Abstract
Sequencing mitochondrial and chloroplast genomes has become an integral part in understanding the genomic machinery and the phylogenetic histories of green algae. Previously, only three chloroplast genomes (Oltmannsiellopsis viridis, Pseudendoclonium akinetum, and Bryopsis hypnoides) and two mitochondrial genomes (O. viridis and P. akinetum) from the class Ulvophyceae have been published. Here, we present the first chloroplast and mitochondrial genomes from the ecologically and economically important marine, green algal genus Ulva. The chloroplast genome of Ulva sp. was 99,983 bp in a circular-mapping molecule that lacked inverted repeats, and thus far, was the smallest ulvophycean plastid genome. This cpDNA was a highly compact, AT-rich genome that contained a total of 102 identified genes (71 protein-coding genes, 28 tRNA genes, and three ribosomal RNA genes). Additionally, five introns were annotated in four genes: atpA (1), petB (1), psbB (2), and rrl (1). The circular-mapping mitochondrial genome of Ulva sp. was 73,493 bp and follows the expanded pattern also seen in other ulvophyceans and trebouxiophyceans. The Ulva sp. mtDNA contained 29 protein-coding genes, 25 tRNA genes, and two rRNA genes for a total of 56 identifiable genes. Ten introns were annotated in this mtDNA: cox1 (4), atp1 (1), nad3 (1), nad5 (1), and rrs (3). Double-cut-and-join (DCJ) values showed that organellar genomes across Chlorophyta are highly rearranged, in contrast to the highly conserved organellar genomes of the red algae (Rhodophyta). A phylogenomic investigation of 51 plastid protein-coding genes showed that Ulvophyceae is not monophyletic, and also placed Oltmannsiellopsis (Oltmannsiellopsidales) and Tetraselmis (Chlorodendrophyceae) closely to Ulva (Ulvales) and Pseudendoclonium (Ulothrichales).
Introduction
The green algae are comprised of two main clades, Chlorophyta and Streptophyta. Chlorophyta includes a wide diversity of marine, freshwater and terrestrial green algae. Streptophyta includes the freshwater charophyte green algae along with the land plants [1],[2]. Chlorophyta is currently composed of the paraphyletic prasinophytes along with five classes recently coined as the “core chlorophytes” (Chlorodendrophyceae, Chlorophyceae, Pedinophyceae, Trebouxiophyceae, and Ulvophyceae) [2],[3]. Several investigations have suggested that Ulvophyceae and Trebouxiophyceae are not monophyletic and further genomic investigations with an increased taxon sampling are still needed to resolve higher taxonomic ranks [3],[4],[5],[6].
In order to help answer the phylogenetic questions that remain for the green algae and to gain a better understanding of the organellar genomic composition of these organisms, plastid and mitochondrial genomes have been sequenced throughout most of the major chlorophyte lineages, and many more organellar genomes are expected to be published in the near future. Green algae have shown to have a wide range of organellar genome sizes, content (e.g. GC%, number of genes, and number of introns), and organization of genes [2],[7],[8]. Indeed, the mitochondrial genomes (mtDNA) have ranged from as small as 13 Kbp in Polytomella capuana [9] to as large as 201.8 Kbp in Chlorokybus atmophyticus [10], and the chloroplast genomes (cpDNA) have shown even larger size differences from 37.5 Kbp in the nonphotosynthetic and parasitic Helicosporidium sp. [11] to 521 Kbp in Floydiella terrestris [12] and 525 Kbp in Volvox carteri [13], or possibly as big as 2 Mbp in dasycladalean green algae [14],[15]. Members of the Chlorophyceae are known to have a reduced-derived state, where most of the mitochondrial genes have been transferred to the nucleus. Conversely, mtDNAs of the Ulvophyceae and Trebouxiophyceae have shown to have an expanded pattern from the hypothesized ancestral state due to an increase of intergenic spacers and introns [8]. An expanded pattern has also been seen in the mitochondrial genomes of land plants, suggesting that convergent evolution has taken place in the two major lineages of Viridiplantae [8],[16],[17].
Ulvophyceae is well represented by marine macroalgae (known as green seaweeds) [2],[5] and contains more than 1600 species [18]. However, only three complete chloroplast genomes, Oltmannsiellopsis viridis [19], Pseudendoclonium akinetum [20], and Bryopsis hypnoides [21], and two mitochondrial genomes, O. viridis [17] and P. akinetum [18], have been published from organisms that are currently classified in the Ulvophyceae. Additionally, early structural data of the chloroplast genomes have been published for Codium fragile [15] and Caulerpa sertulariodes [22], and incomplete DNA sequence data has been published for Caulerpa filiformis [23]. It is important to point out that some of these algae could potentially belong to completely different higher taxonomic ranks in the near future. For example, Fučíková et al. [3] recovered Oltmannsiellopsis as sister to Tetraselmis, a member of the Chlorodendrophyceae and early diverging core chlorophyte, with strong support in multi-gene phylogenies mostly based on genomic plastid data.
Species of the green algal genus Ulva are distributed worldwide, where they are ecologically important members in marine and brackish environments and sometimes can be found in freshwater streams and lakes. Some Ulva species are notorious for forming harmful algal blooms called green tides in eutrophic conditions [24],[25]. These macroalgae are either monostromatic and tubular, distromatic blades, or distromatic with hollow monostromatic margins [26]. Morphology-based species level identifications of Ulva are notably challenging due to the lack of diagnostic morphological characters, morphological plasticity [27], and cryptic diversity [28]. There are currently 105 Ulva species accepted [18]. However, this number is likely to change as molecular investigations continue to reveal the true identities of these algae [24],[27],[28],[29],[30],[31],[32],[33],[34].
Transcriptomic studies have been performed on Ulva prolifera [35] and U. linza [36], which have helped reveal some of the genomic mechanisms that these algae have to survive in the harsh intertidal zone such as having land-plant specific genes [36]. Further, U. prolifera showed to potentially have a C3-C4 photosynthetic pathway, which could explain the ability of these species to rapidly grow during green tides [35]. However, complete organellar genomes of Ulva have not previously been published.
In this study, we present the first complete chloroplast and mitochondrial genomes of Ulva. These genomes were sequenced, assembled, and annotated as circular-mapping DNA molecules. Additionally, we compared the Ulva sp. genomes to the previously published chlorophyte genomes in order to gain a better understanding of the evolution of these organellar genomes. Furthermore, we performed a phylogenomic investigation to assess the monophyly of the Ulvophyceae and relationships of ulvophytes with other core chlorophytan clades.
Materials and Methods
Sampling and Species Identification
A monostromatic and tubular Ulva specimen was collected in August 2011 from a jetty near Dauphin Island Sea Lab, Alabama, USA (30º14’50.1”N, 88º04’29.1”W). Since Dauphin Island Sea Lab is a part of The University of Alabama (UA) consortium and a source for learning at several levels, no specific permission was required to collect algal samples. The specimen in this study did not involve an endangered or protected species. The specimen was preserved in silica gel, and a herbarium voucher was deposited at The University of Alabama Herbarium (UNA00071828). An image of the herbarium voucher can be viewed in Supporting Information (S1 Fig).
Due to the difficulty of identifying Ulva species based on morphology, species identification was based on analyses of the chloroplast-encoded rbcL and tufA genes. These genes were selected since large amounts of data are currently available for these molecular markers. An NCBI-blastn of the genes helped find sequences with high identities. Sequences with an identity of 99% or greater were used with other previously published sequences to make alignments of rbcL (15 sequences; 1257 bp) and tufA (12 sequences; 738 bp) using MUSCLE [37]. A neighbor-joining tree was made in Geneious R7 (http://www.geneious.com; [38]) for each alignment with the Jukes-Cantor genetic distance model and 1000 bootstrap replicates (see S2 and S3 Figs, and S1 Table in Supporting Information for gene trees and taxa used, respectively). Monostroma grevillei (GU183089; rbcL) and Monostroma sp. (HQ610262; tufA) were used as outgroups. Additionally, the pairwise distances (bp) were calculated using MEGA 6 [39] to aid in the identification of this Ulva species (see S4 and S5 Figs in Supporting Information).
The rbcL sequence of Ulva sp. UNA00071828 clustered with Ulva sp. OTU1 from the Hawaiian Islands (GU138253; [31]). The 1257 bp alignment showed that these sequences are 100% identical, and thus, likely the same species (S4 Fig in Supporting Information). The closest tufA sequence that Ulva sp. UNA00071828 clustered with was an unidentified Ulva specimen from Australia (KF195535; [40]). These sequences varied by six bp (S5 Fig in Supporting Information), and further investigations would be necessary to determine whether these organisms are conspecific or distinct entities. Due to the difficulty of identifying Ulva species based on morphology and the inability of relating this organism with other sequence data, we are refraining from assigning this organism a species name as O’Kelly et al. [31] also opted for. It is possible that this organism has previously been described since many Ulva species lack molecular data, or Ulva sp. UNA00071828 may represent an undescribed species. Further molecular studies in combination with morphological investigations need to be performed for an accurate identification of this Ulva species.
