated mice (Fig. 4I). The total Tau level, which was detected using the anti-tau 5 antibody, was not altered by any treatment (Fig. 4H).
Figure 4. Quercetin decreases tauopathy in AD mouse brains.
(A) Representative images of AT8 (anti-tau pSer202/Thr205) immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non Tg and 3xTg-AD mice treated with vehicle or quercetin. Magnification: 10x; scale bar: 50 μm. For the insets, magnification: 40x; scale bar: 120 μm. (B) The values in the bar graph are expressed in densitometric relative units (RU) of AT8 immunoreactivity in the CA1 area, (C) the subiculum, (D) the EC and (E) the amygdala. (F) Representative bands and densitometric intensities of PHF-1, (G) AT-8 and (H) Tau 5 protein expression in the hippocampal lysates and (I) of PHF-1 in the amygdalar lysates. Tubulin and β-actin were used as loading controls. Veh: vehicle (DMSO); Qc: quercetin; S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=4. *p: <0.05; **p: <0.01; ***p: <0.001.
3.4. Quercetin reduces astrogliosis and microgliosis in 3xTg-AD mice
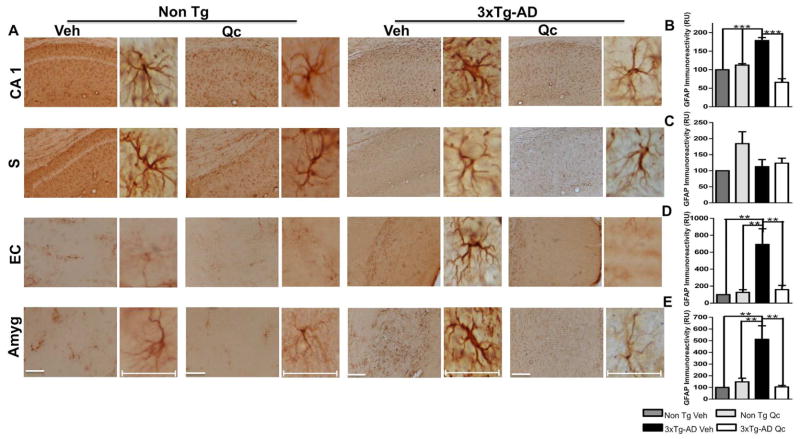
Astrocyte activation is indicated by the appearance of a hypertrophic soma and processes and is often accompanied by an increase in the expression of GFAP, a major intermediate filament protein specific to astrocytes (Furman et al., 2012). Consistent with previous results in an AD mouse model (Meraz-Rios et al., 2013), our data showed a significant increase in GFAP immunoreactivity in the 3xTg-AD mice compared to the Non Tg mice (Fig. 5A–E). However, quercetin treatment significantly reduced GFAP immunoreactivity in the CA1 hippocampal area, the EC and the amygdala compared to vehicle treatment. We did not obtain modifications in subiculum. And Quercetin treatment did not affect the Non Tg animals (Fig. 5A–E).
Figure 5. Quercetin decreases astrogliosis in 3xTg-AD mice.
(A) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non Tg and 3xTg-AD mice treated with vehicle or quercetin. Magnification: 10x; scale bar: 50 μm. For the insets, magnification: 40x; scale bar: 10 μm. (B) The values in the bar graphs are expressed in densitometric relative units (RU) of GFAP immunoreactivity in the CA1 area, (C) the subiculum, (D) the EC and (E) the amygdala. Veh: vehicle (DMSO); Qc: quercetin; S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. **p: <0.01; ***p: <0.001.
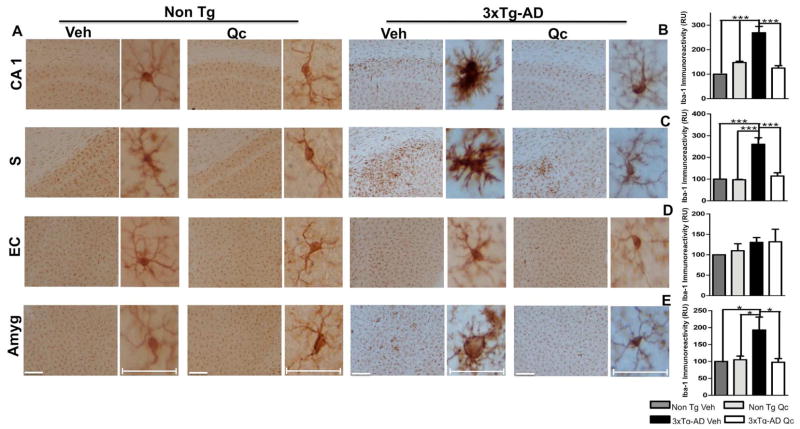
In addition, microglial activation is associated with the distribution of βA plaques and neurofibrillary tangles, which has been correlated to neurodegeneration, dementia progression and AD severity (Mrak, 2012; Thangavel et al., 2012). Microglial activation is a hallmark in AD models and is decreased in neuropathological reversion (Furman et al., 2012; Lee et al., 2013). In our study, we found that the quercetin-treated 3xTg-AD mice displayed microglial immunoreactivity that was significantly decreased in the CA1 area, in the subiculum and in the amygdala compared to the vehicle-treated 3xTg-AD mice and that was similar to that in the Non Tg mice (Fig. 6A–E). However, we did not find changes in the EC.
