Abstract
Background
The global burden of disease is increasingly dominated by non-communicable diseases.These diseases are less amenable to curative and preventative interventions than communicable disease. This presents a challenge to medical practice and medical research, both of which are experiencing diminishing returns from increasing investment.
Objective
Our aim was to (1) review how medical knowledge is generated, and its limitations, (2) assess the potential for emerging technologies and ideas to improve medical research, and (3) suggest solutions and recommendations to increase medical research efficiency on non-communicable diseases.
Methods
We undertook an unsystematic review of peer-reviewed literature and technology websites.
Results
Our review generated the following conclusions and recommendations. (1) Medical knowledge continues to be generated in a reductionist paradigm. This oversimplifies our models of disease, rendering them ineffective to sufficiently understand the complex nature of non-communicable diseases. (2) Some of these failings may be overcome by adopting a “Systems Medicine” paradigm, where the human body is modeled as a complex adaptive system. That is, a system with multiple components and levels interacting in complex ways, wherein disease emerges from slow changes to the system set-up. Pursuing systems medicine research will require larger datasets. (3) Increased data sharing between researchers, patients, and clinicians could provide this unmet need for data. The recent emergence of electronic health care records (EHR) could potentially facilitate this in real-time and at a global level. (4) Efforts should continue to aggregate anonymous EHR data into large interoperable data silos and release this to researchers. However, international collaboration, data linkage, and obtaining additional information from patients will remain challenging. (5) Efforts should also continue towards “Medicine 2.0”. Patients should be given access to their personal EHR data. Subsequently, online communities can give researchers the opportunity to ask patients for direct access to the patient’s EHR data and request additional study-specific information. However, selection bias towards patients who use Web 2.0 technology may be difficult to overcome.
Conclusions
Systems medicine, when combined with large-scale data sharing, has the potential to raise our understanding of non-communicable diseases, foster personalized medicine, and make substantial progress towards halting, curing, and preventing non-communicable diseases. Large-scale data amalgamation remains a core challenge and needs to be supported. A synthesis of “Medicine 2.0” and “Systems Science” concepts into “Systems Medicine 2.0” could take decades to materialize but holds much promise.
Keywords: gene-environment interaction, systems theory, electronic health records, epidemiology, online social networks, crowd-sourcing, Web 2.0
Current Limitations in the Study and Management of Chronic Disease
Medical science has brought clear and dramatic improvements to health over the past 150 years. This has included effective cures and preventions as well as important public health measures. As a consequence of many of these advancements, the leading causes of death and disability have shifted from infectious diseases to more complex non-communicable diseases. In the past 50 years, expenditure on health research and health care has increased dramatically to meet the new challenges that treating and preventing these multifaceted diseases presents. However, combating chronic disease has proven significantly more difficult and costly than infectious disease, with it becoming increasingly difficult to continue raising life expectancy and healthy life expectancy [1]. Many spheres of academia and clinical practice have shifted away from an intent to cure, to an attempt to slow down pathological processes and prevent complications. This is in part due to the inability of science to determine which individuals will suffer the most from any given risk factor, or who will benefit most from specific interventions.
Current medical science is largely conducted using the reductionist paradigm, which assumes that complex entities are best understood by breaking them down into smaller, simpler components. Detailed analysis of the weaknesses of this assumption has been done elsewhere [2], but principally, reductionism limits our ability to understand how multiple variables interact with one another to create emergent effects.
The reductionist approach does offer a useful first step for the understanding of a complex system because it helps identify key components. However, a strong and enduring emphasis on the reductionist approach risks over-simplification (focusing only on a handful of major factors with the biggest effect, while the sum of minor factors may be considerable) and generalization (assuming that a common cause-effect relationship applies equally in all cases). Moreover, an excessive focus on a limited number of pathways may impede our ability to understand both the behavior of the system as a whole, as well as system variability between individuals. Our etiological models of seemingly disparate chronic diseases include a striking number of common pathways. For example, alterations of the tumor necrosis factor alpha (TNF-alpha) gene have been implicated in 88 clinically distinct diseases [3]. However, it remains unclear under what circumstances increased TNF-alpha levels cause an individual to develop rheumatoid arthritis, atherosclerosis, or a septic cytokine cascade. It is assumed that many such pathways interact to produce disease outcomes. Rarely can we adequately describe why a disease develops in an individual, their prognosis, the effect of risk factor modification on an individual, or the likelihood of family being affected by a similar disease.
The type of drugs dispensed to patients further reflects our limited understanding of the true cause of disease. While some drugs can reverse the original disease process to provide a near cure (eg, antibiotics, chemotherapy), many more provide only temporary relief to the current physiological imbalance and fail to cure (eg, thyroxin, antidepressants, anticonvulsants, steroids, diuretics). Others still simply dampen the body’s capacities to exacerbate the condition in a symptomatic or palliative [4] fashion (eg, beta blockers, warfarin) with significant side effects. Another weakness in our management of chronic diseases stems from the way research studies and clinical guidelines consider each disease in isolation, while in reality most patients have at least one comorbidity. This leaves patients with an expanding burden of treatment, polypharmacy, increased side effects, unintended drug interactions, and reduced adherence.
This pattern of diminishing returns necessitates a reevaluation of the approach that medical science has taken in the study and management of chronic diseases. As chronic diseases are substantially more complex than infectious diseases, additional approaches are probably required to overcome the limitations of reductionism. In this essay, we aim to (1) document medical science’s first steps in moving away from reductionism towards more complex models, (2) assess the potential benefit of introducing Systems Science into medical science and evaluate the relative strengths and weaknesses of various technologies in facilitating this (such as Medicine 2.0), and (3) present our suggestions of how to best increase medical research efficiency. To achieve this, we combine an unsystematic review of peer-reviewed literature, with a review of two websites known for the dissemination of novel technologies and ideas (Wired, TED). We then try to bring together these disparate lines of thought, across a range of disciplines and industries, into a subjective but hopefully thought provoking synthesis.
Supplementing the Reductionist Paradigm
Should the prevalent theoretical model, framework, or paradigm find it increasingly difficult to account for experimental data, alternatives to the prevalent model should be considered [5]. This can be done by replacing the old model or by adding supplements to the existing model [6]. We argue that three such supplements have recently been added to the core of reductionism. First, select chemotherapy agents were found to work particularly well for subtypes of breast cancers and leukemia, which corresponded to genetic subtypes of the disease. This reveals that diseases may look similar on the outside but can function very differently on the inside. As our knowledge of this etiome [3] (the precise etiological pathways by which genetic and environmental agents cause a disease) grows, it becomes useful to name each diagnostic subcategory with appropriate subdivisions. The term “intermediate pathophenotype” [7] has been suggested to capture these subdivisions. Linguistically, this denotes a focus on end-state pathology, the likely object of interest for pathologists and systems biologists. Clinicians, epidemiologists, and biomedical scientists are, however, more interested in upstream etiology and the narration of an individual’s past and likely future. Perhaps adopting the term “etiphenotype” instead would make Systems Medicine more accessible to clinicians (or alternatively, letting the term “etignosis” complement “diagnosis”).
