Abstract
OBJECTIVE:
The objective of this study was to evaluate the diagnostic reliability of home respiratory polygraphy (HRP) in children with a clinical suspicion of OSA-hypopnea syndrome (OSAS).
METHODS:
A prospective blind evaluation was performed. Children between the ages of 2 to 14 years with clinical suspicion of OSAS who were referred to the Sleep Unit were included. An initial HRP followed by a later date, same night, in-laboratory overnight respiratory polygraphy and polysomnography (PSG) in the sleep laboratory were performed. The apnea-hypopnea index (AHI)-HRP was compared with AHI-PSG, and therapeutic decisions based on AHI-HRP and AHI-PSG were analyzed using intraclass correlation coefficients, Bland-Altman plots, and receiver operator curves (ROCs).
RESULTS:
Twenty-seven boys and 23 girls, with a mean age of 5.3 ± 2.5 years, were studied, and 66% were diagnosed with OSAS based on a PSG-defined obstructive respiratory disturbance index ≥ 3/h total sleep time. Based on the availability of concurrent HRP-PSG recordings, the optimal AHI-HRP corresponding to the PSG-defined OSAS criterion was established as ≥ 5.6/h The latter exhibited a sensitivity of 90.9% (95% CI, 79.6%-100%) and a specificity of 94.1% (95% CI, 80%-100%).
CONCLUSIONS:
HRP recordings emerge as a potentially useful and reliable approach for the diagnosis of OSAS in children. However, more research is required for the diagnosis of mild OSAS using HRP in children.
Respiratory sleep disorders, and particularly OSA-hypopnea syndrome (OSAS), are common during the pediatric age range. The true prevalence of OSAS in children is unclear, but is estimated to range between 0.2% and 4.1%.1 Numerous studies have shown that OSAS in children is associated with significant morbidity,2‐11 and, therefore, it is critical to recognize and diagnose this condition in a timely manner to reduce the magnitude of OSAS-induced adverse consequences and the attendant increases in direct and indirect health-care costs.12
Overnight polysomnography (PSG) remains the gold standard for the diagnosis of OSAS.6,11 Thus, the number of patients diagnosed with OSAS is critically dependent on the availability and accessibility of PSG. However, access to in-laboratory PSG is complicated not only by the inconvenience to children and their families but also by the insufficient capacity of most clinical sleep programs and the relative scarcity of professionals with the appropriate expertise in pediatric sleep disorders. Therefore, simpler diagnostic approaches are required and should preferentially allow for home-based diagnostic capabilities. Home respiratory polygraphy (HRP) is currently accepted and used as an effective diagnostic technique for OSAS in adults.13 We have validated respiratory polygraphy (RP) in children by performing PSG and in-laboratory RP (LRP) simultaneously in the sleep laboratory and found LRP to provide a useful alternative to full-fledged PSG in the diagnosis of OSAS in children.14 The major aim of the present study was to evaluate the potential usefulness of a diagnostic HRP in children by conducting simultaneous LRP and PSG as well as an HRP on a separate night in children being evaluated for suspected OSAS.
Materials and Methods
The present study was conducted using a prospective and blinded approach in which children being evaluated for clinical suspicion of OSAS were randomly selected to participate. Children included in the study were those who met inclusion criteria (see later) and who arrived to the Sleep Unit (SU) on the days of the week selected from a random number generator table.
The study included 50 children of both sexes, between the ages of 2 and 14 years, who were referred to the SU by their pediatricians for clinical suspicion of OSAS as suggested by the presence of habitual snoring and/or nocturnal respiratory pauses as reported by their parents or caretakers. All children included in the study lived in the urban area of Burgos, and their residential conditions were suitable for HRP studies. Those children suffering from serious chronic medical or psychiatric comorbidities, those who required urgent treatment, and those with symptoms suggestive of sleep disorders other than OSAS (eg, parasomnias, narcolepsy, or periodic leg movements) were excluded.
The study was approved by the Burgos University Hospital Clinical Research Ethics Committee (approval number CEIC 936), and an informed consent was obtained from the parents of all children included prior to their enrollment. The confidentiality of the data was guaranteed by using deidentification procedure-based codes and by completely dissociating all clinical and personal data from the recordings.
A clinical and sleep history was obtained on all children enrolled in the study as well as a general physical, and ear, nose, and throat (ENT) examination. An HRP was conducted, and an LRP and PSG were simultaneously performed in the sleep laboratory within a period of 1 to 2 weeks from the HRP. The results of each of the recordings were scored and interpreted by independent investigators not involved in the clinical management of the subjects and by sleep technologists who were blinded to the identity and aims of the study.
