Abstract
Occupational exposure to aerosolized particles of oil-based metalworking fluid was recently linked to deaths from ischemic heart disease. The current recommended exposure limits might be insufficient. Studying cardiovascular mortality is challenging because symptoms can induce sicker workers to reduce their exposure, causing healthy-worker survivor bias. G-estimation of accelerated failure time models reduces this bias and permits comparison of multiple exposure interventions. Michigan autoworkers from the United AutoWorkers–General Motors cohort (n = 38,666) were followed from 1941 through 1994. Separate binary variables indicated whether annual exposure exceeded a series of potential limits. Separate g-estimation analyses for each limit yielded the total number of life-years that could have been saved among persons who died from specific cardiovascular causes by enforcing that exposure limit. Banning oil-based fluids would have saved an estimated 4,003 (95% confidence interval: 2,200, 5,807) life-years among those who died of ischemic heart disease. Estimates for cardiovascular disease overall, acute myocardial infarction, and cerebrovascular disease were 3,500 (95% confidence interval: 1,350, 5,651), 2,932 (95% confidence interval: 1,587, 4,277), and 917 (95% confidence interval: −80, 1,913) life-years, respectively. A limit of 0.01 mg/m3 would have had a similar impact on cerebrovascular disease but one only half as great on ischemic heart disease. Analyses suggest that limiting exposure to metalworking fluids could have saved many life-years lost to cardiovascular diseases in this cohort.
Keywords: cardiovascular mortality, epidemiologic methods, healthy worker effect, occupational exposures, particulate matter
Editor's note:An invited commentary on this article appears on page 571.
Both long-term and recent short-term exposures to ambient particulate air pollution are known to cause cardiovascular disease and death. Cardiovascular outcomes are specifically linked to fossil fuel combustion and the particles it produces in the fine size fraction (particulate matter with an aerodynamic diameter of 2.5 µm or less (PM2.5)), which can penetrate the alveoli, and the ultrafine size fraction (aerodynamic diameter less than 0.1 µm), which can bypass macrophages and enter the bloodstream (1, 2). Three biologic pathways, which probably co-occur, have been established: pulmonary oxidative stress leading to systemic inflammatory response; activation of receptors in the lungs that connect to the autonomous nervous system resulting in changes to the vasculature, blood, and heart function; and direct interaction between ultrafine particles and blood cells or the endothelium (2). In a recent systematic review and meta-analysis, Hoek et al. (3) estimated a relative risk of 1.15 (95% confidence interval: 1.04, 1.27) for each 10-µg/m3 increase in long-term PM2.5 concentration.
Workplace exposures to particulate matter (PM) often occur at concentrations an order of magnitude higher than ambient levels, but occupational epidemiology has not historically focused on cardiovascular outcomes (4). In many industrial settings, PM with an aerodynamic diameter less than 3.5 µm, rather than PM2.5, is measured as an estimate of the alveolar fraction (5). One source of occupational exposure to PM is the spraying of metalworking fluids to cool and lubricate machining operations such as cutting and grinding. Although combustion does not occur during these processes, the fluids become hot and aerosolize, creating PM that workers inhale. Oil-based (straight) metalworking fluids are made from petroleum and potentially contain polycyclic aromatic hydrocarbons. Metals are contaminants of these fluids (6), and the machining process additionally creates metal particles that might dissolve and accumulate in the fluids (7–9). The (solid and liquid) particles generated by these machining processes thus share many properties with traffic-related PM, as both include alveolar and ultrafine particles that contain polycyclic aromatic hydrocarbons and metals (10).
One obstacle to detecting an effect of occupational PM on cardiovascular outcomes is the fact that symptoms might cause workers to leave their jobs or reduce their exposures while at work, leading to an apparent protective or null association when data are analyzed using traditional regression methods (11). This healthy-worker survivor effect can be overcome (when appropriate variables are measured) by analyzing the data using g-estimation or the parametric g-formula (12, 13). In the present article, we focused on g-estimation of a structural nested model.
In the first application of g-estimation to an occupational study, Chevrier et al. (14) compared its results to those obtained using traditional Cox models. The analyses concerned relationships between duration of exposure to straight metalworking fluids and several mortality outcomes in a cohort of automobile-manufacturing workers. One striking result was the contrast between the null findings when using a Cox model to examine the association between straight metalworking fluids and ischemic heart disease (IHD) and the hazard ratio (for 5 years of exposure) of 1.15 (95% confidence interval: 1.11, 1.19) when using g-estimation of an accelerated failure time model.
