Abstract
Amino acids identified in the Murchison chondritic meteorite by molecular and isotopic analysis are thought to have been delivered to the early Earth by asteroids, comets, and interplanetary dust particles where they may have triggered the appearance of life by assisting in the synthesis of proteins via prebiotic polycondensation reactions [Oró, J. (1961) Nature 190, 389–390; Chyba, C. F. & Sagan, C. (1992) Nature 355, 125–132]. We report the identification of diamino acids in the Murchison meteorite by new enantioselective GC-MS analyses. dl-2,3-diaminopropanoic acid, dl-2,4-diaminobutanoic acid, 4,4′-diaminoisopentanoic acid, 3,3′-diaminoisobutanoic acid, and 2,3-diaminobutanoic acid were detected in the parts per billion range after chemical transformation into N,N-diethoxycarbonyl ethyl ester derivatives. The chiral diamino acids show a racemic ratio. Laboratory data indicate that diamino acids support the formation of polypeptide structures under primitive Earth conditions [Brack, A. & Orgel, L. E. (1975) Nature 256, 383–387] and suggest polycondensation reactions of diamino acids into early peptide nucleic acid material as one feasible pathway for the prebiotic evolution of DNA and RNA genomes [Joyce, G. F. (2002) Nature 418, 214–221]. The results obtained in this study favor the assumption that not only amino acids (as the required monomers of proteins) form in interstellar/circumstellar environments, but also the family of diamino monocarboxylic acids, which might have been relevant in prebiotic chemistry.
Chondritic meteorites, in particular the CM-type carbonaceous chondrites, make up a unique subset of primitive meteorites, which are of particular interest in the context of origins of life because of their relatively high carbon content and because most of this carbon is present as organic matter. This material is a diverse mixture of compounds that in particular includes carboxylic acids, dicarboxylic acids, hydroxy acids, sulfonic acids, phosphonic acids, and amino acids in the form of monoamino alkanoic acids and monoamino alkandioic acids (1). Among these classes of compounds, a very small fraction of the meteoritic isomers is believed to support prebiotic evolutionary processes. We analyzed hydrolyzed hot-water extracts of a fresh sample of the Murchison CM-chondrite by GC-MS with a chiral column. With an efficient derivatization method and an improved detection technique we focused on compounds with more than two functional groups.
Methods
A 1.19-g sample (MPI 320/14) was taken from the interior of the Murchison meteorite by using a stone crusher. The sample provided fresh fracture surfaces. The sample was powdered for 2 × 2 min at 600 rpm by a planetary micro mill (Pulverisette 7, Fritsch, Idar-Oberstein, Germany) in a positive-pressure “class 100” clean room. A 5-mg aliquot of the sample was imaged by a raster electron microscope (CS44, CamScan, Cranberry Township, PA), showing a homogeneous particle size distribution in the low micrometer range. A cold-water (water for organic trace analysis, Fluka) extraction was performed with a 130-mg aliquot that was subjected to diamino acid analysis. In parallel, 347 mg of the powdered sample was extracted with 700 μl of water for 20 h at 100°C. After centrifugation (Eppendorf safe-lock tubes in Biopur-quality), the liquid phase was split. One hundred microliters was taken for direct derivatization by using the procedure described ref. 2, leading to N,N′-diethoxycarbonyl diamino acid ethyl ester (ECEE) derivatives. Another aliquot of 100 μl was hydrolyzed in 6 M HCl (hydrochloric acid for amino acid analysis, ampoule, Fluka) at 110°C for 24 h (3). After evaporation of the 6 M HCl, the residue was dissolved in 0.1 M HCl and derivatized by the above protocol. The ECEE derivatives obtained in this way were subjected to enantioselective GC-MS (Varian Chrompack Chirasil-l-Val capillary column; 12 m × 0.25-mm inner diameter, 0.12-μm film thickness, 250°C inlet temperature, pulsed splitless injection, 1.5 ml·min-1 constant flow of He carrier gas). The oven temperature program applied for the solvent trapping mode started at 50°C with 10°C·min-1 up to 90°C and then with 2°C·min-1 to 110°C, then was 10°C·min-1 to 180°C, where it was held constant for 39 min. The Agilent 6890/5973 GC-MSD system was used. The identities of the diamino acid peaks obtained via enantioselective GC-MS were verified by comparing the retention times and the mass spectra with literature data (4) and external standards purchased from Fluka. A serpentine sample was taken as a blank (5).