The information obtained from the clinical encounter included age, sex, height, BMI (weight in kg/height in m2), and BMI percentile based on age and sex.15 BP was also measured at rest during the morning following the nocturnal PSG.16 ENT examination was performed by visual inspection. Tonsillar hypertrophy was classified according to the intertonsillar space, as previously described.17
Respiratory Polygraphy
The HRP was performed in the child’s home using the eXim Apnea Polygraph (Bitmed, SIBEL Group). Oronasal flow was recorded by oronasal thermistor, and a nasal cannula was used to assess pressure (nasal pediatric size, Pro-Flow Plus, Pro-Tech), individual chest and abdomen movements and their sum by impedance plethysmography, body position by sensor position, snoring by microphone, and heart rate and blood oxygen saturation by means of pulse oximetry (3 Hz sampling rate with four-beat pulse rate averaging). A nurse trained in pediatric sleep techniques went to the child’s home and explained to the child and caregiver how the polygraph recorder worked, as well as how to position, replace, and remove the sensors. The nocturnal HRP recording was performed unsupervised after the nurse placed the sensors, assessed the signals, and started recordings. The following morning, the caregiver removed the polygraph and returned it to the SU together with a nocturnal diary in which the sleep quality and any incidents during the night were documented.
An LRP as well as a simultaneously supervised PSG were performed in the sleep laboratory with the same HRP equipment. Scoring of LRP and HRP were performed independently by the same investigators, who were blinded to the identity of the recordings. The supervised nocturnal PSG and LRP were performed in the sleep laboratory. For the overnight PSG, the Deltamed Coherence 3NT Polysomnograph, version 3.0 system (Diagniscan S.A.U., Group Werfen) was used and recorded EEG, right and left electrooculogram, tibial and submental (leg and chin) electromyogram, ECG, oronasal flow by thermistor, chest-abdomen movements with bands, body position by a position sensor, oxygen saturation using pulse oximetry (Spo2) (Nellcor Puritan Bennett, NPB-290), snoring and airflow by means of a nasal cannula, and a continuous transcutaneous recording of CO2. The American Academy of Sleep Medicine criteria were used to evaluate sleep states and respiratory events18 in the PSG. Both HRP and LRP were scored manually, with the American Academy of Sleep Medicine criteria being used to evaluate respiratory events. In this study, hypopnea in the RP was defined as a decrease > 50% in the amplitude of the nasal pressure or alternative signal compared with the preevent baseline excursion and the fall in the nasal pressure signal amplitude lasting ≥ 90% of the entire respiratory event compared with the signal amplitude preceding the event, and accompanied by an Spo2 decrease ≥ 3% for at least the duration equivalent to two respiratory cycles. Flow limitation events were defined as a discernible drop in the amplitude of the nasal cannula signal < 50% compared with the baseline level and/or a flattening of the nasal pressure waveform, accompanied by snoring, noisy breathing, or visual evidence of respiratory effort, lasting at least two respiratory cycles. The apnea-hypopnea index was defined as the number of apneas and hypopneas, including central apneas per hour of sleep in the PSG (per recorded hour in the RP). The obstructive apnea-hypopnea index (OAHI) was defined as the number of obstructive apneas and hypopneas per hour of sleep in the PSG (per recorded hour in the RP), without taking into account central apneas. The flow limitation index was defined as the total number of flow limitation events per hour of sleep in the PSG (per recorded hour in the RP). The respiratory disturbance index (RDI) was defined as the number of apneas (including central apneas), hypopneas, respiratory-related arousals, and flow limitations per hour of sleep in the PSG. Since no arousals can be objectively identified during RP, the RDI included only respiratory events per recorded hour. The obstructive respiratory disturbance index (ORDI) was defined as the number of obstructive apneas, hypopneas, and flow limitation events per hour of sleep in the PSG (per recorded hour in the RP), without taking into account central apneas.
The interruption of the oronasal flow that frequently follows body movements and the artifacts secondary to movements were not accounted for either in the PSG or the RP. An uninterpretable airflow signal was defined as no airflow during 30 s of normal respiration, whereas respiratory motion signals and Spo2 signal and values remained unchanged. Data were excluded from analysis if > 60% of the airflow signal was uninterpretable.
Statistical Analysis
Descriptive analysis was performed using frequency distributions for the qualitative variables and mean and SD for the quantitative variables. The comparison of means was performed using the general linear model for repeated measurements. The interclass correlation coefficient (ICC) was calculated to assess agreement, and Bland-Altman graphs were constructed. Diagnostic efficiency receiver operating characteristic (ROC) curves were calculated, and the following cutoff points were used for the validity analysis: RDI ≥ 1, ≥ 3, and ≥ 5; ORDI ≥ 1, ≥ 3, and ≥ 5; and OAHI ≥ 1, ≥ 3, and ≥ 5.11,14 The significance level was set at 5%, and 95% CIs were calculated. The data were processed and analyzed with the SPSS statistics package version 14.0 (IBM).