The ultimate goal of occupational epidemiology is prevention: to determine the health effects caused by specific levels of workplace exposures so that appropriate limits can be adopted to protect workers. In 1998, the National Institute for Occupational Safety and Health recommended an exposure limit of 0.4 mg/m3 (thoracic particulate mass) for metalworking fluids (15). However, this limit is unenforceable. Furthermore, the Occupational Safety and Health Administration's 2001 decision to not set a standard did not take into account more recent scientific evidence (16). In the present study, we expanded on prior studies of autoworkers by taking advantage of the available quantitative annual exposure information to consider several cardiovascular mortality outcomes from a public health (rather than etiological) perspective. Effect estimates are presented as the numbers of years of life that could have been saved by enforcing hypothetical workplace exposure limits (17).
METHODS
The United AutoWorkers–General Motors cohort included all workers at 3 General Motors plants in Michigan (Detroit, Ypsilanti, and Saginaw) with a hire date between 1938 and 1982 and an employment duration of at least 3 years (18). For our analysis, follow-up of 38,666 workers began 3 years after they were hired and continued regardless of employment status until the earliest of 3 possible end points: death, the end of 1994, or age 95 years. Outcomes studied included all-cause mortality (all International Classification of Disease, Ninth Revision codes) and deaths due to cardiovascular disease (codes 390–459), IHD (codes 410–414), acute myocardial infarction (AMI; code 410), and cerebrovascular disease (codes 430–438).
Exposures and intermittent time off work were determined by combining detailed employment records with a job-exposure matrix developed in an extensive exposure assessment (18, 19). Industrial hygienists measured exposures to 3 types of metalworking fluids (straight oils, soluble oils, and synthetic fluids) as PM in several size fractions. Here, PM with a diameter of 3.5 µm or less composed of straight metalworking fluids was analyzed as the exposure of interest, with the other 2 fluid types treated as potential confounders.
Statistical methods
For each outcome, we followed the approach introduced by Picciotto et al. (17). We created a series of binary exposure variables, each equal to 0 if the quantitative exposure was below a specified cutoff value and 1 if the exposure exceeded the cutoff. Each binary exposure variable corresponds to an intervention in which the annual average daily exposure never exceeds the cutoff. G-estimation is then run separately for each binary exposure variable. The structural accelerated failure time model relates the observed survival time and the observed duration of exposure above the cutoff to the counterfactual survival time that would have been observed if never exposed (above the cutoff). This relationship is quantified by an unknown coefficient to be estimated (see Web Appendix 1, available at http://aje.oxfordjournals.org/).
Unlike traditional regression, g-estimation can adjust correctly for time-varying confounders that are affected by prior exposure (12). In our cohort, employment status and leaves of absence are variables that, in standard analyses, would result in bias whether or not they were included in regression models predicting the outcome. By contrast, g-estimation adjusts for confounders by including them in a model that predicts exposure. All studies, regardless of statistical method, require the assumption that we have measured all confounders, that is, that the variables measured are sufficient to create strata in which survival time if never exposed is statistically independent of observed exposure. G-estimation uses optimization procedures to estimate the value of the coefficient that achieves this statistical independence. For details, see Web Appendix 1 and Web Table 1 (14, 20, 21).
For each person who experienced the outcome of interest (case), the coefficient estimate for a cutoff is transformed into an estimate of the individual's survival time under the corresponding intervention (see Web Appendix 1) (17). The difference between this counterfactual survival time and the observed survival time represents the estimated number of years of that person's life that would have been saved under the intervention, assuming that he or she did not die of some other cause during this interval. The sum (over all cases) of these years of life saved represents an estimate of the burden of disease attributable to the failure to enforce such an occupational exposure limit in this cohort. Unexposed workers would have been unaffected by any of the interventions and therefore contribute 0 to the total number of years of life saved among the cases. (However, they contribute person-time to the analysis in the g-estimation step.)
For a given outcome, we can compare results from different cutoffs because this measure compares observed outcomes, which are the same for all cutoffs, to counterfactual outcomes under each intervention. Furthermore, this metric is based on the quantity modeled (survival time), so it does not require the additional assumptions that would allow us to convert our results to hazard ratios.
Data analysis
Quantitative annual average daily exposures to straight metalworking fluids were transformed into a series of binary variables, each indicating whether the exposure level exceeded a different cutoff value. Cutoffs were selected based on the exposure distribution: They were closely spaced for the smaller values, where most of the data were concentrated, with a maximum near the 75th percentile of nonzero exposures. We estimated the total number of years of life that would have been saved among the cases if exposure to straight fluids had never been permitted to exceed limits of 0 (i.e., a ban), 0.01, 0.02, 0.03, 0.04, 0.05, 0.10, and 0.15 mg/m3.
