Abstract
Acute and repeated exposure to cocaine induces long-lasting alterations in neural networks that underlie compulsive drug seeking and taking. Cocaine exposure triggers complex adaptations in the brain that are mediated by dynamic patterns of gene expression that are translated into enduring changes. Recently, epigenetic modifications have been unveiled as critical mechanisms underlying addiction that contribute to drug-induced plasticity by regulating gene expression. These alterations are also now linked to the heritability of cocaine-induced phenotypes. This review focuses on how changes in the epigenome, such as altered DNA methylation, histone modifications, and microRNAs, regulate transcription of specific genes that contribute to cocaine addiction.
Keywords: epigenetics, cocaine, DNA methylation, histone modifications, micro RNA, transgenerational inheritance
Introduction
Addiction is a chronic, relapsing disorder that is characterized by compulsive drug seeking and taking accompanied by unfavorable consequences (Mendelson and Mello 1996). Acute and repeated exposure to cocaine induces changes in the plasticity of the mesocorticolimbic circuitry (Schmidt and Pierce 2010). The brain regions involved in this circuit include the nucleus accumbens (NAc) and ventral tegmental area (VTA), important for the rewarding effects of cocaine; the amygdala and hippocampus, involved in memories related to learning cues and context; the prefrontal cortex (PFC) and anterior cingulate cortex, critical for cognition and executive function; and finally the mediodorsal thalamus, connected to abnormal learning disorders (Everitt and Robbins, 2005; Gu et al., 2011). Specifically, alterations in gene expression profiles within this reward circuitry underlie the switch from recreational to chronic drug use (Nestler 2001; Koob and Volkow 2010; Maze and Nestler 2011; Schmidt et al., 2013). Therefore, unveiling the molecular mechanisms that promote stable changes in gene expression and ultimately lead to drug-seeking will assist in identifying new therapeutic targets for the treatment of addiction. Recently, epigenetic mechanisms have been shown to contribute to the heritability of addictive phenotypes as well as drug-induced structural, synaptic, and behavioral plasticity by coordinating the expression of gene networks in specific brain nuclei (Renthal and Nestler 2008; Russo et al. 2010; Schmidt et al., 2013). In this review, specific epigenetic mechanisms, such as DNA methylation, chromatin remodeling through histone acetylation and methylation, as well as microRNAs, will be discussed as mechanisms that regulate gene networks and contribute to cocaine addiction.
Role of Epigenetics in Cocaine Addiction
Epigenetics includes both heritable and stable changes in gene expression that occur without altering the DNA sequence (Bird, 2007; Siegmund et al., 2007; Tsankova et al., 2007). Epigenetic mechanisms translate environmental stimuli into stable alterations in chromatin that either activate or repress gene transcription (Jaenisch and Bird, 2003). Recently, these mechanisms have been shown to contribute to drug-induced changes in the brain by coordinating the expression of gene networks in specific brain regions that are critical for addiction (Renthal and Nestler, 2008; Russo et al., 2010; Schmidt et al., 2013). Specifically, repeated exposure to cocaine leads to addictive phenotypes by enhancing gene expression changes via epigenetic regulation of those specific genes (Nestler, 2013). In addition, exposure to cocaine can induce epigenetic changes in the germline, which are passed on to offspring thereby altering their vulnerability to cocaine (Vassoler et al., 2013; Vassoler & Sadri-Vakili, 2013; Nestler, 2013). Thus, the epigenome provides a direct mechanism whereby drugs of abuse such as cocaine can influence gene expression patterns involved in the development and heritability of addiction. The following sections will focus on specific epigenetic mechanisms and how each modification plays a role in cocaine addiction.
Cocaine induces alterations in DNA methylation
Environmental stimuli such as cocaine exposure can be translated to changes in gene expression and long-lasting behavioral phenotypes by enzymatic modifications to the DNA sequence. One such modification is DNA methylation during which DNA methyltransferases (DNMTs) add methyl groups to the C5 position of cytosine which predominantly resides in cytosine-guanine dinucleotides (CpG) in the genome (Suzuki and Bird 2008). Methylation of CpG islands impedes transcription factor binding to DNA sequences by recruiting co-repressor complexes (Jaenisch and Bird 2003). These methylated DNA regions are bound by methylbinding domain-containing proteins such as methyl CpG-binding protein 2 (MeCP2) that recruit co-repressors such as histone deacetylases (HDACs) and methyltransferases to gene promoters (Figure 1). CpG methylation was originally thought to lead to gene repression, however, it is now known to be a dynamic process that can either promote or repress gene expression (Suzuki and Bird 2008). DNA methylation is critical for imprinting, X chromosome inactivation, and cell differentiation. In addition, it can be altered at specific loci in germ cells by exposure to environmental factors such as toxins (Guerrero-Bosagna et al., 2010) and stress (Franklin et al., 2010), and can then be inherited by offspring over multiple generations. Thus, DNA methylation is a conceivable mechanism for the transmission and maintenance of epigenetic alterations in response to the environment. Altered DNA methylation patterns also contribute to disease and are observed in response to drugs of abuse (Alegria-Torres et al., 2011; Graff et al., 2011).
Figure 1. Schematic representation of DNA methylation.