DNA Sequencing and Assembly
Total genomic DNA was extracted using a QIAGEN DNEasy Plant Mini Kit (QIAGEN, Valencia, CA, USA). The genomic DNA was fragmented into 350 bp and sequenced using Illumina HiSeq 2000 technology at Cold Spring Harbor Laboratory, USA. The sequencing run produced ca. 23 million paired-end reads of 2 x 101 bp. Poor quality sequences and sequencing adapters were removed using Trim Galore! v0.3.7 [41], leaving 13,742,730 trimmed reads. De novo assemblies were run using Geneious, Velvet v1.2.10 [42], and CLC Genomics Workbench v6 [43] with the trimmed sequences. Following the assemblies, putative chloroplast and mitochondrial contigs were identified using a local blast in Geneious using a suite of 2,433 chloroplast and 123 mitochondrial genes from previously published chlorophyte genomes. After the contigs with blast hits for the chloroplast and mitochondrial genes were found, another de novo assembly was run separately for contigs containing chloroplast and mitochondrial genes. This resulted in one contig of ca. 100,000 bp for the chloroplast genome and another contig of ca. 73,000 bp for the mitochondrial genome. Trimmed reads were mapped iteratively to the putative chloroplast and mitochondrial contigs. This resulted in longer contigs with ca. 150 bp overlap in both contigs, which allowed the genomes to be closed.
Annotation of the Chloroplast and Mitochondrial Genomes
Open reading frames (ORFs) of 150 bp or greater were found in Geneious using a bacterial genetic code. Protein-coding genes and rRNA genes from previous published ulvophycean chloroplast and mitochondrial genomes were then mapped to the reference chloroplast and mitochondrial contigs using the ‘Map to Reference’ option in Geneious. Annotations of the mapped genes were transferred to the reference sequence, and identities of the genes were confirmed using NCBI-blastx. Further, a blastx of other ORFs without mapped hits helped find other putative genes. A blastx hit with an E-value cut off of 10-10 was considered as a significant hit. To find putative tRNA genes, the chloroplast and mitochondrial contigs were submitted to tRNAscan-SE v1.21 using the default search mode and Mito/Chloroplast model [44],[45]. A comparison of tRNAs present in Ulva and other ulvophytes can be found in the Supporting Information (S2 and S3 Tables). Introns were also annotated and intron-exon boundaries were located by aligning the sequences of genes containing introns with those of intronless homologs. A blastx of the intronic ORFs (if present) was used to search for conserved motifs. Intron insertion sites were compared among the ulvophytes. Insertion sites of introns in protein-coding genes were determined by the nucleotide before the insertion of the intron. Insertion sites of rRNAs and tRNAs were based on alignments of 16S (NC_004431) and 23S (NC_004431) rDNA of E. coli and trnL(UAA) (NC_002186) of Mesostigma viride (NC_002186), respectively. Inverted and tandem repeats were detected using einverted and equicktandem of the EMBOSS suite [46]. Gene maps were made with OGDRAW (http://ogdraw.mpimp-golm.mpg.de/; [47]). GenBank Accession Numbers of the chloroplast and mitochondrial genomes are as follows: KP720616, KP720617, respectively.
Rearrangements in Chlorophyte Plastid and Mitochondrial Genomes
The double-cut-and-join (DCJ) [48] genome distances were calculated with UniMoG [49],[50] to estimate the number of rearrangements between the selected genomes. The DCJ values were calculated with all genes, and a separate analysis was run without tRNAs (S4 and S5 Tables). Genome alignments with previously published chlorophyte organellar genomes were performed with the Mauve Genome Alignment v2.3.1 [51] Geneious Plugin and the progressive Mauve algorithm [52]. These alignments were made to show similarities in gene clusters called local collinear blocks (LCBs). LCBs also allow for visualizing major rearrangements as the LCBs are connected with lines in the alignment, and also display inverted regions (IR). The beginning of rbcL and cox1 were selected as the starting positions in the chloroplast and mitochondrial genomes, respectively, for the Mauve analyses (S6 and S7 Figs).
Phylogenomic Analyses
Phylogenomic analyses were based on chloroplast genome data of 52 members of Chlorophyta, largely based on Lemieux et al. [4],[53] and Fučíková et al. [3] (S6 Table). 51 chloroplast protein-coding genes were selected from Fučíková et al. [3]: atpA, atpB, atpE, atpF, atpH, atpI, infA, petA, petB, petG, psaA, psaB, psaC, psaJ, psaM, psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbN, psbT, rbcL, rpl2, rpl5, rpl14, rpl16, rpl20, rpl23, rpl32, rpl36, rps3, rps4, rps7, rps8, rps9, rps11, rps12, rps14, rps18, rps19, tufA, ycf3, ycf4 and ycf12. The concatenated alignment consisted of 50,595 positions and was 94% filled at the nucleotide level. Taxa with low gene coverage included Acetabularia_acetabulum (37 genes), Cephaleuros parasiticus (17 genes), Codium decorticatum (32 genes), Halimeda cylindracea (33 genes), Tetraselmis_sp. (11 genes), and Trentepohlia annulata (18 genes). We did not use the mitochondrial genome data because fewer complete mitochondrial genomes have been sequenced in the Chlorophyta and because phylogenomic analysis based on mitochondrion multigene data only weakly resolves relationships within the Chlorophyta [54].
DNA sequences were aligned for each gene separately using the ClustalW translational alignment function [55] in Geneious using a BLOSUM cost matrix, gap open penalty 10 and gap extend cost 0.1. Poorly aligned positions were removed using the Gblocks server (http://molevol.cmima.csic.es/castresana/Gblocks_server.html; [56]) using the least stringent settings: allowing smaller final blocks, gap positions within the final blocks, less strict flanking positions and many contiguous non-conserved positions. Gblocks removed 22,428 of the total 50,595 positions, leaving a nucleotide alignment of 28,167 positions. The resulting alignment was translated to obtain the amino acid alignment of 9389 positions. For the phylogenetic analysis of the DNA sequence alignment, only the first two codon positions were used.
Maximum likelihood and Bayesian trees were inferred from the DNA and amino acid (AA) alignments using RAxML v.7.3.5 [57] and MrBayes v.3.2.1 [58], respectively. For the DNA sequences, we specified a GTRCAT (RAxML) or GTR+Γ (MrBayes) model, and a partitioning strategy in which codon positions were separated (two partitions). The MrBayes analysis was run for five million (nucleotide alignment) or two million (AA alignment) generations. Two independent runs were performed for each data set. The first 10% of samples were discarded as burn-in. Convergence of the runs and stability of parameters were assessed using Tracer v.1.5 [59].
Results and Discussion
The Chloroplast Genome of Ulva sp.
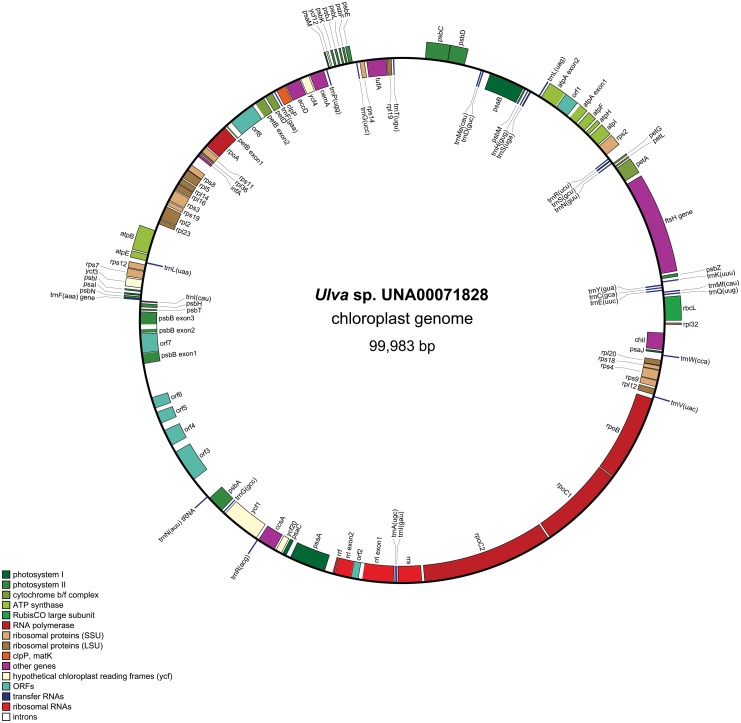
A total of 1,210,556 trimmed reads combined to form a 99,983 bp circular-mapping chloroplast genome of Ulva sp. UNA00071828 (Fig 1). The depth of coverage at each position was a mean of 1202.2 bp, and the whole assembly had a pairwise identity of 99.8%. Table 1 compares general characteristics of the plastid genomes of Ulva with other core chlorophytes. To date, the chloroplast genome of Ulva sp. is the smallest ulvophycean chloroplast genome to be sequenced. However, this is only compared to the three complete ulvophycean genomes: Oltmannsiellopsis viridis (151.9 Kbp; [19]), Pseudendoclonium akinetum (195.8 Kbp; [20]), and Bryopsis hypnoides (153.4 Kbp; [21]). The percent of coding DNA (81.8%) ranks among the highest found in green algae and especially the core chlorophytes. The high coding percentage of this genome is by far the most gene compact ulvophycean genome published, compared to O. viridis (60.4%), P. akinetum (62.4%), and B. hypnoides (59.4%). The cpDNA of Ulva sp. ranks among the highest AT content (74.7%) compared with the core chlorophytes published thus far [2],[7]. An IR, which is commonly found in other green algae and has been found in the plastid genomes of P. akinetum [20] and O. viridis [19], was absent in this Ulva genome, B. hypnoides [21], and the genomes of Codium fragile [15] and Caulerpa sertularioides [22]. Thus, the Ulva cpDNA is not a quadripartite structure.
