Abstract
Aims
The present study is designed to consider a role for the circadian clock protein Per1 in the regulation of the endothelin axis in mouse kidney, lung, liver and heart. Renal endothelin-1 (ET-1) is a regulator of the epithelial sodium channel (ENaC) and blood pressure (BP), via activation of both endothelin receptors, ETA and ETB. However, ET-1 mediates many complex events in other tissues.
Main methods
Tissues were collected in the middle of murine rest and active phases, at noon and midnight, respectively. ET-1, ETA and ETB mRNA expressions were measured in the lung, heart, liver, renal inner medulla and renal cortex of wild type and Per1 heterozygous mice using real-time quantitative RT-PCR.
Key findings
The effect of reduced Per1 expression on levels of mRNAs and the time-dependent regulation of expression of the endothelin axis genes appeared to be tissue-specific. In the renal inner medulla and the liver, ETA and ETB exhibited peaks of expression in opposite circadian phases. In contrast, expressions of ET-1, ETA and ETB in the lung did not appear to vary with time, but ET-1 expression was dramatically decreased in this tissue in Per1 heterozygous mice. Interestingly, ET-1 and ETA, but not ETB, were expressed in a time-dependent manner in the heart.
Significance
Per1 appears to regulate expression of the endothelin axis genes in a tissue-specific and time-dependent manner. These observations have important implications for our understanding of the best time of day to deliver endothelin receptor antagonists.
Keywords: Endothelin-A receptor, Endothelin-B receptor, ETA, ETB, Gene regulation
Introduction
The circadian clock regulates a variety of physiological processes such as metabolism, immune response, sleep–wake cycles, renal function, and blood pressure (BP) (reviewed in Richards and Gumz, 2012, 2013; Stow and Gumz, 2011). On the molecular level, the circadian clock consists of multiple proteins. Four are considered the core proteins that interact with one another to affect transcription of circadian target genes (Dibner et al., 2010). These proteins are Period (Per: homologs 1–3), Cryptochrome (Cry: homologs 1–2), BMAL1, and CLOCK. CLOCK and BMAL1 form a heterodimer, and then bind E-box DNA response elements to transcriptionally regulate CLOCK-controlled genes, including the genes Per and Cry. In the canonical model, Per and Cry presumably interact to repress the transcriptional activity of CLOCK and BMAL1 (Albrecht and Eichele, 2003).
Endothelin-1 (ET-1) is a peptide hormone expressed in multiple tissues and mediates its actions through two receptors: endothelin-A (ETA) and endothelin-B (ETB) receptors. ET-1 was first characterized as a potent vasoconstrictor; however, it is now known that ET-1 action is much more complex (reviewed in Kohan et al., 2011). ET-1 in the renal collecting duct is a potent inhibitor of epithelial Na channel (ENaC) activity through both ETA and ETB receptors (Bugaj et al., 2012; Ge et al., 2006, 2008; Lynch et al., 2013). This inhibition appears to occur via a nitric oxide-dependent mechanism (Bugaj et al., 2008; Stricklett et al., 2006) (reviewed in Kohan, 2013). The ET-1 gene (Edn1) is regulated by epigenetic factors (Welch et al., 2013) and transcription is controlled by mineralocorticoid action (Stow et al., 2009), calcium via the nuclear factor of activated T-cells (NFAT) (Strait et al., 2010), and a variety of other mechanisms (reviewed in Stow et al., 2011). Emerging evidence has demonstrated that Edn1 is also regulated post-transcriptionally (reviewed in Jacobs et al., 2013; Welch et al., 2013). Our laboratory has demonstrated that ET-1 peptide expression varies in a time-dependent manner in the renal cortex and medulla (Stow et al., 2012). The mechanism of this effect appears to be transcriptional. Indeed, we have previously shown that Per1 interacts with a non-canonical E-box from the Edn1 promoter (Stow et al., 2012). Per1 is a repressor of renal ET-1 mRNA and peptide levels, and Per1 knockout (KO) animals have elevated levels of ET-1 peptide in the kidney cortex and medulla (Richards et al., 2013; Stow et al., 2012).
Although ET-1 plays an integral role in a variety of physiological processes, circadian regulation of ET-1 and the receptors by the circadian clock and Per1 has not been studied outside of the kidney. Therefore, the goal of this study was to characterize the time-dependence of ET-1, ETA and ETB (the “endothelin axis”) mRNA expressions and to test the hypothesis that Per1 plays a role in the regulation of the endothelin axis mRNA in the liver, heart, kidney, and lung. It is well established that the circadian clock plays an integral role in the regulation of liver, heart, kidney and lung functions (reviewed in Richards and Gumz, 2013) but the role of Per1 in the regulation of the endothelin axis in these tissues has not been investigated. For the first time, we demonstrate that the endothelin axis is regulated by time and by Per1 in a manner that is unique to each of the tissues tested.