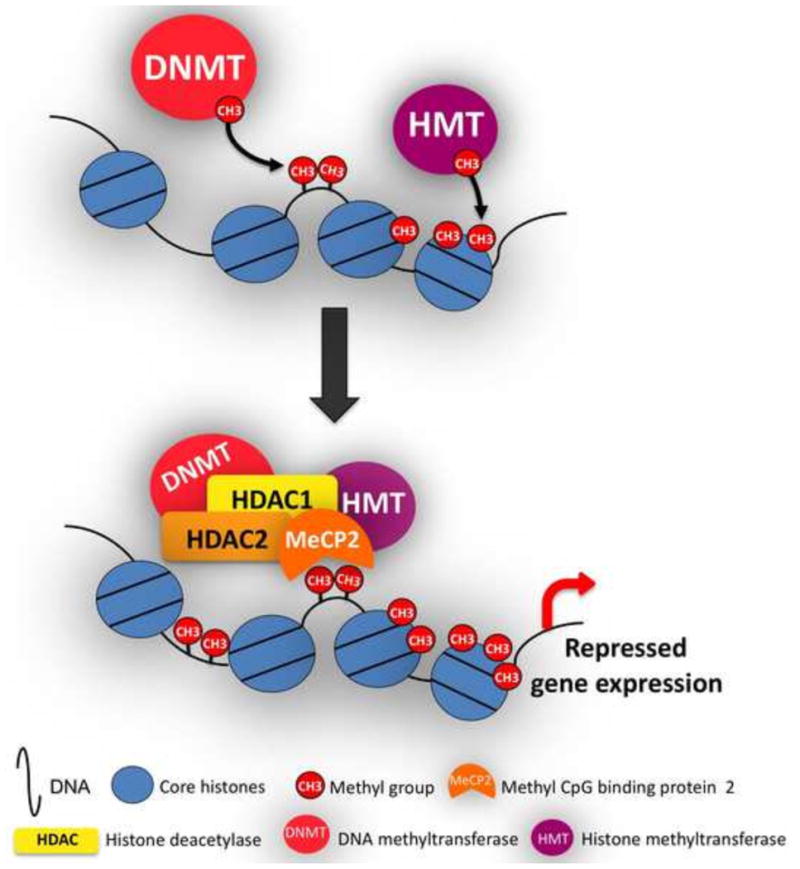
DNA methyltransferases (DNMTs) add methyl groups (CH3) to the C5 position of cytosine in the genome. Histone methyltransferases (HMT) add CH3 to the N-terminal tails of histones. Methylation of cytosines in CpG islands inhibits transcription factor binding to DNA sequences through the recruitment of co-repressor complexes. Methyl-binding domain-containing proteins such as methyl CpGbinding protein 2 (MeCP2) bind methylated DNA regions and recruit co-repressors such as histone deacetylases (HDACs), histone mehtyltransferases (HMT), and DNMTs.
Cocaine exposure has been shown to alter gene expression by modifying DNA methylation that may underlie cocaine-induced behavioral responses. Acute cocaine administration increases DNA methylation, DNMT3A and DNMT3B levels, and binding of MeCP2 to specific gene promoters that decreases gene expression in the NAc (Anier et al. 2010). Importantly, sensitization to the locomotor activating effects of cocaine is delayed in rats treated with a DNMT inhibitor (Anier et al. 2010) or pretreated with S-adenosylmethionine (SAM) (Anier et al., 2013), a methyl group donor, and coincides with altered DNA methylation at gene promoters (Anier et al. 2010). Furthermore, the acquisition or expression of cocaine-induced conditioned place preference (CPP) is blocked by DNMT inhibitors administered into brain regions involved in learning and memory (Han et al., 2010). Importantly, these changes correlated with decreases in DNMT3a and -3b expression in the NAc (Anier et al., 2013). These studies demonstrate the importance of DNA methylation in cocaine-induced behaviors. Courage and colleagues demonstrated that inhibition of DNMT decreases cocaine-induced DNA hypermethylation and attenuates drug-induced down-regulation of gene expression in the NAc (Carouge et al. 2010). Prolonged periods of drug abstinence in cocaine-experienced animals, increases DNMT3a expression (LaPlant et al. 2010), while decreased DNMT3a function enhances the behavioral response to cocaine supporting the hypothesis that decreased DNA methylation promotes increased gene transcription and contributes to drug-induced behavioral plasticity (LaPlant et al. 2010).
Importantly, in addition to functional plasticity, DNA methylation is a critical epigenetic mechanism underlying cocaine-induced structural plasticity. For example, DNMT3a is critical for cocaine-induced increases in dendritic spine density (LaPlant et al. 2010). Specifically, there was a selective increase in thin dendritic spines in the NAc that was dependent on DNA methylation. In addition, repeated cocaine treatment increases DNMT and DNA methylation associated with the β-catalytic subunit of the protein phosphatase type-1 (Ppp1cb), a gene involved in synaptic and structural plasticity (Ceulemans and Bollen, 2004; Mansuy and Shenolikar, 2006), decreasing its expression (Pol Bodetto et al., 2013). Ppp1cb and its endogenous inhibitor Darpp32 (dopamine- and cyclic amphetamine-regulated phosphoprotein) are key players in cocaine's effects (Svenningsson et al., 2004). Therefore epigenetic alterations associated with the regulation of these genes specifically may underlie cocaine's long-lasting structural effects.