Results
A total of 50 children were recruited and completed all of the recordings. The cohort included 27 boys (54%) and 23 girls, with a mean age of 5.3 ± 2.5 years (range, 3-13 years), height of 112 ± 15.8 cm, weight of 21.5 ± 8.8 kg, BMI of 16.5 ± 2.5 kg/m2 corresponding to a BMI percentile of 47.5 ± 31.2 (range, 3-99), systolic BP of 98.3 ± 11.1 mm Hg, and diastolic BP of 59.3 ± 7.7 mm Hg. The most frequent presenting symptoms were nocturnal snoring and mouth breathing in 98% and 94%, respectively. Other symptoms recorded were nocturnal breathing pauses (78%), night sweating (60%), restless nighttime sleep (50%), daytime tiredness and fatigue (42%), nocturnal enuresis (24%), daytime sleepiness (20%), attention deficits (14%), irritability (10%), and hyperactive behaviors (4%). ENT examination revealed decreased pharyngeal space due to tonsillar hypertrophy in 100% of the cases. The degree of tonsillar hypertrophy was grade I in 2%, grade II in 32%, grade III in 48%, and grade IV in 18%.
The total recording time of the HRP was 531.5 ± 49.7 min and 442.1 ± 43.3 min for PSG and LRP. All the PSG and HRP studies were valid for subsequent analysis, and there were no recordings with > 60% of uninterpretable flow signal. The mean characteristics of the parameters obtained in the PSG are shown in Table 1. The prevalence of OSAS according to the indexes and cutoff values selected ranged from 44% to 78%, as follows: RDI ≥ 3: 78%, RDI ≥ 5: 64%; ORDI ≥ 3: 66%, ORDI ≥ 5: 54%; OAHI ≥ 3: 52%, and OAHI ≥ 5: 44%. The respiratory variables obtained in the HRP, LRP, and PSG are shown in Table 2. No significant differences were found in the total number of apneas or hypopneas between the studies. However, there were significant differences in the total number of respiratory events and flow limitation events (Table 2).
TABLE 1 ] .
Polysomnographic Measures in the 50 Children Enrolled in the HRP-LRP-PSG Comparative Study
| Measure | Mean ± SD |
| Time in bed, min | 515.0 ± 28.9 |
| TST, min | 442.1 ± 43.3 |
| LNREM, min | 27.6 ± 17.7 |
| LREM, min | 113.4 ± 62.2 |
| Stage N1, % TST | 13.2 ± 7.0 |
| Stage N2, % TST | 35.9 ± 8.8 |
| Stage N3, % TST | 28.5 ± 8.6 |
| Stage REM, % TST | 22.4 ± 5.5 |
| Arousal index, per h TST | 10.3 ± 5.5 |
| PLMS index, per h TST | 0.04 ± 0.3 |
HRP = home respiratory polygraphy; LNREM = non-rapid eye movement sleep latency; LREM = rapid eye movement sleep latency; LRP = in-laboratory respiratory polygraphy; PLMS = periodic leg movements during sleep; PSG = polysomnography; TST = total sleep time.
TABLE 2 ] .
LRP, HRP, and PSG Findings Among 50 Children Referred for Evaluation of OSAS
| Findings | PSG | HRP | LRP | Difference of Means |
| General Linear Model, P Value | ||||
| No. respiratory events | 101.0 ± 114.7 | 126.6 ± 110.9 | 151.8 ± 140.1 | .038 |
| Obstructive apneas | 37.3 ± 59.7 | 48.0 ± 56.4 | 56.1 ± 79.1 | .235 |
| Central apneas | 6.9 ± 8.1 | 10.1 ± 10.2 | 8.4 ± 7.4 | .069 |
| Mixed apneas | 0.3 ± 1.1 | 0.1 ± 0.7 | 0.1 ± 0.2 | .709 |
| Hypopneas | 34.6 ± 44.5 | 23.5 ± 37.5 | 25.3 ± 39.8 | .744 |
| Flow limitation events | 22.2 ± 23.5 | 45.1 ± 34.3 | 60.9 ± 56.3 | .021 |
| RDI | 13.9 ± 16.6 | 14.5 ± 13.2 | 16.5 ± 15.3 | .003 |
| ORDI | 13.0 ± 16.1 | 13.3 ± 13.0 | 15.5 ± 15.2 | .002 |
| OAHI | 9.9 ± 13.8 | 8.4 ± 10.3 | 9.2 ± 11.0 | .337 |
| Spo2 nadir, % | 87.4 ± 5.7 | 89.3 ± 4.1 | 87.5 ± 4.3 | .023 |
| Spo2 mean, % | 96.5 ± 1.5 | 96.9 ± 1.3 | 96.5 ± 1.0 | .018 |
| CT90% | 0.9 ± 2.9 | 0.7 ± 1.9 | 0.8 ± 2.0 | .616 |
| Oxygen desaturation index | 4.0 ± 5.8 | 4.1 ± 5.4 | 4.0 ± 5.6 | .967 |
| Peak CO2, mm Hg | 47.9 ± 7.2 | … | … | … |
| Ptcco2 > 50 mm Hg, % | 10.9 ± 23.3 | … | … | … |
Data are shown as mean ± SD. CT90% = cumulative percentage of sleep time with oxygen saturation as measured by pulse oximetry < 90%; OAHI = obstructive apnea-hypopnea index; ORDI = obstructive respiratory disturbance index; Ptcco2 = transcutaneous CO2; RDI = respiratory disturbance index; Spo2 = oxygen saturation as measured by pulse oximetry. See Table 1 legend for expansion of other abbreviations.