Figure 6. Quercetin ameliorates microgliosis in 3xTg-AD mice.
(A) Representative images of GFAP immunoreactivity in the CA1 area, the subiculum, the EC and the amygdala of Non Tg and 3xTg-AD mice treated with vehicle or quercetin. Magnification: 10x; scale bar: 50 μm. For the insets, magnification: 40x; scale bar: 10 μm. (B) The values in the bar graphs are expressed in densitometric relative units (RU) of Iba-1 immunoreactivity in the CA1 area, (C) the subiculum, (D) the EC and (E) the amygdala. Veh: vehicle (DMSO); Qc: quercetin; S: subiculum of the hippocampus; EC: entorhinal cortex; Amyg: amygdala. The data are expressed as the means ± SEM. n=3–4. **p 0.01; ***p: <0.001.
3.5. Quercetin improves the spatial learning and memory task performance of 3xTg-AD mice
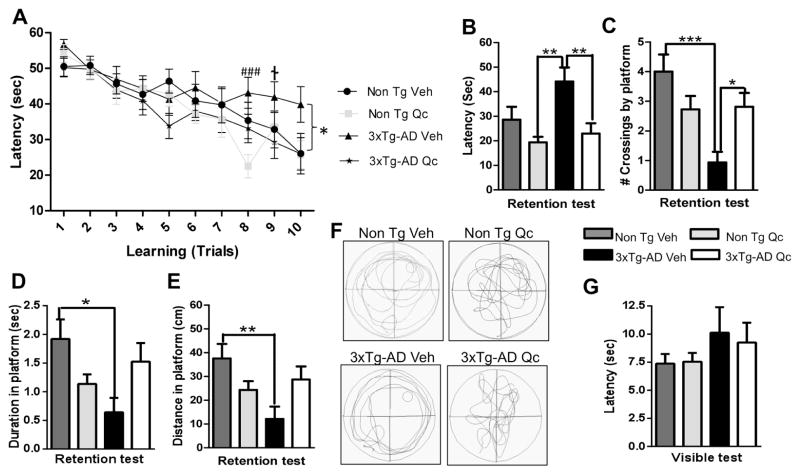
We found that quercetin administration for 3 months in 21–24 month-old 3xTg-AD mice significantly reduced their latency to locate the platform on the MWM, and improvement in the spatial learning tasks occurred after 8–10 trials. 3xTg-AD mice exhibit synaptic dysfunction and long-term potentiation (LTP) deficits (Oddo et al., 2003b; Parachikova et al., 2010). In addition, Parachikova et al. found that the 3xTg-AD mice exhibit a higher latency to locate the platform in the “MWM” test (Parachikova et al., 2010). Confirming these findings, the 3xTg-AD animals exhibited a longer latency to locate the platform than the quercetin- or vehicle-treated Non Tg mice. However, treatment of the 3xTg-AD mice with quercetin clearly decreased their latency on the final trial on the learning test to a level similar to that of treatment of Non Tg mice with quercetin or vehicle (Fig. 7A). Furthermore, the Non Tg mice and the 3xTg-AD mice treated with quercetin exhibited a higher retention performance at 48 hours after the final trial on the learning test than the 3xTg-AD mice treated with vehicle (Fig. 7B). This was confirmed by an increase in the number of crossings of the quadrant corresponding to the initial platform location by quercetin-treated 3xTg-AD mice, in which the vehicle-treated 3xTg-AD mice exhibited fewer crossings than the Non Tg mice (Fig. 7C). In addition, the time and distance to reach the platform were lower in the vehicle-treated 3xTg-AD mice than in the quercetin-treated 3xTg-AD and in the Non Tg mice (Fig. 7D–E). These findings are supported by representative images of the routes of travel during the retention test (Fig. 7F), which demonstrated that the animals treated with quercetin remained closer to the hidden platform and the surrounding area than the animals treated with vehicle. The visible test did not reveal any visual, motor or motivational deficit in any experimental group (Fig. 7G).
Figure 7. Quercetin protects spatial learning and memory function in 3xTg-AD mice.
(A) Mean latency in reaching the hidden platform on the spatial learning task. (B) The latency to reach, (C) the number of crossings of, (D) the duration in and (E) the distance to reach the platform quadrant on the retention test. (F) Representative images of the route of travel during the retention test. (G) No differences were detected in the visual, motor or motivational skills of the animals during the visible test between the experimental groups. The data are expressed as the means ± SEM. n=8–16. *Difference between the 3xTg-AD-Veh group and the other groups; †Difference between the 3xTg-AD-Veh and 3xTg-AD-Qc groups; ###Difference between the 3xTg-AD-Veh and Non Tg-Qc groups. *, † p: <0.05; **<p: 0.01; ***, ### p: <0.001.