We adjusted analyses for the following covariates by including them in the pooled logistic models for exposure: current age, race, sex, manufacturing facility, an indicator for calendar year (before or after 1970, when exposures dramatically decreased (18)), prior exposure history (whether exposure exceeded the cutoff value or not in previous years), prior exposures to the other 2 fluid types, and intermittent time off work (percent of each year). G-estimation also requires adjustment for a variable that represents each individual's maximum observable survival time (equal to the length of time from cohort entry to the end of 1994 or to the worker's 95th birthday, whichever occurs sooner; this quantity is known at cohort entry). Because exposure never occurs after employment termination, this model was fitted only on the actively employed person-time, thus controlling for employment status by conditioning on it. However, outcomes are not censored at termination of employment; the survival times in the structural accelerated failure time model include post-employment time (22).
We adjusted for loss to follow-up (<4%) and censoring by death from competing risks (13% for cardiovascular disease overall, 17% for IHD, 20% for AMI, and 23% for cerebrovascular disease) using inverse probability of censoring weights (see Web Appendix 1) (21). Weights ranged from 0.88 to 1.44.
We ran 200 bootstraps and used the standard deviation of the bootstrap estimates to construct 95% confidence intervals (see Web Appendix 1). Analyses were conducted in SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina). The study was approved by the University of California, Berkeley Committee for the Protection of Human Subjects.
RESULTS
Tables 1 and 2 provide demographic characteristics for the mostly male, mostly white cohort. Approximately 8% of workers who died of cerebrovascular disease were women; only 4% of workers who died of AMI were women.
Table 1.
Demographic and Employment Characteristics of the United AutoWorkers–General Motors Cohort, 1941–1994
| Characteristic | No. | % | Person-Years |
|---|---|---|---|
| Total cohort | 38,666 | 100 | 972,476 |
| Race | |||
| Black | 7,144 | 18 | 169,065 |
| White | 31,522 | 82 | 803,411 |
| Sex | |||
| Male | 33,907 | 88 | 869,585 |
| Female | 4,759 | 12 | 102,891 |
| Manufacturing facility | |||
| 1 | 24 | 151,901 | |
| 2 | 40 | 248,722 | |
| 3 | 36 | 224,545 | |
| Active years of employment with no time taken off | 72 | 447,244 | |
| Cardiovascular disease deaths | 4,153 | 11 | |
| Ischemic heart disease deaths | 2,612 | 7 | |
| Acute myocardial infarction deaths | 1,699 | 4 | |
| Cerebrovascular disease deaths | 501 | 1 | |
| All-cause mortality deaths | 9,539 | 25 |
Table 2.
Additional Demographic and Employment Characteristics of the United AutoWorkers–General Motors Cohort (n = 38,666), 1941–1994
| Characteristic | Mean (SD) |
|---|---|
| Age at baseline, years | 30.8 (9.1) |
| No. of years worked | 16.2 (9.5) |
| Length of follow-up, years | 24.5 (11.2) |
| Age at death from cardiovascular disease, years | 64.9 (12.2) |
| Age at death from ischemic heart disease, years | 64.4 (11.7) |
| Age at death from acute myocardial infarction, years | 62.7 (11.6) |
| Age at death from cerebrovascular disease, years | 67.0 (12.9) |
| Age at death from any cause, years | 62.5 (13.5) |
| Proportion of year taken off (among person-years) | 0.097 (0.244) |
| Proportion of year taken off if >0 (among person-years) | 0.341 (0.354) |
Abbreviation: SD, standard deviation.
Table 3 summarizes the exposure distribution. The average duration of exposure was slightly longer among cases. The distribution of exposure level (among exposed person-years) was mostly low, with a long tail to the right. Exposures exceeded the current recommended limit of 0.4 mg/m3 in a total of 14,949 person-years for 2,732 distinct workers.
Table 3.
Distribution of Exposure to Oil-Based Metalworking Fluids Among Ever-Exposed Workers in the United AutoWorkers–General Motors Cohort (n = 38,666), 1941–1994a
| Exposure Group | No. | % | Mean (SD) |
|---|---|---|---|
| Workers ever exposed | 20,202 | 52 | |
| Exposed person-years | 157,512 | 16 | |
| Duration of exposure, years | 4.1 (6.6) | ||
| Duration of exposure among ever-exposed workers who died of cardiovascular disease, years | 4.3 (6.9) | ||
| Duration of exposure among ever-exposed workers who died of ischemic heart disease, years | 4.5 (7.1) | ||
| Duration of exposure among ever-exposed workers who died of acute myocardial infarction, years | 4.4 (7.0) | ||
| Duration of exposure among ever-exposed workers who died of cerebrovascular disease, years | 4.8 (7.1) | ||
| Duration of exposure among ever-exposed workers who died of any cause, years | 4.2 (6.8) | ||
| Annual average daily exposure among exposed person-years, mg/m3 | 0.2 (0.4) | ||
| Interquartile range of annual average daily exposure, mg/m3 | 0.01, 0.12b | ||
| Median annual average daily exposure among exposed person-years, mg/m3 | 0.03 |
Abbreviation: SD, standard deviation.
a The total number of person-years was 972,476.
b Interquartile range (25th percentile to 75th percentile).