The effects of cocaine exposure are not limited to alterations in DNA methylation or DNMTs, changes in MeCP2 also occur in brain areas that contribute to reward and addiction. MeCP2 phosphorylation at Ser421 increases with both acute and repeated administration of cocaine or amphetamine and, importantly, it is selective for a subset of neurons in the striatum, NAc and PFC (Deng et al. 2010; Mao et al., 2011). Importantly, MeCP2 Ser421Ala knock-in mice display enhanced sensitivity to the reinforcing effects of self-administered cocaine (Deng et al., 2014), demonstrating that phosphorylation of MeCP2 functions to decrease drug-induced plasticity within the NAc. Findings from our group demonstrated that following abstinence from chronic cocaine exposure MeCP2 dissociation from brain-derived neurotrophic factor (BDNF protein; Bdnf gene) promoter IV in the PFC is correlated with increases in BDNF expression (Sadri-Vakili et al. 2010). In addition, MeCP2 is increased in the striatum and PFC of rats in response to cocaine self-administration (Im et al. 2010; Host et al. 2011; Pol Bodetto et al., 2014) and MeCP2 knockdown in striatum is associated with impaired cocaine-dependent increases in BDNF levels (Im et al. 2010). This study along with the one from our group indicate that increases in MeCP2 expression are associated with Bdnf regulation following exposure to cocaine, however, it is unclear whether it is required. It is important to note that MeCP2 alterations do not necessarily point to a role for changes in DNA methylation since MeCP2 could have different functions. Together, these results suggest that dynamic changes in DNA methylation or methyl-binding proteins such as MeCP2 may be important epigenetic mechanisms underlying cocaine-induced behavioral effects.
Histone modifications associated with cocaine exposure
The second epigenetic mechanism that plays a role in cocaine addiction is the regulation of gene transcription by post-translational modifications of histone proteins. The nucleosome is comprised of DNA wrapped around an octamer of histone proteins that is further compacted to form chromatin. Chromatin is found in two basic states characterized by different levels of condensation. Euchromatin (open chromatin) is associated with active gene transcription due to a relaxed chromatin structure and accessible DNA sequences, while heterochromatin (condensed chromatin) is associated with inactive gene transcription due to tight packaging of DNA around histone cores (Berger 2007; Schmidt et al., 2013). Combinations of posttranslational modifications of histone proteins change the affinity of DNA for histones, thereby positively or negatively regulating transcription (Strahl and Allis 2000). Therefore, chromatin remodeling through post-translational modifications of histones is a required mechanism for gene expression.
The N-terminal tails of histones contain specific amino acid residues that are sites for several post-translational modifications (Strahl and Allis 2000) including methylation, acetylation, phosphorylation, ubiquitination, and sumoylation, to name a few (Renthal and Nestler, 2008; Schmidt et al., 2013). Specific enzymes add or remove associated histone marks, indicating that histone modifications are reversible (Kouzarides 2007). Altogether, dynamic histone signatures at individual genes and throughout the genome form a “Histone Code” that regulates transcription (Strahl and Allis 2000). Two histone modifications, namely lysine acetylation and methylation, have been implicated as important mechanisms in cocaine addiction and will be focused on in this review.
Cocaine-induced changes in histone acetylation
Histone acetylation has been studied the most extensively in models of cocaine addiction. Acetylation of lysine residues on N-terminal tails of histones decreases the electrostatic interactions between histones and negatively charged DNA (Kouzarides 2007). Thus, hyperacetylation of promoters is associated with increased gene expression, whereas hypoacetylation correlates with decreased gene expression (Kurdistani et al. 2004). Histone acetyltransferases (HATs) are enzymes that catalyze the addition of acetyl groups to lysines on histone tails, creating an open chromatin configuration that enhances gene activation. In contrast, histone deacetylases (HDACs) are enzymes that remove acetyl groups from histones, thereby promoting condensation of chromatin and inhibiting gene transcription (Marks et al. 2003). Together, HATs and HDACs modify chromatin structure to regulate gene expression (Figure 2). Similar to other disorders, drugs of abuse alter histone acetylation in the brain, and these alterations may underlie some of the functional abnormalities observed in addiction (Renthal and Nestler 2008; McQuown and Wood 2010; Schmidt et al., 2013).
Figure 2. Schematic representation of HAT and HDAC activity.
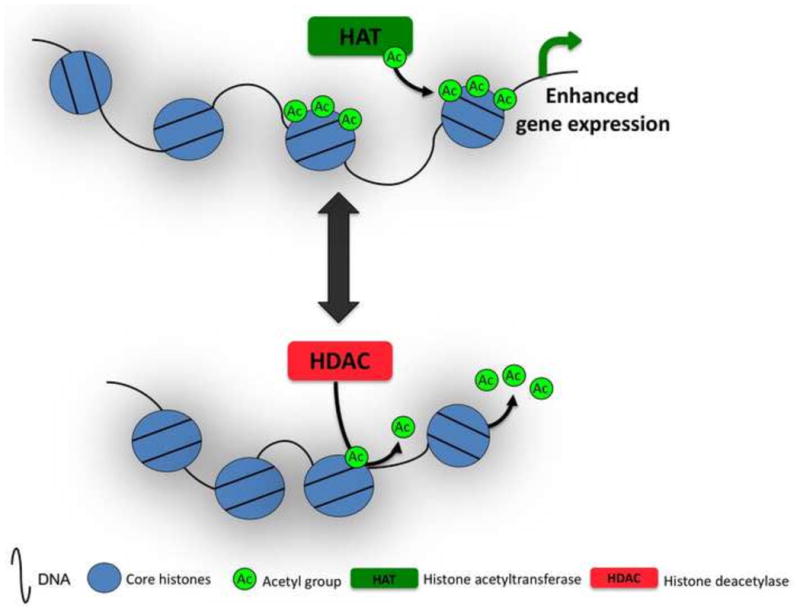
HATs add acetyl groups to N-terminal tails of histone proteins, decreasing the electrostatic interactions between histones and negatively charged DNA, thereby creating an open chromatin confirmation. HDACs remove acetyl groups from histone tails, thereby promoting condensation of chromatin and inhibiting gene transcription.
