Abstract
The development and function of B lymphocytes is regulated by numerous signaling pathways, some emanating from the B-cell antigen receptor (BCR). The spleen tyrosine kinase (Syk) plays a central role in the activation of the BCR, but less is known about its contribution to the survival and maintenance of mature B cells. We generated mice with an inducible and B-cell-specific deletion of the Syk gene and found that a considerable fraction of mature Syk-negative B cells can survive in the periphery for an extended time. Syk-negative B cells are defective in BCR, RP105 and CD38 signaling but still respond to an IL-4, anti-CD40, CpG or LPS stimulus. Our in vivo experiments show that Syk-deficient B cells require BAFF receptor and CD19/PI3K signaling for their long-term survival. These studies also shed a new light on the signals regulating the maintenance of the normal mature murine B-cell pool.
Keywords: BAFF receptor, B-cell antigen receptor, CD19, mb1-CreERT2, Syk
See also: S Kőnigsberger & F Kiefer (April 2015)
Introduction
B lymphocytes play an essential role in the establishment of humoral immunity. Their production and maintenance requires the expression of a functional BCR, as well as signals from the B-cell environment (Dorshkind, 1990; Lam et al, 1997). In the adult mouse, B-cell development occurs first in the bone marrow (BM) in the presence of the chemokine CXCL12 and interleukin IL-7 (Grabstein et al, 1993; Nagasawa, 2007). The expression of a pre-BCR on the surface of B-cell precursors marks the transition from the pro- to the pre-B-cell stage (Grabstein et al, 1993; Pelanda et al, 2002a). The pre-BCR is an autonomously active receptor, signals of which drive clonal expansion and differentiation of pre-B cells into immature, BCR-expressing B cells (Kohler et al, 2008; Herzog et al, 2009). This signaling requires the interaction of the pre-BCR with the kinase Syk (Cheng et al, 1995; Reth & Nielsen, 2014). Therefore, B cells with defective genes for Syk or any of the four pre-BCR components have a complete or partial block in development (Turner et al, 1997; Otipoby et al, 2001; Yang & Reth, 2010).
Immature B cells migrate from the BM to the spleen, where they develop further through transitional B-cell stages into either marginal zone (MZ) or mature follicular B cells. In addition to mature B cells, the peritoneum of the mouse also harbors B1 B cells that are polyreactive and seem to require continuous BCR expression and stimulation for their survival (Hayakawa et al, 1985; Sindhava & Bondada, 2012). Therefore, the deletion of genes encoding components of the BCR signaling pathway frequently results in the loss of this B-cell type. In its monomeric form, the BCR is a 1:1 complex between the membrane-bound immunoglobulin molecule (mIg) and the Igα/Igβ heterodimer. On resting mature B cells, the BCR forms autoinhibited oligomers which prevent continuous signaling (Yang & Reth, 2010). Upon exposure to cognate antigens, some BCR oligomers are opened and phosphorylated on tyrosines in the immunoreceptor tyrosine-based activation motif (ITAM) of Igα and Igβ by the tyrosine kinases Lyn and Syk (Reth, 1989; Johnson et al, 1995). This results in the binding of Syk to the BCR via its tandem SH2 domains, the rapid phosphorylation of neighboring BCR oligomers and the amplification of the BCR signal via a Syk-mediated inside-out signaling process (Klasener et al, 2014).
Once bound to the BCR, Syk becomes fully activated and phosphorylates several downstream signaling elements, including the adaptor protein SLP65/BLNK. This organizes the calcium (Ca2+) signalosome, leading to Ca2+ mobilization and activation of the MAP kinase pathway (Johnson et al, 1995; Sada et al, 2001; Xu et al, 2005). Syk can also phosphorylate the inhibitory receptor CD22, whereas the BCR coreceptor CD19 is dominantly phosphorylated in a Syk-independent manner by Src-family kinases including Lyn (Fujimoto et al, 2000; Otipoby et al, 2001; Xu et al, 2002). Phosphorylated CD19 is associated with and activates several signaling components, most prominently the p85/p110 PI3K heterodimer. Therefore, together with the adaptor protein BCAP, CD19 is the dominant activator of the PI3K pathway leading to B-cell survival and proliferation (Brunet et al, 1999; Datta et al, 1999; Monroe, 2006; Aiba et al, 2008; Srinivasan et al, 2009).
The B-cell-activating factor belonging to the TNF family (BAFF) and its receptor BAFF-R provide another important survival signal in mature B cells (Mackay et al, 2010; Harless et al, 2001). Both PI3K and BAFF-R signals regulate the homeostasis of mature B cells. Mice deficient for BAFF or BAFF-R have reduced follicular and MZ B-cell numbers, and the same holds true for defects in PI3K (Sasaki et al, 2004; Durand et al, 2009). Another factor implicated in the survival of mature B cells is the BCR itself. Indeed, the lack of the mIgM heavy chain in mature B cells results in the loss of BCR expression and a strong reduction in mature B-cell numbers (Lam et al, 1997). Whether this is due to the absence of a tonic pro-survival signaling or the induction of a death signal in mIgM-negative B cells is not yet clear. A tonic BCR signal may employ different signaling components than those involved in B-cell activation or it may use the standard BCR signaling components at reduced levels (Monroe, 2006). Of interest in this context is the recent finding that the deletion of the Syk gene in all hematopoietic cells of the mouse also results in strongly reduced B-cell numbers (Schweighoffer et al, 2013). Based on the finding that the Syk-negative B cells respond less well to BAFF in culture, the authors suggest that Syk is an essential intermediate between the BAFF-R and the tonic signal of the BCR. We have revisited this issue in a mouse strain, which permits an inducible deletion of the Syk gene specifically in the B-cell lineage. We find that up to 25% of Syk-negative B cells survive for long time periods in the periphery of these mice. While the study by Schweighoffer et al focuses on the death of B cells in the absence of Syk, we have examined Syk-independent survival signals in B cells. We find that in the absence of Syk, B cells are still able to respond to BAFF and depend on its presence for their survival. Thus, Syk is not required for the B-cell response to BAFF. Furthermore, survival of Syk-deficient B cells requires CD19 and its activation of the PI3K pathway as Syk-deficient B cells do not survive in the absence of CD19 and survive better when they lack FoxO1. In summary, both the BAFF and CD19—PI3K pathways provide important signals for the survival of B cells in the absence of Syk.
Results
Efficient, inducible deletion of the Syk gene in B cells of mb1-CreERT2;Sykfl/fl mice
To investigate the function of genes in mature B cells, we generated the mouse line mb1-CreERT2 allowing for an inducible and B-cell-specific Cre activity. We inserted a cDNA encoding the CreERT2 recombinase into the mb-1 gene, which is primarily expressed in B cells. The CreERT2 construct, a gift from P. Chambon, encodes a fusion protein consisting of the Cre recombinase and a mutated ligand-binding domain of the estrogen receptor (ER), which binds the estrogen analog tamoxifen (Tam) but not estrogen itself (Brocard et al, 1998; Indra et al, 1999). The mb1-CreERT2 allele contains the promoter region of the mb-1 gene followed by exon I with a mutated start codon, the complete intron I, the CreERT2 cDNA inserted into exon II behind a newly generated start codon and an SV40 polyA signal (Fig1A). We have previously demonstrated that an mb1-Cre allele generated by the same strategy can drive B-cell-restricted Cre expression and deletion of floxed genes at all B-cell developmental stages (except plasma cells) starting from the pro/pre-B-cell stage (Hobeika et al, 2006; Liu et al, 2007). We found that mb1-CreERT2 mice expressed CreERT2 only in cells of the B-cell lineage and that the protein was localized to the nucleus when cells were exposed to Tam (data not shown).
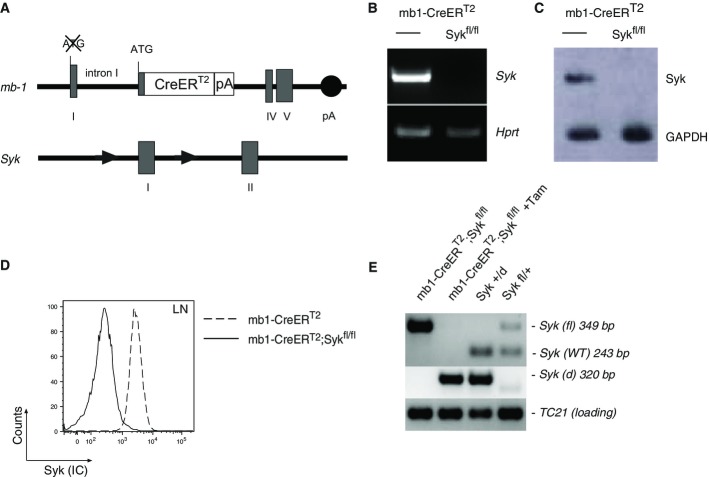
Figure 1.