Fig 1. Gene map of Ulva sp. UNA00071828 chloroplast genome using OGDRAW.
Genes in the clockwise direction are on the inside of the map, and genes in the counterclockwise direction are on the outside of the map. Annotated genes are colored according to the functional categories shown in the legend (bottom left).
Table 1. A comparison of core chlorophyte cpDNAs.
| GenBank Accession | Genome size (Kbp) | AT (%) | Total genes a | Protein-coding genes | tRNA genes | rRNA genes | Free-standing ORFs b | Coding DNA (%) c | Introns d | Intronic ORFs | IR size (Kbp) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulvophyceae | ||||||||||||
| Ulva sp. UNA00071828 | KP720616 | 100 | 74.7 | 102 | 71 | 28 | 3 | 4 | 81.8 | 5 | 4 | none |
| Pseudendoclonium akinetum | NC_008114 | 195.8 | 62.3 | 105 | 73 | 29 | 3 | 12 | 62.4 | 27 | 19 | 6 |
| Oltmannsiellopsis viridis | NC_008099 | 151.9 | 59.5 | 104 | 75 | 26 | 3 | 3 | 60.4 | 5 | 5 | 18.5 |
| Bryopsis hypnoides e | NC_013359 | 153.4 | 66.9 | 132 | 78 | 51 | 3 | 7 | 59.4 | 12 | 5 | none |
| Trebouxiophyceae | ||||||||||||
| Chlorella vulgaris | NC_001865 | 150.6 | 68.4 | 112 | 76 | 33 | 3 | 18 | 54.5 | 3 | 1 | none |
| Parachlorella kessleri | NC_012978 | 124 | 70 | 112 | 79 | 29 | 3 | 2 | 64.8 | 1 | 0 | 10.9 |
| Coccomyxa sp. | NC_015084 | 175.7 | 49.3 | 114 | 79 | 32 | 3 | 1 | 45.8 | 1 | 0 | none |
| Leptosira terrestris | NC_009681 | 195.1 | 72.7 | 107 | 76 | 28 | 3 | 1 | 47.3 | 4 | 1 | none |
| Chlorophyceae | ||||||||||||
| Acutodesmus obliquus | NC_008101 | 161.5 | 73.1 | 99 | 70 | 26 | 3 | 1 | 65.1 | 10 | 7 | 12 |
| Dunaliella salina | NC_016732 | 269 | 76.9 | 100 | 69 | 28 | 3 | 0 | 56.8 | 26 | 14 | 14.4 |
| Volvox carteri | GU084820 | 461 | 57.6 | 97 | 66 | 26 | 5 | 1 | 23.9 | 8 | 5 | 16 |
| Gonium pectorale | NC_020438 | 222.6 | 70.2 | 99 | 67 | 27 | 5 | 0 | 44.3 | 3 | 1 | 14.8 |
| Chlamydomonas reinhardtii | NC_005353 | 203.8 | 65.5 | 99 | 67 | 27 | 5 | 2 | 49.9 | 5 | 0 | 22.2 |
| Oedogonium cardiacum | NC_011031 | 196.5 | 70.5 | 102 | 70 | 29 | 3 | 2 | 77.1 | 23 | 11 | 35.5 |
| Floydiella terrestris | NC_014346 | 521.2 | 65.5 | 99 | 69 | 27 | 3 | 5 | 27.7 | 30 | 1 | none |
| Schizomeris leibleinii | NC_015645 | 182.8 | 72.8 | 101 | 68 | 30 | 3 | 2 | 71.4 | 37 | 8 | none |
| Stigeoclonium helveticum | NC_008372 | 223.9 | 71.1 | 98 | 67 | 28 | 3 | 2 | 54.6 | 25 | 10 | none |
| Pedinophyceae | ||||||||||||
| Pedinomonas minor | NC_016733 | 98.3 | 65.2 | 105 | 74 | 28 | 3 | 5 | 74.4 | 0 | 0 | 10.3 |
a A sum of protein-coding genes, tRNA genes, rRNA genes. Duplicated genes only counted once
b ORFs > 300 bp
c % of genome consisting of conserved genes (including introns) and ORFs > 300 bp
d Duplicated genes with introns only counted once
e Re-annotated by Frederik Leliaert
Gene Content
A total of 102 identifiable genes were annotated in the cpDNA of Ulva sp., including 71 protein-coding genes ranging from 93 bp (psaM) to 8067 bp (rpoC2), 28 tRNA genes, and three rRNA genes (rrf, rrl, and rrs). All of the protein-coding genes were also found in at least one of the previously published ulvophycean genome [19],[20],[21] (Table 2). The 28 tRNAs present were able to complete the full genetic code and two tRNA genes were found for trnG, trnF, trnI, trnL, trnM, trnN, trnR, and trnS (S2 Table in Supporting Information). In addition to the 102 identified genes, one freestanding ORF and three intronic ORFs had significant blastx hits in NCBI’s non-redundant protein database. The freestanding orf3 (2019 bp) had top hits of the NAD-dependent DNA ligase LigA found in several Rickettsia species and other species of bacteria; however, with low identities (e.g. 38% identical to R. montanensis, E-value 1e-115, WP_014409548; 37% identical to R. massiliae, E-value 5e-116, WP_014365478; 37% identical to R. rhipicephali, 1e-115, WP_014408984).
Table 2. A comparison of the gene content of the Ulva sp. cpDNA with 17 other core chlorophytes (excluding tRNAs).
| Ulvophyceae | Trebouxiophyceae | Chlorophyceae | Ped. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. | Pseu akin | Oltm viri | Bryo hypn | Chlo vulg | Para kess | Cocc sube | Lept terr | Acut obli | Duna sali | Volv cart | Goni pect | Chla rein | Oedo card | Floy terr | Schi leib | Stig helv | Ped mino | |
| accD | x | x | x | X | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| ccsA | x | x | x | x | x | x | x | - | x | x | x | x | x | x | x | x | x | x |
| chlB | - | - | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | - |
| chlI | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - | - | - | x |
| chlL | - | - | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | - |
| chlN | - | - | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | - |
| cysA | - | - | - | x | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| cysT | - | - | - | x | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| infA | x | x | x | x | x | x | x | x | x | - | - | - | - | x | x | - | - | - |
| minD | - | x | x | - | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| petA | x | x | x | x | x | x | x | x | x | x | x | x | x | - | - | - | - | x |
| psaI | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| psaM | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | x | x | x |
| rpl12 | x | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - | x |
| rpl19 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - | - | x |
| rpl32 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | x | x | x |
| tilS | - | x | - | x | - | x | x | x | - | - | - | - | - | - | - | - | - | - |
| ycf12 | x | x | x | x | x | x | x | - | x | x | x | x | x | x | x | x | x | x |
| ycf20 | x | x | x | - | - | x | x | x | - | - | - | - | - | - | - | - | - | x |
| ycf47 | - | - | - | x | - | x | x | - | - | - | - | - | - | - | - | - | - | - |
Twenty-four genes (not shown in table) were shared by all 19 taxa (atpA, atpB, atpE, atpF, atpH, atpI, cemA, clpP, ftsH, petB, petD, petG, petL, psaA, psaB, psaC, psaJ, psbA, psbB, psbC, psbD, psbE, psbF, ycf1). Fifty-seven other genes (not shown in table) present in Ulva sp. were also present in most but not all of the other 18 taxa (psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ, rbcL, rpl14, rpl16, rpl2, rpl20, rpl23, rpl36, rpl5, rpoA, rpoB, rpoC1, rpoC2, rps11, rps12, rps14, rps18, rps19, rps2, rps3, rps4, rps7, rps8, rps9, tufA, ycf3, ycf4, rrf, rrl, rrs, trnA, trnC, trnD, trnE, trnF, trnG, trnH, trnI, trnK, trnL, trnM, trnN, trnP, trnQ, trnR, trnS, trnT, trnV, trnW, trnY). An “x” represents the presence of a gene.