Materials and methods
Animals
All animal-use protocols were approved by the University of Florida and North Florida/South Georgia Veterans Administration Institutional Animal Care and Use Committee in accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals. Per1 KO and wild type (WT) mice (129/sv) were originally provided by Dr. David Weaver (University of Massachusetts (Bae et al., 2001)). WT and Per1 heterozygote (het) mice were bred in house by UF Animal Care Services Staff. Animals were maintained on a normal 12 h light: dark cycle. Mice were fed normal lab chow and given free access to water. At noon and midnight, mice were anesthetized and tissues were collected and snap frozen in liquid nitrogen. Kidneys were later dissected and cortex removed for protein or RNA isolation.
Isolation of IMCD
Isolations of inner medullary collecting ducts (IMCDs) were prepared as described below. WT and Per1 het mice were euthanized at midnight and inner medulla was dissected from both kidneys. The inner medulla was minced longitudinally and then digested at 37 °C in a buffer containing 250 mM sucrose, 10 mM Triethanolamine, 3 mg/mL of Collagenase type I, and 2 mg/mL of Hyaluronidase type IV for 30 min with gentle inversion. DNAse I was then added at a concentration of 0.1 mg/mL and incubated for an additional 10 min. The mixture was then filtered over a 100 μm filter and the resulting supernatant spun at 600 ×g for 3 min. The pellet was then suspended in a buffer containing 250 mM sucrose and 10 mM Triethanolamine and spun again. The pellet was then suspended in Hanks Buffered Salt Solution (HBSS—10 mM HEPES pH 7.4) and spun at 600 ×g for 5 min.
Endothelin-1 ELISA
ET-1 peptide levels were determined as previously described (Stow et al., 2012). Cytosolic extracts were isolated using NE-PER kit (Pierce). Immunoreactive ET-1 peptide was detected by chemiluminescent ELISA (R&D Systems) and normalized to total protein content as determined by BCA assay (Pierce).
RNA isolation and qPCR
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer’s instructions. RNA (10 μg) was treated with DNA-free DNaseI (Ambion). DNaseI-treated RNA (2 μg) samples were used as template for reverse transcription with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resulting cDNAs (20 ng) were then used as template in quantitative real-time PCR (qPCR) reactions (Applied Biosystems) to evaluate changes in ET-1, ETA, and ETB mRNA levels. Cycle threshold (Ct) values were normalized against β-actin and relative quantification was performed using the ΔΔCt method (Livak and Schmittgen, 2001). Fold change values were calculated as the change in mRNA expression levels relative to the control. TaqMan primer/probe sets were purchased from Applied Biosystems.
Statistical analysis
All data are represented as mean ± standard error of the mean (SEM). Statistics were performed with Graphpad Prism v6. All graphs and plots were made with Graphpad Prism v6. The effects of time and genotype were analyzed by two-way ANOVA with post-hoc Student– Newman–Keuls test. All p values less than 0.05 were considered significant.
Results
Per1 regulates ET-1 expression but not ETA and ETB mRNA expression in the kidney
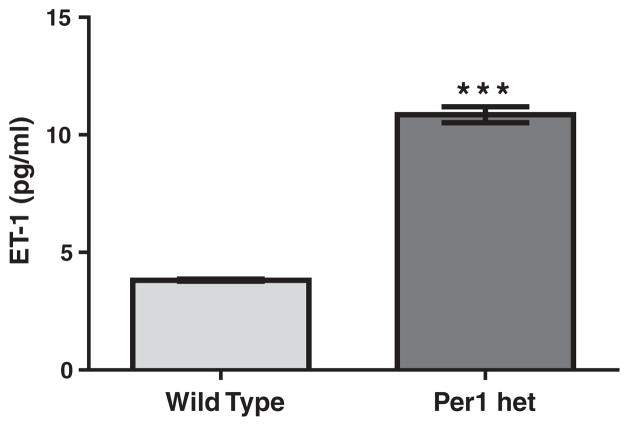
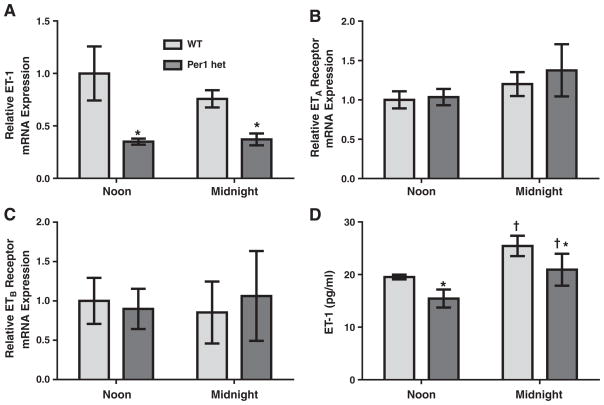
We have previously shown that ET-1 peptide expression varies with time in murine renal cortex and inner medulla, with peak expression during the inactive period. In mice completely lacking the Per1 protein (Per1 knockout), renal ET-1 levels were increased (Stow et al., 2012). We have previously shown that Per1 heterozygous (het) mice have an approximate 50% reduction in Per1 protein expression in the kidney and liver (Richards et al., 2013) and that these mice exhibit a renal sodium wasting phenotype and reduced plasma aldosterone levels (Richards et al., 2013). To determine the effect of reduced Per1 expression on ET-1 expression, renal ET-1 peptide levels were measured in Per1 hetmice and compared to wild type (WT). ELISA was used to measure ET-1 peptide levels ex vivo in inner medullary collecting ducts (IMCD) isolated from WT and Per1 het mice at midnight. ET-1 peptide levels were significantly higher in IMCD from Per1 het mice than WT mice (Fig. 1).