- Schematic representation of the targeted mb-1 locus harboring the CreERT2 construct and the “floxed” Syk locus. The construct is inserted between exons I and IV. The filled rectangles represent the exons of the mb-1 allele. The open rectangle represents the CreERT2 construct followed by a poly-adenylation (pA) site. The black circle represents the endogenous pA site of the mb-1 allele. The “truncated” version of mb-1 exon I shown here lacks the ATG start codon and is followed by the complete mb-1 intron I with its splice donor and acceptor sites. Intron I was maintained to provide intron/exon splicing in the transcript and as a source of possible transcription regulatory sequences. The targeted Syk locus was targeted with 2 LoxP sites and has been described in Saijo et al (2003).
- RT-PCR performed on cDNA from RNA isolated from splenic B cells derived from Tam-treated mb1-CreERT2 (left lane) or mb1-CreERT2;Sykfl/fl mice (right lane); the Syk- and Hprt-specific amplification products were identified by agarose gel electrophoresis with Hprt used as an endogenous loading control.
- Immunoblot analysis of proteins isolated from B cells derived from spleens of mb1-CreERT2 (left lane) or mb1-CreERT2;Sykfl/fl (right lane) mice both treated with Tam as described; blots were probed with anti-Syk and anti-GAPDH Ab, GAPDH being used as a loading control.
- Intracellular flow cytometric analysis for Syk expression in B cells derived from the LN of Syk-deficient and control mice.
- Genomic DNA analysis of Tam-treated mb1-CreERT2 or mb1-CreERT2;Sykfl/fl mice (as indicated). Upper row: amplification of floxed (fl) and wt (+) alleles. Middle row: amplification of the deleted (d) allele. Lower row: loading control (TC21, a gene unaffected by the deletion of Syk). Established control DNA was used to visualize the individual bands (as indicated).
To investigate the role of Syk in the activation and survival of mature B cells, we crossed mb1-CreERT2 mice with Sykfl/fl mice, in which the exon I of the Syk gene is flanked by cis-orientated loxP sites (floxed) (Saijo et al, 2003). The resulting mb1-CreERT2;Sykfl/fl mice were treated 5× with Tam every third day and analyzed on day 5 after the last treatment, 20 days after the beginning of the treatment. Splenic B cells isolated from the Tam-treated mice lacked both Syk transcripts and protein as demonstrated by a RT-PCR and a Western blot analysis (Fig1B and C). Additionally, flow cytometric analysis revealed that lymph node (LN)-derived mature B cells from Tam-treated mb1-CreERT2;Sykfl/fl mice were devoid of intracellular Syk expression, in contrast to LN-derived B cells from mb1-CreERT2 control mice which also received Tam (Fig1D). The efficient deletion of the floxed Syk allele in Tam-treated mb1-CreERT2;Sykfl/fl mice was further confirmed by PCR analysis of genomic DNA (Fig1E). Notably, the floxed Syk gene is the most efficient Cre target we have studied, indicating that in B cells this gene locus is highly accessible to the Cre recombinase.
The B-cell populations of Tam-treated mb1-CreERT2;Sykfl/fl mice
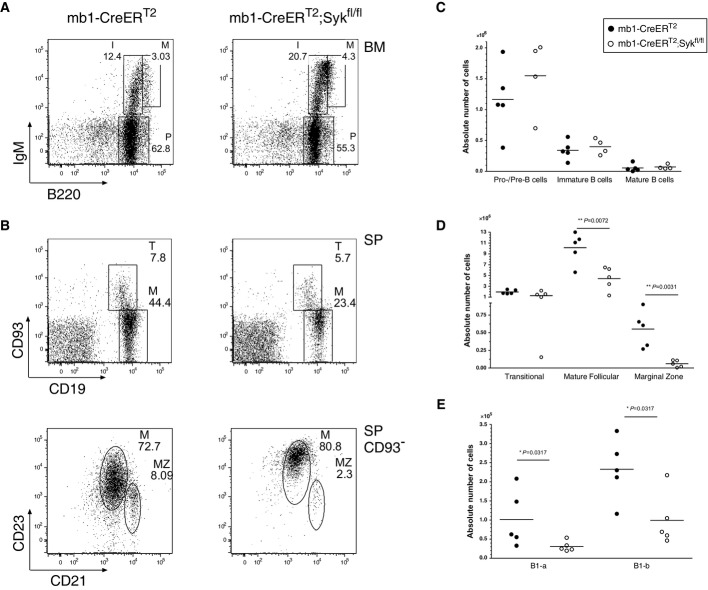
Five days after the last Tam treatment, the frequencies and absolute cell numbers of pro-/pre- and immature B cells in the BM did not differ significantly between mb1-CreERT2 control and mb1-CreERT2;Sykfl/fl mice while recirculating B cells were slightly reduced (Fig2A and C). In the spleen (SP) of Tam-treated mb1-CreERT2;Sykfl/fl mice, however, the relative and absolute numbers of mature follicular (M) and marginal zone (MZ) B cells were reduced threefold and tenfold, respectively (Fig2B and D). In other lymphatic organs such as the peritoneal cavity (PC), the B1 cell numbers were also reduced in the Tam-treated mb1-CreERT2;Sykfl/fl mice (Fig2E). B1 B cells proliferate constitutively, and this might contribute to a selection against Syk-negative B cells. We tested this by intracellular Syk staining in flow cytometric analysis and found that all B1 B cells in the PC of mb1-CreERT2;Sykfl/fl mice were Syk negative (Supplementary Fig S1). Interestingly, the transitional B-cell numbers in the spleen of the Tam-treated mice were not altered (Fig2B), suggesting that this transitional B-cell pool is rapidly replenished with Syk-sufficient transitional B cells after the discontinuation of the Tam treatment. This notion is supported by a intracellular flow cytometric analysis of transitional and immature B cells in the BM of Tam-treated mb1-CreERT2;Sykfl/fl mice showing the appearance of Syk-positive B cells at day 5 but not day 2 after induction (Supplementary Fig S2).
Figure 2.
- A, B Flow cytometric analysis of B cells from (A) the BM and (B) the SP of mb1-CreERT2 control (left) and mb1-CreERT2;Sykfl/fl mice (right) treated with Tam as described in the Materials and Methods section. The BM cells were stained with anti-IgM and anti-B220, and the SP cells with anti-CD19 and anti-CD93 or anti-CD23 and anti-CD21. The gated regions in the dot blots correspond to individual B-cell populations: (A) Bone marrow: gate P (B220+ IgM−) pro-/pre-B cells, gate I (B220lo IgM+) immature B cells, gate M (B220hi IgM+) mature B cells; (B) spleen: gate T (CD19+ CD93+) transitional (T) B cells, gate M (CD19+ CD93−) mature B cells; in CD93− splenic B cells, gate M (CD23hiCD21int CD93−) mature follicular B cells and gate MZ (CD23lo CD21hi CD93−) marginal zone B cells. The numbers in the dot plots indicate the mean relative frequency of cells in the gate.
- C-E Statistical analysis of absolute cell numbers per Tam-treated mouse: filled circles indicating cells obtained from mb1-CreERT2 control mice and open circles from mb1-CreERT2;Sykfl/fl mice. (C) Statistical analysis of the absolute cell numbers of pro-/pre-, immature and mature recirculating B cells in the BM. (D) Absolute cell numbers of transitional, M and MZ B cells found in the SP. (E) Statistical analysis of the absolute cells numbers of peritoneal B cell subsets B1-a and B1-b. An asterisk (*) marks statistically significant differences (P < 0.05), two asterisks (**) indicate P < 0.01, P-values were obtained using two-tailed Student's t-test. Cell numbers of four to five mice per group are shown, with each dot representing an individual animal.
A partial signaling defect in Syk-deficient mature B cells
The presence of a pool of Syk-negative B cells in the spleen of Tam-treated mb1-CreERT2;Sykfl/fl mice allowed us to study the signaling behavior of mature B cell in the absence of Syk. We first analyzed the expression of the BCR and coreceptors on the surface of splenic B cells derived from Tam-treated mb1-CreERT2 control and mb1-CreERT2;Sykfl/fl mice. In comparison with the control B cells, the Syk-negative B cells displayed slightly increased amounts of IgM, CD19 and CD23 on their surface (Fig3A) even though the Syk-negative B cells were smaller than control B cells (data not shown).
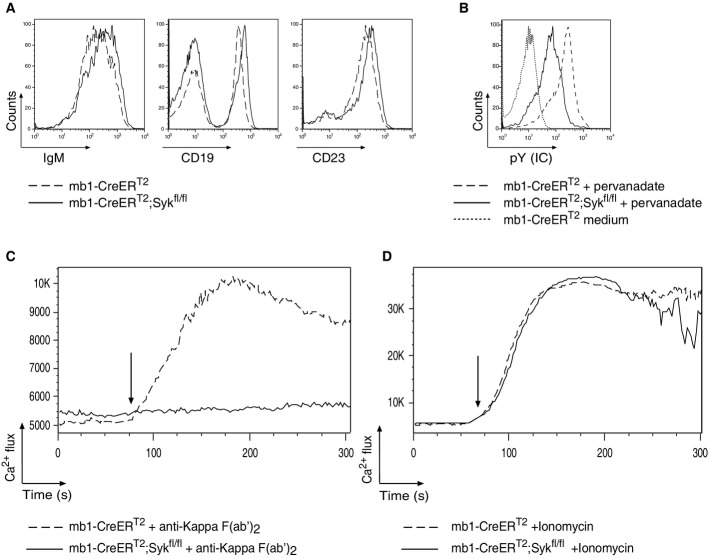
Figure 3.