The second supplement to reductionism came from the observation that most patients have more than one disease at any given time and that some diseases tend to cluster together. For example, people with diabetes have a greater risk of developing certain other diseases, such as stroke. Recent evidence has identified numerous early commonalities, such as “the metabolic syndrome” or “diabesity”, but our scientific language and modes of thought struggle to describe the interconnectedness of these tightly intertwined etiological processes [8]. Reductionism is ill-suited to deal with the question of comorbidity, prompting a search for alternative models [9]. Third, in many cases it appears better to treat complex diseases with a cocktail of drugs administered simultaneously. This has been applied in the treatment of infections (eg, tuberculosis and human immunodeficiency virus), as well as for chronic conditions (eg, acute coronary syndrome, exacerbations of chronic obstructive pulmonary disease) [10]. Advances in such complex, combined interventions have prompted the Medical Research Council to issue guidance on how they should be monitored and evaluated [11]. We believe that this will give only modest improvements, as intervention development will still remain somewhat blind and ignorant of the intricacies of etiological processes.
Modifying the Reductionist Paradigm With Network Medicine
A transitionary model of medical research is emerging that attempts to reconcile some of the discrepancies outlined above. There is much hope that network medicine could be better suited to understand the basic biological processes that culminate in health and disease [12,13]. This framework suggests that complex diseases are the emergent result of perturbations in multiple genes that are interconnected to one another to create a disease module (Figure 1). A hypothetical study of diabetes could begin by mapping the system of proteins that are responsible for healthy function like glucose regulation (Figure 1, panel a), also known as the interactome of proteins. Next, existing literature is used to identify a candidate gene or protein critical in etiology (Figure 1, panel b). This protein is mapped onto the interactome to identify a smaller set of proteins that directly influence the candidate protein (Figure 1, panel c), to identify a suspected diabetes disease module (colored red). Concurrent mapping of comorbid diseases (such as stroke in blue) can identify structural reasons for comorbidity as well as common premorbid states (such as the metabolic syndrome, marked by the red-blue proteins). Models like this can account for the effectiveness of combined interventions (Figure 1, panel d, dark blue proteins) and identify novel drug targets (Figure 1, panel d, light blue). Such models have been used to identify novel genes across a range of cancers [14-16]. It has also allowed drugs to be re-positioned for other diseases, such as using the anti-ulcer drug cimetidine in the treatment of lung cancer [17].
Figure 1.
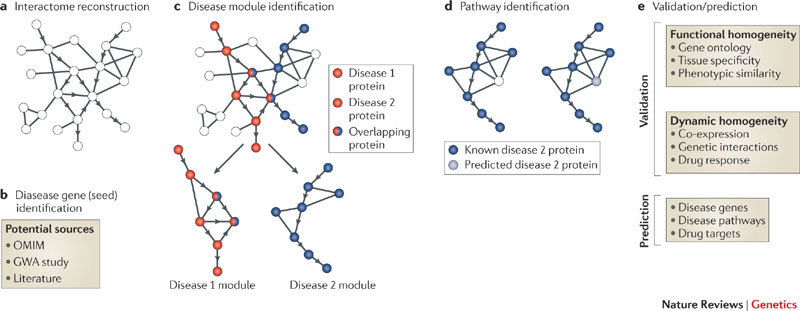
The methodological steps by which network medicine identifies disease modules and predicts novel protein targets for intervention.
We believe that the network medicine approach can achieve half the paradigm shift required toward systems medicine. However, such models remain incomplete because of the following weaknesses [18]. (1) When considering disease, network medicine looks only at snapshots of end-stage illness. The model cannot be applied to longitudinal data to describe the gradual shift that takes place when healthy states slowly transform into diseased states. This weakness stems from the model’s implicit assumption that disease processes (such as glucose dysregulation) are merely broken flipsides of healthy processes (glucose regulation). The cascade of events that caused the shift from healthy state to diseased state are irrelevant and not investigated [19]. (2) Network medicine focuses exclusively on the intracellular level of proteins and genes. Researchers interested in higher order risk factors (such as how weight gain is influenced by childhood nutrition, family upbringing, socioeconomic status, or the physical environment of green spaces and fast food outlets) struggle to utilize network medicine models [20]. (3) Simplistic causal modeling, wherein diseased genes and proteins are thought to exert their effects uniformly across a range of interindividual and interenvironmental variation. Thus network medicine struggles to model how genetic risk interacts with environmental risk to create disease. We feel that these three weaknesses will keep our models of disease largely incomplete, thus perpetuating many of the shortcomings outlined in our introduction.
Replacing the Reductionist Paradigm With Systems Medicine
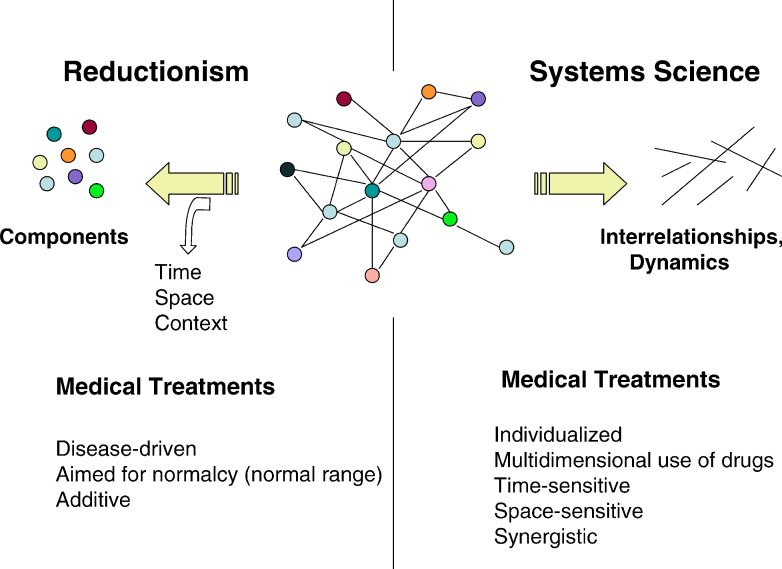
It helps to begin by clarifying our core terms. A System can be defined as a set of components that are related to one another in a meaningful way (centerpiece, Figure 2). Systems Science is the formal study of systems [21]. Systems Thinking describes modes of thought that focus on the connectedness and interrelationships of components, rather than focusing on the components themselves. Systems Theory is a set of theories that try to derive generalizable organizing principles that apply to all systems. For example, complex systems can maintain robust performance during external perturbations, thanks to system-wide properties such as modularity and built-in redundancy (ie, having two kidneys), features that are seen in biological and non-biological systems alike [19]. Systems Biology is the study of how biological functions emerge from the interactions between the components of living systems and how these emergent properties in turn influence the behavior of lower-level components [22]. The field developed quickly during the past decade, due to technological advances in generating large amounts of high-throughput data very quickly, along with the interdisciplinary ability to compute, model, and make sense of this data.
Figure 2.
Schematic illustration of the core differences between reductionism and systems science, when analyzing the properties of a system.
To date, little of the above paradigms have been incorporated into medical research. In part, this may be due to difficulties in amalgamating large-scale clinical datasets. However, as such technical barriers begin to fall away, increasing attribution can be placed on lack of knowledge about Systems Science among the medical research community. Indeed, those looking for a working definition of Systems Medicine may be baffled by the diversity of opinions out there [19,23,24]. Historically, Systems Medicine has been defined as the clinical application of Systems Biology approaches to medicine, where traditional model-driven experiments are informed by data-driven models in an iterative manner [25]. We see Systems Medicine as the long-term objective of a wider paradigm shift in medical science, at the end of which a range of different models and approaches will coexist under the Systems Medicine umbrella. All of these models will be substantially more complex than the models used in Reductionism or Network Medicine. We suggest that Systems Medicine models should include two or more of the following organizing principles of the human body: non-linearity, multi-agency, multi-levelness, or adaptivity [26].
