Abstract
Transgenerational effects of infection have a huge potential to influence the prevalence and intensity of infections in vectors and, by extension, disease epidemiology. These transgenerational effects may increase the fitness of offspring through the transfer of protective immune factors. Alternatively, however, infected mothers may transfer the costs of infection to their offspring. Although transgenerational immune protection has been described in a dozen invertebrate species, we still lack a complete picture of the incidence and importance of transgenerational effects of infection in most invertebrate groups. The existence of transgenerational infection effects in mosquito vectors is of particular interest because of their potential for influencing parasite prevalence and intensity and, by extension, disease transmission. Here we present what we believe to be the first study on transgenerational infection effects in a mosquito vector infected with malaria parasites. The aim of this experiment was to quantify both the benefits and the costs of having an infected mother. We find no evidence of transgenerational protection in response to a Plasmodium infection. Having an infected mother does, however, entail considerable fecundity costs for the offspring: fecundity loss is three times higher in infected offspring issued from infected mothers than in infected offspring issued from uninfected mothers. We discuss the implications of our results and we call for more studies looking at transgenerational effects of infection in disease vectors.
Keywords: Culex pipiens, Plasmodium relictum, immunity, transgenerational effects
1. Introduction
The environmental conditions experienced by an organism can have a drastic effect on the outcome of infection in its offspring. Factors such as nutritional availability, crowding conditions or the temperature experienced by mothers can affect the ability of their offspring to withstand an infection [1,2]. When such transgenerational maternal effects result in a net increase in the fitness of offspring, they are usually assumed to be adaptive [3]. One particular instance of such adaptive transgenerational maternal effects occurs when infected mothers transfer some level of disease protection to their offspring. In vertebrates such transgenerational disease protection has been extensively investigated and shown to be largely mediated through the transfer of immunoactive compounds such as antibodies [4]. Demonstrating the existence of transgenerational immune protection in invertebrates has, however, been much more slow. Invertebrates have been long thought to have the most basic of immune systems, allowing them to defend themselves against a wide range of pathogens, but lacking the two defining characteristics of the vertebrate immune system: specificity and memory. In the last decade this paradigm has, however, been entirely overhauled.
The first reported instances of transgenerational immune protection in invertebrates, published over 10 years ago, constituted a paradigm shift in our understanding of how the invertebrate immune system works [5]. Since then, transgenerational immune protection has been reported in a range of invertebrate species, including several insects (electronic supplementary material, table S1). This form of immune protection is, however, not expected to be widespread in invertebrates. Following a well-established evolutionary biology maxim, transgenerational immune protection should evolve only if the benefits of immune transfer (protection against pathogens) outweigh its costs. Two sine qua non conditions for the evolution of transgenerational protection are that (i) the pathogen induces a fitness cost on the offspring and (ii) the offspring have a high probability of encountering the same pathogen as their parents. As a result, the overwhelming majority of transgenerational protection studies have focused on aquatic, low-dispersing or eusocial invertebrates (electronic supplementary material, table S1). We therefore lack a complete picture of the incidence and importance of transgenerational protection in other invertebrate groups. More generally, we also lack information about what other transgenerational effects may be passed on from infected mothers to their offspring. There are numerous examples of maternal effects decreasing, as opposed to increasing, offspring fitness [3], resulting in offspring that may be more easily infected or less able to withstand an infection. Either way, it is clear that maternal effects of infection can have drastic consequences on disease dynamics.
Many invertebrates act as vectors of human and animal diseases. Transgenerational effects of infection have a huge potential to influence the prevalence and intensity of infections in vectors and, by extension, disease epidemiology. Despite this, to our knowledge no study has investigated whether having an infected mother influences the outcome of infection in any vector of disease. Here we investigate this question by focusing on the avian malaria parasite Plasmodium relictum and its natural vector, the mosquito Culex pipiens. Recent work has indeed shown that a Plasmodium infection triggers a long-lived response in mosquitoes [6], opening up the possibility that this memory-like response is transmitted to the offspring. Transgenerational immune protection against avian malaria may be expected to evolve in C. pipiens mosquitoes because this species is highly ornitophylic and avian malaria infections are extremely prevalent in the field: in some areas, the proportion of infected birds can be as high as 90% (R. Pigeault et al. 2014, unpublished data). In addition, avian malaria incurs fitness costs in C. pipiens mosquitoes: infected females lay, on average, 30% fewer eggs than uninfected ones [7].