- A Flow cytometric analysis of IgM, CD19 and CD23 expression of splenic B cells from Tam-treated mb1-CreERT2 control (dashed line) and mb1-CreERT2;Sykfl/fl mice (solid line).
- B Flow cytometric analysis of tyrosine phosphorylation (pY) in splenic B cells from Tam-treated mb1-CreERT2 and mb1-CreERT2;Sykfl/fl mice stimulated with medium (dotted line) or 50 μM pervanadate (dashed and solid lines).
- C, D Flow cytometric analysis of the intracellular Ca2+ influx in purified splenic B cells derived from Tam-treated mice. mb1-CreERT2 or mb1-CreERT2;Sykfl/fl were treated with (C) 10 μg/ml anti-Kappa F(ab′)2 fragments or (D) 1 μM ionomycin.
Exposure of mature B cells to the phosphatase inhibitor pervanadate results in increased tyrosine phosphorylation (pY) (Wienands et al, 1996). We treated splenic B cells with pervanadate for 5 min and determined the intracellular pY levels by flow cytometry. In comparison with control B cells, the Syk-deficient B cells showed eightfold lower pY (Fig3B). To test whether Syk-deficient B cells could mobilize intracellular Ca2+ upon BCR engagement, we stimulated isolated splenic B cells from Tam-treated mb1-CreERT2 control and mb1-CreERT2;Sykfl/fl mice with an anti-kappa F(ab′)2 antibody. Only the control B cells, but not the Syk-negative B cells, mobilized Ca2+ upon treatment with this stimulus (Fig3C). Both B-cell populations responded equally well to ionomycin in this assay, indicating that the absence of a Ca2+ response in Syk-negative B cells is due to a defect of BCR signaling and not a problem of Ca2+ release per se (Fig3D). In addition, no Ca2+ release was detected in Syk-negative B cells upon stimulation with anti-IgM F(ab′)2, anti-IgD or latrunculin (Supplementary Fig S3). Together, these experiments confirm the requirement of Syk for the Ca2+ release observed upon BCR activation, in line with data obtained from the Syk-deficient chicken B-cell line DT40 (Takata et al, 1994).
As another readout for BCR-dependent or BCR-independent B-cell signaling, we analyzed mTOR activation and the chemotactic behavior of B cells from Tam-treated mb1-CreERT2 control and mb1-CreERT2;Sykfl/fl mice. Using the phosphorylation of ribosomal protein S6, an established indicator for mTOR activity, control B cells but not Syk-deficient mature B cells activated mTORC1, when stimulated with anti-kappa F(ab′)2 (Supplementary Fig S4).
In transwell migration experiments with the chemokines CCL21, CXCL12 and CXCL13, Syk-deficient B cells migrated less well than control B cells in response to the chemokine CXCL12 (Supplementary Fig S5A), although the surface expression of the corresponding CXCL12 receptor, namely CXCR4, is slightly higher compared to the control B cells (Supplementary Fig S5B). These results are in line with the finding that by phosphorylating SWAP-70, Syk increases the chemotaxis and polarization of B cells particularly to CXCL12 (Pearce et al, 2011).
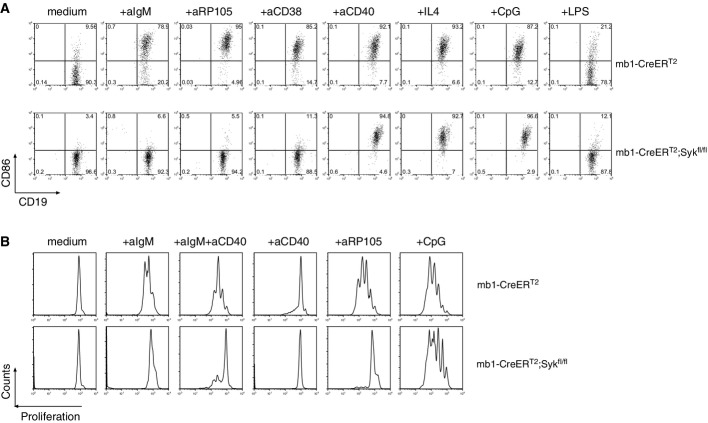
B-cell activation increases the expression of CD86 on the surface of stimulated mature B cells (Lenschow et al, 1993). We exposed B cells from Tam-treated mb1-CreERT2 control or mb1-CreERT2;Sykfl/fl mice to different stimuli and monitored their CD86 expression by flow cytometry. Contrary to control B cells, the Syk-negative B cells failed to upregulate CD86 when stimulated with anti-IgM F(ab′)2, anti-CD38 and anti-RP105, indicating that direct and indirect signaling via the BCR is defective in the absence of Syk (Fig4A). Increased CD86 expression was, however, detected when Syk-negative B cells were stimulated with anti-CD40, IL-4, CpG, and to a lesser extent with LPS. This shows that the latter receptors do not require Syk for signaling in mature B cells.
Figure 4.
- Ex vivo activation assay of splenic mature B cells from Tam-treated mb1-CreERT2 (upper row) or Tam-treated mb1-CreERT2;Sykfl/fl mice (lower row). Purified splenic B cells were either left unstimulated (medium) or were stimulated with the indicated stimuli. The cells were analyzed by flow cytometry after 24 h. Shown are CD19 versus CD86 dot plots. 7-AAD was included to distinguish dead from viable cells. The numbers in the quadrants indicate the relative frequencies of cells in the gate.
- Ex vivo proliferation assay with mature B cells from Tam-treated mb1-CreERT2 (upper row) or Tam-treated mb1-CreERT2;Sykfl/fl mice (lower row). The cells were incubated with the indicated stimuli for 90 h.
The results of a B-cell proliferation assay mirrored those of the CD86 expression analyses. Syk-negative B cells underwent fewer cell division cycles when cultured in the presence of anti-IgM F(ab′)2 or anti-RP105 antibodies (Fig4B). Simultaneous signaling via BCR and CD40 exacerbated the differences in proliferation between Syk-negative and Syk-expressing B cells, whereas treatment of B cells with TLR9-activating CpG led to a similar rate of proliferation in both types of B cells (Fig4B). The absence of Syk in the proliferating mb1-CreERT2;Sykfl/fl-derived B-cell population was verified by flow cytometric analysis of intracellular Syk expression (Supplementary Fig S6).
A fraction of Syk-negative B cells can survive for extended times in vivo
The partial loss of mature B cells in Tam-treated mb1-CreERT2;Sykfl/fl mice could be due to increased death of Syk-negative mature B cells and/or to a reduced input into the mature B-cell compartment. To distinguish between these possibilities, we injected Rag2−/−;γc−/− mice, which cannot produce B or T cells, with equal numbers of mature splenic T or B cells from either mb1-CreERT2 or mb1-CreERT2;Sykfl/fl mice. The recipient mice were then treated a total of 5 times with Tam at 3-day intervals, and T- or B-cell cellularity was monitored 20 days later by flow cytometry (Fig5A). While the number of T cells from mb1-CreERT2;Sykfl/fl mice was not altered by the Tam treatment, compared to the control, the number of mature B cells derived from the mb1-CreERT2;Sykfl/fl mice was reduced by approximately threefold (Fig5B). Similar results were obtained from mice, which were injected after the last Tam treatment with anti-IL-7 receptor antibodies to block the migration of newly generated B cells from the bone marrow. In these mice, more than 40% of the mature splenic B cells survived without Syk for more than 40 days (Supplementary Fig S7), showing again that a considerable fraction of mature B cells can survive without Syk.
Figure 5.