Introns
Five introns were present in four genes (atpA, psbB, petB, and rrl) of the Ulva chloroplast genome. AtpA, rrl, and intron 1 in psbB contained an intronic ORF with a LAGLIDADG conserved motif, and an intronic ORF was present in petB with a reverse transcriptase conserved motif. Intronic ORFs 1 (atpA), 2 (rrl), 7 (psbB), and 8 (petB) were 1138 bp, 767 bp, 2306 bp, and 2211 bp, respectively. An intronic ORF was not present in intron 2 in psbB. A total of 27, 5, and 12 introns were previously found in the chloroplast genomes of Pseudendoclonium akinetum [20] Oltmannsiellopsis viridis [19], and Bryopsis hypnoides [21], respectively. DNA alignments showed that the intron in atpA of Ulva is at the same position as the first intron in P. akinetum. Additionally, the intron in rrl of Ulva and the first intron in O. viridis are at homologous positions. A comparison of insertion sites of all of the ulvophyte introns can be found in Table 3.
Table 3. Intron insertion sites in ulvophyte mtDNA genes.
| Gene | Insertion Sites | Ulva sp. | Pseu akin | Oltm viri | Bryo hypn |
|---|---|---|---|---|---|
| atpA | 489 | 1138 | 1637 | - | - |
| 513 | - | 344 | - | - | |
| 684 | - | - | - | 1005 | |
| petB | 69 | 2211 | - | - | - |
| 534 | - | - | 1322 | - | |
| psaA | 1601 | - | 520 | - | - |
| 1794 | - | - | - | 2235 | |
| psaB | 291 | - | 1368 | - | - |
| 1047 | - | 1488 | - | - | |
| 1579 | - | 1272 | - | - | |
| psbA | 179 | - | 1068 | - | - |
| 271 | - | 1120 | - | - | |
| 384 | - | 1452 | - | - | |
| 408 | - | 1082 | - | - | |
| 525 | - | 1216 | 1127 | - | |
| 548 | - | 978 | - | - | |
| 570 | - | 1073 | - | - | |
| 645 | - | 333 | - | - | |
| 760 | - | 1045 | - | - | |
| 898 | - | 314 | - | - | |
| psbB | 489 | - | 966 | - | - |
| 600 | 1306 | - | - | - | |
| 689 | - | - | - | 1104 | |
| 772 | 351 | - | - | - | |
| 1022 | - | 1282 | - | - | |
| 1352 | - | 1230 | - | - | |
| psbC | 543 | - | 887 | - | - |
| 708 | - | 907 | - | - | |
| 882 | - | 960 | - | - | |
| 973 | - | 1051 | - | - | |
| psbD | 740 | - | 1118 | - | - |
| 1034 | - | 921 | - | - | |
| rbcL | 699 | - | 1682 | - | - |
| 834 | - | - | - | 2469 | |
| rpl2 | 326 | - | - | - | 837 |
| rpl23 | 30 | - | - | - | 392 |
| rpl5 | 161 | - | - | - | 354 |
| rrl | 1931 | 767 | - | 830 | - |
| 2500 | - | - | 1129 | - | |
| 2593 | - | 816 | 767 | - | |
| rrs | 510 | - | - | - | 971 |
| 794 | - | - | - | 882 | |
| tilS | 471 | - | - | - | 71 |
| trnL | 36 | - | - | - | 206 |
| ycf3 | 182 | - | - | - | 369 |
Insertion sites for protein-coding genes were based on the position relative to the homologous genes in Mesostigma viride (NC_002186). Insertion sites of rRNAs were based on 16S (NC_004431) and 23S (NC_004431) of Escherichia coli. Duplicated genes with introns are only shown once. The base pair before the intron is presented. The length of an intron (bp) is given if present at a specific site.
The Mitochondrial Genome of Ulva sp.
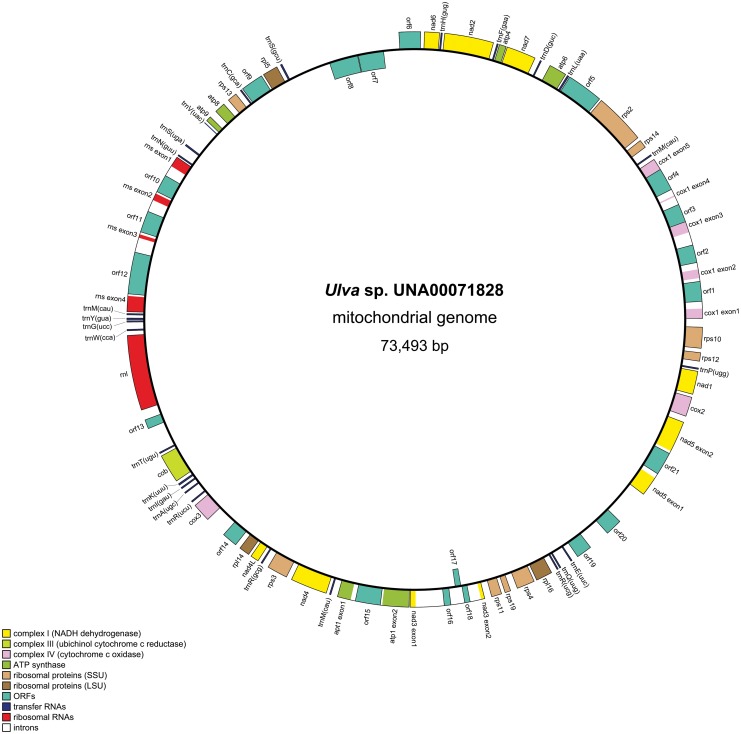
A total of 299,728 trimmed reads combined to form a 73,493 bp circular-mapping mitochondrial genome of Ulva sp. UNA00071828 (Fig 2). The depth of coverage at each site was a mean of 402.5 bp with a pairwise identity of 99.8%. Table 4 compares the overall characteristics of the Ulva sp. mtDNA with the previously published core chlorophyte mitogenomes. The mtDNA of Ulva sp. follows the expanded-derived pattern of the hypothesized ancestral state due to the presence of introns and/or increases in intergenic space as seen in the previously published ulvophyceans [8],[16],[17] and trebouxiophyceans [54],[60],[61],[62]. The “expanded” nature of the mtDNA has also been noted in the streptophyte lineage, suggesting the two major lineages of Viridiplantae have evolved expanded mitochondrial genomes through convergent evolution [16],[17]. However, the land plants have far larger mitochondrial genomes compared to green algae [63],[64]. Indeed, the embryophyte genomes are typically several hundred Kbp in length, and some are even over 1Mbp such as the 1.68 Mbp mtDNA of Cucumis sativus [65]. Thus far, all chlorophyte mtDNAs sequenced are under 100 Kbp [8]. The percent of coding DNA in the mtDNA of Ulva sp. was 75.8%, which is higher than the other two ulvophyceans, P. akinetum (56.7%) and Oltmannsiellopsis viridis (66.2%). More similar percentages of coding regions have been seen in the trebouxiophyceans, Helicosporidium sp. (75.7%), Trebouxiophyceae sp. MX-AZ01 (68.2%), and Prototheca wickerhamii (70.6%). The AT-content of the Ulva sp. mtDNA genome (67.6%) falls within the normal range of most green algae [8].
Fig 2. Gene map of Ulva sp. UNA00071828 mitochondrial genome using OGDRAW.
Genes in the clockwise direction are on the inside of the map, and genes in the counterclockwise direction are on the outside of the map. Annotated genes are colored according to the functional categories shown in the legend (bottom left).
Table 4. A comparison of core chlorophyte mtDNAs.