The first studies examining alterations in histone acetylation in response to cocaine demonstrated that acute cocaine administration increased histone H4 acetylation at the promoters of the immediate early genes Fos and Fosb in the NAc (Kumar et al. 2005; Levine et al. 2005). Notably, the time course of histone acetylation following acute cocaine exposure was consistent with the induction kinetics for the Fos and Fosb genes (Renthal and Nestler 2008). In addition, acute cocaine exposure globally increased histone H3 phospho-acetylation and histone H4 acetylation in the striatum (Brami-Cherrier et al. 2005; Kumar et al. 2005). Chronic cocaine exposure also promotes histone acetylation by decreasing HDAC5 activity in the NAc and increasing expression of HDAC5 target genes (Renthal et al. 2007). It was also associated with increased histone acetylation at specific gene promoters. For example, repeated cocaine administration increased histone H3 acetylation at the Cdk5 and Bdnf promoters thereby increasing gene expression (Kumar et al. 2005). The effects of chronic cocaine exposure on histones associated with the Bdnf promoter are region specific. While increases in Bdnf expression are associated with increased acetylation of histone H3 at Bdnf exon I-containing promoters in the NAc (Cleck et al. 2008) and VTA (Schmidt et al. 2012), histone H3 acetylation is increased at the Bdnf exon IV promoter in the medial PFC (Sadri-Vakili et al. 2010). During abstinence from cocaine, histone H3 acetylation and corresponding changes in transcription are stable (Freeman et al. 2008) suggesting that cocaine-induced chromatin remodeling leads to persistent changes in gene expression that may underlie relapse.
The role of histone acetylation in cocaine-taking behavior is complex. Both pharmacological inhibition and genetic manipulation of HDACs alter the behavioral responses to cocaine. Inhibiting HDACs promotes behavioral responses in animals self-administering cocaine, however, these effects are highly dependent upon the timing of HDAC inhibitor administration and the route of delivery. Systemic and intra-accumbens administration of HDAC inhibitors enhances cocaine-induced locomotor activity and CPP (Kumar et al. 2005; Renthal et al. 2007). Whereas the systemic administration of an HDAC inhibitor before daily cocaine selfadministration sessions are initiated decreases the number of infusions self-administered, suggesting that histone acetylation decreases the reinforcing efficacy of cocaine (Romieu et al. 2008). Consistent with these results, viral-mediated overexpression of HDAC5 in the NAc decreases histone acetylation and attenuates cocaine-induced CPP (Renthal et al. 2007). Similarly, overexpressing HDAC4 in the NAc shell decreases cocaine self-administration on a progressive ratio schedule (Wang et al. 2010). Mice with targeted HDAC1 deletions in the NAc demonstrate attenuated behavioral responses to cocaine (Kennedy et al., 2013) and deletion of HDAC3 in the NAc facilitates extinction of cocaine place conditioning (Malvaez et al., 2013). These behavioral effects correspond with increased histone acetylation and suggest that alterations in chromatin structure and transcription during drug withdrawal may prevent craving and relapse (Malvaez et al. 2010). In contrast, cocaine taking increases when animals that are stably self-administering cocaine are pretreated with an HDAC inhibitor, suggesting that histone acetylation increases the reinforcing effects of cocaine (Sun et al. 2008). Administration of an HDAC inhibitor directly into the NAc increases an animal's motivation to self-administer cocaine and is associated with increased histone H3 acetylation (Wang et al. 2010). Moreover, HDAC inhibitor treatment facilitated extinction of cocaine-CPP and attenuated the reinstatement of cocaine-seeking behavior (Malvaez et al. 2010; Romieu et al. 2011). While these results provide evidence for histone acetylation as an epigenetic mechanism that underlies cocaine-taking behavior, the exact temporal sequence of histone acetylation and gene expression in relation to cocaine exposure and, importantly, behavioral outcomes remains unknown.
While most studies have focused on the role of HDACs in cocaine addiction, HATs have also been shown to contribute. Mice deficient in the HAT, CREB binding protein (CBP), have reduced histone H4 acetylation and decreased sensitivity to cocaine (Levine et al. 2005). Furthermore, our previous findings, together with those from other groups, demonstrate that cocaine-induced increases in Bdnf transcription are associated with increases in histone acetylation at several Bdnf promoters (Kumar et al. 2005; Schroeder et al. 2008; Sadri-Vakili et al. 2010; Schmidt et al. 2012). Histone acetylation at BDNF promoters is associated with binding of CBP (Schmidt et al. 2012) as well as HDAC1 and HDAC2 (Guan et al. 2009) to promoter sequences. These studies indicate that CBP is involved in cocaine addiction, but the role of other HATs has yet to be elucidated. Together, these results indicate that cocaine-induced behavioral plasticity is mediated, in part, by alterations in both HATs and HDACs that work in concert to alter acetylation of histones associated with gene networks. Thus, identifying intracellular signaling pathways that mediate histone acetylation will provide a mechanism for how this specific epigenetic modification facilitates the effects of cocaine.