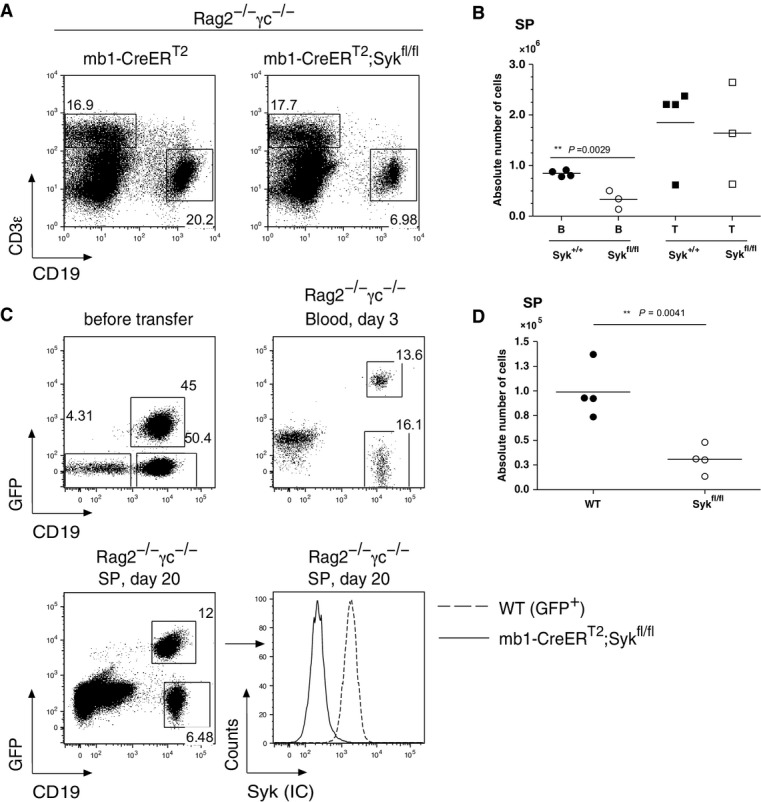
- Flow cytometric analysis of splenic B cells from Rag2−/−;γc−/− mice. CD19 versus CD3ε dot plots are shown. The mice were injected i.v. with 5 × 106 splenic (CD19+ CD93−) B cells and (CD3ε+) T cells from Tam-untreated mb1-CreERT2 control or mb1-CreERT2;Sykfl/fl mice. The recipient mice were treated with Tam beginning 1 day after transfer as described in the Materials and Methods section.
- Quantitative analysis of B and T cells from Rag2−/−;γc−/− mice repopulated with mb1-CreERT2 or mb1-CreERT2;Sykfl/fl splenocytes. Each symbol represents an individual mouse. Filled circles and squares represent Syk+/+ B and T cells, respectively; open circles and squares represent Sykfl/fl B and T cells.
- Flow cytometric analysis of Rag2−/−; γc −/− mice repopulated with mature splenic B cells from WT mice (1 × 107) carrying a GFP reporter construct or mature splenic B cells from mb1-CreERT2;Sykfl/fl mice (1 × 107) at a ratio of 1:1. The recipient mice were treated with Tam as described in the Materials and Methods section. Shown are GFP versus CD19 dot plots to distinguish GFP+ WT and GFP− Sykfl/fl B cells before transfer (upper left panel), in the blood of the recipient mice at day 3 after transfer (upper right panel) or in the spleen of the recipient mice at day 20 after transfer (lower left panel). The cells were stained for the intracellular expression of Syk (lower right panel).
- Quantitative analysis of WT or Sykfl/fl B cells. Absolute number of cells is shown. Filled circles represent WT B cells and open circles the Sykfl/fl B cells.
To study the survival of Syk-negative B cells in the presence of wild-type (WT) B cells, we injected Rag2−/−;γc−/− mice with equal numbers of mature B cells derived from the spleen of either mb1-CreERT2;Sykfl/fl mice or mice expressing EGFP specifically in B cells (Fig5C). B cells in the injected mice were harvested 5 days after the last Tam treatment and analyzed by flow cytometry (Fig5C). We found that one-third of the surviving B cells were Syk deficient (Fig5D). The results obtained from the competitive adoptive transfer experiment are in line with the analysis of Tam-treated mice left for 2 months without treatment. In these mice, 20% of the mature B cells were still Syk negative (Supplementary Fig S8). In summary, these experiments indicate that a portion (20–30%) of the Syk-negative B cells are able to survive for prolonged times in the periphery, even when competing with wild-type B cells and despite having apparent signaling defects restricting their “fitness”.
Syk-negative B cells require BAFF for their survival
To test whether Syk-negative B cells can respond to the pro-survival factor BAFF, we cultured B cells from Tam-induced mb1-CreERT2 or mb1-CreERT2;Sykfl/fl mice in the absence or presence of BAFF (Fig6A). Without BAFF, most B cells die within 3 days, whereas in the presence of BAFF, the B-cell cultures can be maintained for longer times. After 8 days of culture, 60% of the control and only 30% of the Syk-negative B cells were still viable (Fig6A). This difference in the response to BAFF could not be due to an altered BAFF-R expression as the B cells from both mouse strains expressed similar amounts of BAFF-R on their surface (Fig6B).
Figure 6.
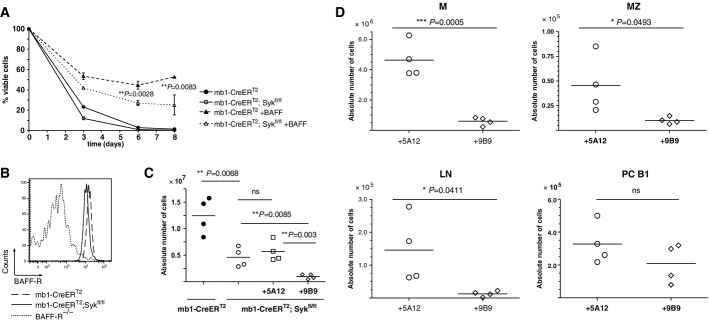
- Ex vivo survival assay performed on mature splenic B cells isolated from Tam-treated mb1-CreERT2 or mb1-CreERT2;Sykfl/fl mice. The cells were cultured in the presence (dashed lines) or absence (solid lines) of 100 ng/ml human recombinant BAFF and harvested at the indicated time points. Viable cells were identified by flow cytometry using CD19 versus 7-AAD staining. Shown are means ± SD from a triplicate experiment representative of at least three independent experiments.
- Histogram representation of the BAFF-R surface staining on mature B cells from Tam-treated mb1-CreERT2;Sykfl/fl (solid line) or control mb1-CreERT2 mice (dashed line). Splenic transitional B cells derived from the BAFF-R−/− mouse strain served as a negative control (dotted line).
- Quantitative analysis of absolute splenic B-cell numbers from Tam-treated mb1-CreERT2 (filled circles) or mb1-CreERT2;Sykfl/fl (open circles). The animals were treated either with the isotype control, non-blocking anti-BAFF-R antibody clone 5A12 (open squares) or the anti-BAFF-R-blocking antibody clone 9B9 (open diamonds). Mice were analyzed 2 weeks after antibody injection. Each circle represents an individual mouse.
- Absolute B-cell numbers representing the different B lymphocyte subsets from different lymphoid organs are depicted. Each open circle represents an individual mouse from the mb1-CreERT2;Sykfl/fl plus control Ab group while each open diamond a mouse from the mb1-CreERT2;Sykfl/fl plus BAFF-R-blocking Ab group.
We next studied the BAFF dependence of Syk-negative B cells in vivo by injecting Tam-treated mb1-CreERT2;Sykfl/fl mice with either 5A12 or 9B9 anti-BAFF-R antibodies, only the latter of which blocks BAFF binding to the receptor, and thus BAFF-R-mediated survival signals (Fig6C). A recent study revealed that treatment of WT mice with the 9B9 antibody resulted in the elimination of 80% of the mature B cells (Rauch et al, 2009). Similarly, injection of Tam-induced mb1-CreERT2;Sykfl/fl mice with the 9B9 antibody resulted in a tenfold reduction in the absolute number of splenic B cells. The non-blocking 5A12 antibody injection has no effect on the B-cell numbers. The reduction in Syk-negative B cells in anti-9B9-treated mice was observed to a different extent in different lymphatic tissues, including the SP, LN and PC (Fig6D). A study of the mature follicular B-cell frequencies in the blood from control and B-cell-specific Syk-deficient mice revealed no significant difference in the kinetics of B-cell elimination at several time points (day 3 to day 15) after anti-BAFF-R treatment (Supplementary Fig S9).
These data indicate that Syk-negative B cells are, to a certain extent, responsive to BAFF and depend on BAFF–BAFF-R interactions for their long-term in vivo survival. The Syk family kinase ZAP-70 can partially replace Syk function in Syk-deficient pre-B cells (Schweighoffer et al, 2003). Therefore, one possibility for the long-term survival of Syk-negative mature B cells could be the expression of ZAP-70. To test this, B cells from mb1-CreERT2;Sykfl/fl mice were subjected to immunoblot and flow cytometric analyses. However, no ZAP-70 expression was detected in these cells (Supplementary Fig S10). In line with these data, the Syk-negative mature B-cell population is not affected in mice lacking ZAP-70 (data not shown).