| GenBank Accession # | Genome size (Kbp) | Conformation | AT (%) | Total genes a | Protein-coding genes | tRNA genes | rRNA genes | Free-standing ORFs b | Coding DNA (%) c | Introns | Intronic ORFs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulvophyceae | ||||||||||||
| Ulva sp. UNA00071828 | KP720617 | 73.5 | circular | 67.6 | 56 | 29 | 25 | 2 | 9 | 75.8 | 10 | 12 |
| Oltmannsiellopsis viridis | NC_008256 | 56.8 | circular | 66.6 | 54 | 27 | 24 | 3 | 5 | 66.2 | 3 | 4 |
| Pseudendoclonium akinetum | NC_005926 | 95.9 | circular | 60.7 | 57 | 30 | 25 | 2 | 15 | 56.7 | 7 | 6 |
| Trebouxiophyceae | ||||||||||||
| Prototheca wickerhamii | NC_001613 | 55.3 | circular | 74.2 | 58 | 29 | 26 | 3 | 5 | 70.6 | 5 | 2 |
| Helicosporidium sp. | NC_017841 | 49.3 | circular | 74.4 | 60 | 32 | 25 | 3 | 2 | 75.7 | 3 | 3 |
| Trebouxiophyceae sp. | NC_018568 | 74.4 | circular | 46.6 | 56 | 30 | 23 | 3 | 3 | 68.2 | 10 | 8 |
| Chlorella sorokiniana | NC_024626 | 52.5 | circular | 66.6 | 60 | 32 | 25 | 3 | 1 | 64.1 | 0 | 0 |
| Coccomyxa sp. | NC_015316 | 65.5 | circular | 46.8 | 59 | 30 | 26 | 3 | 0 | 59.1 | 5 | 1 |
| Chlorophyceae | ||||||||||||
| Polytomella magna | NC_023091 | 24.4 | linear | 65.2 | 40 | 14 | 2 | 24 | 0 | 87.1 | 0 | 0 |
| Polytomella capuana | NC_010357 | 13 | linear | 42.8 | 20 | 7 | 1 | 12 | 0 | 82 | 0 | 0 |
| Chlamydomonas reinhardtii | EU306620 | 19 | linear | 55.4 | 23 | 8 | 3 | 12 | 0 | 84.2 | 3 | 3 |
| Chlamydomonas eugametos | NC_001872 | 22.9 | circular | 65.4 | 20 | 7 | 4 | 9 | 0 | 84.5 | 9 | 7 |
| Dunaliella salina | NC_012930 | 28.3 | circular | 69.7 | 19 | 7 | 3 | 9 | 0 | 69.7 | 18 | 2 |
| Gonium pectorale | NC_020437 | 16 | circular | 61.3 | 23 | 7 | 4 | 12 | 0 | 19 | 1 | 1 |
| Pleodorina starrii | NC_021108 | 20.4 | circular | 62 | 22 | 7 | 3 | 12 | 0 | 80.6 | 3 | 3 |
| Acutodesmus obliquus | NC_002254 | 42.8 | circular | 63.8 | 47 | 14 | 27 | 6 | 4 | 51.9 | 3 | 2 |
| Bracteacoccus aerius | NC_024755 | 47.2 | circular | 52.9 | 43 | 13 | 24 | 6 | 2 | 48 | 3 | 2 |
| Pedinophyceae | ||||||||||||
| Pedinomonas minor | NC_000892 | 25.1 | circular | 77.8 | 23 | 11 | 9 | 3 | 0 | 60.5 | 1 | 0 |
a A sum of protein-coding genes, tRNA genes, rRNA genes
b ORFs > 300 bp
c % of genome consisting of conserved genes (including introns) and ORFs > 300 bp
Gene Content
A total of 56 identifiable genes were annotated in the mitochondrial genome, including 29 protein-coding genes ranging from 193 bp (rps3) to 6876 bp (cox1), 25 tRNA genes, and two rRNA genes (rrs and rrl). The 29 protein-coding genes were also found in at least one of the previously published ulvophycean genome (Table 5). The Ulvophyceae also have more genes in common with Trebouxiophyceae than with Chlorophyceae (Table 5), as most of the chlorophycean mitochondrial genes have been transferred to the nucleus [8]. The mtDNA of Ulva sp. contains 25 tRNAs that completed the full genetic code. Two trnL genes were present in addition to three trnM and three trnR genes. The tRNAs found in Ulva sp. and other ulvophyceans are shown in S3 Table in the Supporting Information. One freestanding and 12 intronic ORFs were also annotated. The freestanding orf7 (1131 bp) had a significant blastx hit as a DNA-directed RNA polymerase in the land plants Phoenix dactylifera (31% identical, E-value 4e-11, YP_005090363) and Daucus carota (28% identical, E-value 2e-12, AAS15052).
Table 5. A comparison of the gene content of the Ulva sp. mtDNA with 13 other core chlorophytes (excluding tRNAs).
| Ulvophyceae | Trebouxiophyceae | Chlorophyceae | Ped. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. | Pseuakin | Oltm viri | Prot wick | Heli sp. | Treb sp. | Chlo soro | Cocc sp. | Poly magn | Chla rein | Duna sali | Goni pect | Pleo star | Acut obli | Brac mino | Pedi mino | |
| apt1 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| atp4 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| atp6 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | x |
| atp8 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | x |
| atp9 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | - |
| cox2 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | - |
| cox3 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | - |
| nad3 | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | x |
| nad4L | x | x | x | x | x | x | x | x | - | - | - | - | - | x | x | x |
| nad7 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| nad9 | - | - | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps2 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps3 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps4 | x | x | - | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps7 | - | - | - | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps10 | x | x | - | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps11 | x | x | x | x | x | - | x | - | - | - | - | - | - | - | - | - |
| rps12 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps13 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps14 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rps19 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rpl2 | - | - | x | - | - | - | - | - | - | - | - | - | - | - | - | - |
| rpl5 | x | x | - | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rpl6 | - | - | - | x | x | - | x | - | - | - | - | - | - | - | - | - |
| rpl14 | x | x | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| rpl16 | x | x | x | x | x | x | x | x | - | - | - | - | - | - | - | - |
| rrn5 | - | - | x | x | x | x | - | x | - | - | - | - | - | - | - | - |
| tatC | - | x | x | - | x | x | x | - | - | - | - | - | - | - | - | - |
Nine genes (not shown in table) were present in all core chlorophytes (nad1, nad2, nad4, nad5, nad6, cob, cox1, rrs, rrl). An “x” represents the presence of a gene.
Introns
A total of 10 introns were present in 5 genes (atp1, cox1, nad3, nad5, and rrs) in the mitochondrial genome of Ulva sp. Multiple introns were present in cox1 (4) and rrs (3). The presence of introns in cox1 indeed confirms why amplifying this gene in PCR has been such a difficult task [32]. Intronic ORFs with a LAGLIDADG conserved motif were found in atp1 (orf15: 1345 bp), nad5 (orf21: 1309 bp), each of the four introns in cox1 (orf1: 1287 bp, orf2: 1590 bp, orf3: 1162 bp, and orf4: 1240 bp, respectively), and intron 1 and intron 2 in rrs (orf10: 1319 bp and orf11: 1534 bp, respectively). Intron 3 in rrs contained an intronic ORF (orf12: 1734 bp) with a reverse transcriptase conserved motif. The intron in nad3 had two intronic ORFs (orf16: 288 bp; orf17: 156 bp) with a reverse transcriptase conserved motif and 1 intronic ORF (orf18: 240 bp) with a group II maturase conserved motif (GIIM). A total of 7 were previously annotated in the mtDNA of Pseudendoclonium akinetum [16] and 3 introns in Oltmannsiellopsis viridis [17]. DNA alignments showed that Ulva sp. and P. akinetum have a few introns at homologous positions. These include the intron in apt1, introns 1 and 2 in cox1, and intron 3 in Ulva and intron 4 in P. akinetum. Intron insertion sites in the ulvophyte mtDNAs can be found in Table 6.
Table 6. Intron insertion sites of ulvophyte mtDNA genes.
| Gene | Insertion Sites | Ulva sp. | Pseu akin | Oltm viri |
|---|---|---|---|---|
| atp1 | 540 | 1345 | 1424 | - |
| cob | 474 | - | - | 1226 |
| 865 | - | 911 | - | |
| cox1 | 429 | 1287 | 2048 | - |
| 751 | 1590 | 1595 | - | |
| 773 | - | 1659 | - | |
| 781 | - | - | 2414 | |
| 1149 | 1162 | 1731 | - | |
| 1167 | 1240 | - | - | |
| nad3 | 218 | 2727 | - | - |
| nad5 | 664 | 1309 | - | - |
| rrs | 521 | 1319 | - | - |
| 801 | 1534 | - | - | |
| 915 | 2449 | - | - | |
| rrl | 2500 | - | - | 1258 |
Insertion sites for protein-coding genes were based on the position relative to the homologous genes in Mesostigma viride (NC_002186). Insertion sites of rRNAs were based on 16S (NC_004431) and 23S (NC_002186) of Escherichia coli. The base pair before the inserted intron is given. If an intron was present at a specific site, the length of the intron (bp) is given if present.
Rearrangements in the Chlorophyte cpDNA and mtDNA
The number of rearrangements between organisms from lineages throughout Chlorophyta was estimated by calculating the double-cut-and-join (DCJ) values. The DCJ values calculated for the cpDNAs and mtDNAs with tRNAs are shown in Tables 7 and 8, respectively. The DCJ values calculated for cpDNAs and mtDNAs without tRNAs can be found in the Supporting Information (S4 and S5 Tables, respectively). Mauve alignments of the selected chlorophyte genomes can also be found in the Supporting Information (S6 and S7 Figs for cpDNA and mtDNA, respectively).
Table 7. DCJ values for chlorophyte cpDNAs of chlorophytes (includes tRNAs).
| Ulva sp. | Pseu akin | Oltm viri | Bryo hypn | Chlo vulg | Cocc sp. | Acut obli | Duna sali | Oedo card | Pedi mino | Pycn prov | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. | 0 | 37 | 58 | 63 | 55 | 64 | 76 | 74 | 76 | 57 | 57 |
| Pseu akin | - | 0 | 59 | 67 | 58 | 67 | 75 | 72 | 78 | 56 | 56 |
| Oltm viri | - | - | 0 | 65 | 56 | 66 | 75 | 78 | 81 | 54 | 58 |
| Bryo hypn | - | - | - | 0 | 67 | 73 | 78 | 79 | 84 | 65 | 61 |
| Chlo vulg | - | - | - | - | 0 | 62 | 74 | 69 | 76 | 47 | 57 |
| Cocc sp. | - | - | - | - | - | 0 | 77 | 75 | 79 | 58 | 60 |
| Acut obli | - | - | - | - | - | - | 0 | 55 | 76 | 69 | 72 |
| Duna sali | - | - | - | - | - | - | - | 0 | 72 | 68 | 71 |
| Oedo card | - | - | - | - | - | - | - | - | 0 | 75 | 76 |
| Pedi mino | - | - | - | - | - | - | - | - | - | 0 | 58 |
| Pycn prov | - | - | - | - | - | - | - | - | - | - | 0 |
The GenBank accession numbers used are as follows: Ulva sp. (KP720616), Pseudendoclonium akinetum (NC_008114), Oltmannsiellopsis viridis (NC_008099), Bryopsis hypnoides (NC_013359), Chlorella vulgaris (NC_001865), Coccomyxa sp. C-169 (NC_015084), Acutodesmus obliquus (NC_008101), Dunaliella salina (NC_016732), Oedogonium cardiacum (NC_011031), Pedinomonas minor (NC_016733), Pycnococcus provasolii (NC_012097).