Fig. 1.
ET-1 peptide levels are elevated in IMCDs of mice with reduced levels of Per1. Wild type (WT) (light bars) and Per1 het (dark bars) mice were euthanized at midnight. IMCDs were isolated as described in the Materials and methods. ELISA was used to measure ET-1 peptide levels in WT and Per1 het mice. Data are presented relative to WT, ±SEM. ***P < 0.05 vs. genotype. N = 3.
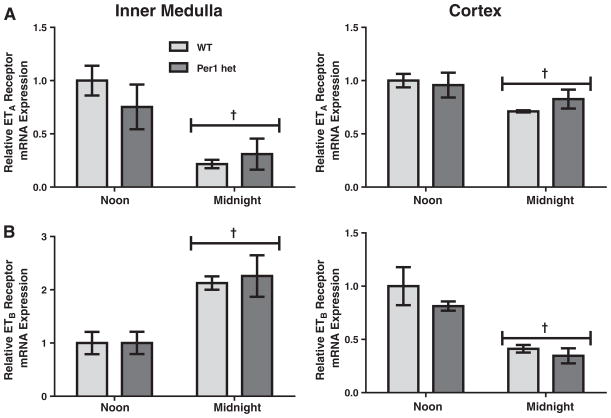
To determine the effects of time and reduced Per1 protein levels on expression of the endothelin receptors, mRNA levels of ETA and ETB were assessed by qPCR in WT and Per1 het mice at noon and midnight, the middle of murine rest and active phases, respectively. In wild-type mice, ETA mRNA levels were significantly lower at midnight compared to noon in both the renal cortex and inner medulla (Fig. 2A), similar to the timed regulation of ET-1 peptide levels that we have previously observed (Stow et al., 2012). ETB mRNA levels also changed with time; however, the pattern was remarkably different between renal inner medulla and renal cortex (Fig. 2B). ETB mRNA levels were significantly higher at midnight in the renal inner medulla, but lower in the renal cortex at the same time. Per1 het mice had similar patterns of ETA and ETB mRNA expression with no apparent differences when compared to WT.
Fig. 2.
Time-dependent regulation of ETA and ETB mRNA expressions in the renal inner medulla and cortex. Wild type (light bars) or Per1 het (dark bars) mice were euthanized at noon or midnight. Total RNA was isolated from dissected inner medulla and cortex and converted to cDNA. Real time quantitative RT-PCR (qPCR)was performed to evaluate mRNA expressions of ETA (Panel A) and ETB (Panel B). Data are presented relative to the WT at noon, ±SEM. †P < 0.05 vs. time, n= 3–4.
Timed regulation and Per1-dependence of ET-1, ETA, and ETB mRNA expression in other tissues
To examine timed regulation of the endothelin axis in non-renal tissues, mRNA levels of ET-1, ETA, and ETB were assessed in WT and Per1 het mice at noon and midnight in the heart, liver, and lung. In contrast to what was seen in the kidney, ET-1 mRNA levels in heart tissue from WT mice were significantly higher at midnight than at noon (Fig. 3A). Interestingly, the Per1 het mice exhibited elevated levels of ET-1 mRNA at both time points. ETA mRNA levels mirrored ET-1 mRNA in both genotypes (Fig. 3B). ETB mRNA levels remained constant at both time points for either genotype (Fig. 3C).
Fig. 3.
Regulation of ET-1, ETA and ETB mRNA expression in the heart by time and Per1. Wild type or Per1 hetmice were euthanized at noon or midnight. Total RNA was isolated from the heart and converted to cDNA. Real time quantitative RT-PCR (qPCR)was performed to evaluate in ET-1 (Panel A), ETA (Panel B), and ETB (Panel C). Data are presented relative to the WT at noon, ±SEM. *P < 0.05 vs. genotype; †P < 0.05 vs. time, n= 3–4.