Non-linearity means that the independent variables interact with one another and modify each other’s effects on the dependent variable. This makes the dependent variable exhibit emergent properties that can be understood only when all of the independent variables are assessed concurrently. Such interactions have been documented between behavioral risk factors, between two genes [27], between transcribed mRNA and regulatory microRNA [28], between single nucleotide polymorphisms (SNP) and expression quantitative trait loci [29], as well as between transcription factors [30]. Perhaps most important of all are gene-environment interactions, which have begun to emerge for obesity [31], coronary artery disease [32], asthma [33], colorectal cancer [34], depression [35], eczema [36], Alzheimer’s disease [37], and multiple sclerosis [38] to name a few. Models of systems medicine need to be built and empirical data assembled so that the detection of interactive effects and non-linear dynamics is facilitated. Contrast this with the stance taken in most introductory epidemiology and medical statistics courses, where interactive effects are seen as “nuisance” phenomena and students are discouraged against opening such cans of worms because of seemingly unmanageable type 1 errors. Regrettably, the study of interaction phenomena remains a niche field. This stifles progress at understanding any non-linear mechanisms of the human body.
Non-linearity can be present in relatively simple systems [39]. Consider the example of a system where smoking and drinking alcohol leads to increased stroke risk, through the upregulation of a hypothetical inflammatory cytokine called “smokdrink”. These two risk factors can interact with one another and produce non-linear responses that are quite complex in nature (such as a dramatic increase in smokdrink, but only if you drink more than 3 units a day and have accumulated more than 20 years of smoking damage). Nonetheless, our model remains simply non-linear (Multimedia Appendix 1, frame 1). Next, we can expand the model to account for how stroke is further influenced by another non-linear system, namely the cholesterol system (frame 2). If we view smokdrink and cholesterol as our two agents of stroke, then we have built a multi-agent model (frame 3). This concept is useful to bear in mind as the outcome of complex systems is rarely determined by one agent, but rather is the interaction between multiple agents (Multimedia Appendix 1, red arrows at the top of frame 3). Many of these agents remain directly unmeasurable, but their parameters can be estimated. For example, even before we discover the inflammatory cytokine smokdrink, we can calculate that 20 cigarettes + 3 pints a day has a similar effect on stroke, as does 10 cigarettes + 5 pints a day. Constructing intermediary agents in silico (such as smokdrink and cholesterol) facilitates our conceptual and mathematical understanding of the nature of dynamic disease processes. Such constructs could also include the inflammatome [40], the Metabolic Syndrome scale, the Hypothalamus-Pituitary-Axis dysregulation scale, or the allostatic load scale [41].
Multi-level models account for how people often form natural groups, which in turn influence individual behavior and disease outcomes. A grouping variable, such as “living in a deprived neighborhood or not”, can exert its effect on the disease outcome directly just like any other risk factor (Multimedia Appendix 1, frame 4, dotted blue line), or indirectly by modifying the effect of a lower order risk factor (such as drinking alcohol; Multimedia Appendix 1, red dotted lines). Although it may be tempting to enter “living in a deprived neighborhood” into the model alongside the other variables, this violates certain statistical assumptions. Accordingly, models known interchangeably as nested, hierarchical, or multiscale models, are useful to take account of the multi-levelness of such real-life phenomena.
Finally, all the models presented so far compare states of full health against states of full illness. The sequence of events over which full health deteriorates towards full illness remains unknown. To understand this process, we must build dynamic models of the human body. The core principle here is adaptivity, wherein after a certain stimulus, the system modifies its own response pattern in anticipation of similar stimuli in the future. It is through processes such as down-regulation, long-term potentiation, habituation, and synaptic learning, that the body modifies its definitions of “an ideal state” or “an ideal response”. Thus the rules that explain the behavior of homeostatic systems are nested in larger systems, called homeodynamic systems [41]. Imagine an individual whose homeostatic system is trying to keep its blood glucose level around 6, and Body Mass Index (BMI) around 22 (also known as an attractor zone). The system tolerates and shows resilience in the face of a range of perturbations in the short-term (such as skipping a meal, met by glycogenolysis), medium-term (entering Ramadan, met by lipolysis), or permanent (increased energy demands, met by increasing appetite). Whatever happens, the system will try to return to the initial attractor zone. This is easy to do if the person’s state meanders only slightly in the immediate phase space around the central attractor zone, also known as the system’s basin (eg, glucose 4-8, BMI 20-24). However, if the person’s state moves far away enough from the basin (eg, glucose 2, BMI 10), the integrity of the system is at stake and death from starvation may follow. In other diseases, the attractor zone may shift to BMI 26 (diagnosed as overweight) or to glucose 12 (diagnosed with diabetes) over a few decades. Other diseases still may show less gradual but more sudden shifts with bifurcation into a far away attractor zone (as seen in an acute epileptic seizure [42], many other acute presentations, but also in agent-based models of community interventions [43]). Other diseases can show cyclical flip-flop behavior between two attractor zones (such as is seen in bipolar disorder [44]). Dynamical Systems Theory focuses on understanding how and why these attractor zones change over time. Chaos Theory describes a type of system change that is particularly sensitive to initial conditions (such as seen in the Barker and Hygiene hypotheses). Similar models have been used to understand cell fates [45], endothelial function [46], cytokine function [47], heart rate variability [48], atrial fibrillation [49], septic shock [50], and multiple organ dysfunction syndrome [51]. Understanding these dynamic processes may lead to targeted interventions of preventative [52] or curative nature. For example, animal models suggest that infection with helmliths can reprogram autoimmune disease states towards healthier ones in colitis [53], gastric atrophy [54], multiple sclerosis [55], and diabetes [56].
Analytical Approaches to Systems Medicine
Conducting systems medicine research will require numerous changes to how medical scientists and epidemiologists conduct their daily work. Such changes have been poorly addressed in the existing literature. Fundamentally, an entire new approach to data collection and analyses is required. If most risk factors interact with one another to create small interactive effects, many of which are nested in intricate multi-levels of hierarchy, then the detection of such intricacies will require large numbers of patients with large numbers of variables [57].
Once assembled, such high-fidelity datasets could be explored for “hot spots”, or loci of association, between risk factor data (including behavioral, environmental, and sociological risks), molecular data (including genomic, trascriptomic, and proteomic data), and clinical outcomes, in order to identify and treat etiphenotypical subgroups. Some of this exploration will take the narrow shape of testing a priori hypotheses. Other exploration will use high-throughput techniques to scan more broadly and identify novel hypotheses for subsequent scrutiny [58,59]. This may be facilitated by machine learning algorithms [60]. For example, the world’s most advanced supercomputer, IBM Watson, has recently been given access to data on over a million cancer patients, as well as the emerging oncological literature. It is hoped that Watson will support and enhance clinical decision making in real time [61]. This may shatter the traditional view of humans generating hypotheses on which computers calculate the test statistic, as computers like Watson may also become indispensible in hypothesis generation. Our vision of Systems Medicine becomes impossible without seamless collaboration with mathematicians, modelers, and data scientists, so that the right modeling tools can be used and combined to appropriately balance sufficient complexity with practical utility.