Cocaine-induced changes in histone methylation
In addition to histone acetylation, alterations in histone methylation have also been shown in response to cocaine exposure. Addition of methyl groups is a relatively stable modification that occurs on both lysine and arginine residues located on histone tails (Rice and Allis 2001). Methylation is a complex process as it can occur in mono- (me), di- (me2), or tri-methylated (me3) states with each methylation event having distinct, and often opposite, effects on gene expression (Rice and Allis 2001). Histone methylation at gene promoters can promote or repress gene expression depending on the exact amino acid residues being methylated (Maze and Nestler 2011). As an example, tri-methylation of histone H3 lysine residues 4 (H3K4me3) enhances transcription (Rice and Allis 2001). In contrast, di- and tri-methylation of histone H3 lysine residues 9 (H3K9me2/3) decreases gene transcription by recruiting co-repressors that function to increase chromatin condensation (Rice and Allis 2001).
The first study assessing the role of histone methylation in response to cocaine was performed by Black and colleagues and demonstrated that histone H3 methylation is decreased in the medial PFC, altering gene expression in adult rats that were exposed to cocaine during adolescence (Black et al. 2006). Importantly, these adolescent rats developed cognitive impairments in adulthood (Black et al. 2006), suggesting that cocaine exposure during adolescence produces long-lasting changes in gene expression that are mediated by histone methylation.
Numerous studies have demonstrated an important role for G9a, a repressive histone methyltransferase, in cocaine addiction. Maze and colleagues (2010) showed that repeated administration of cocaine in adult mice represses G9a expression thereby decreasing histone methylation in the NAc and enhancing cocaine-induced behavioral responses (Maze et al. 2010). Consistent with these findings, viral-mediated knockdown of G9a increased the expression of G9a target genes in the NAc with chronic cocaine exposure and enhanced cocaine-induced synaptic and behavioral plasticity (Maze et al. 2010). Thus, the inability of G9a to regulate gene transcription following repeated cocaine results in aberrant synaptic plasticity in the NAc (Maze et al. 2010). Subsequently, these investigators showed that repeated cocaine exposure decreased histone H3 methylation in the NAc and produced long-lasting decreases in heterochromatin formation, which suggests that cocaine-induced alterations in histone methylation and the formation of heterochromatin are also important mechanisms in the longterm actions of cocaine (Maze et al. 2011). G9a regulation of histone methylation in the NAc was also shown to play a critical role in drug-induced vulnerability to stress (Covington et al. 2011). A more recent study by Maze and colleagues demonstrated a reduction in G9a expression in both Drd1 and Drd2 cells following repeated cocaine (Maze et al., 2014). This study is the first to implicate G9a as an important regulator of neuronal subtype identity in the adult CNS (Maze et al., 2014). Taken together, these results suggest that chronic cocaine exposure during adolescence and adulthood regulates histone methlyation and thereby gene transcription in the brain that, in turn, may contribute to drug-induced behavioral plasticity.
Genome-wide alterations in histone modifications in response to cocaine
Cocaine-induced alterations in histone modifications have been identified on a genome-wide level using microarrays and next generation sequencing methods. Specifically, several studies have identified the precise genomic loci that are associated with modified histones following exposure to cocaine using genome-wide promoter arrays (ChIP-chip) or parallel DNA sequencing (ChIP-Seq) (Renthal et al. 2009; Maze et al. 2011; Zhou et al. 2011). These highthroughput methods fully characterize the complex cocaine-induced epigenetic signatures that regulate transcription of gene networks that may underlie drug-induced behavioral plasticity. For example, the ChIP-chip analysis of the NAc following chronic cocaine exposure revealed increases in histone H3 and H4 acetylation (to increase transcription), as well as increases in histone H3 dimethyl of K9 or K27 (H3K9me2 or H3K27me2) (to decrease transcription) (Renthal et al. 2009). Furthermore, chronic cocaine exposure also decreased histone H3 methylation (H3K9me3) in the NAc as measured by ChIP-Seq (Maze et al. 2011). Taken together, these studies demonstrate that cocaine alters patterns of gene expression in the NAc through epigenetic mechanisms that lead to stable and enduring changes. Therefore, future studies should focus on genome-wide studies in order to unveil the dynamic chromatin signatures involved in cocaine-taking and -seeking.