The aim of this study was twofold: (i) to test whether the prevalence and/or the intensity of a Plasmodium infection is lower in mosquitoes issued from infected mothers and (ii) to test whether having an infected mother incurs fecundity costs for the offspring.
2. Material and methods
Complete Material and methods are provided in the electronic supplementary material. The experiment spanned two consecutive mosquito generations, henceforth called maternal (or F0) and offspring (or F1) generations. The aim of the experiment was to quantify infection success and fecundity in four kinds of F1 females: uninfected F1 females issued from either uninfected F0 mothers or infected F0 mothers, and infected F1 females issued from either uninfected F0 mothers or infected F0 mothers.
For the maternal (F0) blood meal, mosquitoes were randomly allocated to feed on either a Plasmodium-infected (n = 4) or an uninfected bird (n = 4). The success of the infection was verified by dissecting a subsample of the mosquitoes. The egg rafts laid by mosquitoes within a treatment (infected or uninfected) were pooled together in a tray until the eggs hatched. Larvae issued from infected and uninfected mothers were randomly seeded into 18 plastic trays (nine for each treatment) and reared to adulthood in cages following standard protocols [7].
For the offspring (F1) blood meal, 20 experimental cages were set up, each containing 40 female mosquitoes issued from an infected mother and 40 issued from an uninfected mother randomly sampled from the 18 infected and uninfected cages. Mosquitoes originating from infected and uninfected mothers were marked with different-coloured fluorescent powders for subsequent identification (electronic supplementary material). Cages were then randomly provided with either an infected (n = 10) or an uninfected (n = 10) bird.
To obtain an estimate of the blood meal size, all engorged F1 females were placed individually in numbered plastic tubes until all haematin was excreted. Mosquitoes were then transferred to a tube containing mineral water to allow the females to lay their eggs. We quantified female size, number of eggs laid, larvae emerged and haematin excreted as previously published [7].
To estimate the prevalence and intensity of the Plasmodium infection in the F1 generation, 7 days after the blood meal, 20 females (10 issued from uninfected and 10 from infected mothers) from each of the infected cages were randomly sampled and dissected to count the number of oocysts in their midguts.
(a). Statistical analysis
Analyses were carried out using the R statistical package (v. 3.1.0). The statistical models built to analyse the data (numbered m1–m10) are described in the electronic supplementary material, table S2. Briefly, when the response variable was a proportion, the data were analysed using a linear mixed effects model with a binomial error distribution (lmer). Otherwise, data were log or Box Cox transformed and a normal error distribution was used (lme). Count data were analysed using a glm with a quasi-Poisson error distribution, to correct for overdispersion. Laying date was analysed using Cox proportional hazards mixed effects models (coxme). A posteriori contrasts were carried out by aggregating factor levels together and by testing the fit of the simplified model using a likelihood ratio test.
3. Results and discussion
The aim of this experiment was to quantify both the benefits (protection from infection) and the costs (effects on fecundity) for mosquitoes of having a Plasmodium-infected mother. We found no effect of a Plasmodium infection in the maternal generation on either the probability (m1: 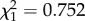 , p = 0.386, figure 1a) or the intensity (m2:
, p = 0.386, figure 1a) or the intensity (m2: 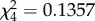 , p = 0.7126, figure 1b) of an infection in the offspring. Our results, therefore, conclusively show no evidence for a transgenerational transfer of protection in response to a Plasmodium infection in mosquitoes.
, p = 0.7126, figure 1b) of an infection in the offspring. Our results, therefore, conclusively show no evidence for a transgenerational transfer of protection in response to a Plasmodium infection in mosquitoes.
Figure 1.
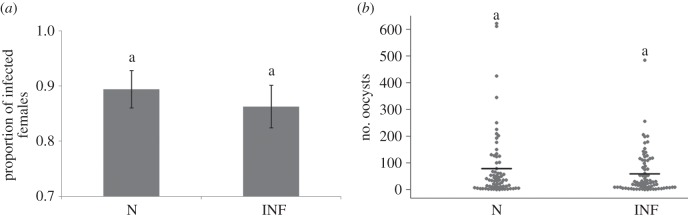
Effect of the infection status of the maternal (F0) generation on the (a) infection prevalence and (b) oocyst burden in the F1 offspring. (a) Mean (±s.e.) proportion of females infected; (b) number (dots) and median number (horizontal line) of oocysts. N, uninfected mothers; INF, infected mothers.