Table 8. DCJ values calculated for mtDNAs of chlorophytes (includes tRNAs).
| Ulva sp. | Pseu akin | Oltm viri | Prot wick | Heli sp. | Chlo vari | Acut obli | Pedi mino | Neph oliv | Ostr taur | Pycn prov | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ulva sp. | 0 | 36 | 39 | 47 | 47 | 47 | 27 | 16 | 48 | 44 | 32 |
| Pseu akin | - | 0 | 45 | 44 | 48 | 45 | 29 | 16 | 49 | 44 | 32 |
| Oltm viri | - | - | 0 | 47 | 46 | 45 | 26 | 16 | 46 | 38 | 28 |
| Prot wick | - | - | - | 0 | 23 | 27 | 27 | 16 | 46 | 42 | 31 |
| Heli sp. | - | - | - | - | 0 | 27 | 28 | 17 | 50 | 45 | 31 |
| Chlo vari | - | - | - | - | - | 0 | 31 | 15 | 45 | 45 | 28 |
| Acut obli | - | - | - | - | - | - | 0 | 11 | 31 | 28 | 22 |
| Pedi mino | - | - | - | - | - | - | - | 0 | 15 | 14 | 12 |
| Neph oliv | - | - | - | - | - | - | - | - | 0 | 24 | 30 |
| Ostr taur | - | - | - | - | - | - | - | - | - | 0 | 26 |
| Pycn prov | - | - | - | - | - | - | - | - | - | - | 0 |
The GenBank accession numbers used are as follows: Ulva sp. (KP720617), Pseudendoclonium akinetum (NC_005926), Oltmannsiellopsis viridis (NC_008256), Prototheca wickerhamii (NC_001613), Helicosporidium sp. (NC_017841), Chlorella variabilis (NC_025413), Acutodesmus obliquus (NC_002254), Pedinomonas minor (NC_000892), Nephroselmis olivacea (NC_008239), Ostreococcus tauri (NC_008290), Pycnococcus provasolii (NC_013935).
The DCJ values and Mauve alignments show that both organellar genomes of chlorophytes are highly rearranged. This is in contrast to the highly conserved organellar genomes of red algae (Rhodophyta) [66],[67]. The cpDNA of Ulva had the lowest DCJ value with Pseudendoclonium (37 with tRNAs and 22 without tRNAs), which was expected since these organisms are more closely related than the other organisms in this analysis. Of the expanded mtDNAs (i.e. Ulvophyceae and Trebouxiophyceae), Ulva also had the lowest DCJ value with Pseudendoclonium (36 with tRNAs and 17 without tRNAs). The lower DCJ values of Ulva compared to Pedinomonas, Acutodesmus, and Pycnococcus according to Table 8 and S7 Table are likely due to the reduced gene count in the mtDNA of these three organisms.
Phylogenomic Analyses
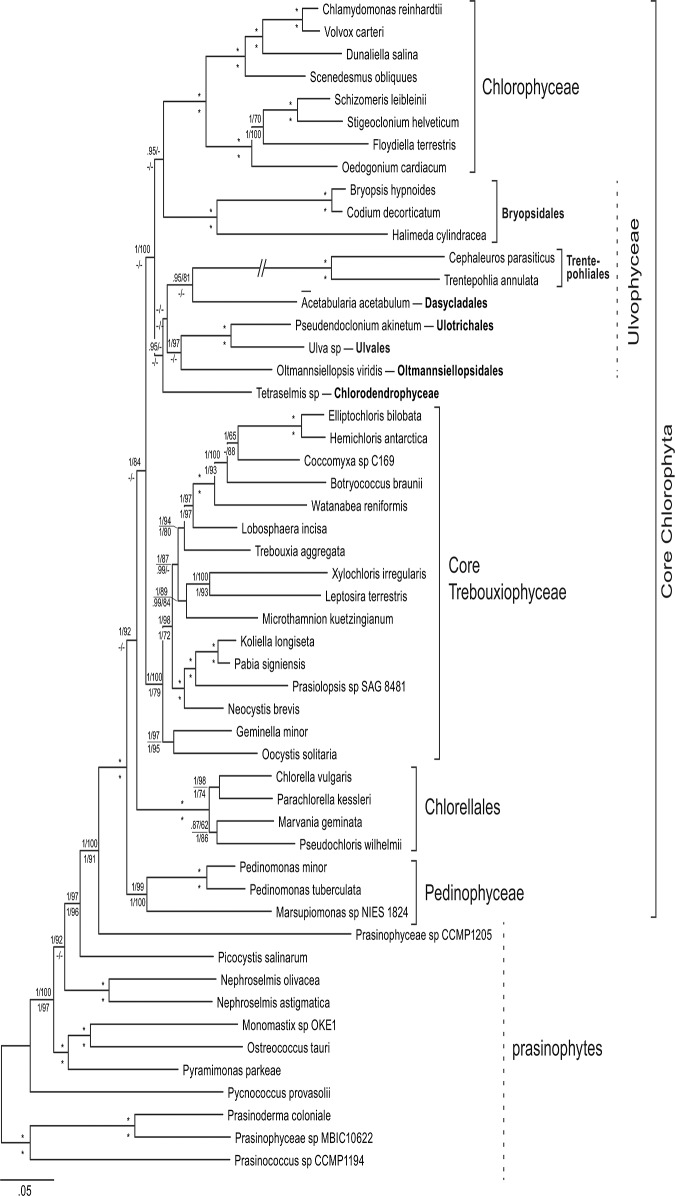
The phylogenetic tree resulting from Bayesian analyses of the DNA alignment, along with branch support from the other analyses (ML and BI of the DNA and AA alignments) is presented in Fig 3. The ML and Bayesian trees based on an amino acid alignment are presented in the Supporting Information (S8 and S9 Figs, respectively).
Fig 3. Bayesian majority rule tree showing all compatible partitions, inference from the nucleotide alignment of 51 concatenated chloroplast genes (first two codon positions: 28,167 nucleotide positions and 52 terminal taxa).
Node support is given as Bayesian posterior probabilities and maximum-likelihood (ML) bootstrap values of the nucleotide analyses (above branches), and the amino acid analyses (below branches); values <0.9 and 50, respectively, are not shown; asterisks indicated full support in both the Bayesian and ML analyses.
As expected, Ulva (Ulvales) and Pseudendoclonium akinetum (Ulotrichales; according to [68]) formed a clade in all analyses. The topology of the tree was in general agreement with previous chloroplast phylogenomic analyses [3],[4],[53] in recovering the Pedinophyceae and the Chlorellales as early branching lineages of the core Chlorophyta, and the Chlorophyceae and core Trebouxiophyceae as strongly supported clades within the core Chlorophyta. Similar to the analysis of Fučíková et al. [3], but in contrast to the analysis of Cocquyt et al. [5], the Ulvophyceae fell apart into several clades, including the Bryopsidales (Bryopsis, Codium, Halimeda), Dasycladales (Acetabularia), Trentepohliales (Cephaleuros, Trentepohlia), and Ulvales (Ulva, Pseudendoclonium). The relationship among these clades was not well supported. A notable difference between our tree and the phylogeny of Fučíková et al. [3] was the position of Oltmannsiellopsis (Oltmannsiellopsidales) and Tetraselmis. Oltmannsiellopsis is currently classified as an ulvophyte, which was supported by chloroplast phylogenomic analyses (e.g. [4];[23]). Tetraselmis is a member of the Chlorodendrophyceae and in nuclear ribosomal DNA based phylogenies has been recovered as an early branching clade of the core Chlorophyta [2], [69]. The analyses of Fučíková et al. [3] unexpectedly supported a clade including Tetraselmis and Oltmannsiellopsis, which was found branching early in the radiation of the core Chlorophyta. In contrast, our analysis places Oltmannsiellopsis and Tetraselmis in the vicinity of the Ulvales-Ulotrichales. Our nucleotide based phylogenetic analysis placed Tetraselmis sister to the Oltmannsiellopsis-Ulvales-Ulotrichales clade (Fig 3), where as the AA based phylogeny recovered a Tetraselmis-Oltmannsiellopsis clade that was sister to the Ulvales-Ulotrichales clade. It should be noted that taxon sampling in the Ulvophyceae is still poor compared to the Trebouxiophyceae and Chlorophyceae, and that gene sampling in Tetraselmis is incomplete. Future studies with additional gene and taxon sampling will undoubtedly shed light on the relationships among the Ulvophyceae and the phylogenetic positions of the Oltmannsiellopsidales and Chlorodendrophyceae.