If straight metalworking fluids had been banned, workers who were ever exposed and who died of cardiovascular disease would have lived an average of 1.58 (95% confidence interval: 0.63, 2.52) years longer. On average, IHD deaths would have happened 2.77 (95% confidence interval: 1.56, 3.98) years later, and those who died of AMI would have lived 3.13 (95% confidence interval: 1.74, 4.53) years longer. Deaths from cerebrovascular disease among exposed workers would have occurred a mean of 3.19 (95% confidence interval: −0.14, 6.51) years later.
Figure 1 shows the estimates of the population effects on cardiovascular mortality under the different exposure limits. Although a ban is estimated to have a reasonably strong impact, the magnitude quickly decreases for exposure limits only slightly above 0.
Figure 1.
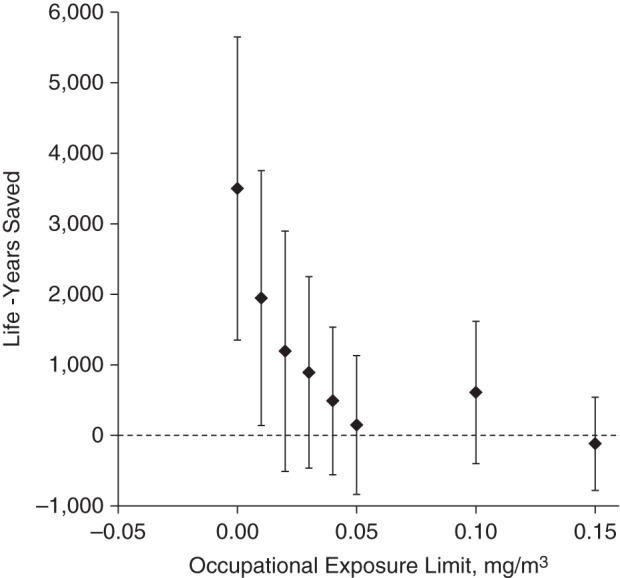
Number of years of life that could have been saved among workers who died of cardiovascular disease by enforcing various occupational exposure limits for oil-based metalworking fluids, United AutoWorkers–General Motors cohort, 1941–1995.
Figure 2 shows estimates of the exposure limits’ impacts on IHD mortality. Although the overall shape is the same as that for cardiovascular disease, all of the limits show higher impacts on IHD, even though there are fewer cases.
Figure 2.
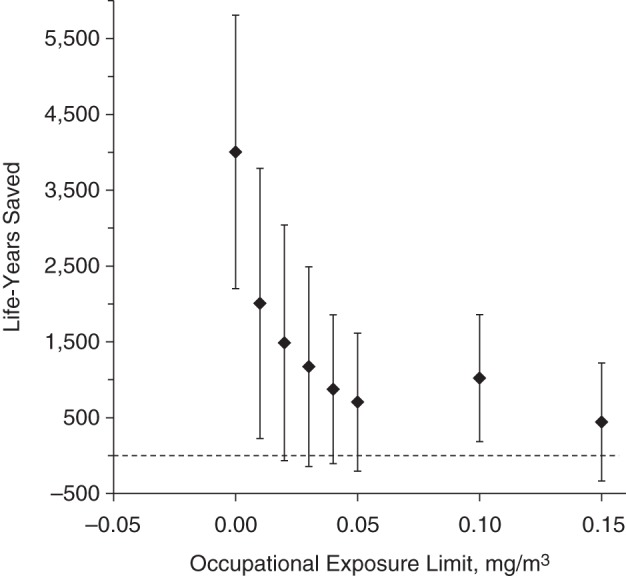
Number of years of life that could have been saved among workers who died of ischemic heart disease by enforcing various occupational exposure limits for oil-based metalworking fluids, United AutoWorkers–General Motors cohort, 1941–1995.
The associations of AMI mortality with exposure (Figure 3) were similar to those for IHD. IHD excluding AMI also showed positive estimates when analyzed separately, whereas cardiovascular death not due to IHD or cerebrovascular disease appeared unrelated to exposures to straight metalworking fluid (data not shown).
Figure 3.