3.6. Quercetin exerts an anxiolytic effect on 3xTg AD mice
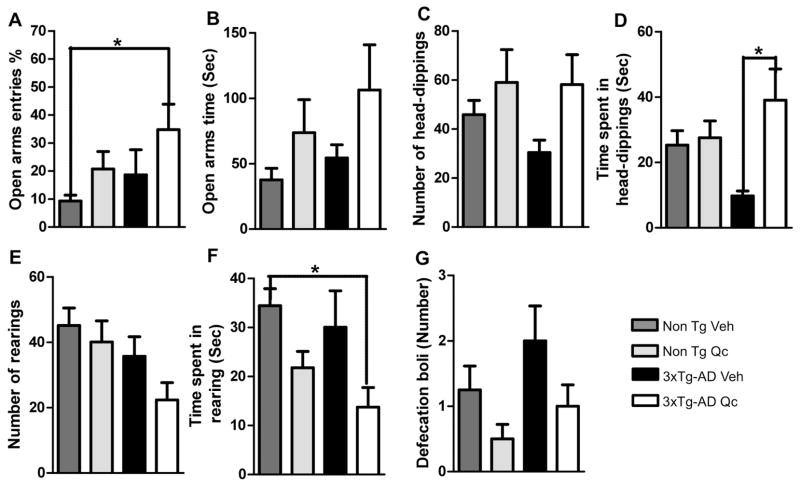
We evaluated the effect of quercetin on the animals using the EPM test. We found that the Non Tg mice treated with quercetin or vehicle rarely visited the open arm, very similar to the 3xTg-AD mice treated with vehicle, and the 3xTg-AD mice treated with quercetin spent more time in the open arm (Fig. 8A). In addition, we detected an interesting tendency toward increased time spent in the open arms by the Non Tg and 3xTg-AD mice treated with quercetin (Fig. 8B). Additionally, treatment of the 3xTg-AD mice with quercetin resulted in a higher frequency and significantly greater time spent performing head-dipping than treatment of the 3xTg-AD mice with vehicle and were similar to the levels detected for treatment of Non Tg mice with either vehicle or quercetin (Fig. 8C–D). This head-dipping behavior was inversely proportional to the time spent rearing, which predominantly occurred in the closed arms. Therefore, the time spent rearing was significantly lower in the quercetin-treated 3xTg-AD mice than in the other mice (Fig. 8E–F). In addition, we evaluated the number of defecation boli at the end of the task, which may indicate an anxious state (Walsh and Cummins, 1976). We found that the number of defecation boli from the vehicle-treated 3xTg-AD mice tended to be increased, which is consistent with previous studies of this model (Gimenez-Llort et al., 2007). However, quercetin treatment decreased the number of boli from the Non Tg mice and tended to reduce the number of boli from the 3xTg-AD mice (Fig. 8G).
Figure 8. Quercetin increases anxiolytic activity and risk assessment in 3xTg-AD mice.
(A–B) The relative frequency of open arm entries and the time spent in the open arms. (C–D) The number of head-dipping behaviors and the time spent head-dipping. (E–F) The number of rearing behaviors and the time spent rearing. (G). The number of defecation boli at the end of the EPM test performed two weeks after the final dose of vehicle or quercetin following three months of administration. The data are expressed as the means ± SEM. n=7–10. *p: <0.05.
4. Discussion
Our study is the first to provide a complete evaluation of the neuroprotective properties of quercetin as a therapeutic compound that ameliorates brain deficits in a triple transgenic AD mouse model. Quercetin treatment correlated to reversed brain levels of β-amyloidosis and tauopathy and ameliorated astroglial and microglial reactivity in the CA1 area, the subiculum, the EC and the amygdala. In addition, treatment of aged 3xTg-AD mice with quercetin induced an improvement in cognitive and emotional behavioral performance compared to treatment of these mice with vehicle.
We found that treatment with quercetin for three months decreased β-amyloidosis in aged 3xTg-AD mice. Our data are supported by previous in vitro studies in which primary hippocampal cultures were pretreated with quercetin, which significantly attenuated the βA 1–42-mediated cytotoxicity and reduced protein oxidation, lipid peroxidation and apoptosis (Ansari et al., 2009). Although, some studies did not present effect of quercetin on Beta-amyloid neurotoxicity in vitro (Bate et al., 2004). And also, its effect was not robustly able to prevent neuronal toxicity at 100 and 200 mg/kg per day (Kaariainen et al., 2008) or it is not considered protective on neurodegenation (Ossola et al 2009). Other recent studies suggest that quercetin-3-O-glucoronide, a brain-targeted polyphenol metabolite in Cabernet Sauvignon that acts as a potent inhibitor of βA aggregation, reduced the βA peptide levels in primary neuron cultures generated from Tg2576 AD model mice (APP KM670/671NL -Swe) and alleviated the deficits in basal synaptic transmission in the hippocampus and in LTP (Ho et al., 2013). In addition, after the inclusion of Cabernet Sauvignon in the diet of Tg2576 mice, there was an improvement in performance on the Barnes maze task, possibly due to the promotion of non-amyloidogenic processing of APP (Wang et al., 2006).