Results and Discussion
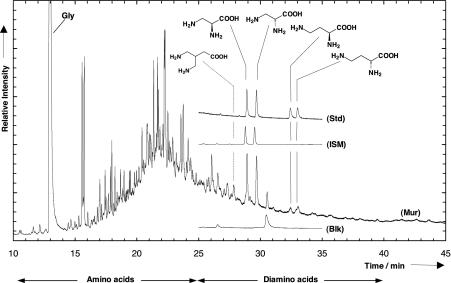
The analytical technique proved to be highly sensitive to the detection of diamino acids, leading to the identification of six diamino acids, i.e., diamino alkanoic acids, in the Murchison meteorite. Fig. 1 shows the chromatogram of a Murchison sample in which dl-2,3-diaminopropanoic acid, dl-2,4-diaminobutanoic acid, and 4,4′-diaminoisopentanoic acid appear. The identification of 3,3′-diaminoisobutanoic acid and 2,3-diaminobutanoic acid is given in Figs. 2 and 3. The left portion of Fig. 1 with retention times (Rt) <25 min shows a wide variety of well known (mono)amino acids including glycine with Rt = 12.95 min. Until now, >70 different (mono)amino acids have been identified in the Murchison meteorite. Most of them show slightly different constitutions of the side chains. Diamino acids have never before been identified in meteorites. Most of the peaks observed with retention times between 25 and 45 min are diamino acids; 2,5-diaminopyrrole is an exception. The presence of diamino acids in Murchison is confirmed by comparison with external standards of diamino acids that have identical mass spectra and retention times. To exclude any contamination processes during the analytical procedure, a serpentine sample was taken as a blank. For this purpose, a sample of serpentine was heated for 4 h at 500°C and submitted to the complete analytical protocol. As depicted in Fig. 1, no diamino acids are present in the blank sample. Apart from this, no other serpentine and epidote samples, regardless of whether they were subjected to heat, contained any diamino acid. These results demonstrate that the identified meteoritic diamino acids were not the result of contamination in the laboratory.
Fig. 1.
Gas chromatogram showing diamino acids identified in a sample of the Murchison meteorite (Mur). Data were obtained after hot-water extraction, 6 M HCl hydrolysis, and ECEE derivatization (2). Detection was in the single-ion monitoring mode of mass trace 175 atomic mass units (amu) typical for specific diamino acid derivatives. (Insets) External standard of the enantiomer separated diamino acids dl-2,3-diaminopropanoic acid and dl-2,4-diaminobutanoic acid (Std) purchased from Fluka and detected in the total ion current; a laboratory sample produced by UV-irradiation of circumstellar/interstellar ice analogues (ISM) under the conditions described in ref. 6 detected in the single-ion monitoring of mass trace 175 amu; and a serpentine blank (Blk) at mass trace 175 amu that had been heated before extraction for 4 h at 500°C and passed through the analytical protocol.
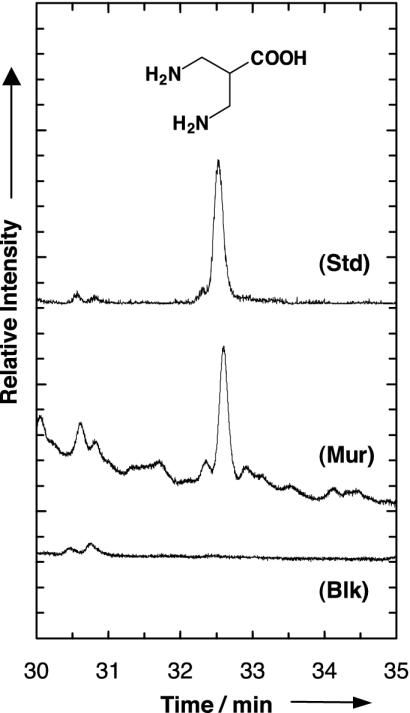
Fig. 2.
Gas chromatogram showing the diamino acid 3,3′-diaminoisobutanoic acid identified in a sample of the Murchison meteorite (Mur). Data were obtained after hot-water extraction, 6 M HCl hydrolysis, and ECEE derivatization by application of the standard GC-MS protocol. Detection was in the single-ion monitoring mode of mass trace 142 amu in deviation from Fig. 1. For comparison, the chromatogram of a sample produced experimentally by photoprocessing of circumstellar ice analogues was used as external standard (Std) in the extract ion mode at m/z = 142. The blank sample chromatogram (Blk) was obtained from a serpentine sample analyzed in the single-ion monitoring mode of mass trace 142 amu.