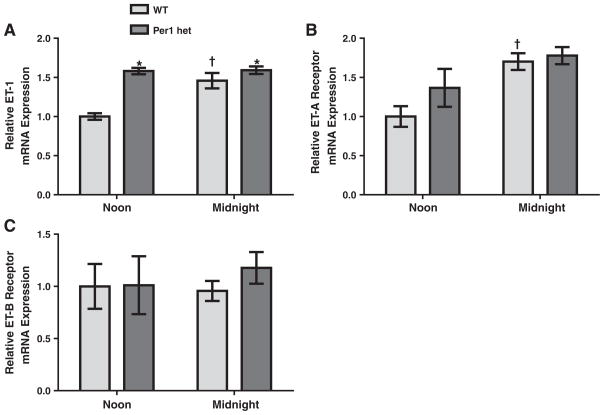
In the liver, ET-1 mRNA levels in WT mice were slightly higher at midnight than at noon (Fig. 4A). This variation in ET-1 mRNA levels with time was abolished in the Per1 het mice, but amore dramatic effect was a substantial increase in ET-1 mRNA levels around the clock compared to WT. ETA mRNA levels were significantly lower at midnight in WT mice when compared to noon (Fig. 4B), and this effect was lost in Per1 het mice. Conversely, ETB mRNA levels were significantly higher at midnight when compared to noon in both genotypes (Fig. 4C).
Fig. 4.
Regulation of ET-1, ETA, and ETB mRNA expressions in the liver by time and Per1. Wild type or Per1 hetmice were euthanized at noon or midnight. Total RNA was isolated from the liver and converted to cDNA. Real time quantitative RT-PCR (QPCR) was performed to evaluate expression of ET-1 (Panel A), ETA (Panel B), and ETB (Panel C). Data are presented relative to the WT at noon, ±SEM. *P < 0.05 vs. genotype; †P < 0.05 vs. time; ϕ = significant interaction by 2-way ANOVA, n= 3–4.
In WT mice lungs, ET-1, ETA and ETB mRNA levels did not significantly change between noon and midnight (Fig. 5). Interestingly, Per1 het mice exhibited significantly lower ET-1mRNA levels at both time points compared to WT mice (Fig. 5A), but no differences were observed in ETA and ETB mRNA levels (Fig. 5B and C). ET-1 peptide levels were measured in lung samples from WT and Per1 het mice euthanized at noon and midnight. ET-1 peptide levels were significantly lower in Per1 het mice at both time points (Fig. 5D) a result that corresponds to the changes observed at the mRNA level. Interestingly, WT ET-1 peptide levels in the lung increased significantly at midnight relative to noon.
Fig. 5.
Regulation of the endothelin axis in the lung by time and Per1. Wild type or Per1 het mice were euthanized at noon or midnight. Total RNA was isolated from the lung and converted to cDNA. Real time quantitative RT-PCR (qPCR) was performed to evaluate expressions of ET-1 (Panel A), ETA (Panel B), and ETB (Panel C). Data are presented relative to the WT at noon, ±SEM. *P < 0.05 vs. genotype; †P < 0.05 vs. time, n=3–4 (Panel D). ELISA was used to measure ET-1 peptide levels in the lung of WT and Per1 hetmice at noon and midnight±SEM. Data were analyzed using 2-way ANOVA, with significant genotype (*P < 0.05) and time (†P < 0.05) effects. There was no significant interaction between genotype and time. N = 3.
Tissue-specific differences in relative expression levels of ETA and ETB mRNA
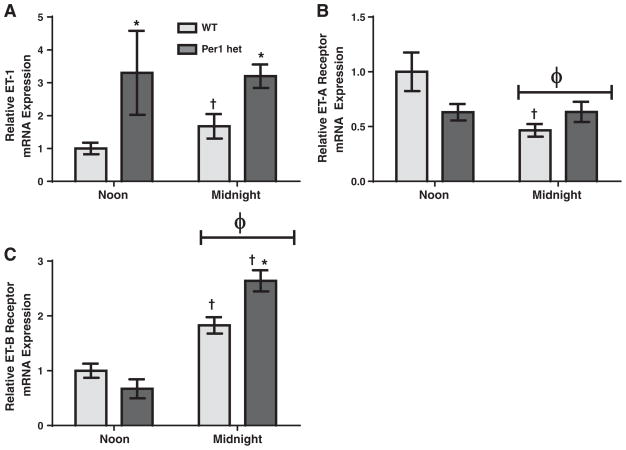
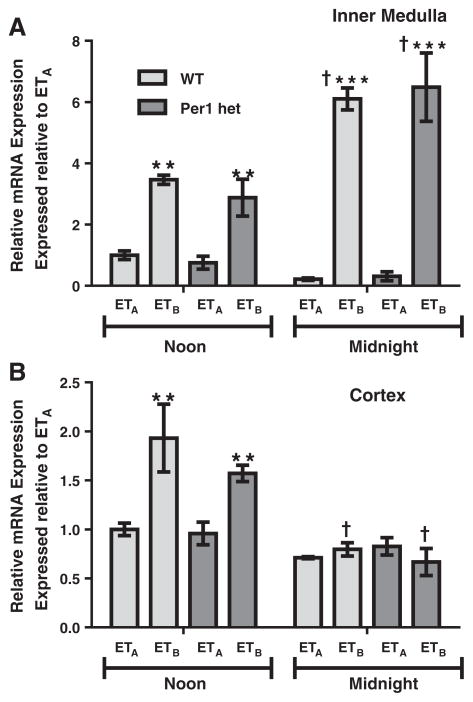
Both ETA and ETB are G protein-coupled receptors, but mediate distinct physiological responses. ETA is mostly associated with vasoconstriction, whereas ETB is normally associated with vasodilation (reviewed in Kohan et al., 2011). Therefore, the time-dependent relative levels of ETA and ETB would be informative for better understanding of ET-1 physiological action. We re-evaluated ETB mRNA expression relative to ETA in each tested tissue type to ascertain relative differences in receptor expression. In the renal inner medulla, mRNA levels of ETB were more than three-fold higher compared to ETA at noon for both genotypes and this difference jumped to six-fold at midnight (Fig. 6A). In the renal cortex at noon, ETB mRNA levels were only about two-fold higher than ETA (Fig. 6B). This difference was not apparent at midnight.