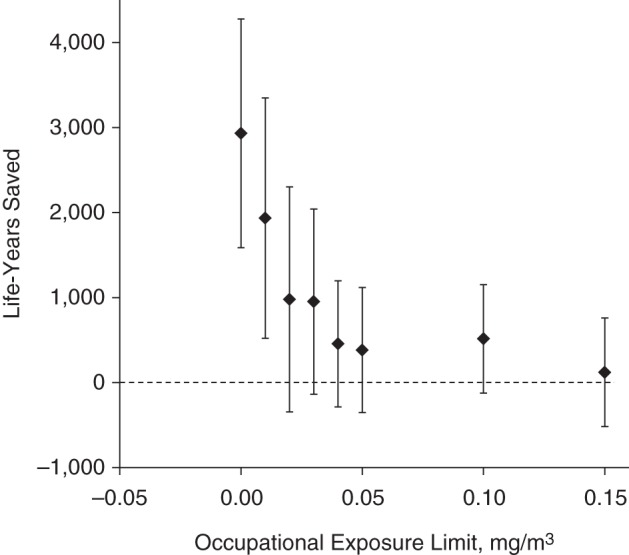
Number of years of life that could have been saved among workers who died of acute myocardial infarction by enforcing various occupational exposure limits for oil-based metalworking fluids, United AutoWorkers–General Motors cohort, 1941–1995.
Figure 4 shows the estimated effects on cerebrovascular mortality. Here, the shape of the curve is different from the shape for the other outcomes. A ban is estimated not to provide substantially more benefit for this outcome than a limit of 0.02 mg/m3, whereas a smaller impact was estimated for a limit of 0.03 mg/m3. For higher exposure limits, the estimated effects drop down to null. Figure 5 shows the estimated effects on all-cause mortality. These larger impacts had narrower confidence intervals.
Figure 4.
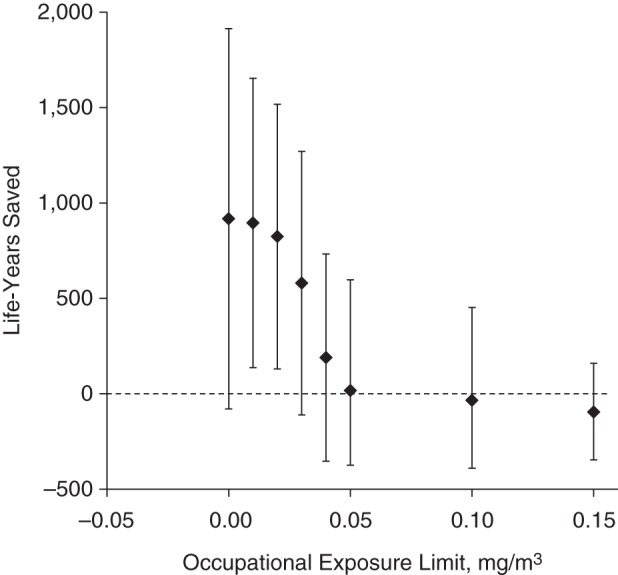
Number of years of life that could have been saved among workers who died of cerebrovascular disease by enforcing various occupational exposure limits for oil-based metalworking fluids, United AutoWorkers–General Motors cohort, 1941–1995.
Figure 5.
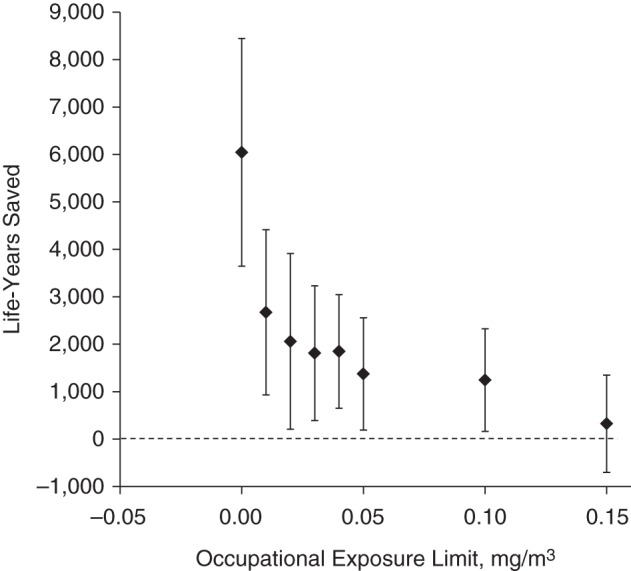
Number of years of life that could have been saved among workers who died of any cause by enforcing various occupational exposure limits for oil-based metalworking fluids, United AutoWorkers–General Motors cohort, 1941–1995.