The survival of Syk- and CD19-negative B cells is rescued by FoxO1 deletion
Recent experiments implicate the PI3K signaling pathway in the survival and maintenance of mature B cells in the periphery (Srinivasan et al, 2009; Jellusova et al, 2013). As the BCR co-receptor CD19 is an important activator of PI3K signaling pathway in B cells, we assessed the survival of Syk-negative B cells in the absence of CD19. We crossed mb1-CreERT2;Sykfl/fl mice with CD19-CreERT2 mice, to produce the mb1-CreERT2;Sykfl/fl;CD19−/− strain. CD19−/− mice show significantly reduced B-cell numbers in peripheral lymphoid tissues and a BCR signaling defect (Del Nagro et al, 2005). In this respect, they resemble the mb1-CreERT2;Sykfl/fl mouse strain (Fig7). After 30 days of Tam treatment of mb1-CreERT2;Sykfl/fl;CD19−/− mice, the survival of mature B cells was severely compromised in comparison with CD19−/− or mb1-CreERT2;Sykfl/fl animals. The relative and absolute B-cell numbers in these mice were strongly reduced in the BM, especially the recirculating B cells (Fig7A and C). A drastic reduction in the mature B cells was also detected in the SP of mb1-CreERT2;Sykfl/fl;CD19−/− mice (Fig7B and D). These results indicate that CD19-derived signals support the survival of Syk-negative B cells and that Syk promotes the survival of CD19-negative B cells. Multiple copies of CreERT2 in the mb1-CreERT2;Sykfl/fl;CD19−/− compound mice lead to accelerated recombination of the Syk locus compared with the control mice on day 6 or 8 after Tam exposure (Supplementary Fig S11). However, at day 10, Syk deletion is complete in the majority of mature B cells of mb1-CreERT2;Sykfl/fl mice, and at day 20, these B cells are entirely devoid of Syk (Supplementary Fig S11B). For this reason, we do not think that gene dosage plays a critical role in our analysis performed at day 35 after Tam treatment.
Figure 7.
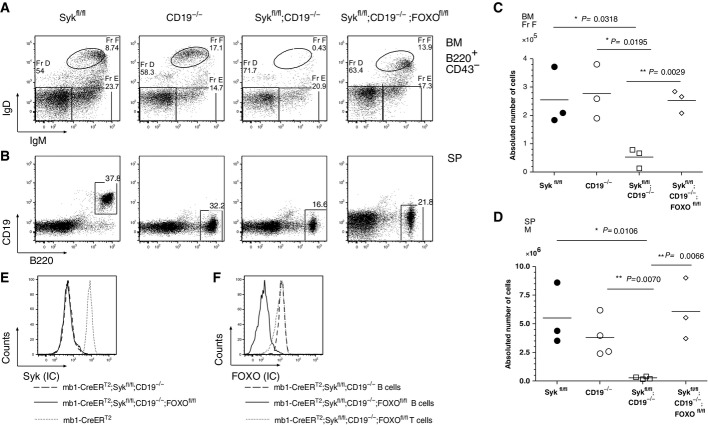
- A, B B lymphocytes derived from mb1-CreERT2;Sykfl/fl (first panel), CD19−/− (second panel), mb1-CreERT2;Sykfl/fl;CD19−/− (third panel) and mb1-CreERT2;Sykfl/fl;CD19−/−; FoxOfl/fl (fourth panel) mice were analyzed by flow cytometry after Tam treatment (see Materials and Methods). (A) Flow cytometric analysis of BM-derived (A) or splenic (B) B cells. IgM versus IgD dot plots representing B220+CD43− BM-derived B cells are depicted in (A). The Fr. D, E, and F are distributed as indicated. A CD19 versus B220 dot plot is depicted in (B).
- C, D Statistical analysis of absolute B-cell numbers derived from the (C) the BM, Fr F and (D) splenic M B cells of mb1-CreERT2;Sykfl/fl (filled circles), mb1-CreERT2;CD19−/− (open circles), mb1-CreERT2;Sykfl/fl;CD19−/− (open squares) and mb1-CreERT2;Sykfl/fl;CD19−/−;FoxOfl/fl (open diamonds). An asterisk (*) marks statistically significant differences (P < 0.05); two asterisks (**) indicate a P < 0.01. P-values were obtained by a two-tailed Student's t-test. Shown are data from three to four mice per group.
- E, F Intracellular flow cytometric analysis of (E) Syk and (F) FoxO1 in mb1-CreERT2;Sykfl/fl;CD19−/− (dashed line) and mb1-CreERT2;Sykfl/fl;CD19−/−;FoxOfl/fl (solid line) B cells. In (E), B cells from mb1-CreERT2 mice served as a positive control for the Syk staining (dotted line). In (F), T cells from mb1-CreERT2;Sykfl/fl;CD19−/−;FoxOfl/fl mice served as a positive control for FoxO1 expression showing that FoxO1 deletion was B cell specific.
One mechanism by which the CD19/PI3K signals can increase the survival of mature B cells is through the phosphorylation and subsequent proteasomal degradation of the pro-apoptotic transcription factor FoxO1 (Limon & Fruman, 2012). This effect of PI3K signaling can be mimicked by the deletion of the FoxO1 gene. To test the effect of FoxO1 deletion in a CD19/Syk double-deficient background, we generated mb1-CreERT2;Sykfl/fl,CD19−/−;FoxO1fl/fl mice. The absence of Syk and FoxO1 proteins was confirmed by flow cytometric analysis (Fig7E and F), indicating efficient Cre-mediated deletion of the floxed Syk and FoxO1 genes in these mice. Interestingly, the ablation of FoxO1 expression restored the numbers of mature B cells to the levels of Syk or CD19 single-deficient B cells in both the BM and the SP (Fig7A and B right panel; Fig7C and D). Thus, the PI3K signaling pathway is indeed likely to be induced by CD19, to support the survival of Syk-negative B cells.
Discussion
We show here that approximately 25% of the mature peripheral B cells can survive in the mouse for at least 2 months without Syk, in a manner that requires BAFF-R and CD19 signaling. In contrast, deletion of the Syk gene in early B cells results in the appearance of a small number of immature IgM+ B cells, which, however, fail to give rise to any mature B cells in the periphery (Cheng et al, 1995). Thus, pre-B and mature B cells have different requirements for Syk. Indeed, the pre-BCR is an autonomously signaling receptor that continuously engages Syk, whereas the BCR forms an autoinhibited oligomer on mature B cells that is not in contact with Syk. This notion is supported by a proximity ligation analysis showing that Syk is localized near the BCR only after BCR activation (Infantino et al, 2010; Klasener et al, 2014).
The presence of large amounts of Syk-negative mature B cells in the induced mb1-CreERT2;Sykfl/fl mice allowed us to analyze the in vivo role of this kinase in the activation of mature B cells. Our finding, that in the absence of Syk, mature B cells have a defective Ca2+ response, is consistent with an earlier study in Syk-negative DT40 B cells (Takata et al, 1994). This signaling defect is explained by the finding that the phosphorylation of the adaptor protein SLP-65/BLNK by Syk is required for the organization of a functional Ca2+ signalosome. Signals from membrane proteins that are functionally connected to the BCR, such as CD38 and RP105, are also defective in Syk-negative B cells (Lund et al, 1996; Chan et al, 1998; Yazawa et al, 2003). Other receptor systems, however, such as CD40 and the innate immunity receptors TLR9 are not affected by the loss of Syk.
While the number of mature B cells is reduced only threefold in the induced mb1-CreERT2;Sykfl/fl mice, other B-cell subpopulation such as MZ B cells are reduced more than tenfold in the absence of Syk. This is surprising as MZ B cells are regarded as a more innate B-cell population that requires exposure to innate receptor stimuli for their maintenance and expansion (Cerutti et al, 2013). However, it is feasible that Syk is not only involved in signaling from the activated BCR, but also in B-cell migration and/or adhesion. In neutrophils and macrophages, Syk is involved in signaling from integrin and chemokine receptors (Mocsai et al, 2010). Indeed, we found that Syk-negative B cells display a defective CXCL12-driven chemotactic behavior (Supplementary Fig S5). Recently, it was shown that Syk phosphorylates the F-actin-binding protein SWAP-70, resulting in altered chemotaxis and polarization of B cells, particularly to CXCL12 (Pearce et al, 2011). Interestingly, SWAP-70−/− mice also show reduced numbers of MZ B cells (Chopin et al, 2010). A loss of Syk might lead to a failure of T2 B cells to migrate to the MZ and thus to lower amounts of MZ B cells (Miosge & Goodnow, 2005).
It is not clear at present why 70% of the mature B cells disappear in the induced mb1-CreERT2;Sykfl/fl mice. Our transfer experiments, and the deletion of Syk in combination with an anti-IL-7R treatment, show that this loss is not primarily due to reduced production of mature B cells but is a feature of mature B cells themselves. Syk is also needed for the migration of transitional T0 B cells into the white pulp region of the spleen, where the cells develop into T1 B cells and receive additional survival signals (Henderson et al, 2010). Thus, a migration and/or adhesion defect, as discussed above, could be the cause of the loss of the mature B2 B cells in the induced mb1-CreERT2;Sykfl/fl mice. For example, if locally defined survival niches exist in the periphery that mature B cells need to visit from time to time to ensure their long-term survival, a defective migration of Syk-negative B cells would result in reduced cell numbers. Alternatively, within the cell, Syk may send a tonic signal to the mTOR pathway that controls the “fitness” of mature B cells. At least in B-cell tumor cells, such a Syk-mTOR connection has been described (Leseux et al, 2006; Fruchon et al, 2012; Carnevale et al, 2013). In line with this, we found that Syk-deficient mature B cells do not activate mTORC1 when stimulated with anti-Kappa F(ab′)2 (Supplementary Fig S4).