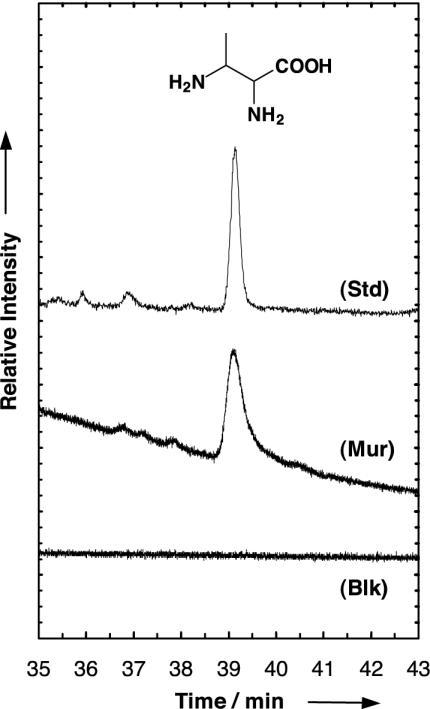
Fig. 3.
Gas chromatogram showing the diamino acid 2,3-diaminobutanoic acid identified in a sample of the Murchison meteorite (Mur). Data were obtained after hot-water extraction, 6 M HCl hydrolysis, and ECEE derivatization by application of the GC-protocol, deviating from the standard protocol on 12-m Chirasil-l-Val stationary phase, with oven temperature programmed for 3 min at 70°C, 5°C·min-1, and 17.5 min at 180°C. Detection was in the single-ion monitoring mode of mass trace 203 amu. For comparison, the chromatogram of a sample produced experimentally by photoprocessing of circumstellar ice analogues was used as external standard (Std). The blank sample chromatogram (Blk) was obtained from a solvent sample that had passed the whole analytical protocol and was detected in the single-ion monitoring mode of mass trace 203 amu.
Table 1 lists the molecules detected and the mass traces of the corresponding ECEE derivatives. The diamino acid retention times are given along with their corresponding quantities. Quantification of the diamino acids was performed by comparison of the peak areas with the diamino acid external standards. The concentrations of diamino acids we observed are lower than those of monoamino acids in the Murchison carbonaceous chondrite (1, 7). In general, the abundances of amino acids in carbonaceous chondrites decrease logarithmically with increasing carbon number. In the reported analyses of meteoritic material, the absolute abundance of amino acids generally is enhanced by antecedent 6 M HCl hydrolysis (8). In the present study the concentration of diamino acids in the Murchison meteorite was also observed to increase after acid hydrolysis. Without a hydrolysis step, d-2,3-diaminopropanoic acid was detected at 8.8 ± 2.2 ppb, l-2,3-diaminopropanoic acid was detected at 8.3 ± 2.1 ppb, d-2,4-diaminobutanoic acid was detected at 18.7 ± 2.6 ppb, and l-2,4-diaminobutanoic acid was detected at 16.3 ± 2.5 ppb. The acid hydrolysis increased the amount of free diamino acids by factors between 1.7 and 6.0 relative to the extraction with hot water at 100°C. After cold-water extraction, no diamino acids were detected. These data indicate that these products must have been originally formed as molecules of higher molecular mass and released as free diamino acids after hydrolysis.
Table 1. Diamino acid identification in the Murchison meteorite.
| Diamino acid | Mass trace, amu | Retention time, min | Quantity, ppb |
|---|---|---|---|
| (2,5-Diaminopyrrole) | 168, 96 | 26.10 | 39.5 ± 3.7 |
| 4,4′-Diaminoisopentanoic acid | 142, 116 | 27.79 | 32.2 ± 3.3 |
| d-2,3-diaminopropanoic acid | 175, 129, 102 | 28.92 | 49.9 ± 4.2 |
| l-2,3-diaminopropanoic acid | 175, 129, 102 | 29.69 | 49.8 ± 4.2 |
| d-2,4-diaminobutanoic acid | 175, 116 | 32.47 | 31.6 ± 3.3 |
| 3,3′-Diaminoisobutanoic acid | 188, 142, 115 | 32.58 | 48.6 ± 4.1 |
| l-2,4-diaminobutanoic acid | 175, 116 | 33.06 | 29.9 ± 3.2 |
| d-ornithine | 142, 129 | 43.26 | <5 |
| l-ornithine | 142, 129 | 44.54 | <5 |
| 2,3-Diaminobutanoic acid* | 203, 157, 85 | 38.62 | 89.9 ± 6.2 |
| (A-unidentified)*† | 216 | 20.19 | 7.1 ± 2.0 |
| (B-unidentified)*† | 216 | 20.39 | 7.2 ± 2.0 |
Numbers shown in boldface are the main mass fragments. ppb, parts per billion, i.e., ng analyte per g Murchison. The diamino acid quantities were calculated by comparison of the peak areas with external diamino acid standards. The uncertainties were calculated from a calibration of the analytical procedure.