Quercetin-3-O-glucoronide may exert a neuroprotective effect in the brain; however, studies must be developed to determine the bioavailability of quercetin metabolites. Although it is known that glucuronide and/or sulfate conjugates with or without O-methylation exclusively circulate in the human bloodstream following intake of a quercetin-containing diet, it has been shown that quercetin-3-O-glucoronide in the bloodstream it translocates to the CNS under conditions of oxidative stress and that quercetin-3-O-glucoronide is more effective than the aglycone form of the molecule (Terao et al., 2011). This activity is related to the “paradox of flavonoids”, including quercetin, in which although their protective effects on various diseases have been demonstrated, these substances are not found in plasma after oral administration. These results suggest that hydrophilic bioactive agents as well as detoxified metabolites of quercetin are produced by the enzyme β-glucuronidase (Perez-Vizcaino et al., 2012). Thus, glucuronidation appears to play a crucial role in the bioavailability and the biological effects of the quercetin aglycone (Boonpawa et al., 2014).
Another important marker of AD is tauopathy, which begins in the hippocampus of the 3xTg-AD mice. In particular, tauopathy is detected in the pyramidal neurons of the CA1 area at 15 months old (Oddo et al., 2003b), but in the current colony this pathological hallmark is clearly accentuated in all homozygous mice at 18 months of age (Castro-Alvarez et al., 2014; Piedrahita et al., 2010) leading to degenerative symptoms, including significant deficits in performance on hippocampal-dependent cognitive tasks, such as spatial learning and memory, followed by the progression of tauopathy to other areas (Oddo et al., 2003b; Yamin, 2009). In the present study, we showed that quercetin administration to aged 3xTg-AD mice correlated with reversion of PHF levels based on the decrease in the phosphorylation of AT-8 tau in the CA1 area, the subiculum and the amygdala and decreased PHF-1 and AT-8 protein levels in the hippocampal and amygdala lysates. These data are consistent with the findings described using other flavonoids, such as myricetin and epicatechin, which inhibit tau fibril formation and disrupt the formation of PHFs and neurofibrillary tangles (George et al., 2013; Ksiezak-Reding et al., 2012). Although there are no reports of the mechanism of action of quercetin regarding tauopathy, it has been found that epigallocatechin-3-gallate (EGCG), a potent antioxidant structurally related to quercetin, induced a reduction in potentially toxic sarkosyl-soluble phospho-tau isoforms (Rezai-Zadeh et al., 2008). Additionally, EGCG-inducible phosphorylation sites on GSK-3β have been demonstrated in vitro (Miyai et al., 2010). This mechanism has been recognized to underlie the Morin-flavonoid-induced reduction of tau hyperphosphorylation (Gong et al., 2011). Futhermore, we have reported that the silencing of CDK5 is effective on the reduction of neurofibrillary tangles in those 3xTg-AD mice (Piedrahita et al., 2010). In studies using quercetin and species rich in flavonoids, such as Rhus parviflora has been demonstrated the inhibition of CDK5 in vitro and in silico (Shrestha et al., 2013; Zapata-Torres et al., 2004). Also, Yao et al., reported neuroprotection by quercetin in cerebral ischemia mediated by Akt (Yao et al., 2012), and quercetin restored ERK/CREB/BDNF signaling pathway in Aβ25–35-induced amnesic mice (Liu et al., 2013b). In the context of AD with transgenic mice (APP-PS1) treated with EGCG for 4 weeks improved the memory skills and this could be explained by increase of pSer 9 GSK-3β, pSer 473 Akt and decrease of p-JNK (Jia et al., 2013). Despite those results, we did not obtain significant changes in the analyzed kinases (CDK5, GSK3β, MAPK), which are close related to tauopathy, or we did not find any significant modification in some members of survival pathways (pAkt, p38, pStat3) and plasticity (pCREB) at least in the used experimental design, 11 days after last doses of quercetin (supplementary figure 1). Maybe because there was a modulation in the signal cascades, without lost the accumulated effect on the reversion of histopathological hallmarks, and/or maybe suggesting an additional alternative mechanism, such as regulation of oxidative stress and anti-inflammatory process, which must be evaluated in the future.
Additionally, we did not detect immunoreactivity in the EC of the 3xTg-AD mice, which was in accord with the absence or very minor immunoreactivity of phospho-h Tau (Thr231) and PHF in 26-month-old mice (Mastrangelo and Bowers, 2008). Whose absence of staining could be supported by studies that show a higher correlation between microglial activation and tauopathy (Morales et al., 2013, Yoshiyama et al., 2007). However, these results differ from those of other reports of the EC using different transgenic models and in humans, in which neurofibrillary tangles were described to begin to appear during the early stages of AD (de Calignon et al., 2012; Khan et al., 2014; Velayudhan et al., 2013).
In contrast, it has been found that the proliferation of astrocytes and microglia accompanies βA deposition in the brain of AD model mice (Furman et al., 2012; Oddo et al., 2003a) and in AD human brains (Venneti et al., 2009). Interestingly, we detected a significant decrease in GFAP immunolabeling in the CA1 area, the EC and the amygdala but no change in GFAP immunolabeling in the subiculum. Epidemiological studies suggest that the long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with reduced AD risk (Lim et al., 2001). Additionally, quercetin displays anti-inflammatory activity due to its inhibition of iNOS, COX-2 and IL-1β (de Pascual-Teresa et al., 2004; Garcia-Mediavilla et al., 2007; Martinez-Florez et al., 2005; Sharma et al., 2007), and it exerts a potent anti-inflammatory effect on atherosclerotic disease in a dose dependent-mode (Kleemann et al., 2011). Furthermore, quercetin increases the GSH levels in astrocytes and neurons, contributing to a decrease in oxidative stress (Lavoie et al., 2009), which could be related to the reduction in the βA and tau levels following quercetin treatment in the present study.