Based on the finding that Syk-negative B cells respond less well to BAFF in culture, Schweighoffer et al suggested that Syk is an essential common component of the BCR and the BAFF-R, both of which mediate B-cell survival signaling (Schweighoffer et al, 2013). Our study confirms that in culture, Syk-negative B cells respond less well to the pro-survival factor BAFF. However, we also found that Syk-negative B cells still respond to BAFF in vivo and require this factor for their long-term survival in the mouse. Thus, an unresponsiveness to BAFF is not the reason for the reduction of the Syk-negative B cells in induced mb1-CreERT2;Sykfl/fl mice. Another discrepancy between these two studies is that we, in contrast to Schweighoffer et al, find a significant reduction in the B1 B-cell population in the induced mb1-CreERT2;Sykfl/fl mice. B1 B cells are polyreactive, slowly renewing cells that are strongly affected by the loss of positive BCR signaling components (Berland & Wortis, 2002). The reduction in the B1 B-cell numbers in the absence of Syk is in line with these studies. We have found that under certain conditions, B1 B cells can escape Cre-mediated deletion of the floxed Syk gene (Alsadeq et al, 2014). An incomplete Syk gene deletion may be the reason for this discrepancy. We verified by intracellular staining that Syk was indeed deleted in the B1 B-cell population that was still present in the induced mb1-CreERT2;Sykfl/fl mice.
The PI3K pathway can promote the survival of WT and Syk-deficient B cells. The importance of this pathway for B-cell survival was recently demonstrated in mice with a B-cell-specific activation of PI3K signaling via a dominant-active form of the p110α subunit (Srinivasan et al, 2009). In these mice, B cells survive, even in the absence of BCR expression. Schweighoffer et al have shown that the survival of Syk-deficient B cells can be rescued, at least in part by the activation of the PI3K pathway (by deleting PTEN). The BCR coreceptor CD19 is a well-known PI3K activator, and we find that its expression is required for the survival of Syk-deficient B cells. The cytoplasmic tail of CD19 is phosphorylated by the Src-family kinase Lyn, thus allowing CD19 to connect to the p85/p110 PI3K module. It is thus not a surprise that CD19 can still be phosphorylated and function in the absence of Syk, although it was shown that Syk can also function upstream of PI3K in BCR signaling (Beitz et al, 1999). We recently found that CD19 is in close association with the IgD-BCR on resting B cells (Klasener et al, 2014). However, whether CD19 inducibly or constitutively signals in Syk-deficient B cells remains to be determined. One important downstream target of the PI3K pathway is the transcription factor FoxO1, whose phosphorylation by Akt1 targets it for destruction. PI3K signaling can thus be mimicked by a FoxO1 gene deletion, and we found that such a deletion rescues mature Syk;CD19 double-deficient B cells from elimination. This provides a direct proof that the survival of Syk-deficient B cells involves not only BAFF but also PI3K signaling. In conclusion, our study suggests that two different receptor systems, namely BAFF-R and CD19, synergize to signal the survival not only of Syk-deficient but also of wild-type B cells.
Materials and Methods
Syk knockout mice
To delete Syk in mature B cells, the Sykfl/fl mouse strain, kindly provided by Prof. A. Tarakhovsky, was mated with mice expressing CreERT2 in a B-cell-specific manner (mb1-CreERT2). The CreERT2 insert was kindly provided by Prof. P. Chambon (Feil et al, 1997). The resulting mb1-CreERT2;Sykfl/fl mice express a Tam-inducible form of the Cre recombinase under the control of the mb-1 promoter, the mb-1 gene (encoding the Ig-α protein) being B-cell-specific and expressed throughout B-cell development. In these mice, Tam treatment induces Cre activity and deletion of Syk in all B-cell subsets that have matured beyond the early pro-B-cell stage.
The CD19−/− strain has been described previously (Yasuda et al, 2013) and was kindly provided by Dr. M. Schmidt-Supprian. The FoxO1f/f mouse strain was described in Paik et al (2007). All animal studies were absolved randomly and blindly on mice aged between 8 and 12 months and were carried out in accordance with the German Animal Welfare Act, having been reviewed by the regional council and approved under the license # G-09/103.
Tam treatment
Mice were treated by oral gavage with 6 mg of tamoxifen (Tam) citrate (Ratiopharm) dissolved in 20% ClinOleic acid (Baxter). The animals were treated for a total of five times at 3-day intervals and were then sacrificed 5 days after the last treatment, at day 20.
Isolation of genomic DNA and PCR analysis
Genomic (g) DNA was obtained from purified mature splenic and LN-derived B cells or from BM B cells culture using the Quick-gDNA MicroPrep isolation kit (Zymo Research). Amplification of the gDNA was achieved by PCR using following oligonucleotides primers. Syk F (d-band): 5′-GCC CGT TCT GTG CCT ACT GG-3′ and 5′-GCT GGT TCC CTT TTC CTT CC-3′; Syk F2 (f/+-band): 5′-GCC CGT TCT GTG CCT ACT GG-3′ and 5′-TAC CTA ACC AAA CCC ACG GC-3′, TC21 (loading control): 5′-GGA TCA TGT TGT GGA GTT CGT GC-3′; 5′-GTC CAA GAA GAC ATG GAT GGG GG-3′.
mRNA isolation and RT-PCR
Total mRNA was isolated from whole mature B cells using the TRI Reagent kit (Sigma). B cells (5 × 106) were lysed in TRI Reagent, and mRNA was isolated by chloroform/isopropanol precipitation. After ethanol washing and centrifugation, the RNA pellets were air-dried and resuspended in DEPC-treated water. RNA content was measured, and aliquots of 0.1 μg mRNA were reverse-transcribed using the first-strand cDNA synthesis kit (Fermentas) according to the manufacturer's instructions. Amplification of the Syk cDNA was achieved by PCR using the primers Syk-cDNA-for: 5′-TGA GGA CCT GAA GGA GAA CC-3′ and Syk-cDNA-rev: 5′-AGG CTT TGG GAA GGA GTA GG-3′ and running 30 cycles with an annealing step at 60°C. For the amplification of the Hprt-cDNA, the DNA primers HPRT-F: 5′-GCT GGT GAA AAG GAC CTC T-3′ and HPRT-R: 5′-CAC AGG ACT AGA ACA CCT GC-3′ were used. PCR products were then separated and analyzed by agarose gel electrophoresis.
Immunoblot analysis
For immunoblotting, the samples were separated by denaturing SDS-gel electrophoresis, transferred onto a nitrocellulose membrane and probed with anti-Syk (N19; rabbit anti-mouse; Santa Cruz) or anti-ZAP-70 (N15; goat anti-mouse; Santa Cruz). Detection of GAPDH (Calbiochem) was used to demonstrate the amount of total protein loaded. Prior to incubation with the primary antibody (Ab), the membrane was blocked with PBS containing 5% milk powder and subsequently washed. Incubation of the membrane with the primary Ab diluted in PBS supplemented with 0.5% BSA (PAA Laboratories) and 0.1% NaN3 (Sigma) was performed for 1 h at room temperature (RT), followed by washing steps with PBS. Incubation with the secondary Ab, diluted in PBS, was carried out for 1 h at RT. Excess Ab was washed off, and immuno-reactive proteins were detected by the ECL chemoluminescence detection system (Amersham).
Intracellular cell staining
Intracellular cell staining was performed using the ADG fix&perm kit (Dianova) according to the manufacturer's instructions. After fixation and permeabilization, cell suspensions isolated from various lymphoid organs were washed in saponin-based buffer (PBS; 0.5% saponin (Sigma); 0.2% BSA; 0.02% NaN3) and subsequently in FACS buffer (PBS; 3% FCS (PAN Biotech); 0.05% NaN3). Intracellular staining was performed using anti-Syk Ab (BioLegend, clone 5F5 or clone SYK-01) labeled with Zenon Alexa 647 (Invitrogen). Detection of ZAP-70 was performed using anti-ZAP-70-PE (eBioscience, clone 1E7.2). The detection of pY was carried out by staining with directly labeled anti-pY Ab (BioLegend, clone PY20 labeled with Alexa Fluor 647). The detection of FoxO1 was performed using anti-FoxO1 Ab (Cell Signaling, clone C29H4, rabbit anti-mouse IgG) and anti-rabbit IgG DyLight 649 (BioLegend, clone Poly4064). The detection of S6 phosphorylation was performed using a directly labeled anti-pS6 Ser240/244 Ab (Cell Signaling, clone D68F8, labeled with PE). The fixable viability dye eFluor450 (eBioscience) was used in some cell culture experiments.
MACS depletion and cell sorting
Splenic B cells were obtained by MACS-based negative selection using the magnetically labeled B-cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. The cells were separated by AutoMACS (Miltenyi Biotec).
The Abs used in the flow cytometry studies were anti-IgM-PE, anti-IgM-Cy5, anti-IgM-DyLight649, anti-IgD-PE, anti-CD93-APC, anti-CD21-FITC, anti-CD23-PE, anti-CD5-PerCP-Cy5.5, anti-B220-PE, anti-biotin-PE (Becton and Dickinson, eBioscience, or Jackson) and biotinylated anti-BAFF-R (R&D).