Fig. 6.
Relative differences between ETA and ETB mRNA expressions in renal inner medulla and cortex. Wild type or Per1 het mice were euthanized at noon or midnight. RNA was extracted and processed as described in Fig. 1. Real time quantitative RT-PCR (qPCR) was performed to evaluate relative differences in ETA and ETB expressions in renal inner medulla (Panel A) and cortex (Panel B). Data are presented relative to the WT ETA at noon, ±SEM. **p < 0.01, ***p < 0.001 vs. ETA; †P < 0.05 vs. time, n= 3–4.
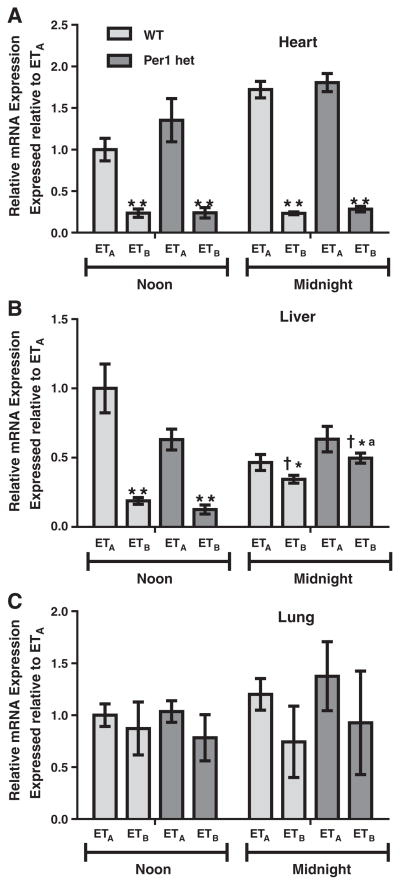
In contrast to the kidney, ETB mRNA expression in the heart is significantly lower than ETA at both noon and midnight (Fig. 7A). There did not appear to be an effect of reduced Per1 expression on the relative expression of the receptors in the heart. Similarly in the liver, significantly lower ETB mRNA levels were observed (Fig. 7B). However, this relative difference appeared to be Per1-dependent and was significantly decreased at midnight compared to noon. In the lung, there were no significant differences between ETA and ETB mRNA levels based on either time or genotype (Fig. 7C).
Fig. 7.
Relative differences between ETA and ETB mRNA expressions in the heart, liver and lung. Wild type or Per1 hetmice were euthanized at noon or midnight. RNA was extracted and processed as described in Figs. 2–4. Real time quantitative RT-PCR (qPCR) was performed to evaluate relative differences in ETA and ETB expressions in the heart (Panel A), liver (Panel B), and lung (Panel C). Data are presented relative to the WT ETA at noon, ±SEM. *P < 0.05, **p < 0.01 vs. ETA; †P < 0.05 vs. time; P < 0.05 vs. genotype n = 3–4.
Discussion
The purpose of this study was to investigate the role of Per1 and time in the regulation of the endothelin axis in the kidney, heart, liver, and lung. Our investigation of mRNA expression in whole tissue samples clearly showed that tissue-specific and time-dependent regulation of ET-1, ETA, and ETB mRNAs occurs. We observed patterns of time-dependent and Per1-mediated regulation of the endothelin axis that were unique to each tissue type tested.