Cocaine-induced alterations in microRNAs
The final epigenetic mechanism implicated in cocaine addiction is regulation of gene expression at the post-transcriptional level by microRNAs (miRNAs), a class of non-protein coding RNA transcripts (∼22 nucleotides) (Ambros 2004). miRNAs have emerged as a new class of epigenetic regulators that are capable of altering synaptic plasticity as well as behavior (Guarnieri and DiLeone 2008). There are over 800 unique miRNA species in humans (Bentwich et al. 2005; Berezikov et al. 2006) that are highly expressed in the brain (Sempere et al. 2004; Lugli et al. 2008). Greater than 33% of the mammalian genome is subject to miRNA regulation and each miRNA is capable of targeting an average of 200 mRNA transcripts (Lewis et al. 2005; Friedman et al. 2009b). A growing body of evidence indicates that miRNAs have multiple effects on transcription that include, mRNA degradation, increased mRNA translation, chromatin remodeling, and DNA methylation. In addition to repressing protein synthesis and directing sequence-specific degradation of complementary mRNA, the miRNA complex has also been shown to lead to increases in gene expression by activating mRNA translation (Vasudevan et al. 2007; Place et al. 2008; Steitz and Vasudevan 2009). miRNAs are also able to alter the expression of genes by remodeling chromatin structure and increasing DNA methylation (Tan et al. 2009). Lastly, miRNAs are able to coordinate the expression of related gene networks involved in synaptic plasticity (Kosik 2006; Schratt et al. 2006) as they have been identified in dendrites, suggesting that miRNAs rapidly translate cellular signals into regulation of mRNA (Ashraf and Kunes 2006; Hobert 2008). Therefore, given the potential role of drug-induced plasticity in the development and persistence of compulsive behaviors associated with addiction (Hyman et al. 2006; Luscher and Malenka 2011; Mameli and Luscher 2011), it is not surprising that miRNAs play a critical role in cocaine addiction (Dreyer 2010; Pietrzykowski 2010; Li and van der Vaart 2011)
Chronic cocaine exposure alters numerous miRNAs in the NAc and striatum (Eipper-Mains et al., 2011) and its effects are mediated, in part, by specific miRNAs. Cocaine selfadministration increases miR-212 expression in the dorsal striatum, which in turn decreases this behavior, suggesting that upregulation of striatal miR-212 is a compensatory mechanism that decreases the motivational effects of cocaine (Hollander et al. 2010). Cocaine-induced increases in striatal miR-212 are attenuated by MeCP2, which is critical for regulating increased cocaine-seeking, however, miR-212 inhibits MeCP2 expression (Im et al. 2010). This finding suggests that an important balance between MeCP2 and miR-212 levels in the striatum regulates compulsive cocaine-taking. The overexpression of miR-212 in the striatum also attenuated the cocaine-induced upregulation of BDNF protein levels (Im et al. 2010). Interestingly, miR-212 amplifies CREB signaling, which also regulates Bdnf transcription (Shieh et al. 1998; Tao et al. 1998), and increases cocaine-induced expression of CREB-target genes including Fos (Hollander et al. 2010; Im et al. 2010). These opposing effects of miR-212 highlight the challenges associated with interpreting the effects of disrupting single regulatory factors within the context of an interconnected transcriptional network.
In addition to miR-212, other miRNAs are affected following chronic cocaine exposure. miR-181a levels are increased, whereas miR-124 and let-7d are decreased, following chronic cocaine administration (Chandrasekar and Dreyer 2009). Increased miR-181a in the NAc enhances, while increased miR-124 and let-7d attenuates, cocaine-induced CPP (Chandrasekar and Dreyer 2011). These opposing results are associated with specific changes in gene expression in the NAc (Chandrasekar and Dreyer 2011). Taken together, these results suggest that complex miRNA pathways alter cocaine-induced behavioral plasticity by directing expression of gene networks. By targeting hundreds of transcripts, a single miRNA is able to coordinate the expression of gene networks that regulate neuronal plasticity and behavior. While miRNAs may represent promising novel targets for the development of therapies for drug addiction, future studies will need to determine the exact role of miRNAs and their specific targets in the mechanisms underlying addiction.
Transgenerational inheritance of cocaine addiction phenotypes
While the field of transgenerational epigenetic inheritance is still in its infancy, multiple studies have demonstrated potential mechanisms of inheritance following exposure to a number of environmental stimuli (Guerrero-Bosagna et al., 2010; Manikkam et al., 2012a; Manikkam et al., 2012b, c, 2013; Tracey et al., 2013; Vassoler et al., 2013). Several studies have focused on the transgenerational effects of stress (Franklin et al., 2010; Franklin et al., 2011; Morgan and Bale, 2011; Dietz et al., 2011; Weiss et al., 2011; Howerton et al., 2013) and diet (Binder et al., 2012a; Binder et al., 2012b; Fullston et al., 2012; Kim et al., 2013). Thus far, alterations in DNA methylation, histone modifications (Figure 3), and non-coding RNAs provide heritable epigenetic mechanisms that are transmitted from parent to offspring (Danchin et al., 2011; Bohacek et al., 2012; Vassoler and Sadri-Vakili, 2014). Specifically, genes involved in spermatogenesis and developmental regulation are associated with alterations in histone and DNA methylation (Hammoud et al., 2009; Brykczynska et al., 2010). Together, these studies demonstrate that exposure to environmental stimuli during gestation induces a permanent epigenetic change in the germline that is transmitted to the offspring and results in the adult-onset of disease.
Figure 3. Post-translational modifications of histone H3 involved in transgenerational inheritance.
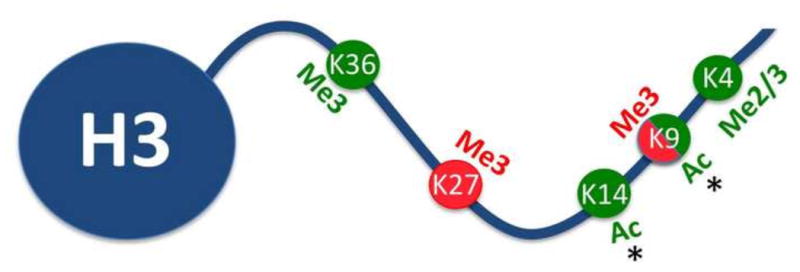
Lysine residues on the N-terminal tail of histone H3 undergo modifications such as acetylation and methylation in response to environmental stimuli. Previously described modifications include, repressive histone marks (H3K9me3, H3K27me3; red) as well as permissive histone marks (H3K4me2/3, H3K9K14ac2, H3K36me3; green). * indicates alterations in response to cocaine exposure.