CD93− (mature) and CD93+ (transitional) B cells were obtained by cell sorting using FACSAria (Becton Dickinson) or Dako Cytomation MoFlo (Dako) cell sorters. After FACSort separation, the B cells were analyzed for purity and viability using an anti-CD19 Ab (BioLegend clone 6D5) and 7-AAD (eBioscience), respectively.
Ca2+ influx measurement
Ca2+ influx in mature CD93− B cells was measured using the intracellular fluorescent dye Indo-1 (Invitrogen) and the LSRII flow cytometer (Becton Dickinson). Cells (0.5–1 × 106) were resuspended in 1 ml plain Iscove's medium (Biochrom AG) supplemented with 1% FCS. The Indo-1 dye was prepared 5 min prior to incubation with the cell samples and consisted of 25 μl Indo-1 (dissolved in DMSO), 25 μl Pluronic F-127 (Invitrogen) and 113 μl FCS. Indo-1 aliquots (15 μl) were then added to each sample, and cell samples were incubated for 45 min at 37°C protected from light. Cells were then centrifuged at 300 × g and 4°C. For analysis, the cell pellets were resuspended in 500 μl of Iscove's medium supplemented with 1% FCS. The stimuli used in the experiments included 10 μg/ml polyclonal anti-IgM F(ab′)2 (Jackson Lab), 10 μg/ml anti-IgD (eBioscience, clone 10.4.22), 1 μM Ionomycin (Enzo) and 1 μM latrunculin A (Cayman).
Transfer of mature B cells into Rag2−/−;γc−/−
Mature splenic B cells (CD19+CD93−) (1 × 107) from mb1-CreERT2 or mb1-CreERT2;Sykfl/fl mice were isolated by negative selection using the B-cell isolation kit (Miltenyi) in combination with anti-CD93 Ab and transferred into Rag2−/−;γc−/−mice intravenously (i.v.). Recipient mice were treated with Tam every third day, and cell populations were analyzed by flow cytometry 20 days after the transfer. For WT versus Sykfl/fl competition experiments, mature splenic B cells (CD19+CD93+) from WT mice (1 × 107) carrying a GFP reporter construct (Pelanda et al, 2002b) or mature splenic B cells mb1-CreERT2;Sykfl/fl mice (1 × 107) were purified as described above and injected i.v. into Rag2−/−;γc−/− mice at a ratio of 1:1. The recipient mice were treated with Tam every third day until day 30 post-transfer. SP was then analyzed by flow cytometry.
Treatment of mice with anti-BAFF-R Ab
The mb1-CreERT2 and mb1-CreERT2;Sykfl/fl mice were treated with Tam 5 times. At day 15 after Tam treatment, the deletion of Syk was confirmed by intracellular flow cytometric analysis as well as in an activation assay, in which Syk-deficient B cells were non-responsive to BCR stimulation (as shown in Fig4A). The mice were then injected i.v. with 0.5 mg of anti-BAFF-R-blocking Ab (rat anti-mouse, clone 9B9) or the isotype control, which binds to BAFF-R but does not interfere with the BAFF–BAFF-R interaction (non-blocking, rat anti-mouse, clone 5A12) (Rauch et al, 2009). Mice were treated with Tam every third day and were analyzed at days 3, 5, 7, 9 and 15 after anti-BAFF-R treatment when lymphocyte subpopulations in the blood, SP, LN, PP, and PC were analyzed by flow cytometry.
Treatment of mice with anti-IL-7R Ab
mb1-CreERT2;Sykfl/fl were treated with Tam for 35 days every third day. After confirming the deletion of Syk by intracellular staining day 20 after Tam treatment, mice were injected i.v. with 1 mg of anti-IL-7R-blocking Ab (rat anti-mouse, clone A7R34, BXCell and homemade). Mice remained under anti-IL-7R treatment every third day until day 40 after anti-IL-7R-treatment when lymphocyte subpopulations in the SP, BM, LN, PP and PC were analyzed by FACS.
Ex vivo activation and proliferation experiments
Splenic B lymphocytes were purified by negative selection after incubation with magnetic beads (see MACS depletion and cell sorting). CD19+CD93− mature B cells (1 × 105) were cultured in 0.2 ml complete Iscove's cell culture medium (Biochrom AG) supplemented with 10% FCS. The cells were either left unstimulated or were incubated in the presence of the following stimuli: 10 μg/ml polyclonal anti-IgM F(ab′)2 (Jackson immunoresearch, goat anti-mouse polyclonal, catalog #115-006-075), 10 μg/ml anti-RP105 (eBioscience, clone RP/14), 10 μg/ml anti-CD38 (eBioscience, clone 90), 10 μg/ml anti-CD40 (eBioscience, clone 1C10), and 10 ng/ml mIL-4 (Miltenye Biotec); 2.5 μg/ml unmethylated CpG oligonucleotides (Invivogen, ODN 1826); and 10 μg/ml LPS (Sigma). The cells were harvested after 24 h of stimulation, and expression of the activation marker CD86 was analyzed by flow cytometry using anti-CD86 staining (eBioscience, clone GL1). To discriminate viable and dead cells, 7-AAD was added to each sample.
Proliferation of mature B cells was measured using the eFluor 670 proliferation dye (eBioscience). Mature CD93− B cells (2 × 106) were labeled with eFluor 670 prior to cultivation according to manufacturer's instruction. Aliquots of 1 × 105 mature B cells were then cultured in 0.2 ml complete Iscove's medium with 10% FCS for 72 h and were either left unstimulated or stimulated with anti-RP105, anti-IgM alone, anti-IgM and anti-CD40, anti-CD40 alone or unmethylated CpG oligonucleotides at the concentrations described above. Proliferation was measured by flow cytometry. Once again, 7-AAD staining was included in the analysis to discriminate between viable and dead cells. Phosphorylation of total intracellular pY was measured after stimulation of purified B cells with 50 μM pervanadate/H2O2 (Sigma/Merck) for 5 min at 37°C. The pervanadate/H2O2 solution was prepared as described (Wienands et al, 1996). Cells were fixed immediately after phosphorylation and subjected to intracellular staining.
Ex vivo survival assays
Purified splenic B cells were cultured in complete Iscove's medium supplemented with 10% FCS in the absence or presence of 100 ng/ml human recombinant (hr) BAFF (R&D) over a period of 8 days. The medium, supplemented with fresh hrBAFF, was exchanged every second day. The cells were harvested and analyzed by flow cytometry. Dead cells were excluded using 7-AAD and Annexin V staining.
Generation of mb1-CreERT2;Sykfl/fl;CD19−/− and mb1-CreERT2;Sykfl/fl,CD19−/−; FoxO1fl/fl mice
To generate the mb1-CreERT2;Sykfl/fl;CD19−/− mouse strain, mb1-CreERT2;Sykfl/fl mice were crossed to CD19-CreERT2 mice expressing CreERT2 from the CD19 locus and therefore lacking CD19 expression in the homozygous state (Mark Schmidt-Supprian, personal communication and Yasuda et al, 2013). To generate the mb1-CreERT2;Sykfl/fl;CD19−/−;FoxO1 fl/fl mouse strain, mb1-CreERT2;Sykfl/fl;CD19−/− mice were crossed to FoxO1fl/fl mice (Paik et al, 2007). The mb1-CreERT2 Sykfl/fl inducible mice (control for the CD19;Syk double-deficient mice) have one copy of CD19-CreERT2. The CD19−/− controls harbor mb1-CreERT2. The animals were treated with Tam for a total of 10 times at 3-day intervals, and the cells were subsequently analyzed 5 days after the last induction by flow cytometry.
Ex vivo stimulation for pS6 detection
The phosphorylation of S6 was measured after stimulation of purified mature B cells (1 × 105 cells) from mb1-CreERT2 and mb1-CreERT2;Sykfl/fl mice at day 20 after Tam treatment. Purified mature Syk-deficient B cells and the control cells were cultured overnight (O/N) in complete Iscove's medium with 3% FCS. The cells were then stimulated with 10 μg/ml anti-Kappa F(ab′)2, for 10 min (goat anti-mouse Polyclonal von Southern Biotech catalog# 1052-14). To assess the specificity of the anti-pS6 Ab, the cells were pre-treated with 500 nM rapamycin for 1 h and were stimulated with anti-Kappa F(ab′)2 for additional 10 min. Stimulation with 50 μM pervanadate/H2O2 (Sigma/Merck) for 5 min at 37°C served as a positive control for the phosphorylation.
In vitro migration assay
Cell migration was measured using 8-μm Transwell inserts (Greiner) for 24-well plates (Greiner). Iscove's medium/3% FCS alone or supplemented with either 1 μg/ml CCL21, 0.2 μg/ml CXCL12 or 1 μg/ml CXCL13 (PeproTech) was added to the wells, and purified mature B cells (2 × 105 cells) from mb1-CreERT2 and mb1-CreERT2;Sykfl/fl mice at day 10 after Tam treatment were added into the inserts. The cells were allowed to migrate for 2.5 h at 37°. The number of migrated cells and the number of cells in the insert were measured by flow cytometry for 1 min. Dead cells were excluded using 7-AAD. The relative migration was calculated, and specific migration was determined by subtracting the relative unspecific migration without stimulus from the relative migration in response to the chemokines. The surface expression of CXCR4 (receptor for CXCL12) and CXCR5 (receptor for CXCL13) was assessed by flow cytometry using anti-CXCR4-BrilliantViolet421 (BioLegend) and anti-CXCR5-PE (BD) Abs.