Gas chromatograms taken in deviation from the standard protocol on 12-m Chirasil-l-Val stationary phase, with oven temperature programmed for 3 min at 70°C, 5°C·min-1, and 17.5 min at 180°C
A, the first eluting enantiomer; B, the second eluting enantiomer of a hitherto unidentified structure, most likely a diamino acid
A tentative detection of interstellar glycine has recently been claimed (9). Stable isotope measurements suggest that at least some of the organic species found in carbonaceous chondrites are of interstellar origin (1, 7). Thus, it is a priori appropriate to make a comparison between our results and the organic refractory material obtained from UV-irradiation of circumstellar/interstellar ice analogues. Such a comparison, however, is not necessarily straightforward, because interstellar ices are believed to be pristine, whereas carbonaceous chondrites experienced a different history and underwent (possibly frequent) alterations through aqueous processing, thermal effects, or shock, which all may have affected their original composition (10, 11). The Murchison meteorite is classified as a CM2-type chondrite, a type that is characterized by minimal alteration relative to other chondrites studied for organic components (1). The diamino acids described here have previously been identified as refractory products of photoprocessing and thermal processing of circumstellar/interstellar ice analogues in the laboratory (6) and hence point to a correlation between the organic matter that might be present on icy grain mantles residing in star-forming regions (and hence likely also in circumstellar disks) and carbonaceous chondrites. The pathways of formation of the amino acids and diamino acids in carbonaceous chondrites hitherto are unknown. Beside the well studied monoamino acids, β-amino acids (12, 13) and diamino acids have been identified in both laboratory simulations of circumstellar/interstellar ice photoprocessing and thermal processing and (as presented here) in carbonaceous chondrites. The latter organic compounds could not have been formed by the known Strecker-cyanohydrin synthesis (7). The results suggest an alternative formation mechanism, namely that amino acids and diamino acids as refractory products of experimental simulations of ice photoprocessing in circumstellar regions and in carbonaceous chondrites are synthesized via complex recombination processes of radicals produced by photochemistry.
The delivery of organic compounds by meteorites, interplanetary dust particles, or comets (14, 15) to the early Earth is one mechanism thought to have triggered the appearance of life on Earth (16, 17). However, so far there is little conclusive evidence on the early development of genetic DNA material and its analogues. The molecular DNA constituent purine (18) and pyrimidine bases (19), as well as sugar-related organic compounds (20), have been identified in carbonaceous meteorites.
In the course of the evolution of genetic material, it is widely accepted that today's “DNA–RNA–protein world” was preceded by a prebiotic system in which RNA oligomers functioned both as genetic materials and as enzyme-like catalysts (21). In recent years, oligonucleotide systems with properties resembling RNA have been studied intensively (22, 23), and interesting candidates for a potential predecessor to RNA were found (24). Among these candidates, a peptide nucleic acid (PNA) molecule is an uncharged analogue of a standard nucleic acid, in which the sugar-phosphate backbone is replaced by a backbone held together by amide bonds (25–34). The backbone can either be composed of N-(2-aminoethyl)glycine (aeg) or its structural analog, leading to aegPNA molecules, or of diamino acids (da), leading to daPNA structures (Fig. 4). The nucleic bases adenine, uracil, guanine, and cytosine are attached via spacers to the obtained PNA structure. N-(2-aminoethyl)glycine can be produced directly in electric discharge reactions from CH4,N2,NH3, and H2O (35). Racemic 2,3-diaminopropanoic acid was synthesized by spark-discharge experiments (36). The diamino acids 2,4-diaminobutanoic acid and ornithine, which had been suggested to be essential constituents of daPNA monomers (24), were detected in the Murchison meteorite in this work. Ornithine was detected tentatively.