A Roadmap to Systems Medicine
Although multiple challenges impede the integration of systems approaches within medical research, we prioritize five that deserve attention first. (1) Existing models that view the human body as complex adaptive systems are limited and need specialist development. Our review of the literature suggests that while textbooks exist on modeling for Systems Science and Systems Biology, little consensus exists on modeling for Systems Medicine. (2) While large-scale research projects (such as the Human Genome Project, Wellcome Trust Case Control Consortium, Virtual Physiological Human, ECell, Cancer Genome Atlas, Human Brain Project) are developing at a promising rate, the majority of research continues to be conducted in an almost proprietary-like mindset of non-sharing, with individual teams collecting and holding data in small silos. This makes it difficult to create the large datasets required for systems analysis. (3) While some organizations (like IBM, Sage Bionetworks, and Farr Institute) can foster multidisciplinary teams between medical researchers and information specialists, this collaboration gap remains large in most areas of public research. (4) Although research funders are beginning to recognize and invest in long-term and larger research endeavors, particularly in database curation, the vast majority of funds continue to be spent on low-complexity projects with short-term deliverables and seemingly low risk. Many such projects are underpowered in breadth, depth, and complexity to sufficiently address the problems they seek to address, with sometimes negligible subsequent improvement in our knowledge base. (5) The obstacles above will yield with sufficient energy and leadership; however, progress at each front continues to be slow due to the prevalent reductionist culture in the medical research community and general risk aversion to engage with new technology (particularly those that may alter existing power relations). Therefore, greater discussion, awareness, and education of differences between systems medicine and reductionism are required across the board, in order to promote and facilitate interest and activity in this important transition.
Sharing Data
The medical research community itself is a complex adaptive system. Shifting this toward a more efficient, systems-science configuration will require time and effort to be applied at multiple levels. We believe that the second obstacle mentioned above, creating large datasets, is most pressing so we discuss this in detail. There are two possible non-competing solutions: (1) sharing data between researchers or (2) using data from electronic health records (EHR) for research purposes. Although data sharing appears to be increasing, most research data are not shared or recycled outside the original project team. A range of factors discourage data sharing. Project-specific data are often collected using context-specific priorities, definitions, and measurement tools that are rarely compatible with other peer-researchers, let alone researchers looking at the picture from a higher or lower level of order [62]. Data access requests are often cumbersome and slow, and some researchers may be wary of giving up their competitive edge [63]. Ultimately, even if all research groups pool their data successfully, a rich trove of clinical process and outcome data will continue to be held by health care providers who are not involved in research.
Health care data are never assembled for research purposes, which substantially hampers its transferability. For example, patients with borderline disease states are often “upcoded” into more severe disease states, as this brings financial rewards to the health care provider. Some EHR data may always be absent or of insufficient quality to be useful in medical research (eg, tracking the daily improvement of inpatients with cellulitis or fluid overload, which is difficult to quantify). However, other EHR data lend themselves very well to scientific analyses, particularly as drug prescription data are of exceptional quality. Furthermore, as health care is carried out on real patients, studying the real-life effects of drugs is preferable to unrepresentative clinical trials, particularly in understanding how one drug can accidentally influence two disparate diseases [64,65]. Future work could clarify which health care data are transferrable to medical research, and develop some tools to facilitate this. For example, should some severe diagnoses, when seen in conjunction with other hospital laboratory parameters, be downcoded back to a more truthful level, to correct for financial upcoding in EHR? The integration of health care and research data will create its own technical, logistical, and legal challenges, and at present we cannot tell if this will be worthwhile. If attempted, we suggest that groups of patients could be subdivided empirically based on their differential risk factor profiles, disease trajectories, or molecular data [66], and these subgroups subsequently explored in more detail for evidence of etiphenotypical subgroups.
Unlocking Health Care Data
There is growing support for the collation of EHRs for secondary research purposes [67]. Transmission of data is typically thought to proceed with researchers asking health care providers for access to anonymized data [68]. Added value can be gained by integrating data from multiple providers [69]. Overall, this approach offers substantial productivity gains. However, this benefit must be considered against the drawback of reduced data accuracy.
As an alternative, we suggest an approach where researchers ask patients directly for their data, who in turn have to ask hospitals for their data. The advantage here is that routine medical data could be augmented by study-specific scientific tests (such as genotyping). The disadvantage is that samples will not be representative of the wider patient group, but biased towards those comfortable with sharing their personal data with scientists over the Internet, a concept that probably frightens most patients today. It also requires EHRs to be personally accessible and personally controlled—a feature that is still under development [70], but with some proof of concept from private settings [71].
Online Communities
Web 2.0 technology is creating a social Web, where users create content, share useful information with interested peers, and moderate each other’s activities (eg, Facebook, eBay, ReseacherGate) [72]. Web 2.0 has been combined with the power of crowdsourcing (the force behind projects like Wikipedia) to foster Science 2.0 (also known as Open Science or Cyberscience 2.0) [73]. Here, online platforms facilitate large-scale data sharing between researchers who can merge or re-analyze each other’s data (eg, HapMap project, Sage Bionetworks). Some of these projects (eg, GalaxyZoo) are also enlisting the power of citizens in the scientific endeavor. A seminal article by Eysenbach in 2008 [74] detailed how the application of these Web 2.0 technologies to personally controlled health care records will create “Medicine 2.0”. This will be a world where the collaboration gap between researcher, clinician, and patient will narrow. Online patient communities such as PatientsLikeMe are not only useful to facilitate peer transfer of knowledge [75] but also in scientifically testing new drug applications [76].
To the best of our knowledge, a major gap in the current literature is the fact that systems medicine researchers have never suggested that their vision could be achieved quicker using Medicine 2.0 tools. Similarly, although Medicine 2.0 enthusiasts have begun to associate their ideas to Big Data, Web Science, Health Web Science, and the Semantic Web [77], none have spoken of the potential for Medicine 2.0 to further our understanding of the human body as a complex adaptive system. We urge these two important communities to begin collaboration on what we suggest could be called “Systems Medicine 2.0” (Figure 3). Understandably, few patients will trust their most sensitive secrets with an amorphous, faceless research community. An online community with detailed researcher profiles, faces, and credentials (eg, ReseacherGate) could be augmented by allowing participants and researchers to interact with one another. Reliable feedback systems (such as those seen in Amazon and eBay) will be central to creating sufficient trust. To date, most sites have focused on fostering researcher-researcher collaboration [78,79], or patient-patient collaboration. The next step would be to foster bi- or tri-directional links (also to clinicians), with the added challenge of increased knowledge asymmetries.
Figure 3.
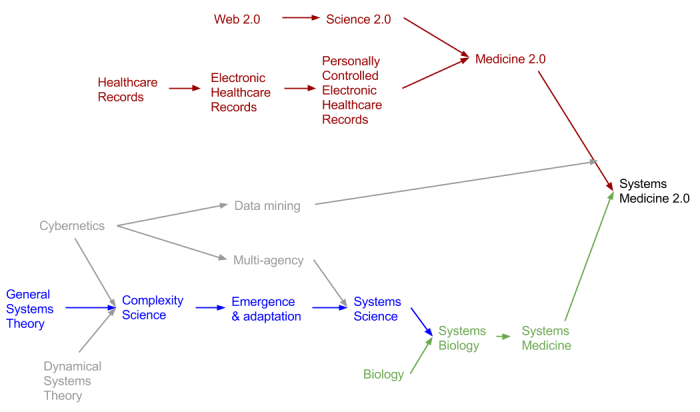
The historical origins of “Systems Science” and “Medicine 2.0”, and the potential for their combination into “Systems Medicine 2.0”.
Another challenge for Systems Medicine 2.0 is how health care data has very poor interoperability, both nationally and internationally. We welcome the work of the Joint Initiative on SDO Global Health Informatics Standardization to facilitate the rapid adoption of common health informatics standards. A related challenge is how various Systems Medicine research teams will initially create their own datasets, analytical programs, and models. Reporting these via traditional journals will hamper progress. Datasets, programs, and models should be shared, scrutinized, and developed, perhaps by making use of the efficiency tools perfected by the Open Source movement [80]. Open access [81], open standards, open source software [82], and open competitions [83] can foster faster innovation and drive efficiency. Ideally, the entire Systems Medicine community could see themselves as working on the same project, akin to the global communities who brought us Linux, Mozilla Firefox, Wikipedia, HUGO, and Systems Biology.