Statistical analysis
Unpaired two-tailed Student's t-tests (with n between three and five mice per group) were carried out using Prism 4 software (GraphPad Software Inc) to determine the statistical relevance of differences between groups. In all the carried experiments, the results are representative of at least three independent experiments.
Acknowledgments
We are grateful to Prof. K. Rajewsky for the CD19−/−, Prof. A. Tarakhovsky for the “floxed” Syk and Prof. R. De Pinho for the “floxed” FoxO1 mice. We thank Prof. A. Rolink for the anti-BAFF-R Ab. In addition, we thank Dr. P. Nielsen for reading the manuscript and for helpful scientific discussions. This study was supported by the Excellence Initiative of the German Federal and State Governments (EXC 294), by ERC-grant 32297, by the Deutsche Forschungsgemeinschaft through SFB746, TRR130, EXC294 and the CCI program “the German Federal Ministry of Education and Research” (BMBF 01 EO 0803). The authors are responsible for the contents of this publication.
Author contributions
EH, EL-Z, VA, AA, SA, and RPo conducted the experiments. MWD and RPe provided materials, EH and MR designed the experiments, and EH, EL-Z, RPo and MR wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Figures S1–S11
Supplementary Figure Legends
Review Process File
References
- Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- Alsadeq A, Hobeika E, Medgyesi D, Klasener K, Reth M. The role of the syk/shp-1 kinase-phosphatase equilibrium in B cell development and signaling. J Immunol. 2014;193:268–276. doi: 10.4049/jimmunol.1203040. [DOI] [PubMed] [Google Scholar]
- Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274:32662–32666. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- Brocard J, Feil R, Chambon P, Metzger D. A chimeric Cre recombinase inducible by synthetic, but not by natural ligands of the glucocorticoid receptor. Nucleic Acids Res. 1998;26:4086–4090. doi: 10.1093/nar/26.17.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Carnevale J, Ross L, Puissant A, Banerji V, Stone RM, DeAngelo DJ, Ross KN, Stegmaier K. SYK regulates mTOR signaling in AML. Leukemia. 2013;27:2118–2128. doi: 10.1038/leu.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan VW, Mecklenbrauker I, Su I, Texido G, Leitges M, Carsetti R, Lowell CA, Rajewsky K, Miyake K, Tarakhovsky A. The molecular mechanism of B cell activation by toll-like receptor protein RP-105. J Exp Med. 1998;188:93–101. doi: 10.1084/jem.188.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- Chopin M, Quemeneur L, Ripich T, Jessberger R. SWAP-70 controls formation of the splenic marginal zone through regulating T1B-cell differentiation. Eur J Immunol. 2010;40:3544–3556. doi: 10.1002/eji.201040556. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Del Nagro CJ, Otero DC, Anzelon AN, Omori SA, Kolla RV, Rickert RC. CD19 function in central and peripheral B-cell development. Immunol Res. 2005;31:119–131. doi: 10.1385/IR:31:2:119. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Durand CA, Hartvigsen K, Fogelstrand L, Kim S, Iritani S, Vanhaesebroeck B, Witztum JL, Puri KD, Gold MR. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673–5684. doi: 10.4049/jimmunol.0900432. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fruchon S, Kheirallah S, Al Saati T, Ysebaert L, Laurent C, Leseux L, Fournie JJ, Laurent G, Bezombes C. Involvement of the Syk-mTOR pathway in follicular lymphoma cell invasion and angiogenesis. Leukemia. 2012;26:795–805. doi: 10.1038/leu.2011.248. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Fujimoto Y, Poe JC, Jansen PJ, Lowell CA, DeFranco AL, Tedder TF. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity. 2000;13:47–57. doi: 10.1016/s1074-7613(00)00007-8. [DOI] [PubMed] [Google Scholar]
- Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RB, Grys K, Vehlow A, de Bettignies C, Zachacz A, Henley T, Turner M, Batista F, Tybulewicz VL. A novel Rac-dependent checkpoint in B cell development controls entry into the splenic white pulp and cell survival. J Exp Med. 2010;207:837–853. doi: 10.1084/jem.20091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino S, Benz B, Waldmann T, Jung M, Schneider R, Reth M. Arginine methylation of the B cell antigen receptor promotes differentiation. J Exp Med. 2010;207:711–719. doi: 10.1084/jem.20091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellusova J, Miletic AV, Cato MH, Lin WW, Hu Y, Bishop GA, Shlomchik MJ, Rickert RC. Context-specific BAFF-R signaling by the NF-kappaB and PI3K pathways. Cell Rep. 2013;5:1022–1035. doi: 10.1016/j.celrep.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- Klasener K, Maity PC, Hobeika E, Yang J, Reth M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. eLife. 2014;3:e02069. doi: 10.7554/eLife.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leseux L, Hamdi SM, Al Saati T, Capilla F, Recher C, Laurent G, Bezombes C. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:228. doi: 10.3389/fimmu.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE, Yu N, Kim KM, Reth M, Howard MC. Signaling through CD38 augments B cell antigen receptor (BCR) responses and is dependent on BCR expression. J Immunol. 1996;157:1455–1467. [PubMed] [Google Scholar]
- Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- Miosge LA, Goodnow CC. Genes, pathways and checkpoints in lymphocyte development and homeostasis. Immunol Cell Biol. 2005;83:318–335. doi: 10.1111/j.1440-1711.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- Nagasawa T. The chemokine CXCL12 and regulation of HSC and B lymphocyte development in the bone marrow niche. Adv Exp Med Biol. 2007;602:69–75. doi: 10.1007/978-0-387-72009-8_9. [DOI] [PubMed] [Google Scholar]
- Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem. 2001;276:44315–44322. doi: 10.1074/jbc.M105446200. [DOI] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Audzevich T, Jessberger R. SYK regulates B-cell migration by phosphorylation of the F-actin interacting protein SWAP-70. Blood. 2011;117:1574–1584. doi: 10.1182/blood-2010-07-295659. [DOI] [PubMed] [Google Scholar]
- Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-alpha and Ig-beta. J Immunol. 2002a;169:865–872. doi: 10.4049/jimmunol.169.2.865. [DOI] [PubMed] [Google Scholar]
- Pelanda R, Hobeika E, Kurokawa T, Zhang Y, Kuppig S, Reth M. Cre recombinase-controlled expression of the mb-1 allele. Genesis. 2002b;32:154–157. doi: 10.1002/gene.10070. [DOI] [PubMed] [Google Scholar]
- Rauch M, Tussiwand R, Bosco N, Rolink AG. Crucial role for BAFF-BAFF-R signaling in the survival and maintenance of mature B cells. PLoS ONE. 2009;4:e5456. doi: 10.1371/journal.pone.0005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- Reth M, Nielsen P. Signaling circuits in early B-cell development. Adv Immunol. 2014;122:129–175. doi: 10.1016/B978-0-12-800267-4.00004-3. [DOI] [PubMed] [Google Scholar]
- Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. 2003;18:523–533. doi: 10.1016/s1074-7613(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, Tybulewicz VL. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38:475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhava VJ, Bondada S. Multiple regulatory mechanisms control B-1 B cell activation. Front Immunol. 2012;3:372. doi: 10.3389/fimmu.2012.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Gulbranson-Judge A, Quinn ME, Walters AE, MacLennan IC, Tybulewicz VL. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186:2013–2021. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci USA. 1996;93:7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Beavitt SJ, Harder KW, Hibbs ML, Tarlinton DM. The activation and subsequent regulatory roles of Lyn and CD19 after B cell receptor ligation are independent. J Immunol. 2002;169:6910–6918. doi: 10.4049/jimmunol.169.12.6910. [DOI] [PubMed] [Google Scholar]
- Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Yang J, Reth M. The dissociation activation model of B cell antigen receptor triggering. FEBS Lett. 2010;584:4872–4877. doi: 10.1016/j.febslet.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Wirtz T, Zhang B, Wunderlich T, Schmidt-Supprian M, Sommermann T, Rajewsky K. Studying Epstein-Barr virus pathologies and immune surveillance by reconstructing EBV infection in mice. Cold Spring Harb Symp Quant Biol. 2013;78:259–263. doi: 10.1101/sqb.2013.78.020222. [DOI] [PubMed] [Google Scholar]
- Yazawa N, Fujimoto M, Sato S, Miyake K, Asano N, Nagai Y, Takeuchi O, Takeda K, Okochi H, Akira S, Tedder TF, Tamaki K. CD19 regulates innate immunity by the toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374–1380. doi: 10.1182/blood-2002-11-3573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S11
Supplementary Figure Legends
Review Process File