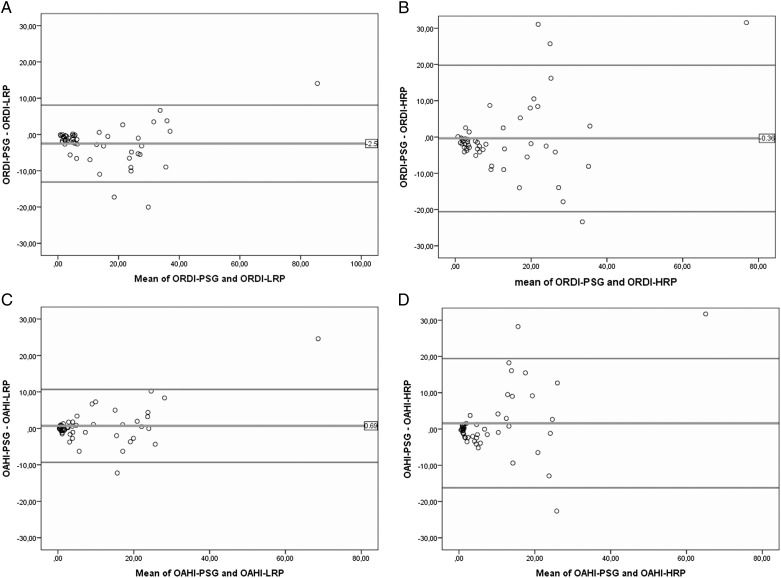
The agreement between RP (HRP and LRP) and PSG using the ICC was > 80% in all cases and higher for LRP when compared with HRP (Table 3). The agreement between ORDI in PSG and ORDI in LRP and HRP was 96.5% (95% CI, 92.3%-98.2%) and 86.7% (95% CI, 76.5%-92.5%), respectively. Bland-Altman plots for ORDI and OAHI obtained to assess the agreement between both diagnostic techniques (Fig 1) showed a bias between −2.51 and 1.57, with almost all of the values falling within the limits of agreement.
TABLE 3 ] .
ICCs and 95% CIs for Several Respiratory Cutoff Values Used as OSAS Diagnostic Criteria
| PSG | LRP | HRP |
| RDI ≥ 3 | 96 (91.8-97.9) | 85.9 (75.2-92) |
| ORDI ≥ 3 | 96.5 (92.3-98.2) | 86.7 (76.5-92.5) |
| OAHI ≥ 3 | 95.8 (92.6-97.6) | 84.3 (72.5-91.1) |
Figure 1 –
Bland-Altman plots showing the agreement between RP and PSG. A, Bland-Altman plots of differences in the ORDI-PSG and ORDI-LRP as a function of the mean ORDI derived from the two methods. B, Bland-Altman plots of differences in the ORDI-PSG and ORDI-HRP as a function of the mean ORDI derived from the two methods. C, Bland-Altman plots of differences in the OAHI-PSG and OAHI-LRP as a function of the mean OAHI derived from the two methods. D, Bland-Altman plots of differences in the OAHI-PSG and OAHI-HRP as a function of the mean OAHI derived from the two methods. HRP = home respiratory polygraphy; LRP = in-laboratory respiratory polygraphy; OAHI = obstructive apnea-hypopnea index; ORDI = obstructive respiratory disturbance index; PSG = polysomnography; RP = respiratory polygraphy.