Previous work has shown that the offspring of Aedes aegypti mosquitoes primed using negatively charged Sephadex beads were no better at melanizing beads than the offspring of naive ones [8], a result that seems to agree with the findings of our study. Whether the quantification of a single immune pathway suffices to prove the existence, or lack thereof, of transgenerational immune protection is, however, debatable. The use of a few immune assays as a proxy for parasite resistance has come under increased scrutiny, as evidence accumulates that they are not necessarily correlated with each other [9]. This question is highly relevant because the overwhelming majority of published studies have used immune measurements taken from the offspring of infected mothers to infer resistance (electronic supplementary material, table S1). Further studies are, in our opinion, needed to establish whether the outcome of these assays translates into increased protection against parasites.
Having an infected mother does not have any measurable benefits in terms of parasite protection, but it does, however, entail considerable costs. Offspring of infected and uninfected mothers have the same probability of laying an egg raft (m3: 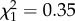 , p = 0.5509), but infected offspring issued from uninfected mothers lay on average 10 ± 3 (mean ± s.e.) fewer eggs per egg raft than uninfected ones, which represents a fecundity loss of ca 8%. This fecundity loss is tripled in infected offspring issued from infected mothers: infected offspring lose as many as 38 ± 2 eggs (25%) compared with uninfected ones (m4:
, p = 0.5509), but infected offspring issued from uninfected mothers lay on average 10 ± 3 (mean ± s.e.) fewer eggs per egg raft than uninfected ones, which represents a fecundity loss of ca 8%. This fecundity loss is tripled in infected offspring issued from infected mothers: infected offspring lose as many as 38 ± 2 eggs (25%) compared with uninfected ones (m4: 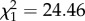 , p < 0.0001, figure 2a). As a result, the highest fecundity costs are paid by infected offspring issued from infected mothers. A similar trend, albeit of smaller amplitude, was observed for the number of hatched larvae (m6:
, p < 0.0001, figure 2a). As a result, the highest fecundity costs are paid by infected offspring issued from infected mothers. A similar trend, albeit of smaller amplitude, was observed for the number of hatched larvae (m6: 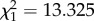 , p = 0.0002). One potential explanation for these results is that the offspring of infected mothers avoided feeding on the infected birds, which could be equated to a form of transgenerational behavioural protection. We have, however, shown that both infected and uninfected mosquitoes have a preference for feeding on infected birds [10], making this explanation unlikely, although worth testing in the future.
, p = 0.0002). One potential explanation for these results is that the offspring of infected mothers avoided feeding on the infected birds, which could be equated to a form of transgenerational behavioural protection. We have, however, shown that both infected and uninfected mosquitoes have a preference for feeding on infected birds [10], making this explanation unlikely, although worth testing in the future.
Figure 2.
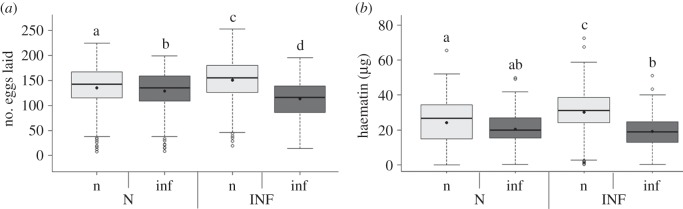
Effect of the infection status of the maternal (F0) generation on the fecundity (a) and blood meal size (b) of uninfected (light grey) and infected (dark grey) F1 offspring issued from uninfected (N) or infected (INF) F0 mothers. Boxplots represent the means (points) and medians (horizontal lines). Boxes above and below the medians show the first and third quartiles, respectively. Dashed lines delimit 1.5 times the inter-quartile range, above and below which individual counts are considered outliers and marked as empty circles.
Previous work on this system has shown that Plasmodium-infected mosquitoes lay their eggs significantly earlier than uninfected ones, possibly to compensate for the strong and progressive decrease in the quality of eggs laid by these females [11]. Our results agree with this: infected females lay egg rafts earlier than uninfected ones (m7: 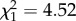 , p = 0.047), although this effect is independent of the infection status of the mother (m7:
, p = 0.047), although this effect is independent of the infection status of the mother (m7: 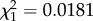 , p = 0.8931).
, p = 0.8931).