Many studies examining the behavioral, physiological, and molecular consequences of cocaine exposure exist, however those investigating the probable mechanisms underlying the heritability of cocaine addiction remain limited. The first study implicating a potential transmission mechanism was performed by He and colleagues and demonstrated that cocaine selfadministration decreased DNMT-1 levels in the seminiferous tubules of the testes (He et al., 2006a). Given the critical role of DNMT-1 for maintaining methyl groups on imprinted genes in germ cells, a reduction in its expression could provide a potential mechanism of transgenerational inheritance (He et al., 2006a). A recent study from our group and our colleagues demonstrated that cocaine self-administration increased histone acetylation in the sperm and testes and that this modification was transmitted to the offspring (Vassoler et al., 2013). Specifically, increased acetylated histone H3 (H3K9K14ac2) associated with the Bdnf promoter in the PFC was observed in sperm and histone H3 was hyperacetylated in the testes of cocaine-experienced rats as well as their male offspring (Vassoler et al., 2013). This study is the first to demonstrate that paternal cocaine self-administration causes changes in histone acetylation within sperm associated with a specific gene that is subsequently transmitted to the offspring. While there is little doubt that addiction is influenced by genetic and environmental factors, this data needs to be interpreted with caution as many unanswered questions still remain and further work is required to elucidate the exact mechanisms involved in this phenomenon.
Future Studies
As outlined above both acute and repeated exposure to cocaine lead to changes in epigenetic marks and thereby gene expression in the corticostriatal circuit. To date, a number of epigenetic alterations have been described in selective brain regions involved in drug abuse and addiction however these studies also raise a number of issues that will need to be addressed by future studies. The first issue is the identification of specific gene networks that are regulated by alterations in epigenetic marks. To date, there is no correlation between genome-wide alterations in specific epigenetic modifications and gene expression profiles. This lack in consistency could be due to alterations in epigenetic signatures as opposed to single epigenetic modifications associated with genes. It is conceivable that combinations of epigenetic alterations converging on selective genes are responsible for enhancing or attenuating gene expression. More in depth genome-wide approaches employing novel genome- and epigenomewide methods of analysis will help to assess and identify the sets of genes and epigenetic mechanisms that are involved in these processes. Another important and similar area where additional experimentation is required is identifying the specific transcripts that are targeted by miRNAs. In addition, as we gain better understanding of the different subtypes of non-coding RNAs and their involvement in the epigenetic regulation of gene expression, it becomes pertinent to assess these effects in response to drugs of abuse such as cocaine. Finally, even though numerous alterations in specific epigenetic marks have been described, whether these changes are long-lasting or the time-course of their emergence are unclear. While a number of epigenetic marks are transient, others may be important for enduring effects and perhaps even the inherited effects of exposure to drugs of abuse. Therefore, future work should focus on determining the time course of genome-wide epigenetic and mRNA changes in brain regions involved in addiction.
Concluding Remarks
The studies summarized here provide increasing evidence for the epigenetic regulation of cocaine-induced gene expression profiles within the corticostriatal circuit. Alterations in specific epigenetic marks in discrete brain regions are associated with drug taking and seeking behavior in animal models of addiction. Therefore, characterizing these molecular events involved in the alteration of chromatin structure and thereby gene transcription following chronic cocaine exposure is likely to unveil novel targets for the treatment of drug craving and relapse. However, it remains to be determined how lasting alterations in specific transcripts as well as gene networks throughout the brain relate to produce long-term changes in the epigenome and ultimately cocaine addiction.
Highlights.
Cocaine exposure triggers complex adaptations in the brain.
Epigenetic modifications are critical mechanisms underlying addiction.
Alterations in epigenome is also linked to the heritability of drug-induced phenotypes.
Acknowledgments
G.S-V. is supported by DA033641.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegria-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3(3):267–77. doi: 10.2217/epi.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(12):2450–2461. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anier K, Zharkovsky A, Kalda A. S-adenosylmethionine modifies cocaine-induced DNA methylation and increases locomotor sensitization in mice. Int J Neuropsychopharmacol. 2013;16(9):2053–66. doi: 10.1017/S1461145713000394. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, Kunes S. A trace of silence: memory and microRNA at the synapse. Current opinion in neurobiology. 2006;16(5):535–539. doi: 10.1016/j.conb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature genetics. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28(2):328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH. Diversity of microRNAs in human and chimpanzee brain. Nature genetics. 2006;38(12):1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Binder NK, Hannan NJ, Gardner DK. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One. 2012a;7:e52304. doi: 10.1371/journal.pone.0052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Mitchell M, Gardner DK. Parental diet-induced obesity leads to retarded early mouse embryo development and altered carbohydrate utilisation by the blastocyst. Reprod Fertil Dev. 2012b;24:804–812. doi: 10.1071/RD11256. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(38):9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Simon AJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25(49):11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, Beisel C, Schubeler D, Stadler MB, Peters AH. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17(6):679–87. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- Carouge D, Host L, Aunis D, Zwiller J, Anglard P. CDKL5 is a brain MeCP2 target gene regulated by DNA methylation. Neurobiology of disease. 2010;38(3):414–424. doi: 10.1016/j.nbd.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiolo Rev. 2004;84(1):1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Molecular and cellular neurosciences. 2009;42(4):350–362. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(6):1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 2008;201(1):15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, Dietz DM, Lobo MK, Ghose S, Mouzon E, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71(4):656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nature neuroscience. 2010;13(9):1128–1136. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Wan Y, Wang X, Cohen S, Wetsel WC, Greenberg ME, Kenny PJ, Calakos N, West AE. MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci. 2014;34(13):4519–27. doi: 10.1523/JNEUROSCI.2821-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010;2(12):92. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains JE, Kiraly DD, Palakodeti D, Mains RE, Eipper BA, Graveley BR. microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA. 2011;17(8):1529–43. doi: 10.1261/rna.2775511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thony B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(8):1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2009a;106(19):7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009b;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, Lane M. Dietinduced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27:1391–1400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59(6):947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Kim D, Dobbin MM, Tsai LH. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev. 2011;91(2):603–49. doi: 10.1152/physrev.00012.2010. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri DJ, DiLeone RJ. MicroRNAs: a new class of gene regulators. Annals of medicine. 2008;40(3):197–208. doi: 10.1080/07853890701771823. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Li Y, Wang D, Wei C, Yang X, Sui N. Effect of 5-aza-2-deoxycytidine microinjecting into hippocampus and prelimbic cortex on acquisition and retrieval of cocaine-induced place preference in C57BL/6 mice. Eur J Pharmacol. 2010;642(1-2):93–8. doi: 10.1016/j.ejphar.2010.05.050. [DOI] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006a;28:198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319(5871):1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466(7303):197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J. Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. Journal of psychopharmacology. 2011;25(2):222–229. doi: 10.1177/0269881109348173. [DOI] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci U S A. 2013;110:5169–5174. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature neuroscience. 2010;13(9):1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Feng J, Robinson AJ, Maze I, Badimon A, Mouzon E, Chaudhury D, Damez-Werno DM, Haggarty SJ, Han MH, Bassel-Duby R, Olson EN, Nestler EJ. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16(4):434–40. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Kim KN, Choi YJ, Chang N. Effects of paternal folate deficiency on the expression of insulin-like growth factor-2 and global DNA methylation in the fetal brain. Mol Nutr Food Res. 2013;57:671–676. doi: 10.1002/mnfr.201200558. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117(6):721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature neuroscience. 2010;13(9):1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102(52):19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li MD, van der Vaart AD. MicroRNAs in addiction: adaptation's middlemen? Molecular psychiatry. 2011 doi: 10.1038/mp.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. Journal of neurochemistry. 2008;106(2):650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biological psychiatry. 2010;67(1):36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, Rusche JR, Wood MA. HDAC3-selective inhibitor enhances extinction of cocaineseeking behavior in a persistent manner. Proc Natl Acad Sci USA. 2013;110(7):2647–52. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Luscher C. Synaptic plasticity and addiction: Learning mechanisms gone awry. Neuropharmacology. 2011;61(7):1052–1059. doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012a;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012b;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Miller T, Richon VM. Histone deacetylases. Current opinion in pharmacology. 2003;3(4):344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Feng J, Wilkinson MB, Sun H, Shen L, Nestler EJ. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. The epigenetic landscape of addiction. Annals of the New York Academy of Sciences. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Chaudhury D, Dietz DM, Von Schimmelmann M, Kennedy PJ, Lobo MK, Sillivan SE, Miller ME, Bagot RC, Sun H, Turecki G, Neve RL, Hurd YL, Shen L, Han MH, Shaefer A, Nestler EJ. G9a influences neuronal subtype specification in striatum. Nat Neurosci. 2014;17(4):533–9. doi: 10.1038/nn.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England journal of medicine. 1996;334(15):965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nester EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology Pt B. 2013:259–68. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ. The role of microRNAs in drug addiction: a big lesson from tiny molecules. International review of neurobiology. 2010;91:1–24. doi: 10.1016/S0074-7742(10)91001-5. [DOI] [PubMed] [Google Scholar]
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(5):1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol Bodetto S, Carouge D, Fonteneau M, Dietrich JB, Zwiller J, Anglard P. Cocaine represses protein phosphatase-1Cβ through DNA methylation and Methyl-CpG Binding Protein-2 recruitment in adult rat brain. Neuropharmacology. 2013;73:31–40. doi: 10.1016/j.neuropharm.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Pol Bodetto S, Romieu P, Sartori M, Tesone-Coelho C, Majchrzak M, Barbelivien A, Zwiller J, Anglard P. Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food. Int J Neuropsychopharmacol. 2014;17:1–14. doi: 10.1017/S1461145714000972. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28(29):7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Current opinion in cell biology. 2001;13(3):263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J. The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr Neuropharmacol. 2011;9(1):21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(38):9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33(6):267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(35):11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes, brain, and behavior. 2008;7(8):906–914. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, Sadri-Vakili G. Increased BDNF Expression in the Ventral Tegmental Area During Cocaine Abstinence is Associated with Increased Histone Acetylation at BDNF Exon IContaining Promoters. Journal of neurochemistry. 2011 doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. Epigenetics and Psychostimulatn addiction. Cold Spring Harb Perspect Med. 2013;3(3):a012047. doi: 10.1101/cshperspect.a012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(12):2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20(4):727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2(9):e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz JA, Vasudevan S. miRNPs: versatile regulators of gene expression in vertebrate cells. Biochemical Society transactions. 2009;37(Pt 5):931–935. doi: 10.1042/BST0370931. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang L, Jiang B, Hui B, Lv Z, Ma L. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine- and sucrose-maintained selfadministration in rats. Neuroscience letters. 2008;441(1):72–76. doi: 10.1016/j.neulet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–96. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tan Y, Zhang B, Wu T, Skogerbo G, Zhu X, Guo X, He S, Chen R. Transcriptional inhibiton of Hoxd4 expression by miRNA-10a in human breast cancer cells. BMC molecular biology. 2009;10:12. doi: 10.1186/1471-2199-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–7. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, Ma L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(4):913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Franklin TB, Vizi S, Mansuy IM. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci. 2011;5:3. doi: 10.3389/fnbeh.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yuan Q, Mash DC, Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6626–6631. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]