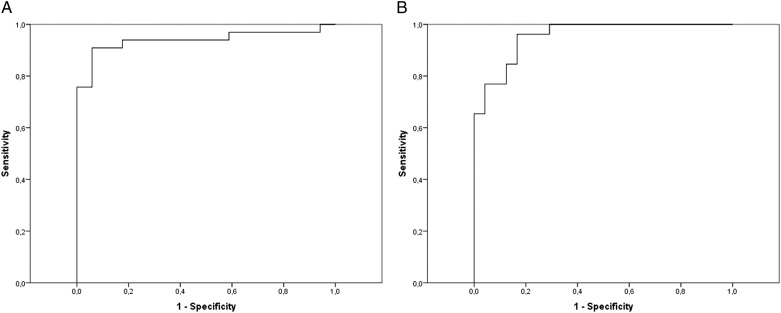
Based on such findings, the diagnostic efficiency curves (ROC) were calculated for the different diagnostic criteria used: RDI ≥ 3, ORDI ≥ 3, and OAHI ≥ 3, both in the sleep laboratory and at home. The RP in the sleep laboratory had an area under the ROC curve of 93.5% (85.5%-100%) for RDI ≥ 3, 96.5% (92.1%-100%) for ORDI ≥ 3, and 95.5% (90.6%-100%) for OAHI ≥ 3; and at home, 93.5% (86.8%-100%) for RDI ≥ 3, 93.5% (87%-100%) for ORDI ≥ 3, and 92.9% (85.9%-100%) for OAHI ≥ 3 (Fig 2).
Figure 2 –
Receiver operating characteristic (ROC) curves in HRP. A, ROC curve for ORDI in HRP with the criterion for diagnosis of OSA-hypopnea syndrome (OSAS) in children set as an ORDI ≥ 3 in PSG. B, ROC curve for OAHI in HRP with the criterion for diagnosis of OSAS in children set as an OAHI ≥ 3 in PSG. See Figure 1 legend for expansion of other abbreviations.
For an ORDI ≥ 3 in the PSG, the best cutoff point in the LRP was ORDI ≥ 4.6, with a sensitivity of 100% (95% CI, 98.5%-100%) and a specificity of 88.4% (95% CI, 70%-100%). For the HRP, an ORDI ≥ 5.6 exhibited a sensitivity of 90.9% (95% CI, 79.6%-100%) and a specificity of 94.12% (95% CI, 80%-100%). The different cutoff points obtained and their validity indexes are shown in Table 4, whereas validity indexes for cutoff values of RDI ≥ 1 and 5, ORDI ≥ 1 and 5, and OAHI ≥ 1 and 5 are shown in Table 5.
TABLE 4 ] .
Diagnostic Validity Indexes for LRP and HRP Compared With In-Laboratory PSG
| Index | PSG RDI ≥ 3 | PSG ORDI ≥ 3 | PSG OAHI ≥ 3 | |||
| LRP RDI ≥ 4.7 | HRP RDI ≥ 5.6 | LRP ORDI ≥ 4.6 | HRP ORDI ≥ 5.6 | LRP OAHI ≥ 3 | HRP OAHI ≥ 4 | |
| Sensitivity, % | 92.3 | 87.1 | 100 | 90.9 | 96.1 | 92.3 |
| Specificity, % | 90.9 | 90.9 | 88.2 | 94.1 | 83.3 | 83.3 |
| Validity index, % | 92 | 88 | 96 | 92 | 90 | 88 |
| Positive predictive value, % | 97.3 | 97.1 | 94.3 | 96.8 | 86.2 | 85.7 |
| Negative predictive value, % | 76.9 | 66.7 | 100 | 84.2 | 95.2 | 90.9 |
| Prevalence, % | 78 | 78 | 66 | 66 | 52 | 52 |
| Positive likelihood ratio | 10.2 | 9.6 | 8.5 | 15.5 | 5.8 | 5.5 |
| Negative likelihood ratio | 0.08 | 0.14 | … | 0.10 | 0.05 | 0.09 |
TABLE 5 ] .