As previously reported by (Rodriguez et al., 2013), we found increased microglial immunostaining in the hippocampus and the amygdala of the 3xTg-AD mice. However, we did not detect microglial immunoreactivity in the EC, nor did we detect neurofibrillary tangles in mice at 21–24 months of age. However, one report has shown activated microglia in the EC of 3xTg-AD mice at 6 months of age (Janelsins et al., 2005). Interestingly, quercetin treatment attenuated microglial activation in the hippocampus and the amygdala of the AD model mice, and a similar result was observed in a 3-nitropropionic acid-induced Huntington’s disease model (Chakraborty et al., 2014). A similar protective effect on dopaminergic neurons has been demonstrated using other flavonoids, such as baicalein and genistein (Li et al., 2005; Wang et al., 2005).
In the present study, administration of quercetin (25 mg/kg) every 48 hours for three months significantly ameliorated impairments in learning and memory performance in aged 3xTg-AD mice without affecting the Non Tg mice, suggesting that quercetin may represent an effective therapy for cognitive function in AD animal models. One recent study describes that APPswe/PS1De9 mice treated with quercetin 40 mg/kg for 16 weeks showed decreased escape latency compared with that APPswe/PS1De9 control mice (Wang et al., 2014). In addition, quercetin has been used at doses of up to 300 mg/kg via oral liposomes and has been shown to decrease the latency to reach the platform on the MWM test (Priprem et al., 2008). Moreover, quercetin acts as a neuroprotective agent to reduce white matter damage in chronic cerebral ischemia models, improving memory and learning abilities (Huang et al., 2012; Yao et al., 2010), and protects cognitive functions in an βA 25–35 i.c.v. amyloidosis model based on improved performance on a memory test (Liu et al., 2013b). Our data and those of other studies suggest quercetin as a therapeutic agent to reverse the histopathological hallmarks of and memory deficits in neurodegenerative diseases.
The amygdala is a component of the limbic system and is involved in emotion and a variety of cognitive functions, such as attention, perception, emotional memory, declarative memory and explicit memory, due to its extensive connections to many brain areas, such as the sensory cortices, the hippocampus, and the prefrontal cortex (LeDoux, 2007; Yao et al., 2013). Our results showed a reduction in β-amyloidosis, PHFs and NFTs in the amygdala. Therefore, we evaluated the performance of these mice on the EPM. Interestingly, the quercetin-treated 3xTg-AD mice exhibited increased frequency of entry into the open arms of the EPM compared to the Non Tg mice treated with quercetin or vehicle. Additionally, the quercetin-treated 3xTg-AD mice spent more time in head-dipping, less time and rearing and showed a tendency to fewer boli depositions than the Non Tg mice and the vehicle-treated 3xTg-AD mice, considering that these findings were obtained nine days after the last doses of quercetin administration (Figure 1). These results suggest a that the quercetin-treated 3xTg-AD mice exhibited reduced anxiety or a “risk assessment” behavior when they visit the open arms of the maze (Walf and Frye, 2007) compared to the vehicle-treated 3xTg-AD mice, which may exhibit a higher level of anxiety than control mice (Gimenez-Llort et al., 2007; Sterniczuk et al., 2010). In fact, the flavonol quercetin exerts antidepressant and/or anxiolytic effects based on several studies of oral administration of quercetin to both rats (Abdalla et al., 2014; Merzoug et al., 2014; Priprem et al., 2008) and C57BL/6 mice (Vissiennon et al., 2012). Furthermore, quercetin has been demonstrated to act as a monoamine oxidase inhibitor (Chimenti et al., 2006). Additionally, other flavonoids have been shown to affect GABAA receptor-favoring mechanisms, thereby contributing to their anxiolytic effect (Anderson et al., 2012; Karim et al., 2011; Kumar et al., 2014). However, the risk assessment and anxiolytic effect of quercetin on 3xTg-AD mice has not yet to be reported.
5. Conclusions
To conclude, our data suggest that quercetin reverses the histopathological hallmarks of AD and ameliorates cognitive and emotional impairments in 3xTg-AD mice without exerting adverse effects on Non Tg mice. Determining the pharmacokinetics and the signaling mechanism underlying the protective effect of quercetin and developing preventive studies using this and other neurodegenerative models will enable future translational research using this promising natural compound.
Supplementary Material
Representative bands of CDK 5 (A), GSK3 pSer9 (B), MAP Kinase activity (C), pAkt ser473 (D), p38 MAPK Thr 180/Tyr 182 (E), pStat-3 Tyr705 (F), and pCREB (G) and densitometric quantification from hippocampal lysates analyzed by Western blotting are shown. Tubulin and actin were used as loading control. The data are expressed as the mean ± SEM. n=4–5.
Highlights.