Parental infection has been reported to impact several life-history traits of the offspring [12]. To our knowledge, however, the actual mechanisms underlying changes in life-history traits in offspring have never been investigated. To deepen our insight into the mechanisms underlying the large differences in fecundity costs in offspring issued from infected and uninfected mothers, we analysed the blood meal size (haematin) of mosquitoes. Blood meal size is a strong predictor of fecundity in this system [7]. Offspring that feed on infected birds secrete significantly less haematin than those that feed on uninfected ones (m8: 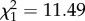 , p = 0.0035). This result may be due to smaller blood meals and/or to the confounding effect of anaemia [7]. The difference in haematin between infected and uninfected offspring is, however, much more marked when the mother is infected (m9:
, p = 0.0035). This result may be due to smaller blood meals and/or to the confounding effect of anaemia [7]. The difference in haematin between infected and uninfected offspring is, however, much more marked when the mother is infected (m9: 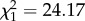 , p < 0.0001, figure 2b). These differences in blood meal size cannot be explained by differences in body size (m10:
, p < 0.0001, figure 2b). These differences in blood meal size cannot be explained by differences in body size (m10: 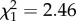 , p = 0.328). When haematin was added as a covariate in our statistical model, the significance of the F0 and F1 infection treatment on fecundity was maintained (m5:
, p = 0.328). When haematin was added as a covariate in our statistical model, the significance of the F0 and F1 infection treatment on fecundity was maintained (m5: 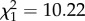 , p = 0.0014), implying that the fecundity costs observed are not entirely mediated through an alteration of blood meal size and that other, as yet unknown, mechanisms are at play.
, p = 0.0014), implying that the fecundity costs observed are not entirely mediated through an alteration of blood meal size and that other, as yet unknown, mechanisms are at play.
4. Conclusion
Whether the absence of transgenerational immune protection we report is specific to this particular mosquito–parasite combination or whether it is a general attribute of mosquito vectors requires further investigation, because of its potential epidemiological consequences [13]. Transgenerational acquired immunity has thus far been described in a dozen invertebrate species, the large majority of which are either aquatic, eusocial or stored-product species (electronic supplementary material, table S1), life histories that favour a common mother–offspring environment and thus the evolution of an immune transfer across generations. Whether the scarcity of published negative results indicates that transgenerational protection is the rule rather than the exception in the invertebrate world or whether it reflects a publication bias deserves further consideration. Either way a more complete picture of the transgenerational effects of infection across the invertebrate world is needed.
Supplementary Material
Ethics statement
Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors’ institution (permit number CEEA- LR-1051).
Data accessibility
Data for this study are available at Dryad (doi:10.5061/dryad.ht79k).
Author contributions
R.P., J.V., S.G. and A.R. conceived and designed the experiment. R.P. and A.N. performed the experiment. R.P. analysed the data. R.P., S.G. and A.R. wrote the paper.
Funding statement
S.G. was funded through an ERC starting grant EVOLEPID 243054.
Competing interests
We declare we do not have any competing interest.
References
- 1.Mitchell SE, Read AF. 2005. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B 272, 2601–2607. ( 10.1098/rspb.2005.3253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boots M, Roberts KE. 2012. Maternal effects in disease resistance: poor maternal environment increases offspring resistance to an insect virus. Proc. R. Soc. B 279, 4009–4014. ( 10.1098/rspb.2012.1073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 4.Boulinier T, Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288. ( 10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 5.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492. ( 10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. 2010. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355. ( 10.1126/science.1190689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vézilier J, Nicot A, Gandon S, Rivero A. 2012. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. R. Soc. B 279, 4033–4041. ( 10.1098/rspb.2012.1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voordouw MJ, Lambrechts L, Koella J. 2008. No maternal effects after stimulation of the melanization response in the yellow fever mosquito Aedes aegypti. Oikos 117, 1269–1279. ( 10.1111/j.0030-1299.2008.16741.x) [DOI] [Google Scholar]
- 9.Adamo SA. 2004. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 68, 1443–1449. ( 10.1016/j.anbehav.2004.05.005) [DOI] [Google Scholar]
- 10.Cornet S, Nicot A, Rivero A, Gandon S. 2013. Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar. J. 12, 179 ( 10.1186/1475-2875-12-179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vézilier J, Nicot A, Gandon S, Rivero A. 2015. Plasmodium infection brings forward mosquito oviposition. Biol. Lett. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moret Y. 2006. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 273, 1399–1405. ( 10.1098/rspb.2006.3465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tidbury HJ, Best A, Boots M. 2012. The epidemiological consequences of immune priming. Proc. R. Soc. B 279, 4505–4512. ( 10.1098/rspb.2012.1841) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are available at Dryad (doi:10.5061/dryad.ht79k).


