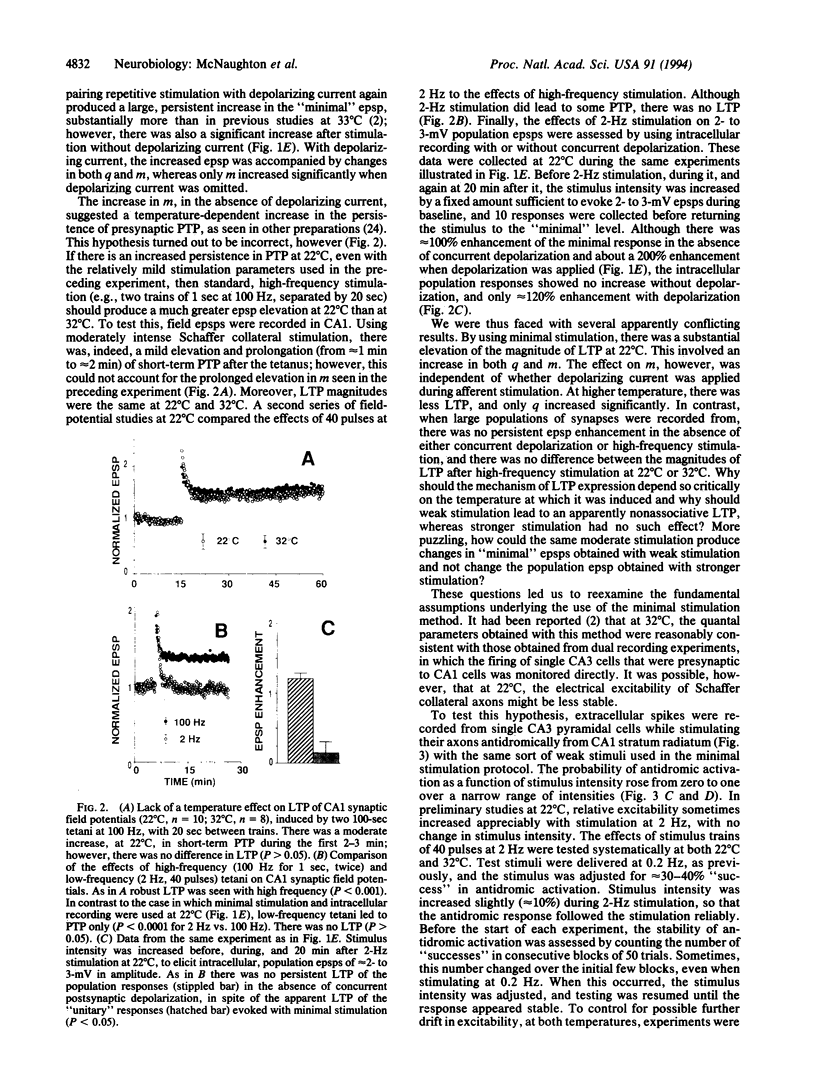
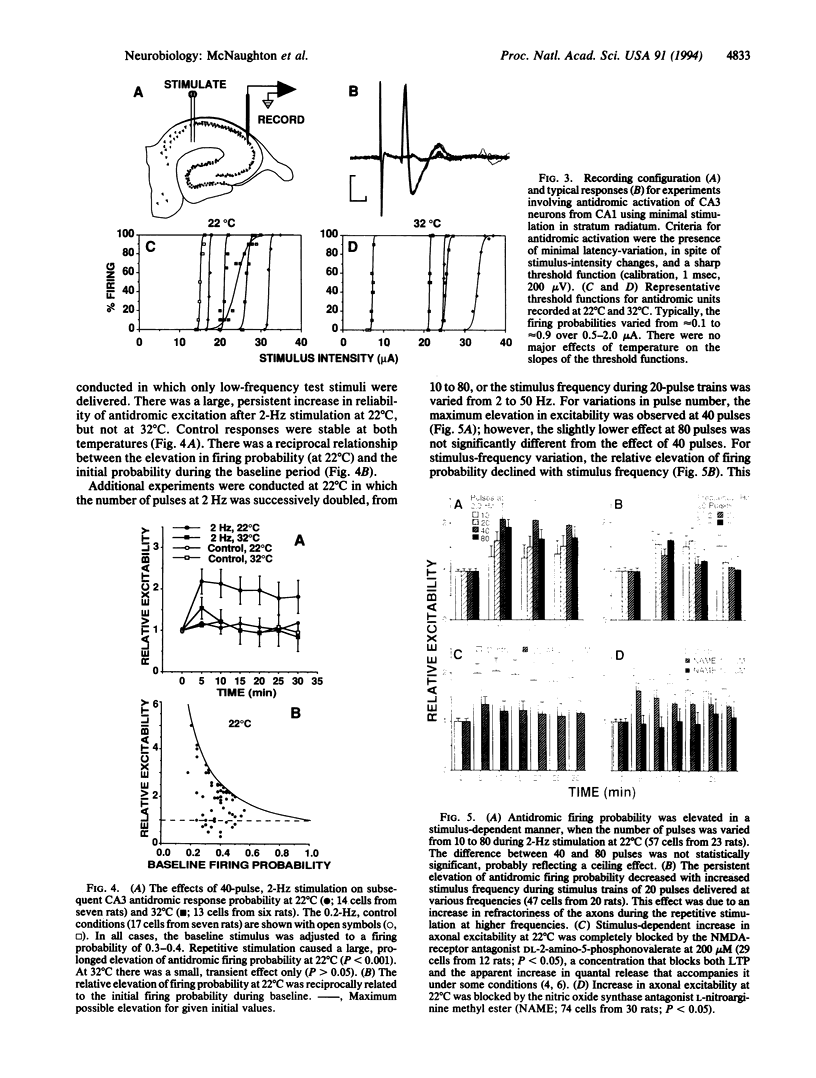
Abstract
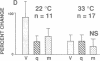
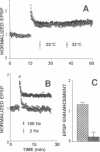
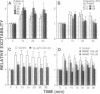
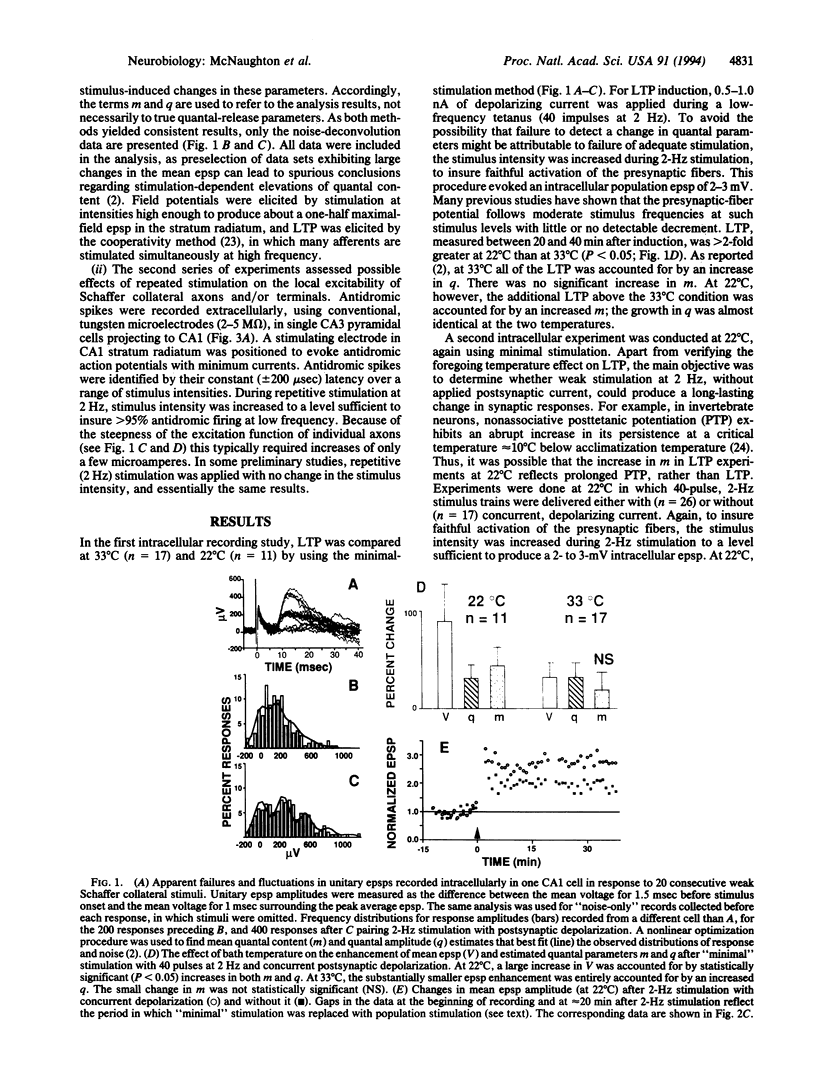
The electrical excitability of Schaffer collateral axons and/or terminals was studied in hippocampal slices by monitoring single, CA3 pyramidal neurons activated antidromically from CA1 stratum radiatum. At 22 degrees C, weak, repetitive stimulation with as few as 10 impulses at 2 Hz led to a robust lowering of the antidromic activation threshold that lasted > 30 min. The effect was completely absent at 32 degrees C and was blocked by both the N-methyl-D-aspartate receptor antagonist, 2-amino-5-phosphonovalerate and the inhibitor of nitric-oxide synthase, L-nitro-arginine methyl ester. Such threshold lowering would alter the variance of synaptic responses from axons stimulated in the variable excitation region of their input-output functions. These results thus raise important doubts about the interpretation of experiments in which the so-called minimal-stimulation method has been used at reduced temperature to infer changes in quantal transmission during hippocampal long-term potentiation. In the present experiments, no changes were observed in the estimate of excitatory postsynaptic potential quantal content in long-term potentiation experiments