Recent work on the role of the endothelin axis in the kidney sheds light onto the novel function ET-1 plays in the regulation of sodium transport in the distal nephron. ET-1 is a potent inhibitor of ENaC activity via both the ETA and ETB receptors (Bugaj et al., 2012; Ge et al., 2008; Lynch et al., 2013), presumably through a nitric oxide-cyclic GMP-dependent mechanism (Bugaj et al., 2008; Stricklett et al., 2006). We have previously shown that ET-1 peptide levels vary in a time-dependent manner, peaking at noon, and that Per1 KO animals have increased ET-1 peptide in the renal cortex and inner medulla (Stow et al., 2012). In the present study, in both the renal cortex and inner medulla, ETA mRNA was higher at noon than at midnight, coinciding with our previous report that ET-1 peptide levels were higher at noon than at midnight in mice (Stowet al., 2012). In contrast, ETB mRNA levels exhibit opposite expression patterns in the inner medulla and cortex; ETB mRNA levels are higher at midnight in the inner medulla, but lower in the cortex. However, ETB and ETA mRNA expression did not appear to be affected by reduced levels of Per1. Although the receptors do not appear to be regulated in a Per1-dependent manner in this tissue, the timed regulation of ETA and ETB receptor expressions in the kidney should be investigated further to determine if there is a role for the ET receptors in the circadian control of sodium regulation.
ETB mRNA levels are much higher than ETA mRNA levels in both the renal inner medulla and cortex, and this observation is supported by previous reports using positron emission tomography which demonstrated that ET-1 binding to ETB was significantly higher than ETA binding in the rat kidney (Johnstrom et al., 2005). Also, a recent study demonstrated that renal collecting duct-specific ETA KO mice have complete blunting of ETA antagonist-mediated fluid retention (Stuart et al., 2013). This effect was not seen in cardiac-specific ETA KO and was only partially evident in vascular smooth muscle-specific ETA KO, confirming the tissue-specific expression/action of the receptor types.
In the heart, ET-1 and ETA mRNA levels from WT mice were elevated at midnight as compared to noon. ETA was found to be much higher than ETB at all times. Activation of ETA is primarily associated with increased contractility (MacCarthy et al., 2000), most likely through increases in intracellular calcium concentration (Talukder et al., 2001). It is possible that the increased ETA levels at midnight act as a positive ionotrope for the increased activity during this time period.
ET-1 has also been shown to induce cardiac hypertrophy, presumably through an ERK1/2-dependent cascade (reviewed in Vignon-Zellweger et al., 2012). Recent findings have suggested that ETB may play a role in this process as well. Cardiomyocyte-specific ETA KO mice develop similar cardiac hypertrophy in response to angiotensin II infusion as WT controls (Kedzierski et al., 2003). Moreover, a recent study demonstrated that activation of the nuclear-membrane bound form of the ETB receptor by intracellular ET-1 can result in inositol 1,4,5-triphosphate (IP3) receptor-mediated calcium release, which is implicated in cardiachypertrophy (Merlen et al., 2013). Here ETB mRNA was found to be constant and independent of circadian control. In contrast, both ET-1 and ETA mRNAs were significantly increased in Per1 hetmice at both time points tested, suggesting loss of a negative regulatory mechanism. It should be noted that ETA mRNA was significantly higher than ETB mRNA expression, corroborating a previous study in rats (Fareh et al., 1996). These findings may imply that an alteration in the ETA:ETB ratio, possibly through defects in negative regulatory mechanisms as in this study, or increased trafficking of the ETB receptor to the nuclear membrane could be involved in cardiac hypertrophy.
Much less is known about the physiological role of the endothelin axis in the liver. However, its role in liver pathogenesis is more apparent. ET-1 can promote activation of hepatic stellate cells (HSC), leading to increased cell proliferation, contraction, and survival (Pinzani et al., 1996; Rockey et al., 1998). HSCs, which express both ETA and ETB receptors, are pericytes found in the perisinusoidal space that once activated, lead to liver fibrosis and injury (reviewed in Yin et al., 2013) (Housset et al., 1993; Pinzani et al., 1996). Angiotensin II has been shown to induce ET-1 production in HSCs (He et al., 2013). Another recent study demonstrated that interferon-gamma, a cytokine produced by T-cells that was previously shown to inhibit HSC proliferation and fibrogenesis, negatively regulates ET-1 expression (Li et al., 2012).
In normal liver function, ET-1 may act through an ETB-nitric oxide-dependent mechanism to increase expression of critical bile secretory genes and regulate choleresis, the secretion of bile into the gallbladder (Rodriguez et al., 2013). Here liver ET-1 mRNA increased somewhat at midnight and was substantially elevated in the livers of Per1 het mice at both time points. It will be interesting to see if Per1 knockout mice have an increased incidence of liver fibrosis or deregulation of bile secretion.