Validity Indexes for HRP Compared With PSG for Various Common PSG-Derived Diagnostic Criteria
| Index | PSG RDI ≥ 1 | PSG ORDI ≥ 1 | PSG OAHI ≥ 1 | PSG RDI ≥ 5 | PSG ORDI ≥ 5 | PSG OAHI ≥ 5 |
| HRP RDI ≥ 5.0 | HRP ORDI ≥ 4.5 | HRP OAHI ≥ 3.0 | HRP RDI ≥ 8.0 | HRP ORDI ≥ 5.6 | HRP OAHI ≥ 6.7 | |
| Sensitivity, % | 74.5 | 80.0 | 72.5 | 81.3 | 85.2 | 81.8 |
| Specificity, % | 66.7 | 100.0 | 90.0 | 83.3 | 95.6 | 92.9 |
| Validity index, % | 74.0 | 82.0 | 76.0 | 82.0 | 90.0 | 88.0 |
| Positive predictive value, % | 97.2 | 100.0 | 96.7 | 89.7 | 95.8 | 90.0 |
| Negative predictive value, % | 14.3 | 35.7 | 45.0 | 71.4 | 84.6 | 86.7 |
| Prevalence, % | 94.0 | 90.0 | 80.0 | 64.0 | 54.0 | 44.0 |
| Positive likelihood ratio | 2.2 | … | 7.3 | 4.9 | 19.6 | 11.5 |
| Negative likelihood ratio | 0.3 | 0.2 | 0.3 | 0.2 | 0.1 | 0.2 |
Discussion
This study shows that HRP provides a reasonably valid alternative to in-laboratory PSG for the diagnosis of OSAS in children clinically referred with a high index of clinical suspicion for the presence of OSAS. This frequent and highly prevalent pediatric condition is associated with adverse consequences and excessive and costly use of health-care services.2‐12,19 Overnight PSG requires specialized equipment and personnel in a sleep laboratory,6,18 and for this reason, the ability to diagnose risk of OSAS in children depends on the availability of and accessibility to such usually scarce clinical resources. Accordingly, alternative diagnostic approaches have been sought aiming to expand the accessibility and identify and treat children with OSAS in a timely manner. Such approaches have included questionnaires, audio-visual recordings, pulse oximetry recordings, daytime nap PSG, and home-based PSG.20 Questionnaires generally exhibit low sensitivity and specificity, which enables their use for screening but clearly precludes their routine use for diagnostic purposes.21‐23 Pulse oximetry and nap-based PSG display high specificity but low sensitivity, such that a negative result mandatorily implies the need for a PSG.7,8
The Tucson Children’s Assessment of Sleep Apnea (TuCASA) study20 showed that high-quality unattended multichannel PSG was possible in children aged 5 to 12 years in the context of a research protocol. Similar results have been reported by another recent study involving 201 children who were evaluated by unattended ambulatory PSG for research purposes. In this contextual setting, a technically satisfactory recording was obtained in 91% of cases.24
Although RP approaches are widely accepted and commonly used in clinical settings as an alternative for the diagnosis of OSAS in adults,13 they have not been critically appraised in the most recent guidelines issued by the American Academy of Pediatrics, such that recommendations regarding their use are currently lacking.7,8 However, several studies have been published on the validity of RP in children with discordant results.25‐31 A systematic review concluded that although there is limited evidence concerning diagnostic alternatives to PSG for identifying OSA in children, polygraphy may be a valid test, a conclusion that will require confirmation in subsequent studies.32 We have evaluated LRP by performing concomitant RP and PSG.14 This study showed that an RDI cutoff ≥ 4.6 exhibited excellent sensitivity and specificity for identifying OSAS in children and was useful in both the diagnosis and follow-up of children with OSAS.14,33 The present study confirms our previous findings on the LRP cutoff value of the RDI (ie, 4.6). Of note, a recent study using LRP and standard diagnostic criteria suggested that use of LRP may significantly affect clinical management decisions, particularly in children with mild and moderate OSAS.31 Several years ago, a consensus panel reached the decision to adjudicate the presence of a PSG-based ORDI ≥ 3 as the diagnostic criterion of pediatric OSAS in Spain.11 Based on such an approach, the agreement between HRP and PSG was good, albeit less than the LRP ROC characteristics. It is possible, however, that night-to-night variability may account for some of the discrepancies in performance currently identified between LRP and HRP recordings.34 Because other diagnostic PSG-based criteria are used around the world, we sought to reconcile our current findings with frequently used PSG criteria for diagnosis and severity classification elsewhere.35‐38 For this reason, we assessed the validity indexes for RDI ≥ 1, ≥ 3, and ≥ 5; ORDI ≥ 1, ≥ 3, and ≥ 5; and OAHI ≥ 1, ≥ 3, and ≥ 5 and thus enable comparisons across multiple studies. The area under the curve performance of HRP remained good for all the cutoff values considered and was > 90%. Furthermore, we defined the HRP cutoff ORDI as ≥ 5.6, and this approach exhibited excellent sensitivity and specificity. Thus, the current study findings strongly support the diagnostic usefulness of the HRP and provide initial criteria for its use in the convenient and child-friendly home environment, thereby avoiding laboratory overnight stay.