Quercetin treatment reverses β-amyloidosis in 3xTg-AD mice
Quercetin decreases tauopahty in 3xTg-AD mice
Quercetin reduces astrogliosis and microgliosis in 3xTg-AD mice
Quercetin improves cognitive function of aged 3xTg-AD mice
Quercetin exerts an anxiolytic effect on aged 3xTg-AD mice
Acknowledgments
The authors would like to thank the Cellular and Molecular Neurobiology Area of the Neuroscience Group of Antioquia and the Group of Bioactive Substances for their scientific and technical support during the experiments. This research was funded by grants from COLCIENCIAS # 111551928905 (GPC-G), CODI University of Antioquia, Young Investigator Programme 2011–2012 Colciencias (AM S-G) and Project 1 R01 AG029802-01 NIA/NIH, Subcontract 2011–2012 (GPC-G).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
None of the authors has a conflict of interest to declare in relation to the present research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Goncalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Goncalves JF, Mazzanti CM. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na(+), K(+)-ATPase activities. Physiol Behav. 2014;135:152–167. doi: 10.1016/j.physbeh.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Anderson W, Barrows M, Lopez F, Rogers S, Ortiz-Coffie A, Norman D, Hodges J, McDonald K, Barnes D, McCall S, Don JA, Ceremuga TE. Investigation of the anxiolytic effects of naringenin, a component of Mentha aquatica, in the male Sprague-Dawley rat. Holist Nurs Pract. 2012;26:52–57. doi: 10.1097/HNP.0b013e31823c003a. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee T, Van der Vliet A, Ziboh VA. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot Essent Fatty Acids. 2002;66:485–492. doi: 10.1054/plef.2002.0387. [DOI] [PubMed] [Google Scholar]
- Bassil N, Grossberg GT. Novel regimens and delivery systems in the pharmacological treatment of Alzheimer’s disease. CNS Drugs. 2009;23:293–307. doi: 10.2165/00023210-200923040-00003. [DOI] [PubMed] [Google Scholar]
- Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1–42. J Neuroinflammation. 2004;1:4. doi: 10.1186/1742-2094-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonpawa R, Spenkelink A, Rietjens IM, Punt A. A physiologically based kinetic (PBK) model describing plasma concentrations of quercetin and its metabolites in rats. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Cao X, Liu M, Tuo J, Shen D, Chan CC. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye Res. 2010;91:15–25. doi: 10.1016/j.exer.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Molecular and cellular neurosciences. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Carvalho KM, Morais TC, de Melo TS, de Castro Brito GA, de Andrade GM, Rao VS, Santos FA. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol Pharm Bull. 2010;33:1534–1539. doi: 10.1248/bpb.33.1534. [DOI] [PubMed] [Google Scholar]
- Castro-Alvarez JF, Uribe-Arias SA, Kosik KS, Cardona-Gomez GP. Long- and short-term CDK5 knockdown prevents spatial memory dysfunction and tau pathology of triple transgenic Alzheimer’s mice. Front Aging Neurosci. 2014;6:243. doi: 10.3389/fnagi.2014.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s Disease. CNS Neurosci Ther. 2014;20:10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti F, Cottiglia F, Bonsignore L, Casu L, Casu M, Floris C, Secci D, Bolasco A, Chimenti P, Granese A, Befani O, Turini P, Alcaro S, Ortuso F, Trombetta G, Loizzo A, Guarino I. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study. J Nat Prod. 2006;69:945–949. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]
- Choi SM, Kim BC, Cho YH, Choi KH, Chang J, Park MS, Kim MK, Cho KH, Kim JK. Effects of Flavonoid Compounds on beta-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons. Chonnam Med J. 2014;50:45–51. doi: 10.4068/cmj.2014.50.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pascual-Teresa S, Johnston KL, DuPont MS, O’Leary KA, Needs PW, Morgan LM, Clifford MN, Bao Y, Williamson G. Quercetin metabolites downregulate cyclooxygenase-2 transcription in human lymphocytes ex vivo but not in vivo. J Nutr. 2004;134:552–557. doi: 10.1093/jn/134.3.552. [DOI] [PubMed] [Google Scholar]
- Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Fiorani M, Guidarelli A, Blasa M, Azzolini C, Candiracci M, Piatti E, Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J Nutr Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mediavilla V, Crespo I, Collado PS, Esteller A, Sanchez-Campos S, Tunon MJ, Gonzalez-Gallego J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and downregulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- George RC, Lew J, Graves DJ. Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2013;36:21–40. doi: 10.3233/JAD-122113. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, Tobena A, LaFerla FM, Fernandez-Teruel A. Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev. 2007;31:125–147. doi: 10.1016/j.neubiorev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Gong EJ, Park HR, Kim ME, Piao S, Lee E, Jo DG, Chung HY, Ha NC, Mattson MP, Lee J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3beta. Neurobiol Dis. 2011;44:223–230. doi: 10.1016/j.nbd.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JJ, Liu X, Wang XQ, Yang LH, Qi DS, Yao RQ. Effects of quercetin on the learning and memory ability of neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:454–457. [PubMed] [Google Scholar]
- Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12:38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Han K, Kong JJ, Zhang XM, Sha S, Ren GR, Cao YP. (−)-Epigallocatechin-3-gallate alleviates spatial memory impairment in APP/PS1 mice by restoring IRS-1 signaling defects in the hippocampus. Mol Cell Biochem. 2013;380:211–218. doi: 10.1007/s11010-013-1675-x. [DOI] [PubMed] [Google Scholar]
- Kaariainen TM, Piltonen M, Ossola B, Kekki H, Lehtonen S, Nenonen T, Lecklin A, Raasmaja A, Mannisto PT. Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced parkinsonian models in rats and dopaminergic cell cultures. Brain Res. 2008;1203:149–159. doi: 10.1016/j.brainres.2008.01.089. [DOI] [PubMed] [Google Scholar]
- Kanter M, Unsal C, Aktas C, Erboga M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol Ind Health. 2013 doi: 10.1177/0748233713504810. [DOI] [PubMed] [Google Scholar]
- Karim N, Gavande N, Wellendorph P, Johnston GA, Hanrahan JR, Chebib M. 3-Hydroxy-2′-methoxy-6-methylflavone: a potent anxiolytic with a unique selectivity profile at GABA(A) receptor subtypes. Biochem Pharmacol. 2011;82:1971–1983. doi: 10.1016/j.bcp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Ho L, Santa-Maria I, Diaz-Ruiz C, Wang J, Pasinetti GM. Ultrastructural alterations of Alzheimer’s disease paired helical filaments by grape seed-derived polyphenols. Neurobiol Aging. 2012;33:1427–1439. doi: 10.1016/j.neurobiolaging.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Kumar P, Choonara YE, Modi G, Naidoo D, Pillay V. Cur(Que)min: A neuroactive permutation of Curcumin and Quercetin for treating spinal cord injury. Med Hypotheses. 2014 doi: 10.1016/j.mehy.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Lara-Guzman OJ, Tabares-Guevara JH, Leon-Varela YM, Alvarez RM, Roldan M, Sierra JA, Londono-Londono JA, Ramirez-Pineda JR. Proatherogenic macrophage activities are targeted by the flavonoid quercetin. J Pharmacol Exp Ther. 2012;343:296–306. doi: 10.1124/jpet.112.196147. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Chen Y, Dalton TP, Gysin R, Cuenod M, Steullet P, Do KQ. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108:1410–1422. doi: 10.1111/j.1471-4159.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee DC, Rizer J, Hunt JB, Selenica ML, Gordon MN, Morgan D. Review: experimental manipulations of microglia in mouse models of Alzheimer’s pathology: activation reduces amyloid but hastens tau pathology. Neuropathol Appl Neurobiol. 2013;39:69–85. doi: 10.1111/nan.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FQ, Wang T, Pei Z, Liu B, Hong JS. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm. 2005;112:331–347. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu CM, Zheng GH, Cheng C, Sun JM. Quercetin protects mouse brain against lead-induced neurotoxicity. J Agric Food Chem. 2013a;61:7630–7635. doi: 10.1021/jf303387d. [DOI] [PubMed] [Google Scholar]
- Liu R, Zhang TT, Zhou D, Bai XY, Zhou WL, Huang C, Song JK, Meng FR, Wu CX, Li L, Du GH. Quercetin protects against the Abeta(25–35)-induced amnesic injury: involvement of inactivation of rage-mediated pathway and conservation of the NVU. Neuropharmacology. 2013b;67:419–431. doi: 10.1016/j.neuropharm.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Siciliano R, Barone E, Preziosi P. Natural substances and Alzheimer’s disease: from preclinical studies to evidence based medicine. Biochim Biophys Acta. 2012;1822:616–624. doi: 10.1016/j.bbadis.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Martinez-Florez S, Gutierrez-Fernandez B, Sanchez-Campos S, Gonzalez-Gallego J, Tunon MJ. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J Nutr. 2005;135:1359–1365. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MA, Bowers WJ. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008;9:81. doi: 10.1186/1471-2202-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzoug S, Toumi ML, Tahraoui A. Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:921–933. doi: 10.1007/s00210-014-1008-y. [DOI] [PubMed] [Google Scholar]
- Mimica N, Presecki P. Side effects of approved antidementives. Psychiatr Danub. 2009;21:108–113. [PubMed] [Google Scholar]
- Miyai S, Yamaguchi A, Iwasaki T, Shamsa F, Ohtsuki K. Biochemical characterization of epigallocatechin-3-gallate as an effective stimulator for the phosphorylation of its binding proteins by glycogen synthase kinase-3beta in vitro. Biol Pharm Bull. 2010;33:1932–1937. doi: 10.1248/bpb.33.1932. [DOI] [PubMed] [Google Scholar]
- Mrak RE. Microglia in Alzheimer brain: a neuropathological perspective. Int J Alzheimers Dis. 2012;2012:165021. doi: 10.1155/2012/165021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugroschl J, Sano M. An update on treatment and prevention strategies for Alzheimer’s disease. Curr Neurol Neurosci Rep. 2009;9:368–376. doi: 10.1007/s11910-009-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Green KN, Hendrix C, LaFerla FM. Formulation of a medical food cocktail for Alzheimer’s disease: beneficial effects on cognition and neuropathology in a mouse model of the disease. PLoS One. 2010;5:e14015. doi: 10.1371/journal.pone.0014015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- Patil CS, Singh VP, Satyanarayan PS, Jain NK, Singh A, Kulkarni SK. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology. 2003;69:59–67. doi: 10.1159/000072357. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2004. [Google Scholar]