Conclusion
Organellar genomes contain valuable information that can be used for understanding the evolution of these organelles as well as phylogenomic inferences of the green algal lineages. Our investigation of the chloroplast and mitochondrial genomes of Ulva sp. has added to the limited amount of genomic data available for the Ulvophyceae. We hope that these genomes will be useful for exploring primer sets for future molecular investigations to aid in species delimitation of Ulva, especially since only limited mitochondrial data is currently available for these taxonomically troublesome algae. As more genomes are published in the near future, we will gain further insights into the evolution of the highly rearranged mitochondrial and chloroplast genomes of green algae, and hopefully answer the major phylogenetic questions that still remain in the green algae tree of life.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(A) Pseudendoclonium akinetum (NC_008114), (B) Oltmannsiellopsis viridis (NC_008099), (C) Bryopsis hypnoides (NC_013359), (D) Chlorella vulgaris (NC_001865), (E) Oedogonium cardiacum (NC_011031), (F) Acutodesmus obliquus (NC_008101), (G) Pedinomonas minor (NC_025530), (H) Pycnococcus provasolii (NC_012097), and (I) Ostreococcus tauri (NC_008289).
(PDF)
(A) Pseudendoclonium akinetum (NC_005926), (B) Oltmannsiellopsis viridis (NC_008256), (C) Chlorella sorokiniana (NC_024626), (D) Prototheca wickerhamii (NC_001613), (E) Helicosporidium sp. (NC_017841), (F) Trebouxiophyceae sp. MX-AZ01 (NC_018568), (G) Pedinomonas minor (NC_000892), (H) Acutodesmus obliquus (NC_002254), (I) Ostreococcus tauri (NC_008290), (J) Nephroselmis olivacea (NC_008239), (K) Micomonas sp. RCC299 (NC_012643), and (L) Pycnococcus provasolii (NC_013935).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Zi-Min Hu and UA undergraduate student David Ward for technical assistance and the reviewers for providing helpful feedback.
Data Availability
All genome sequences are available from the GenBank database: the chloroplast genome of Ulva sp. (KP720616) and the mitochondrial genome of Ulva sp. (KP720617).
Funding Statement
This study was funded by The National Science Foundation under the ATOL Program, through grants DEB 1036495 and 0937978 to JMLB. Additional support was provided by the College of Arts and Sciences (CARSCA Program) and the Office for Research (Research Stimulation Program) at UA to JMLB, and the Department of Biological Sciences (research and travel awards) to JTM.
References
- 1. Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am J Bot 91: 1535–1556. 10.3732/ajb.91.10.1535 [DOI] [PubMed] [Google Scholar]
- 2. Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, et al. (2012) Phylogeny and Molecular Evolution of the Green Algae. Crit Rev Plant Sci 31: 1–46. [Google Scholar]
- 3. Fučíková K, Leliaert F, Cooper ED, Škaloud P, D'hondt S, De Clerck O, et al. (2014) New phylogenetic hypotheses for the core Chlorophyta based on chloroplast sequence data. Front Ecol Evol 2: 63. [Google Scholar]
- 4. Lemieux C, Otis C, Turmel M (2014) Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evol Biol 14: 211 10.1186/s12862-014-0211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cocquyt E, Verbruggen H, Leliaert F, De Clerck O (2010) Evolution and cytological diversification of the green seaweeds (Ulvophyceae). Mol Biol Evol 27: 2052–2061. 10.1093/molbev/msq091 [DOI] [PubMed] [Google Scholar]
- 6. Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG (2014) From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol Biol 14: 23 10.1186/1471-2148-14-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang BF, Nedelcu AM (2012) Plastid Genomes of Algae In: Bock R, Knoop V, editors. Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes: Genomics of Chloroplasts and Mitochondria. pp. 59–87. 10.1007/s11120-013-9921-3 [DOI] [Google Scholar]
- 8. Burger G, Nedelcu AM (2012) Mitochondrial Genomes of Algae In: Bock R, Knoop V, editors. Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes: Genomics of Chloroplasts and Mitochondria. pp. 125–157. 10.1007/s11120-013-9921-3 [DOI] [Google Scholar]
- 9. Smith DR, Lee RW (2008) Mitochondrial genome of the colorless green alga Polytomella capuana: a linear molecule with an unprecedented GC content. Mol Biol Evol 25: 487–496. 10.1093/molbev/msm245 [DOI] [PubMed] [Google Scholar]
- 10. Turmel M, Otis C, Lemieux C (2007) An unexpectedly large and loosely packed mitochondrial genome in the charophycean green alga Chlorokybus atmophyticus . BMC Genomics 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Koning AP, Keeling PJ (2006) The complete plastid genome sequence of the parasitic green alga Helicosporidium sp. is highly reduced and structured. BMC Biology 4: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouard J-S, Otis C, Lemieux C, Turmel M (2010) The exceptionally large chloroplast genome of the green alga Floydiella terrestris illuminates the evolutionary history of the Chlorophyceae. Genome Biol Evol 2: 240–256. 10.1093/gbe/evq014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith DR, Lee RW (2010) The mitochondrial and plastid genomes of Volvox carteri: bloated molecules rich in repetitive DNA. BMC Genomics 10: 132 10.1186/1471-2229-10-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Padmanabhan U, Green BR (1978) The kinetic complexity of Acetabularia chloroplast DNA. BBA—Nucleic Acids and Protein Synthesis 521: 67–73. [DOI] [PubMed] [Google Scholar]
- 15. Manhart JR, Kelly K, Dudock BS, Palmer JD (1989) Unusual characteristics of Codium fragile chloroplast DNA revealed by physical and gene mapping. Mol Gen Genet 216: 417–421. [DOI] [PubMed] [Google Scholar]
- 16. Pombert J-F, Otis C, Lemieux C, Turmel M (2004) The complete mitochondrial DNA sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) highlights distinctive evolutionary trends in the Chlorophyta and suggests a sister-group relationship between the Ulvophyceae and Chlorophyceae. Mol Biol Evol 21: 922–935. [DOI] [PubMed] [Google Scholar]
- 17. Pombert J-F, Beauchamp P, Otis C, Lemieux C, Turmel M (2006) The complete mitochondrial DNA sequence of the green alga Oltmannsiellopsis viridis: evolutionary trends of the mitochondrial genome in the Ulvophyceae. Curr Genet 50: 137–147. [DOI] [PubMed] [Google Scholar]
- 18. Guiry MD, Guiry GM (2014) AlgaeBase World-wide electronic publication, National University of Ireland, Galway: Available: http://www.algaebase.org. Accessed 15 September 2014. [Google Scholar]
- 19. Pombert J-F, Lemieux C, Turmel M (2006) The complete chloroplast DNA sequence of the green alga Oltmannsiellopsis viridis reveals a distinctive quadripartite architecture in the chloroplast genome of early diverging ulvophytes. BMC Biology 4: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pombert J-F, Otis C, Lemieux C, Turmel M (2005) The chloroplast genome sequence of the green alga Pseudendoclonium akinetum (Ulvophyceae) reveals unusual structural features and new insights into the branching order of chlorophyte lineages. Mol Biol Evol 22: 1903–1918. [DOI] [PubMed] [Google Scholar]
- 21. Lü F, Xü W, Tian C, Wang G, Niu J, Pan G, et al. (2011) The Bryopsis hypnoides plastid genome: multimeric forms and complete nucleotide sequence. PLoS ONE 6: e14663 10.1371/journal.pone.0014663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehman RL, Manhart JR (1997) A Preliminary Comparison of Restriction Fragment Patterns in the Genus Caulerpa (Chlorophyta) and the Unique Structure of the Chloroplast Genome of Caulerpa sertulariodes . J Phycol 33: 1055–1062. 9376187 [Google Scholar]
- 23. Zuccarello GC, Price N, Verbruggen H, Leliaert F (2009) Analysis of a plastid multigene data set and the phylogenetic position of the marine macroalga Caulerpa filiformis (Chlorophyta). J Phycol 45: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 24. Leliaert F, Zhang X, Ye N, Malta E- J, Engelen AH, Mineur F, et al. (2009) Identity of the Qingdao algal bloom. Phycol Res 57: 147–151. [Google Scholar]
- 25. Charlier RH, Morand P, Finkl CW, Thys A (2007) Green Tides on the Brittany Coasts. EREM 3: 52–59. [Google Scholar]
- 26. Norris JN (2010) Marine Algae of the Northern Gulf of California: Chlorophyta and Phaeophyceae. Washington, D.C.: Smithsonian Institution Scholarly Press; p. 31. [Google Scholar]
- 27. Hayden H, Blomster J, Maggs CA, Silva PC, Stanhope MJ, Waaland JR (2003) Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur J Phycol 38: 27–294. 12593914 [Google Scholar]
- 28. Hofmann L, Nettleton J, Neefus C, Mathieson A (2010) Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the Great Bay Estuarine System (Atlantic USA): introduced and indigenous distromatic species. Eur J Phycol 45: 230–239. [Google Scholar]
- 29. Heesch S, Broom JES, Neill KF, Farr TJ, Dalen JL, Nelson WA (2009) Ulva, Umbraulva and Gemina: genetic survey of New Zealand taxa reveals diversity and introduced species. Eur J Phycol 44: 143–154. [Google Scholar]
- 30. Kraft LGK, Kraft GT, Waller RF (2010) Investigations Into Southern Australian Ulva (Ulvophyceae, Chlorophyta) Taxonomy and Molecular Phylogeny Indicate Both Cosmopolitanism and Endemic Cryptic Species. J Phycol 46: 1257–1277. [Google Scholar]
- 31. O’Kelly CJ, Kurihara A, Shipley TC, Sherwood AR (2010) Molecular assessment of Ulva spp. (Ulvophyceae, Chlorophyta) in the Hawaiian Islands. J Phycol 46: 728–735. [Google Scholar]
- 32. Saunders GW, Kucera H (2010) An evaluation of rbcL, tufA, UPA, LSU and ITS as DNA barcode markers for the marine green macroalgae. Cryptogam, Algol 31: 487–528. [Google Scholar]
- 33. Kirkendale L, Saunders GW, Winberg P (2012) A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. J Phycol 49: 69–81. [DOI] [PubMed] [Google Scholar]
- 34. Guidone M, Thornber C, Wysor B, O’Kelly CJ (2013) Molecular and morphological diversity of Narragansett Bay (RI, USA) Ulva (Ulvales, Chlorophyta) populations. J Phycol 49: 979–995. [DOI] [PubMed] [Google Scholar]
- 35. Xu J, Fan X, Zhang X, Xu D, Mou S, Cao S, et al. (2012) Evidence of coexistence of C3 and C4 photosynthetic pathways in a green-tide-forming alga, Ulva prolifera . PLoS ONE 7: e37438 10.1371/journal.pone.0037438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang X, Ye N, Liang C, Mou S, Fan X, et al. (2012) De novo sequencing and analysis of the Ulva linza transcriptome to discover putative mechanisms associated with its successful colonization of coastal ecosystems. BMC Genomics 13: 565 10.1186/1471-2164-13-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tamura K, Stecher G, Peterson D, Fiüipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawton RJ, Mata L, de Nys R, Paul NA (2013) Algal bioremediation of waste waters from land-based aquaculture using Ulva: selecting target species and strains. PLoS ONE 8: e77344 10.1371/journal.pone.0077344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trim Galore! v0.3.7. created by Babraham Bioinformatics. Available: http://www.bioinformatics.babraham.ac.uk/.