To start the discussion of how the research community can move towards Systems Medicine, we have compiled a list of suggestions (Textbox 1). This is neither exhaustive nor comprehensive and is designed merely to stimulate discussion, development, and implementation of Systems Medicine.
Initial suggestions for the advancement of systems medicine.
Governments and key research funders:
training systems scientists, big data engineers, and medical researchers
incentivizing and normalizing multidisciplinary collaboration for systems medicine
incentivizing and normalizing the sharing of laboratory data as well as health care data
earmarking research funds for systems medicine
national leadership on systems medicine strategy and implementation
Legislation for the Web 2.0 era:
EHRs to be recognized as patient property
EHR systems must facilitate the simple and free export/import of data by abiding to global standards and/or open standards
anonymized research on EHRs is permitted and automatically facilitated, unless patients opt out
define if and how data can be accessed for commercial purposes and how any proceeds are divided
Scientific community:
opinion leaders to promote the benefits of Systems Science approaches in medicine
disease-specific expert bodies (eg, colleges) to facilitate common standards of data collection in their fields
early Systems Medicine 2.0 academics to focus their papers at one flagship journal (eg, Journal of Medical Internet Research)
Software developers:
develop an online community that links together systems scientists and modelers, big data engineers, medical researchers, and subsequently patients
International co-ordination:
international leadership and co-ordination (eg, World Health Organization) to promote all of the above
an online data-sharing directory listing all large datasets pertaining to Systems Medicine
Conclusion
The prevalent paradigm in medical science is reductionism, whose limitations have become increasingly apparent, resulting in diminishing returns. This paradigm has been supplemented and modified with the framework of network medicine. If further developed with Systems Science approaches, this has the potential to evolve into fully fledged Systems Medicine paradigm. This neatly complements advances in Internet-powered medicine (Medicine 2.0), but as yet the two fields have yet to take advantage of each other’s nascent existence. We hope that our paper can bridge this conceptual gap and advance mutual interest and collaboration between these two, to foster Systems Medicine 2.0. This could lead to significant advances in the prevention and treatment of non-communicable diseases.
Acknowledgments
We wish to thank Michael Baum and Jayant Vaidya for their contributions on how systems science has been applied to breast cancer. We also thank anonymous reviewers at JAMIA and JMIR, as their comments substantially developed the paper. This work was supported by the Wellcome Trust (grant number 097821/Z/11/A), and the lead author has received fellowships from the National Institute of Health Research and the Wellcome Trust.
Abbreviations
- BMI
Body Mass Index
- EHR
Electronic Health Record
Multimedia Appendix 1
A PDF illustrating various aspects of a Systems Medicine model of disease: non-linearity (frame 1), multi-agency (frame 3), multilevelness (frame 4).
Footnotes
Authors' Contributions: TT wrote the first concept paper (in 2009), and the final draft. AG and TT wrote the first draft. GS contributed information about the use of EHRs for secondary research in the United Kingdom. OH contributed information about the use of EHRs in United Arab Emirates and wrote the third draft. AD contributed information about proteomics and metabolomics. PH supervised the research throughout, developed the systems theory concept, and wrote the second draft.
Conflicts of Interest: OH is a Senior Vice President with Healthways International, a global population health and well-being improvement company. As such, he seeks to promote holistic and effective approaches to improving health.
References
- 1.Crimmins E M, Saito Y. Trends in healthy life expectancy in the United States, 1970-1990: gender, racial, and educational differences. Soc Sci Med. 2001 Jun;52(11):1629–41. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahn Andrew C, Tewari Muneesh, Poon Chi-Sang, Phillips Russell S. The limits of reductionism in medicine: could systems biology offer an alternative? PLoS Med. 2006 May;3(6):e208. doi: 10.1371/journal.pmed.0030208. http://dx.plos.org/10.1371/journal.pmed.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Yueyi I, Wise Paul H, Butte Atul J. The "etiome": identification and clustering of human disease etiological factors. BMC Bioinformatics. 2009;10 Suppl 2:S14. doi: 10.1186/1471-2105-10-S2-S14. http://www.biomedcentral.com/1471-2105/10%20Suppl%202/S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildirim Muhammed A, Goh Kwang-Il, Cusick Michael E, Barabási Albert-László, Vidal Marc. Drug-target network. Nat Biotechnol. 2007 Oct;25(10):1119–26. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn TS. The Structure of Scientific Revolutions. Chicago: University Of Chicago Press; 1962. [Google Scholar]
- 6.Lakatos I. Can Theories be Refuted? . Springer: Netherlands; 1975. Falsification and the methodology of scientific research programmes; p. 205. [Google Scholar]
- 7.Loscalzo Joseph, Kohane Isaac, Barabasi Albert-Laszlo. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. http://MSB.embopress.org/cgi/pmidlookup?view=long&pmid=17625512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidalgo César A, Blumm Nicholas, Barabási Albert-László, Christakis Nicholas A. A dynamic network approach for the study of human phenotypes. PLoS Comput Biol. 2009 Apr;5(4):e1000353. doi: 10.1371/journal.pcbi.1000353. http://dx.plos.org/10.1371/journal.pcbi.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May Carl, Montori Victor M, Mair Frances S. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- 10.Csermely Péter, Agoston Vilmos, Pongor Sándor. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005 Apr;26(4):178–82. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Medical Research Council Developing and evaluating complex interventions: new guidance. 2008. http://www.mrc.ac.uk/complexinterventionsguidance/
- 12.Barabási Albert-László, Gulbahce Natali, Loscalzo Joseph. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011 Jan;12(1):56–68. doi: 10.1038/nrg2918. http://europepmc.org/abstract/MED/21164525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loscalzo Joseph, Barabasi Albert-Laszlo. Systems biology and the future of medicine. Wiley Interdiscip Rev Syst Biol Med. 2011;3(6):619–27. doi: 10.1002/wsbm.144. http://europepmc.org/abstract/MED/21928407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang Han-Yu, Lee Eunjung, Liu Yu-Tsueng, Lee Doheon, Ideker Trey. Network-based classification of breast cancer metastasis. Mol Syst Biol. 2007;3:140. doi: 10.1038/msb4100180. http://MSB.embopress.org/cgi/pmidlookup?view=long&pmid=17940530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ergün Ayla, Lawrence Carolyn A, Kohanski Michael A, Brennan Timothy A, Collins James J. A network biology approach to prostate cancer. Mol Syst Biol. 2007;3:82. doi: 10.1038/msb4100125. http://MSB.embopress.org/cgi/pmidlookup?view=long&pmid=17299418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Wenjun, Ma Liye, Lin Liping, Gu Liqiang, Liu Xiaokang, Cai Hui, Yu Yongwei, Tan Xiaojie, Zhai Yujia, Xu Xingxing, Zhang Minfeng, Wu Lingling, Zhang Hongwei, Hou Jianguo, Wang Hongyang, Cao Guangwen. Identification of novel hub genes associated with liver metastasis of gastric cancer. Int J Cancer. 2009 Dec 15;125(12):2844–53. doi: 10.1002/ijc.24699. [DOI] [PubMed] [Google Scholar]