at either temperature, which could not be accounted for by an artificial, temperature-dependent change in the responsiveness of presynaptic axons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T., Staiger V., Aertsen A. Synaptic plasticity in rat hippocampal slice cultures: local "Hebbian" conjunction of pre- and postsynaptic stimulation leads to distributed synaptic enhancement. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Stutzmann J. M., Doble A., Blanchard J. C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991 Jul 9;199(3):379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991 Nov;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. C., McNaughton B. L. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991 Jan;1(1):79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H., Abraham W. C., Huang Y. Y. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987 Mar;7(3):774–780. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Wilcox G. L., Chapman P. F. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992 Feb;8(2):211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- Hirata K., Sawada S., Yamamoto C. Enhancement of transmitter release accompanying with long-term potentiation in synapses between mossy fibers and CA3 neurons in hippocampus. Neurosci Lett. 1991 Feb 11;123(1):73–76. doi: 10.1016/0304-3940(91)90161-l. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Nicoll R. A. Long-term potentiation is associated with increases in quantal content and quantal amplitude. Nature. 1992 May 21;357(6375):240–244. doi: 10.1038/357240a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A., Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol. 1981 Nov;46(5):952–966. doi: 10.1152/jn.1981.46.5.952. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. The mechanism of expression of long-term enhancement of hippocampal synapses: current issues and theoretical implications. Annu Rev Physiol. 1993;55:375–396. doi: 10.1146/annurev.ph.55.030193.002111. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer R. J., Friedlander M. J., Redman S. J. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in the hippocampal slice. J Neurosci. 1990 Mar;10(3):826–836. doi: 10.1523/JNEUROSCI.10-03-00826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer R. J., Redman S. J., Andersen P. Amplitude fluctuations in small EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. J Neurosci. 1989 Mar;9(3):840–850. doi: 10.1523/JNEUROSCI.09-03-00840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapfer W. T., Woodson P. B., Smith G. A., Tremblay J. P., Barondes S. H. Marked prolongation of post-tetanic potentiation at a transition temperature in its adaption. Nature. 1975 Dec 18;258(5536):623–625. doi: 10.1038/258623a0. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]