- Perez-Vizcaino F, Duarte J, Santos-Buelga C. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric. 2012;92:1822–1825. doi: 10.1002/jsfa.5697. [DOI] [PubMed] [Google Scholar]
- Piedrahita D, Hernandez I, Lopez-Tobon A, Fedorov D, Obara B, Manjunath BS, Boudreau RL, Davidson B, Laferla F, Gallego-Gomez JC, Kosik KS, Cardona-Gomez GP. Silencing of CDK5 reduces neurofibrillary tangles in transgenic alzheimer’s mice. J Neurosci. 2010;30:13966–13976. doi: 10.1523/JNEUROSCI.3637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, A. s. D. I. Alzheimer’s Disease International. World Alzheimer Report 2011. In: Prince M, Bryce R, Ferri CA, Jackson J, editors. The benefits of early diagnosis and intervention. Institute of Psychiatry, King’s College London; London: 2009. pp. 2–32. 2011. [Google Scholar]
- Priprem A, Watanatorn J, Sutthiparinyanont S, Phachonpai W, Muchimapura S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomedicine. 2008;4:70–78. doi: 10.1016/j.nano.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A. Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Carcinogenesis. 2007;28:1021–1031. doi: 10.1093/carcin/bgl232. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Noristani HN, Hilditch T, Olabarria M, Yeh CY, Witton J, Verkhratsky A. Increased densities of resting and activated microglia in the dentate gyrus follow senile plaque formation in the CA1 subfield of the hippocampus in the triple transgenic model of Alzheimer’s disease. Neurosci Lett. 2013;552:129–134. doi: 10.1016/j.neulet.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, Palumbo R. Quercetin: A Pleiotropic Kinase Inhibitor Against Cancer. Cancer Treat Res. 2014;159:185–205. doi: 10.1007/978-3-642-38007-5_11. [DOI] [PubMed] [Google Scholar]
- Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Natarajan S, Park JH, Lee DY, Cho JG, Kim GS, Jeon YJ, Yeon SW, Yang DC, Baek NI. Potential neuroprotective flavonoid-based inhibitors of CDK5/p25 from Rhus parviflora. Bioorg Med Chem Lett. 2013;23:5150–5154. doi: 10.1016/j.bmcl.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–1161. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R, Antle MC, Laferla FM, Dyck RH. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 2. Behavioral and cognitive changes. Brain Res. 2010;1348:149–155. doi: 10.1016/j.brainres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Taverna-Chaim K, Morato S. X-Plo-Rat 2005 1.1.0. Ribeirao Preto: Universidade de Sao Paulo; 2008. [Google Scholar]
- Terao J, Murota K, Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2012;38:572–581. doi: 10.1111/j.1365-2990.2011.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L, Proitsi P, Westman E, Muehlboeck JS, Mecocci P, Vellas B, Tsolaki M, Kloszewska I, Soininen H, Spenger C, Hodges A, Powell J, Lovestone S, Simmons A. Entorhinal cortex thickness predicts cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2013;33:755–766. doi: 10.3233/JAD-2012-121408. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wiley CA, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer’s disease. J Neuroimmune Pharmacol. 2009;4:227–243. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissiennon C, Nieber K, Kelber O, Butterweck V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin--are they prodrugs? J Nutr Biochem. 2012;23:733–740. doi: 10.1016/j.jnutbio.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wang DM, Li SQ, Wu WL, Zhu XY, Wang Y, Yuan HY. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem Res. 2014;39:1533–1543. doi: 10.1007/s11064-014-1343-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen S, Ma G, Ye M, Lu G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport. 2005;16:267–270. doi: 10.1097/00001756-200502280-00013. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yamin G. NMDA receptor-dependent signaling pathways that underlie amyloid beta-protein disruption of LTP in the hippocampus. J Neurosci Res. 2009;87:1729–1736. doi: 10.1002/jnr.21998. [DOI] [PubMed] [Google Scholar]
- Yao H, Liu Y, Zhou B, Zhang Z, An N, Wang P, Wang L, Zhang X, Jiang T. Decreased functional connectivity of the amygdala in Alzheimer’s disease revealed by resting-state fMRI. Eur J Radiol. 2013;82:1531–1538. doi: 10.1016/j.ejrad.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- Yao Y, Han DD, Zhang T, Yang Z. Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytother Res. 2010;24:136–140. doi: 10.1002/ptr.2902. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37:1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zapata-Torres G, Opazo F, Salgado C, Munoz JP, Krautwurst H, Mascayano C, Sepulveda-Boza S, Maccioni RB, Cassels BK. Effects of natural flavones and flavonols on the kinase activity of Cdk5. J Nat Prod. 2004;67:416–420. doi: 10.1021/np034011s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative bands of CDK 5 (A), GSK3 pSer9 (B), MAP Kinase activity (C), pAkt ser473 (D), p38 MAPK Thr 180/Tyr 182 (E), pStat-3 Tyr705 (F), and pCREB (G) and densitometric quantification from hippocampal lysates analyzed by Western blotting are shown. Tubulin and actin were used as loading control. The data are expressed as the mean ± SEM. n=4–5.