- 17.Sirota Marina, Dudley Joel T, Kim Jeewon, Chiang Annie P, Morgan Alex A, Sweet-Cordero Alejandro, Sage Julien, Butte Atul J. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci Transl Med. 2011 Aug 17;3(96):96ra77. doi: 10.1126/scitranslmed.3001318. http://stm.sciencemag.org/cgi/pmidlookup?view=long&pmid=21849665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesarovic M, Sreenath SN. Beyond the Flat Earth Perspective in Systems Biology. Biological Theory. 2006 Jan;1(1):33–34. doi: 10.1162/biot.2006.1.1.33. [DOI] [Google Scholar]
- 19.Gross Fridolin. What systems biology can tell us about disease. Hist Philos Life Sci. 2011;33(4):477–96. [PubMed] [Google Scholar]
- 20.Scholes Shaun, Bajekal Madhavi, Norman Paul, O'Flaherty Martin, Hawkins Nathaniel, Kivimäki Mika, Capewell Simon, Raine Rosalind. Quantifying policy options for reducing future coronary heart disease mortality in England: a modelling study. PLoS One. 2013;8(7):e69935. doi: 10.1371/journal.pone.0069935. http://dx.plos.org/10.1371/journal.pone.0069935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klir G. Facets of systems science. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 22.Wolkenhauer Olaf. Why model? Front Physiol. 2014;5:21. doi: 10.3389/fphys.2014.00021. http://dx.doi.org/10.3389/fphys.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Bin, Gaiteri Chris, Bodea Liviu-Gabriel, Wang Zhi, McElwee Joshua, Podtelezhnikov Alexei A, Zhang Chunsheng, Xie Tao, Tran Linh, Dobrin Radu, Fluder Eugene, Clurman Bruce, Melquist Stacey, Narayanan Manikandan, Suver Christine, Shah Hardik, Mahajan Milind, Gillis Tammy, Mysore Jayalakshmi, MacDonald Marcy E, Lamb John R, Bennett David A, Molony Cliona, Stone David J, Gudnason Vilmundur, Myers Amanda J, Schadt Eric E, Neumann Harald, Zhu Jun, Emilsson Valur. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013 Apr 25;153(3):707–20. doi: 10.1016/j.cell.2013.03.030. http://linkinghub.elsevier.com/retrieve/pii/S0092-8674(13)00387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolkenhauer Olaf, Shibata Darryl K, Mesarović Mihajlo D. A stem cell niche dominance theorem. BMC Syst Biol. 2011;5:4. doi: 10.1186/1752-0509-5-4. http://www.biomedcentral.com/1752-0509/5/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet Jean, Anto Josep M, Sterk Peter J, Adcock Ian M, Chung Kian Fan, Roca Josep, Agusti Alvar, Brightling Chris, Cambon-Thomsen Anne, Cesario Alfredo, Abdelhak Sonia, Antonarakis Stylianos E, Avignon Antoine, Ballabio Andrea, Baraldi Eugenio, Baranov Alexander, Bieber Thomas, Bockaert Joël, Brahmachari Samir, Brambilla Christian, Bringer Jacques, Dauzat Michel, Ernberg Ingemar, Fabbri Leonardo, Froguel Philippe, Galas David, Gojobori Takashi, Hunter Peter, Jorgensen Christian, Kauffmann Francine, Kourilsky Philippe, Kowalski Marek L, Lancet Doron, Pen Claude Le, Mallet Jacques, Mayosi Bongani, Mercier Jacques, Metspalu Andres, Nadeau Joseph H, Ninot Grégory, Noble Denis, Oztürk Mehmet, Palkonen Susanna, Préfaut Christian, Rabe Klaus, Renard Eric, Roberts Richard G, Samolinski Boleslav, Schünemann Holger J, Simon Hans-Uwe, Soares Marcelo Bento, Superti-Furga Giulio, Tegner Jesper, Verjovski-Almeida Sergio, Wellstead Peter, Wolkenhauer Olaf, Wouters Emiel, Balling Rudi, Brookes Anthony J, Charron Dominique, Pison Christophe, Chen Zhu, Hood Leroy, Auffray Charles. Systems medicine and integrated care to combat chronic noncommunicable diseases. Genome Med. 2011;3(7):43. doi: 10.1186/gm259. http://www.genomemedicine.com/content/3/7/43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capra F. The web of life: a new synthesis of mind and matter. London: Flamingo; 1996. [Google Scholar]
- 27.Cordell Heather J. Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009 Jun;10(6):392–404. doi: 10.1038/nrg2579. http://europepmc.org/abstract/MED/19434077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziebarth Jesse D, Bhattacharya Anindya, Chen Anlong, Cui Yan. PolymiRTS Database 2.0: linking polymorphisms in microRNA target sites with human diseases and complex traits. Nucleic Acids Res. 2012 Jan;40(Database issue):D216–21. doi: 10.1093/nar/gkr1026. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=22080514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolae Dan L, Gamazon Eric, Zhang Wei, Duan Shiwei, Dolan M Eileen, Cox Nancy J. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010 Apr;6(4):e1000888. doi: 10.1371/journal.pgen.1000888. http://dx.plos.org/10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerke Justin, Lorenz Kim, Cohen Barak. Genetic interactions between transcription factors cause natural variation in yeast. Science. 2009 Jan 23;323(5913):498–501. doi: 10.1126/science.1166426. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=19164747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Yanqing, Zhu Jun, Lum Pek Yee, Yang Xia, Pinto Shirly, MacNeil Douglas J, Zhang Chunsheng, Lamb John, Edwards Stephen, Sieberts Solveig K, Leonardson Amy, Castellini Lawrence W, Wang Susanna, Champy Marie-France, Zhang Bin, Emilsson Valur, Doss Sudheer, Ghazalpour Anatole, Horvath Steve, Drake Thomas A, Lusis Aldons J, Schadt Eric E. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008 Mar 27;452(7186):429–35. doi: 10.1038/nature06757. http://europepmc.org/abstract/MED/18344982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zak Iwona, Niemiec Pawel, Balcerzyk Anna, Krauze Jolanta. Combined "pro-atherosclerotic" variants of the ACE and APOE genes increase the risk of the coronary artery disease associated with the presence of cigarette smoking. Acta Cardiol. 2008 Dec;63(6):741–7. doi: 10.2143/AC.63.6.2033392. [DOI] [PubMed] [Google Scholar]
- 33.Choudhry Shweta, Avila Pedro C, Nazario Sylvette, Ung Ngim, Kho Jennifer, Rodriguez-Santana Jose R, Casal Jesus, Tsai Hui-Ju, Torres Alfonso, Ziv Elad, Toscano Monica, Sylvia Jody Senter, Alioto Mariaelena, Salazar Michael, Gomez Ivan, Fagan Joanne K, Salas Jorge, Lilly Craig, Matallana Henry, Castro Richard A, Selman Moises, Weiss Scott T, Ford Jean G, Drazen Jeffrey M, Rodriguez-Cintron William, Chapela Rocio, Silverman Edwin K, Burchard Esteban González. CD14 tobacco gene-environment interaction modifies asthma severity and immunoglobulin E levels in Latinos with asthma. Am J Respir Crit Care Med. 2005 Jul 15;172(2):173–82. doi: 10.1164/rccm.200409-1232OC. [DOI] [PubMed] [Google Scholar]
- 34.Liu Amy Y, Scherer Dominique, Poole Elizabeth, Potter John D, Curtin Karen, Makar Karen, Slattery Martha L, Caan Bette J, Ulrich Cornelia M. Gene-diet-interactions in folate-mediated one-carbon metabolism modify colon cancer risk. Mol Nutr Food Res. 2013 Apr;57(4):721–34. doi: 10.1002/mnfr.201200180. http://europepmc.org/abstract/MED/22961839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspi Avshalom, Sugden Karen, Moffitt Terrie E, Taylor Alan, Craig Ian W, Harrington HonaLee, McClay Joseph, Mill Jonathan, Martin Judy, Braithwaite Antony, Poulton Richie. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul 18;301(5631):386–9. doi: 10.1126/science.1083968. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=12869766. [DOI] [PubMed] [Google Scholar]