In the lung, it has been demonstrated that ETA receptor activation of pulmonary vascular smooth muscle cells can induce vasoconstriction through a mechanism involving IP3 and calcium (Takuwa et al., 1990), similar to what was observed in the heart. ET-1 was also linked to increasing proliferation of pulmonary vascular smooth muscle cells, through both the ETA and ETB receptors (Chua et al., 1992). However, deregulation of these processes contributes to pathophysiological states, including pulmonary arterial hypertension (PAH) (reviewed in Rubin, 2012). Patients with spontaneous PAH have increased levels of ET-1 in both serum and lung tissue compared to healthy controls (Giaid et al., 1993; Stewart et al., 1991). There are two commercially available endothelin receptor antagonists, ambrisentan and bosentan. Clinical trials have demonstrated the potential therapeutic benefits of these drugs in the treatment of PAH (reviewed in Seferian and Simonneau, 2013). Recently, a clinical trial demonstrated that a new dual ETA and ETB receptor antagonist, macitentan, led to significantly reduced morbidity and mortality among patients with pulmonary arterial hypertension (Pulido et al., 2013). Neither ETA nor ETB mRNA level varied with time of day in WT or Per1 het mice. However, the Per1 het mice did have significantly lower lung ET-1 mRNA expression at both noon and midnight, when compared to WT controls. Although time-dependent regulation was not apparent, reduced Per1 expression led to decreased ET-1 mRNA in the lung. It should be noted that this was a whole lung preparation. It would be interesting to examine the circadian pattern of ET-1 in just the pulmonary artery. Circadian regulation of the ET receptors in this tissue type could be important when timing drug administration for pulmonary arterial hypertension.
It is important to note that work over the last decade has begun to demonstrate potential “cross-talk” between the ETA and ETB receptors, which has arisen based on inhibitory studies that demonstrated inhibition of one of the receptors, which led to compensatory actions of the other (reviewed in Rapoport and Zuccarello, 2011). Multiple studies have shown the potential for ETA and ETB to hetero-dimerize, and that this interaction could be important for the observed “cross-talk” phenomena (reviewed in Watts, 2010). Relative receptor expression levels at a given time of day may affect the study of endothelin receptor cross-talk.
Limitations
The goal of this study was to determine the role of time and Per1 in the regulation of ET-1, ETA and ETB mRNA expression. With the exception of our demonstration that ET-1 peptide levels were significantly increased in IMCD cells but significantly decreased in lung tissue from Per1 het mice relative to WT control mice, our results are focused on changes in mRNA expression. Future studies are needed to determine if the effects of time and Per1 expression on the endothelin axis extend to the level of protein. Determination of relative protein expression at additional time points will be necessary in order to better understand the role of circadian rhythms in the regulation of ET-1, ETA and ETB. Nevertheless, the results of the present study support the hypothesis that Per1 contributes to the regulation of the endothelin axis in a tissue-specific and time-dependent manner.
Conclusions
Results of the present study demonstrate that mRNA expression of the endothelin axis is regulated by Per1 in a tissue-specific and time-dependent manner. Presently, there is increasing interest in targeting the endothelin axis for the treatment of a variety of disorders, such as what is currently being done for the treatment of PAH. Multiple studies have demonstrated the benefit of chrono-pharmacotherapy, or timed administration of drugs to increase efficacy and decrease off-target effects. For example, it has been shown that chrono-pharmacotherapy in the treatment of hypertension leads to a decrease in adverse cardiovascular events (reviewed in Richards and Gumz, 2012). The results presented here suggest that optimization of the best time of day to deliver endothelin receptor antagonists should be considered.
Acknowledgments
This work was supported by NIH DK085193 and DK098460, the ASN Foundation for Kidney Research to MLG, AHA predoctoral fellowship 13PRE16910096 to JR, 2T32HL083810 to AKW, and NIH DK082680 to BDC and CSW.
Footnotes
Conflict of interest statement
None.
References
- Albrecht U, Eichele G. The mammalian circadian clock. Curr Opin Genet Dev. 2003;13:271–7. doi: 10.1016/s0959-437x(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–36. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–70. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol. 2012;302:C188–94. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BH, Krebs CJ, Chua CC, Diglio CA. Endothelin stimulates protein synthesis in smooth muscle cells. Am J Physiol. 1992;262:E412–6. doi: 10.1152/ajpendo.1992.262.4.E412. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Fareh J, Touyz RM, Schiffrin EL, Thibault G. Endothelin-1 and angiotensin II receptors in cells from rat hypertrophied heart. Receptor regulation and intracellular Ca2+ modulation. Circ Res. 1996;78:302–11. doi: 10.1161/01.res.78.2.302. [DOI] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol. 2006;291:F1274–80. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol. 2008;295:F1635–40. doi: 10.1152/ajprenal.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- He C, Miao X, Li J, Qi H. Angiotensin II induces endothelin-1 expression in human hepatic stellate cells. Dig Dis Sci. 2013;58:2542–9. doi: 10.1007/s10620-013-2685-y. [DOI] [PubMed] [Google Scholar]
- Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci U S A. 1993;90:9266–70. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ME, Wingo CS, Cain BD. An emerging role for microRNA in the regulation of endothelin-1. Front Physiol. 2013;4:22. doi: 10.3389/fphys.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstrom P, Fryer TD, Richards HK, Harris NG, Barret O, Clark JC, et al. Positron emission tomography using 18F-labelled endothelin-1 reveals prevention of binding to cardiac receptors owing to tissue-specific clearance by ET B receptors in vivo. Br J Pharmacol. 2005;144:115–22. doi: 10.1038/sj.bjp.0706064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Grayburn PA, Kisanuki YY, Williams CS, Hammer RE, Richardson JA, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23:8226–32. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE. Role of collecting duct endothelin in control of renal function and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2013;305:R659–68. doi: 10.1152/ajpregu.00345.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Shi Z, Rockey DC. Preproendothelin-1 expression is negatively regulated by IFNgamma during hepatic stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G948–57. doi: 10.1152/ajpgi.00359.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynch IJ, Welch AK, Kohan DE, Cain BD, Wingo CS. Endothelin-1 inhibits sodium reabsorption by ETA and ETB receptors in the mouse cortical collecting duct. Am J Physiol. 2013;305:F568–73. doi: 10.1152/ajprenal.00613.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCarthy PA, Grocott-Mason R, Prendergast BD, Shah AM. Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: studies with an intracoronary ET(A) receptor antagonist. Circulation. 2000;101:142–7. doi: 10.1161/01.cir.101.2.142. [DOI] [PubMed] [Google Scholar]
- Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR, et al. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca(2)(+) in adult cardiac myocytes. J Mol Cell Cardiol. 2013;62:189–202. doi: 10.1016/j.yjmcc.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–48. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- Rapoport RM, Zuccarello M. Endothelin(A)-endothelin(B) receptor cross-talk and endothelin receptor binding. J Pharm Pharmacol. 2011;63:1373–7. doi: 10.1111/j.2042-7158.2011.01334.x. [DOI] [PubMed] [Google Scholar]
- Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J. 2012;26:3602–13. doi: 10.1096/fj.12-203554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol. 2013;304:R1053–64. doi: 10.1152/ajpregu.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, All SC, Skopis G, Cheng KY, Compton B, Srialluri N, et al. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. Am J Physiol Regul Integr Comp Physiol. 2013;305:R735–47. doi: 10.1152/ajpregu.00195.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, et al. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–80. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- Rodriguez MR, Soria LR, Ventimiglia MS, Najenson AC, Di Maria A, Dabas P, et al. Endothelin-1 and -3 induce choleresis in the rat through ETB receptors coupled to nitric oxide and vagovagal reflexes. Clin Sci (Lond) 2013;125:521–32. doi: 10.1042/CS20120633. [DOI] [PubMed] [Google Scholar]
- Rubin LJ. Endothelin receptor antagonists for the treatment of pulmonary artery hypertension. Life Sci. 2012;91:517–21. doi: 10.1016/j.lfs.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Seferian A, Simonneau G. Therapies for pulmonary arterial hypertension: where are we today, where do we go tomorrow? Eur Respir Rev. 2013;22:217–26. doi: 10.1183/09059180.00001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension:marker ormediator of disease? Ann Intern Med. 1991;114:464–9. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Gumz ML, Lynch IJ, Greenlee MM, Rudin A, Cain BD, et al. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (Edn1) J Biol Chem. 2009;284:30087–96. doi: 10.1074/jbc.M109.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J. 2011;25:16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, et al. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59:1151–6. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait KA, Stricklett PK, Kohan RM, Kohan DE. Identification of two nuclear factor of activated T-cells (NFAT)-response elements in the 5′-upstream regulatory region of the ET-1 promoter. J Biol Chem. 2010;285:28520–8. doi: 10.1074/jbc.M110.153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol. 2006;290:F1315–9. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- Stuart D, Chapman M, Rees S, Woodward S, Kohan DE. Myocardial, smooth muscle, nephron, and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346:182–9. doi: 10.1124/jpet.113.205286. [DOI] [PubMed] [Google Scholar]
- Takuwa Y, Kasuya Y, Takuwa N, Kudo M, Yanagisawa M, Goto K, et al. Endothelin receptor is coupled to phospholipase C via a pertussis toxin-insensitive guanine nucleotide-binding regulatory protein in vascular smooth muscle cells. J Clin Invest. 1990;85:653–8. doi: 10.1172/JCI114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder MA, Norota I, Sakurai K, Endoh M. Inotropic response of rabbit ventricular myocytes to endothelin-1: difference from isolated papillary muscles. Am J Physiol Heart Circ Physiol. 2001;281:H596–605. doi: 10.1152/ajpheart.2001.281.2.H596. [DOI] [PubMed] [Google Scholar]
- Vignon-Zellweger N, Heiden S, Miyauchi T, Emoto N. Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci. 2012;91:490–500. doi: 10.1016/j.lfs.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Watts SW. Endothelin receptors: what’s new and what do we need to know? Am J Physiol Regul Integr Comp Physiol. 2010;298:R254–60. doi: 10.1152/ajpregu.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch AK, Jacobs ME, Wingo CS, Cain BD. Early progress in epigenetic regulation of endothelin pathway genes. Br J Pharmacol. 2013;168:327–34. doi: 10.1111/j.1476-5381.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–10. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]