DISCUSSION
We estimated that reducing the occupational exposure limit for straight metalworking fluids to 0.01 mg/m3 would have saved 2,000 life-years among autoworkers in this cohort who died of cardiovascular disease. Banning straight metalworking fluids would have saved an estimated 3,500 life-years among those who died of cardiovascular disease and 6,047 life-years overall. Thus, approximately 58% of the impact would be attributable to cardiovascular mortality, which only accounted for 44% of all deaths observed. The results suggest that the most life-years could have been saved per person for exposed workers who died of AMI or cerebrovascular disease. As a subcategory of IHD, AMI was the most specific cause of death studied, and the results for IHD and cardiovascular disease mortality were probably driven largely by the relationship of straight metalworking fluids with AMI.
However, the strongest etiological relationship was estimated for cerebrovascular disease mortality. To our knowledge, associations specifically between exposure to metalworking fluids and cerebrovascular disease have only been reported in 1 study. A higher odds ratio for stroke was reported for exposure to soluble metalworking fluids in an analysis restricted to decedents (23). That study presented little data on straight metalworking fluids.
In a systematic review of occupational studies in which the relationships between various kinds of PM and cardiovascular disease were examined, Fang et al. (24) found only 37 studies in a 20-year period. They did not find strong evidence of an effect of exposure on cerebrovascular outcomes, though they did find some for IHD. However, studies in the review considered exposures to several types of PM with different compositions and toxicities, some of which might not be relevant here.
More recently, in an exposure-response analysis of incident IHD among fabrication workers in the aluminum industry (a group similar to the autoworkers considered here), Costello et al. (25) found that persons exposed to higher levels of PM2.5 had a higher risk of IHD over most of the exposure range. The relationship was approximately linear up to a concentration of almost 1.0 mg/m3, an order of magnitude higher than the limits considered here.
Metalworking fluids are known to irritate the skin and respiratory tract (26). Oil-based metalworking fluids increase the organic carbon and polycyclic aromatic hydrocarbon content of the respirable particle fraction by forming droplets, accumulating on the surfaces of solid particles, or transforming to secondary organic aerosols when heated. Metals that have contaminated the fluids or dissolved in them contribute to respirable particles. These properties have the potential to render PM in these occupational settings at least as toxicologically relevant for the development of cardiovascular disease as ambient PM (1). In particular, particles generated by the use of straight metalworking fluids are likely to contribute to all 3 major modes of action, namely: 1) inducing systemic inflammation and oxidative stress through organic polycyclic aromatic hydrocarbons and metal compounds, 2) activating the autonomic nervous system by irritating the respiratory tract, and 3) exhibiting direct effects by translocation of newly formed ultrafine particles or activated surfaces of particles. Evidence for the link between PM2.5 at ambient levels and cardiovascular disease has further strengthened since 2010 (27, 28), supporting the need to protect workers from cardiovascular disease by lowering the exposure limit to 0.01 mg/m3.
Our methods contribute to the occupational literature in 2 important ways: We minimized healthy-worker survivor bias and also approached the research from a public health perspective. G-estimation provided better control for the healthy-worker survivor effect than is generally possible using traditional analytic methods in occupational studies in which follow-up continues after employment termination. By estimating the number of years of life that would have been saved by enforcing exposure limits, we obtained results with a concrete interpretation. Although banning straight metalworking fluids would have had the greatest effect on all of the cardiovascular mortality outcomes that we considered, a limit of 0.1 mg/m3 could still have saved over 1,000 life-years among those who died of IHD.
Under the assumptions of our analysis, the true number of life-years that would have been saved by enforcing an occupational exposure limit depends on 1) the incidence of the outcome in the study population during follow-up, 2) the distribution of exposure among the cases, and 3) the shape of the exposure-response curve. Thus, the analysis does not address a purely etiological question, but rather estimates the burden of disease that could have been avoided by enforcing a lower exposure limit in the cohort under study. For a given outcome, the estimates of this measure also allow us to compare the potential impacts of the different cutoffs.
Lowering the occupational exposure limit for straight metalworking fluids to any value from 0 mg/m3 to 0.02 mg/m3 would have affected the rate cerebrovascular mortality approximately equally, saving 800–900 years of life for the 501 workers who died of these causes. This seems to suggest a threshold; limits above 0.04 mg/m3 would have had little impact on this outcome. In contrast, although the estimated effects were higher for the other 3 (more prevalent) outcomes, increasing the limit from 0 showed rapidly decreasing impacts: The estimated effects for cardiovascular disease and IHD associated with a limit of 0.01 mg/m3 were half those for a total ban.
These estimates depend on several untestable assumptions. As in any observational study, we must assume that the variables that we measured were sufficient to control for confounding. Plausible unmeasured confounders include job transfer and the use of respirators or other protective equipment, both of which might be mechanisms by which a worker could reduce his or her exposure for health-related reasons. However, any residual confounding by these variables is likely to be toward the null. Furthermore, we lacked data on smoking, so there might be confounding by this important risk factor for cardiovascular disease. However, in a quantitative evaluation of the potential for bias due to uncontrolled confounding by alcohol and tobacco in occupational cohorts, Kriebel et al. (29) estimated that bias would be less than 20%.