Some technical considerations deserve comment. We defined flow limitations in RP as discernible drops in the amplitude of the cannula signal < 50% compared with baseline levels and/or a flattening of the nasal pressure waveform, accompanied by snoring, noisy breathing, or visual evidence of respiratory effort, all of which lasted for at least the time equivalent to two respiratory cycles.18 We believe that this approach enables reliable detection of respiratory events, since no discrepancies could be identified between LRP and PSG in this approach. During RP, it is obviously impossible to establish the occurrence of cortical arousals, and therefore, a proportion of the events will be omitted from the respiratory indexes, a factor that my account for < 100% concurrence between RP and PSG. However, as shown in the Bland-Altman plot in Figure 1, the differences between RP and PSG were similar on both sides of the mean difference, thereby indicating no bias in the measurements. The differences were greater when respiratory disturbance values were higher, such that it is unlikely that they will markedly affect the diagnostic yield of RP in children with OSAS. We should also point out that our study was conducted on a selected population with a high probability of OSAS and that therefore the ROC of HRP may be compromised in lower-risk pediatric populations.
The design of this study included the participation of a nurse skilled in pediatric sleep recordings who was present in the child’s home during the process of placement of the sensors and the initiation of the recordings. In this context, we followed the routine process adopted by the Spanish health-care system, which aims to ensure improved quality of recordings, with reduced frequency of signal losses and minimization of uninterpretable studies. Furthermore, we have conducted a cost-effectiveness study of HRP performed in adult patients in 2000 that supported the implementation of current practices.39 In that study,39 the cost of the PSG was 179.2 Euros (not including overnight stay in hospital), and the cost of home respiratory polygraphy (including the cost of the nurse visit at the patient’s home) was 69.6 Euros. Based on these analyses, and after adjusting for the annual increases in the Consumer Price Index from 2000 to 2013, we now estimate that the current price of the PSG is around 242.1 Euros (not including overnight stay in hospital, the latter current price being 400 Euros), and the current price of HRP is estimated at 94.0 Euros. Clearly, the labor and time required for the hook-up and coordination of any procedure in children is likely to exceed those in adults, such that additional health-care cost studies comparing both approaches in the assessment of pediatric patients at risk for OSA are needed.
In conclusion, we provide initial observations supporting the validity of domiciliary RP in children with a strong history and physical examination suggestive of a high probability for the presence of OSAS. We, therefore, propose that HRP be favorably considered and ultimately incorporated into the diagnostic-therapeutic algorithm of children (≥ 3 years old) with a clinical suspicion of OSAS. We should stress, however, that when inconclusive HRP findings occur, a conventional PSG should be performed, and we further recommend incremental research efforts, particularly for the diagnosis of mild OSA using HRP in children.
Acknowledgments
Author contributions: M. L. A.-A. contributed to concept and design of the study, data collection, drafting the initial manuscript, and approving the final manuscript as submitted; J. T.-S. contributed to drafting the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted; E. O. C. and A. I. N.-E. contributed to data collection and approved the final manuscript as submitted; J. A. C.-G. contributed to analyzing and designing the data collection instruments and coordinated and supervised data collection; L. K.-G. and D. G. contributed to data analysis, edited the manuscript, and approved the final manuscript as submitted.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- ENT
ear, nose, and throat
- HRP
home respiratory polygraphy
- ICC
interclass correlation coefficient
- LRP
in-laboratory respiratory polygraphy
- OAHI
obstructive apnea-hypopnea index
- ORDI
obstructive respiratory disturbance index
- OSAS
OSA-hypopnea syndrome
- PSG
polysomnography
- RDI
respiratory disturbance index
- ROC
receiver operating characteristic
- RP
respiratory polygraphy
- Spo2
oxygen saturation using pulse oximetry
- SU
Sleep Unit
Footnotes
FUNDING/SUPPORT: Funded by the Spanish Respiratory Society (SEPAR) and Ministry of Health Castilla-Leon, Spain, and by National Institutes of Health [Grant HL-65270 to Drs Kheirandish-Gozal and Gozal].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128(1):e85-e92. [DOI] [PubMed] [Google Scholar]
- 3.Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(1):92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourke R, Anderson V, Yang JSC, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011;12(5):489-496. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46(4):313-323. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866-878. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Pediatrics. 2002;109(4):704-712. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-e755. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576-584. [DOI] [PubMed] [Google Scholar]