- 36.Bisgaard Hans, Simpson Angela, Palmer Colin N A, Bønnelykke Klaus, McLean Irwin, Mukhopadhyay Somnath, Pipper Christian B, Halkjaer Liselotte B, Lipworth Brian, Hankinson Jenny, Woodcock Ashley, Custovic Adnan. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008 Jun 24;5(6):e131. doi: 10.1371/journal.pmed.0050131. http://dx.plos.org/10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itzhaki R F, Lin W R, Shang D, Wilcock G K, Faragher B, Jamieson G A. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997 Jan 25;349(9047):241–4. doi: 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- 38.Ramagopalan Sreeram V, Maugeri Narelle J, Handunnetthi Lahiru, Lincoln Matthew R, Orton Sarah-Michelle, Dyment David A, Deluca Gabriele C, Herrera Blanca M, Chao Michael J, Sadovnick A Dessa, Ebers George C, Knight Julian C. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009 Feb;5(2):e1000369. doi: 10.1371/journal.pgen.1000369. http://dx.plos.org/10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins John P. Nonlinear systems in medicine. Yale J Biol Med. 2002;75(5-6):247–60. http://europepmc.org/abstract/MED/14580107. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang I-Ming, Zhang Bin, Yang Xia, Zhu Jun, Stepaniants Serguei, Zhang Chunsheng, Meng Qingying, Peters Mette, He Yudong, Ni Chester, Slipetz Deborah, Crackower Michael A, Houshyar Hani, Tan Christopher M, Asante-Appiah Ernest, O'Neill Gary, Luo Mingjuan Jane, Thieringer Rolf, Yuan Jeffrey, Chiu Chi-Sung, Lum Pek Yee, Lamb John, Boie Yves, Wilkinson Hilary A, Schadt Eric E, Dai Hongyue, Roberts Christopher. Systems analysis of eleven rodent disease models reveals an inflammatome signature and key drivers. Mol Syst Biol. 2012;8:594. doi: 10.1038/msb.2012.24. http://MSB.embopress.org/cgi/pmidlookup?view=long&pmid=22806142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwen Bruce S, Wingfield John C. The concept of allostasis in biology and biomedicine. Horm Behav. 2003 Jan;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 42.Stam C J. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005 Oct;116(10):2266–301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Chalabi Zaid, Lorenc Theo. Using agent-based models to inform evaluation of complex interventions: examples from the built environment. Prev Med. 2013 Nov;57(5):434–5. doi: 10.1016/j.ypmed.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Gottschalk A, Bauer M S, Whybrow P C. Evidence of chaotic mood variation in bipolar disorder. Arch Gen Psychiatry. 1995 Nov;52(11):947–59. doi: 10.1001/archpsyc.1995.03950230061009. [DOI] [PubMed] [Google Scholar]
- 45.Huang Sui, Eichler Gabriel, Bar-Yam Yaneer, Ingber Donald E. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys Rev Lett. 2005 Apr 1;94(12):128701. doi: 10.1103/PhysRevLett.94.128701. [DOI] [PubMed] [Google Scholar]
- 46.Aird William C. Endothelial cell dynamics and complexity theory. Crit Care Med. 2002 May;30(5 Suppl):S180–5. doi: 10.1097/00003246-200205001-00002. [DOI] [PubMed] [Google Scholar]
- 47.Callard R, George A J, Stark J. Cytokines, chaos, and complexity. Immunity. 1999 Nov;11(5):507–13. doi: 10.1016/s1074-7613(00)80125-9. http://linkinghub.elsevier.com/retrieve/pii/S1074-7613(07)00041-6. [DOI] [PubMed] [Google Scholar]
- 48.Lombardi F. Chaos theory, heart rate variability, and arrhythmic mortality. Circulation. 2000;101(1):8–10. doi: 10.1161/01.cir.101.1.8. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=10618296. [DOI] [PubMed] [Google Scholar]
- 49.Garfinkel A, Chen P S, Walter D O, Karagueuzian H S, Kogan B, Evans S J, Karpoukhin M, Hwang C, Uchida T, Gotoh M, Nwasokwa O, Sager P, Weiss J N. Quasiperiodicity and chaos in cardiac fibrillation. J Clin Invest. 1997 Jan 15;99(2):305–14. doi: 10.1172/JCI119159. http://europepmc.org/abstract/MED/9005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchman Timothy G. The community of the self. Nature. 2002 Nov 14;420(6912):246–51. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 51.Seely A J, Christou N V. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med. 2000 Jul;28(7):2193–200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Sherman R C, Langley-Evans S C. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998 Apr;94(4):373–81. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 53.Khan W I, Blennerhasset P A, Varghese A K, Chowdhury S K, Omsted P, Deng Y, Collins S M. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002 Nov;70(11):5931–7. doi: 10.1128/IAI.70.11.5931-5937.2002. http://iai.asm.org/cgi/pmidlookup?view=long&pmid=12379667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox J G, Beck P, Dangler C A, Whary M T, Wang T C, Shi H N, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000 May;6(5):536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 55.Sewell Diane, Qing Zhu, Reinke Emily, Elliot David, Weinstock Joel, Sandor Matyas, Fabry Zsuzsa. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003 Jan;15(1):59–69. doi: 10.1093/intimm/dxg012. http://intimm.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12502726. [DOI] [PubMed] [Google Scholar]
- 56.Weinstock Joel V. Autoimmunity: The worm returns. Nature. 2012 Nov 8;491(7423):183–5. doi: 10.1038/491183a. http://europepmc.org/abstract/MED/23135449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palla Luigi, Higgins Julian P T, Wareham Nicholas J, Sharp Stephen J. Challenges in the use of literature-based meta-analysis to examine gene-environment interactions. Am J Epidemiol. 2010 Jun 1;171(11):1225–32. doi: 10.1093/aje/kwq051. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=20406760. [DOI] [PubMed] [Google Scholar]
- 58.Hubner Norbert, Wallace Caroline A, Zimdahl Heike, Petretto Enrico, Schulz Herbert, Maciver Fiona, Mueller Michael, Hummel Oliver, Monti Jan, Zidek Vaclav, Musilova Alena, Kren Vladimir, Causton Helen, Game Laurence, Born Gabriele, Schmidt Sabine, Müller Anita, Cook Stuart A, Kurtz Theodore W, Whittaker John, Pravenec Michal, Aitman Timothy J. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat Genet. 2005 Mar;37(3):243–53. doi: 10.1038/ng1522. [DOI] [PubMed] [Google Scholar]
- 59.Jensen Peter B, Jensen Lars J, Brunak Søren. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet. 2012 Jun;13(6):395–405. doi: 10.1038/nrg3208. [DOI] [PubMed] [Google Scholar]
- 60.Bilal Erhan, Dutkowski Janusz, Guinney Justin, Jang In Sock, Logsdon Benjamin A, Pandey Gaurav, Sauerwine Benjamin A, Shimoni Yishai, Moen Vollan Hans Kristian, Mecham Brigham H, Rueda Oscar M, Tost Jorg, Curtis Christina, Alvarez Mariano J, Kristensen Vessela N, Aparicio Samuel, Børresen-Dale Anne-Lise, Caldas Carlos, Califano Andrea, Friend Stephen H, Ideker Trey, Schadt Eric E, Stolovitzky Gustavo A, Margolin Adam A. Improving breast cancer survival analysis through competition-based multidimensional modeling. PLoS Comput Biol. 2013;9(5):e1003047. doi: 10.1371/journal.pcbi.1003047. http://dx.plos.org/10.1371/journal.pcbi.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forbes IBM's Watson Gets Its First Piece Of Business In Healthcare. 2013. [2013-10-09]. http://www.forbes.com/sites/bruceupbin/2013/02/08/ibms-watson-gets-its-first-piece-of-business-in-healthcare/
- 62.Leonelli S. Global data for local science: Assessing the scale of data infrastructures in biological and biomedical research. BioSocieties. 2013 Nov 11;8(4):449–465. doi: 10.1057/biosoc.2013.23. [DOI] [Google Scholar]
- 63.Research Information Network Stewardship of digital research data: a framework of principles and guidelines. 2008. [2013-10-09]. http://www.rin.ac.uk/system/files/attachments/Stewardship-data-guidelines.pdf.