Furthermore, we assume consistency, that is, that each person's counterfactual survival time under her or his observed exposure is equal to her or his observed survival time. This holds when the exposure is subject to well-defined interventions. For example, a workplace could comply with an occupational exposure limit in several ways: by improving ventilation, enforcing respirator use, or replacing straight metalworking fluids with other fluid types. We assume that the effects of such different interventions are equivalent if they correspond to the same limit.
A related assumption is that, for each cutoff, exposure being above or below that limit is the relevant exposure classification and that a worker's actual annual average daily exposure level within those categories is not relevant. By studying the potential effects of interventions based on several different cutoffs, we hope to have minimized any bias due to violations of this exposure classification assumption. However, a related technical point is briefly described in Web Appendix 1.
We also assumed correct model specification. Aside from unmeasured confounders, mentioned above, our exposure prediction model could be misspecified if the functional forms of our predictor variables were wrong or if interaction terms should have been included. More importantly, our structural model is based on 2 strong assumptions. First, it assumes there is no effect modification by covariate history. If effect modification (e.g., by (unmeasured) time-varying smoking status) is present, our results might be biased (12).
Second, any structural accelerated failure time model assumes that all individuals who experienced the outcome of interest would have done so under every possible exposure scenario; exposure merely affects the time at which the outcome occurs. This modeling assumption might be realistic for IHD because the underlying process of atherosclerosis is likely to be inflicted by multiple risk factors and accelerated by exposure to straight metalworking fluids. Nevertheless, if the magnitudes of the etiologic effects of metalworking fluids on different outcomes are substantially different, reducing exposure could mean that a worker who actually died of (for example) IHD would have instead died of some other cause, or vice versa. Cardiovascular disease is not a rare outcome, so assuming that those who died of it would have still died of it under other exposure scenarios is more plausible than it might be for a rare cancer. There were fewer cerebrovascular deaths, so those results may be more affected by this issue.
To avoid this problem, one could consider all-cause mortality, for which the modeling assumption is simply the incontrovertible fact that everyone would have died at some point and for which there are no competing risks. Our analysis of all-cause mortality yielded results that looked similar in shape to those for cardiovascular disease, but with greater impacts and confidence intervals that mostly excluded the null. Another way to avoid both this modeling assumption and the need for weights to adjust for competing risks would be to use the parametric g-formula instead of g-estimation (see Web Appendix 1) (13).
In most previous occupational studies, investigators aimed to address an etiologic question. We chose instead to work within a public health framework, attempting to estimate the potential effects of various interventions to limit exposures to metalworking fluid. G-estimation is an analytic method that permits us to answer such questions in an easily interpretable way while also avoiding healthy-worker survivor bias. These analyses suggest that there might be a threshold of 0.02 mg/m3 for exposure to straight metalworking fluids, above which the time to cerebrovascular death is decreased; to our knowledge, ours is the first study to report a relationship between straight fluids and this outcome, so further study is needed. Furthermore, restricting exposures to straight metalworking fluids as much as possible could have saved many years of life among the workers who died of other cardiovascular causes, especially IHD and AMI. Overall, our results indicate that the currently recommended limit of 0.4 mg/m3 does not provide adequate protection for workers.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Environmental Health Sciences, School of Public Health, University of California, Berkeley, Berkeley, California (Sally Picciotto, Ellen A. Eisen); and Institute of Epidemiology II, Helmholtz Zentrum München—German Research Center for Environmental Health, Neuherberg, Germany (Annette Peters).