- 10.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Álvarez ML, Canet T, Cubel-Alarco M, et al. Consensus document on sleep apnea-hypopnea syndrome in children. Arch Bronconeumol. 2011;47(suppl 5):1-18. [PubMed] [Google Scholar]
- 12.Tarasiuk A, Simon T, Tal A, Reuveni H. Adenotonsillectomy in children with obstructive sleep apnea syndrome reduces health care utilization. Pediatrics. 2004;113(2):351-356. [DOI] [PubMed] [Google Scholar]
- 13.Collop NA, Anderson WM, Boehlecke B, et al. ; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737-747. [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso Alvarez ML, Terán Santos J, Cordero Guevara JA, et al. Reliability of respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome in children [in Spanish]. Arch Bronconeumol. 2008;44(6):318-323. [PubMed] [Google Scholar]
- 15.Sobradillo B, Aguirre A, Aresti U, et al. Curvas y tablas de crecimiento (estudio longitudinal y trasversal). In Orbegozo Fundación F., ed. Patrones de crecimientoy desarrollo en España. Atlas de gráfi cas y tablas. Madrid, Spain: Ergon, 2004. [Google Scholar]
- 16.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576. [PubMed] [Google Scholar]
- 17.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13(2):149-156. [DOI] [PubMed] [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Blunden S, Lushington K, Lorenzen B, Ooi T, Fung F, Kennedy D. Are sleep problems under-recognised in general practice? Arch Dis Child. 2004;89(8):708-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin JL, Enright PL, Kaemingk KL, et al. Feasibility of using unattended polysomnography in children for research—report of the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). Sleep. 2001;24(8):937-944. [DOI] [PubMed] [Google Scholar]
- 21.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011;15(1):19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spruyt K, Gozal D. Screening of pediatric sleep-disordered breathing: a proposed unbiased discriminative set of questions using clinical severity scales. Chest. 2012;142(6):1508-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadmon G, Shapiro CM, Chung SA, Gozal D. Validation of a pediatric obstructive sleep apnea screening tool. Int J Pediatr Otorhinolaryngol. 2013;77(9):1461-1464. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Traylor J, Biggs SN, et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10(8):913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob SV, Morielli A, Mograss MA, Ducharme FM, Schloss MD, Brouillette RT. Home testing for pediatric obstructive sleep apnea syndrome secondary to adenotonsillar hypertrophy. Pediatr Pulmonol. 1995;20(4):241-252. [DOI] [PubMed] [Google Scholar]
- 26.Kirk VG, Flemons WW, Adams C, Rimmer KP, Montgomery MD. Sleep-disordered breathing in Duchenne muscular dystrophy: a preliminary study of the role of portable monitoring. Pediatr Pulmonol. 2000;29(2):135-140. [DOI] [PubMed] [Google Scholar]
- 27.Zucconi M, Calori G, Castronovo V, Ferini-Strambi L. Respiratory monitoring by means of an unattended device in children with suspected uncomplicated obstructive sleep apnea: a validation study. Chest. 2003;124(2):602-607. [DOI] [PubMed] [Google Scholar]
- 28.Poels PJP, Schilder AGM, van den Berg S, Hoes AW, Joosten KFM. Evaluation of a new device for home cardiorespiratory recording in children. Arch Otolaryngol Head Neck Surg. 2003;129(12):1281-1284. [DOI] [PubMed] [Google Scholar]
- 29.Moss D, Urschitz MS, von Bodman A, et al. Reference values for nocturnal home polysomnography in primary schoolchildren. Pediatr Res. 2005;58(5):958-965. [DOI] [PubMed] [Google Scholar]
- 30.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan HL, Gozal D, Ramirez HM, Bandla HPR, Kheirandish-Gozal L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep. 2014;37(2):255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brockmann PE, Schaefer C, Poets A, Poets CF, Urschitz MS. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med Rev. 2013;17(5):331-340. [DOI] [PubMed] [Google Scholar]
- 33.Alonso-Álvarez ML, Navazo-Egüia AI, Cordero-Guevara JA, et al. Respiratory polygraphy for follow-up of obstructive sleep apnea in children. Sleep Med. 2012;13(6):611-615. [DOI] [PubMed] [Google Scholar]
- 34.Katz ES, Greene MG, Carson KA, et al. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr. 2002;140(5):589-594. [DOI] [PubMed] [Google Scholar]
- 35.Scholle S, Wiater A, Scholle HC. Normative values of polysomnographic parameters in childhood and adolescence: cardiorespiratory parameters. Sleep Med. 2011;12(10):988-996. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741-753. [DOI] [PubMed] [Google Scholar]
- 37.Lloberes P, Durán-Cantolla J, Martínez-García MÁ, et al. ; Spanish Society of Pulmonology and Thoracic Surgery. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Arch Bronconeumol. 2011;47(3):143-156. [DOI] [PubMed] [Google Scholar]
- 38.Lin CH, Guilleminault C. Current hypopnea scoring criteria underscore pediatric sleep disordered breathing. Sleep Med. 2011;12(7):720-729. [DOI] [PubMed] [Google Scholar]
- 39.Alonso Alvarez ML, Terán Santos J, Cordero Guevara J, et al. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome: analysis of costs [in Spanish]. Arch Bronconeumol. 2008;44(1):22-28. [PubMed] [Google Scholar]