- 64.Brauer Ruth, Smeeth Liam, Anaya-Izquierdo Karim, Timmis Adam, Denaxas Spiros C, Farrington C Paddy, Whitaker Heather, Hemingway Harry, Douglas Ian. Antipsychotic drugs and risks of myocardial infarction: a self-controlled case series study. Eur Heart J. 2014 Jul 8;:-. doi: 10.1093/eurheartj/ehu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quint J K, Herrett E, Bhaskaran K, Timmis A, Hemingway H, Wedzicha J A, Smeeth L. Effect of β blockers on mortality after myocardial infarction in adults with COPD: population based cohort study of UK electronic healthcare records. BMJ. 2013;347:f6650. doi: 10.1136/bmj.f6650. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=24270505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clermont Gilles, Auffray Charles, Moreau Yves, Rocke David M, Dalevi Daniel, Dubhashi Devdatt, Marshall Dana R, Raasch Peter, Dehne Frank, Provero Paolo, Tegner Jesper, Aronow Bruce J, Langston Michael A, Benson Mikael. Bridging the gap between systems biology and medicine. Genome Med. 2009;1(9):88. doi: 10.1186/gm88. http://www.genomemedicine.com/content/1/9/88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Souhami R. Personal data for public good: using health information in medical research. London: Academy of Medical Sciences; 2006. [Google Scholar]
- 68.Mandl Kenneth D, Kohane Isaac S. Tectonic shifts in the health information economy. N Engl J Med. 2008 Apr 17;358(16):1732–7. doi: 10.1056/NEJMsb0800220. [DOI] [PubMed] [Google Scholar]
- 69.Herrett Emily, Shah Anoop Dinesh, Boggon Rachael, Denaxas Spiros, Smeeth Liam, van Staa Tjeerd, Timmis Adam, Hemingway Harry. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. doi: 10.1136/bmj.f2350. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=23692896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tiik Madis, Ross Peeter. Patient opportunities in the Estonian Electronic Health Record System. Stud Health Technol Inform. 2010;156:171–7. [PubMed] [Google Scholar]
- 71.Bourgeois FT, Simons WW, Olson K, Brownstein JS, Mandl KD. Evaluation of influenza prevention in the workplace using a personally controlled health record: randomized controlled trial. J Med Internet Res. 2008;10(1):e5. doi: 10.2196/jmir.984. http://www.jmir.org/2008/1/e5/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kidd Michael R. Personal electronic health records: MySpace or HealthSpace? BMJ. 2008 May 10;336(7652):1029–30. doi: 10.1136/bmj.39567.550301.80. http://europepmc.org/abstract/MED/18460554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lunshof Jeantine E, Chadwick Ruth, Vorhaus Daniel B, Church George M. From genetic privacy to open consent. Nat Rev Genet. 2008 May;9(5):406–11. doi: 10.1038/nrg2360. [DOI] [PubMed] [Google Scholar]
- 74.Eysenbach Gunther. Medicine 2.0: social networking, collaboration, participation, apomediation, and openness. J Med Internet Res. 2008;10(3):e22. doi: 10.2196/jmir.1030. http://www.jmir.org/2008/3/e22/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wicks Paul, Massagli Michael, Frost Jeana, Brownstein Catherine, Okun Sally, Vaughan Timothy, Bradley Richard, Heywood James. Sharing health data for better outcomes on PatientsLikeMe. J Med Internet Res. 2010;12(2):e19. doi: 10.2196/jmir.1549. http://www.jmir.org/2010/2/e19/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wicks Paul, Vaughan Timothy E, Massagli Michael P, Heywood James. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat Biotechnol. 2011 May;29(5):411–4. doi: 10.1038/nbt.1837. [DOI] [PubMed] [Google Scholar]
- 77.Luciano JS, Cumming GP, Wilkinson MD, Kahana E. The emergent discipline of health web science. J Med Internet Res. 2013;15(8):e166. doi: 10.2196/jmir.2499. http://www.jmir.org/2013/8/e166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schleyer Titus, Spallek Heiko, Butler Brian S, Subramanian Sushmita, Weiss Daniel, Poythress M Louisa, Rattanathikun Phijarana, Mueller Gregory. Facebook for scientists: requirements and services for optimizing how scientific collaborations are established. J Med Internet Res. 2008;10(3):e24. doi: 10.2196/jmir.1047. http://www.jmir.org/2008/3/e24/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kahlon Maninder, Yuan Leslie, Daigre John, Meeks Eric, Nelson Katie, Piontkowski Cynthia, Reuter Katja, Sak Rachael, Turner Brian, Weber Griffin M, Chatterjee Anirvan. The use and significance of a research networking system. J Med Internet Res. 2014;16(2):e46. doi: 10.2196/jmir.3137. http://www.jmir.org/2014/2/e46/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bot Brian M, Burdick David, Kellen Michael, Huang Erich S. clearScience: Infrastructure for Communicating Data-Intensive Science. AMIA Jt Summits Transl Sci Proc. 2013;2013:27. [PubMed] [Google Scholar]
- 81.Leonelli S. Why the Current Insistence on Open Access to Scientific Data? Big Data, Knowledge Production, and the Political Economy of Contemporary Biology. Bulletin of Science, Technology & Society. 2013 Aug 20;33(1-2):6–11. doi: 10.1177/0270467613496768. [DOI] [Google Scholar]
- 82.Reynolds Carl J, Wyatt Jeremy C. Open source, open standards, and health care information systems. J Med Internet Res. 2011;13(1):e24. doi: 10.2196/jmir.1521. http://www.jmir.org/2011/1/e24/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plenge Robert M, Greenberg Jeffrey D, Mangravite Lara M, Derry Jonathan M J, Stahl Eli A, Coenen Marieke J H, Barton Anne, Padyukov Leonid, Klareskog Lars, Gregersen Peter K, Mariette Xavier, Moreland Larry W, Bridges S Louis, de Vries Niek, Huizinga Tom W J, Guchelaar Henk-Jan, International Rheumatoid Arthritis Consortium (INTERACT) Friend Stephen H, Stolovitzky Gustavo. Crowdsourcing genetic prediction of clinical utility in the Rheumatoid Arthritis Responder Challenge. Nat Genet. 2013 May;45(5):468–9. doi: 10.1038/ng.2623. http://europepmc.org/abstract/MED/23619782. [DOI] [PMC free article] [PubMed] [Google Scholar]