This work was financially supported by grant R03 OH010202 from the National Institute for Occupational Safety and Health, part of the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
REFERENCES
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;12121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 2.Peters A. Ambient particulate matter and the risk for cardiovascular disease. Prog Cardiovasc Dis. 2011;535:327–333. [DOI] [PubMed] [Google Scholar]
- 3.Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;121:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen MR. Invited commentary: the search for preventable causes of cardiovascular disease—whither work? Am J Epidemiol. 2009;16912:1422–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phalen RF, Hinds WC, John W, et al. Rationale and recommendations for particle size-selective sampling in the workplace. Appl Ind Hyg. 1986;11:3–14. [Google Scholar]
- 6.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear Aromatic Hydrocarbons, Part 2: Carbon Blacks Mineral Oils Lubricant Base Oils and Derived Products and Some Nitroarenes Vol. 33 Lyon, France: IARC; 1984. [PubMed] [Google Scholar]
- 7.Stebbins A. Cobalt exposures enhanced by synthetic coolants. Environ Health News. 1989;3:3–7. [Google Scholar]
- 8.Mosher E, Peterson L, Skold R. The chemical control of cobalt leaching from cemented carbide tooling. Mater Perform. 1986;2510:38–43. [Google Scholar]
- 9.Oxhøj H, Andreasen H, Henius UM. Respiratory symptoms and ventilatory lung function in machine shop workers exposed to coolant-lubricants. Eur J Respir Dis Suppl. 1982;118:85–89. [PubMed] [Google Scholar]
- 10.Costello S, Garcia E, Hammond SK, et al. Ischemic heart disease mortality and PM(3.5) in a cohort of autoworkers. Am J Ind Med. 2013;563:317–325. [DOI] [PubMed] [Google Scholar]
- 11.Eisen EA, Picciotto S, Robins JM. Healthy worker effect in occupational studies. In: El-Shaarawi AH, Piegorsch WW, eds. Encyclopedia of Environmetrics, Second Edition. Vol 2 Chichester, UK: John Wiley & Sons; 2012:1269–1272. [Google Scholar]
- 12.Robins JM, Hernán MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, eds. Longitudinal Data Analysis. New York: Chapman & Hall/CRC; 2009:553–599. [Google Scholar]
- 13.Cole SR, Richardson DB, Chu H, et al. Analysis of occupational asbestos exposure and lung cancer mortality using the g formula. Am J Epidemiol. 2013;1779:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevrier J, Picciotto S, Eisen EA. A comparison of standard methods with g-estimation of accelerated failure-time models to address the healthy-worker survivor effect: application in a cohort of autoworkers exposed to metalworking fluids. Epidemiology. 2012;232:212–219. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services PHS, Centers for Disease Control and Prevention. Criteria for a Recommended Standard for Occupational Exposure to Metalworking Fluids. Cincinnati, OH: National Institute of Occupational Safety and Health; 1998. [Google Scholar]
- 16.Mirer FE. New evidence on the health hazards and control of metalworking fluids since completion of the OSHA advisory committee report. Am J Ind Med. 2010;538:792–801. [DOI] [PubMed] [Google Scholar]
- 17.Picciotto S, Chevrier J, Balmes J, et al. Hypothetical interventions to limit metalworking fluid exposures and their effects on COPD mortality: G-estimation within a public health framework. Epidemiology. 2014;253:436–443. [DOI] [PubMed] [Google Scholar]
- 18.Hallock MF, Smith TJ, Woskie SR, et al. Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med. 1994;265:621–634. [DOI] [PubMed] [Google Scholar]
- 19.Woskie SR, Virji MA, Hallock M, et al. Summary of the findings from the exposure assessments for metalworking fluid mortality and morbidity studies. Appl Occup Environ Hyg. 2003;1811:855–864. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Blevins D, Ritter G, et al. G-estimation of the effect of prophylaxis therapy for Pneumocystis carinii pneumonia on the survival of AIDS patients. Epidemiology. 1992;34:319–336. [DOI] [PubMed] [Google Scholar]
- 21.Hernán MA, Cole SR, Margolick J, et al. Structural accelerated failure time models for survival analysis in studies with time-varying treatments. Pharmacoepidemiol Drug Saf. 2005;147:477–491. [DOI] [PubMed] [Google Scholar]
- 22.Picciotto S, Brown DM, Chevrier J, et al. Healthy worker survivor bias: implications of truncating follow-up at employment termination. Occup Environ Med. 2013;7010:736–742. [DOI] [PubMed] [Google Scholar]
- 23.Park RM. Mortality at an automotive engine foundry and machining complex. J Occup Environ Med. 2001;435:483–493. [DOI] [PubMed] [Google Scholar]
- 24.Fang SC, Cassidy A, Christiani DC. A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int J Environ Res Public Health. 2010;74:1773–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello S, Brown DM, Noth EM, et al. Incident ischemic heart disease and recent occupational exposure to particulate matter in an aluminum cohort. J Expo Sci Environ Epidemiol. 2014;241:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaves IA, Eisen EA, Smith TJ, et al. Respiratory health of automobile workers exposed to metal-working fluid aerosols: respiratory symptoms. Am J Ind Med. 1997;325:450–459. [DOI] [PubMed] [Google Scholar]
- 27.Gold DR, Mittleman MA. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation. 2013;12718:1903–1913. [DOI] [PubMed] [Google Scholar]
- 28.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kriebel D, Zeka A, Eisen EA, et al. Quantitative evaluation of the effects of uncontrolled confounding by alcohol and tobacco in occupational cancer studies. Int J Epidemiol. 2004;335:1040–1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