- 42. Zerbino DR, Birney E (2008) Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18: 821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CLC Genomics Workbench 7.0.3. Available: http://www.clcbio.com.
- 44. Lowe TM, Eddy SR (1997) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schattner P, Brooks AN, Lowe TM (2005) tRNAscan-SE: a Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res 33: 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rice P, Longden I, Bleasby A (2000) EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet 16: 276–277. [DOI] [PubMed] [Google Scholar]
- 47. Lohse M, Drechsel O, Kahlau S, Bock R (2013) OrganellarGenomeDRAW–a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Research 10.1093/nar/gkt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braga MDV, Willing E, Stoye J (2011) Double cut and join with insertions and deletions. J Comput Biol 18: 1167–1184. 10.1089/cmb.2011.0118 [DOI] [PubMed] [Google Scholar]
- 49. Hilker R, Sickinger C, Pedersen CNS, Stoye J (2012) UniMoG–a unifying framework for genomic distance calculation and sorting based on DCJ. Bioinformatics. 28: 2509–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bergeron A, Mixtacki J, Stoye J. A unifying view of genome rearrangements In: Bücher P, Moret BE, editors. Algorithms in Bioinformatics. Springer Berlin; Heidelberg; 2006. pp. 163−173. [Google Scholar]
- 51. Darling AE, Mau B, Blatter FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14: 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Darling AE, Mau B, Perna NT (2010) progressiveMauve: multiple genome alignment with gene gain, loss, and rearrangement. PLoS ONE 5: e11147 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lemieux C, Otis C, Turmel M (2014) Six newly sequenced chloroplast genomes from prasinophyte green algae provide insights into the relationships among prasinophyte lineages and the diversity of streamlined genome architecture in picoplanktonic species. BMC Genomics 15: 857 10.1186/1471-2164-15-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith DR, Burki F, Yamada T, Grimwood J, Grigoriev IV, Van Etten JL, et al. (2011) The GC-rich mitochondrial and plastid genomes of the green alga Coccomyxa give insight into the evolution of organelle DNA nucleotide landscape. PLoS ONE 6: e23624 10.1371/journal.pone.0023624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 56. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552. [DOI] [PubMed] [Google Scholar]
- 57. Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 58. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 59.Rambaut A, Drummond AJ (2007) Tracer v1.4, Available from http://beast.bio.ed.ac.uk/Tracer.
- 60. Servín-Garcidueñas LE, Martínez-Romero E (2012) Complete Mitochondrial and Plastid Genomes of the Green Microalga Trebouxiophyceae sp. Strain MX-AZ01 Isolated from a Highly Acidic Geothermal Lake. Eukaryot Cell 11: 1417–1418. 10.1128/EC.00244-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolff G, Plante I, Lang BF, Kück U, Burger G (1994) Complete sequence of the mitochondrial DNA of the Chlorophyte Alga Prototheca wickerhamii: gene content and genome organization. J Mol Biol 237: 75–86. [DOI] [PubMed] [Google Scholar]
- 62. Pombert J-F, Keeling PJ (2010) The Mitochondrial Genome of the Entomoparasitic Green Alga Helicosporidium . PLoS ONE. 10.1371/journal.pone.0008954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Xue J, Wang B, Li L, Qiu Y-L (2012) Conservative and Dynamic Evolution of Mitochondrial Genomes in Early Land Plants In: Bock R, Knoop V, editors. Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes: Genomics of Chloroplasts and Mitochondria. pp. 159–174. 10.1007/s11120-013-9921-3 [DOI] [Google Scholar]
- 64. Knoop V. Seed Plant Mitochondrial Genomes: Complexity Evolving In: Bock R, Knoop V, editors. Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes: Genomics of Chloroplasts and Mitochondria. Springer; Dordrecht Heidelberg New York London; 2012. pp. 175–200. 10.1007/s11120-013-9921-3 [DOI] [Google Scholar]
- 65. Alverson AJ, Rice DW, Dickinson S, Barry K, Palmer JD (2011) Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell 23: 2499–2513. 10.1105/tpc.111.087189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DePriest MS, Bhattacharya D, Lopez-Bautista JM (2013) The Plastid Genome of the Red Macroalga Grateloupia taiwanensis (Halymeniaceae). PLoS ONE 8: e68246 10.1371/journal.pone.0068246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DePriest MS, Bhattacharya D, Lopez-Bautista JM (2014) The Mitochondrial Genome of Grateloupia taiwanensis (Halymeniaceae, Rhodophyta) and Comparative Mitochondrial Genomic of Red Algae. Biol Bull 227: 191–200. [DOI] [PubMed] [Google Scholar]
- 68. Škaloud P, Nedbalová L, Elster J, Komárek J (2013) A curious occurrence of Hazenia broadyi spec. nova in Antarctica and the review of the genus Hazenia (Ulotrichales, Chlorophyceae). Polar Biol 36: 1281–1291. [Google Scholar]
- 69. Marin B (2012) Nested in the Chlorellales or independent class? Phylogeny and classification of the Pedinophyceae (Viridiplantae) revealed by molecular phylogenetic analyses of complete nuclear and plastid-encoded rRNA operons. Protist 163: 778–805. 10.1016/j.protis.2011.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(A) Pseudendoclonium akinetum (NC_008114), (B) Oltmannsiellopsis viridis (NC_008099), (C) Bryopsis hypnoides (NC_013359), (D) Chlorella vulgaris (NC_001865), (E) Oedogonium cardiacum (NC_011031), (F) Acutodesmus obliquus (NC_008101), (G) Pedinomonas minor (NC_025530), (H) Pycnococcus provasolii (NC_012097), and (I) Ostreococcus tauri (NC_008289).
(PDF)
(A) Pseudendoclonium akinetum (NC_005926), (B) Oltmannsiellopsis viridis (NC_008256), (C) Chlorella sorokiniana (NC_024626), (D) Prototheca wickerhamii (NC_001613), (E) Helicosporidium sp. (NC_017841), (F) Trebouxiophyceae sp. MX-AZ01 (NC_018568), (G) Pedinomonas minor (NC_000892), (H) Acutodesmus obliquus (NC_002254), (I) Ostreococcus tauri (NC_008290), (J) Nephroselmis olivacea (NC_008239), (K) Micomonas sp. RCC299 (NC_012643), and (L) Pycnococcus provasolii (NC_013935).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All genome sequences are available from the GenBank database: the chloroplast genome of Ulva sp. (KP720616) and the mitochondrial genome of Ulva sp. (KP720617).