Fig. 4.
Chemical structure of daPNA (Left) and RNA (Right) molecules. B, nucleic bases, attached qua carbonyl-spacers to the PNA respectively to the RNA backbone; R1–4, rest of the genetic material's molecular chain structure. The backbone monomer of the shown daPNA structure is 2,4-diaminobutanoic acid, which was identified in the Murchison meteorite. Beside 2,4-diaminobutanoic acid, ornithine had been suggested to be essential constituent of a further PNA backbone structure (24). Ornithine was identified in the Murchison meteorite tentatively.
Laboratory syntheses of polypeptides indicate that diamino acids also support the formation of polypeptide structures under primitive Earth conditions (37). Only polypeptide chains that consist of amino acids with alternating hydrophobic and hydrophilic side chains fold spontaneously into a β-sheet secondary structure. This β-sheet structure has the hydrophilic side chains arranged on one side of the sheet and the hydrophobic side chains on the other (38). It was concluded earlier that the first biological systems might have contained only a limited number of different amino acids (39). The results presented here for the Murchison chondrite indicate that the diamino acids are potential candidates as hydrophilic constituents within the β-sheet structure.
In future investigations the stable isotope composition of hydrogen, nitrogen, and carbon in the diamino acids needs to be determined. The δ2H, δ15N, and δ13C values of amino acids in Murchison generally lie outside the ranges of organic matter on Earth (12, 40). A similar shift of the isotopic composition is expected for the diamino acids in the Murchison meteorite. Moreover, different constitutional isomers of diamino acids might be determined in other carbonaceous chondrites in the laboratory, in cometary samples by the GC-MS instrumentation onboard the Rosetta mission (41) and the Stardust project, and possibly by measurements to be performed by Mars missions in the coming years. In addition, the synthetic, bioorganic chemical pathways of PNA structures, which start with the diamino acids like those reported here as meteoritic, must be studied more intensely to elucidate possible origins of life.
Acknowledgments
We thank J. Zipfel (Max-Planck-Institut für Chemie, Mainz, Germany) for providing a fresh sample of the interior of the Murchison meteorite; M. Kölling, M. Zuther, and H. Mai for support in the subsequent sample crushing and imaging; U. Schüssler, W. Balzer, and D. Wöhrle for assistance with laboratory and clean-room environments; F. Goesmann, H. Rosenbauer, and the Max-Planck-Institut für Aeronomie (Katlenburg-Lindau, Germany) for chiral capillary columns; and M. Nuevo, L. d'Hendecourt, and the Institut d'Astrophysique Spatiale (Paris) for a simulated sample of interstellar ices. U.J.M. is grateful to the Deutsche Forschungsgemeinschaft for funding his position, the analytical GC-MS instrumentation, and the research group.
Abbreviations: amu, atomic mass units; ECEE, N,N′-diethoxycarbonyl diamino acid ethyl ester; PNA, peptide nucleic acid; ppb, parts per billion.
References
- 1.Cronin, J. R. & Chang S. (1993) in The Chemistry of Life's Origins, ed. Greenberg, J. M. (Kluwer Academic, Dordrecht, The Netherlands), pp. 209-258.
- 2.Abe, I., Fujimoto, N., Nishiyama, T., Terada, K. & Nakahara, T. (1993) J. Chromatogr. A 722, 221-227. [Google Scholar]
- 3.Cronin, J. R. & Pizzarello, S. (1997) Science 275, 951-954. [DOI] [PubMed] [Google Scholar]
- 4.Huang, Z.-H., Wang, J., Gage, D. A., Watson, J. T. & Sweeley, C. C. (1993) J. Chromatogr. 635, 271-281. [DOI] [PubMed] [Google Scholar]
- 5.Botta, O., Glavin, D. P., Kminek, G. & Bada, J. L. (2002) Origins Life Evol. Biosphere 32, 143-163. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz Caro, G. M., Meierhenrich, U. J., Schutte, W. A., Barbier, B., Arcones Segovia, A., Rosenbauer, H., Thiemann, W. H.-P., Brack, A. & Greenberg, J. M. (2002) Nature 416, 403-406. [DOI] [PubMed] [Google Scholar]
- 7.Cronin, J. R. (1998) in The Molecular Origins of Life Assembling Pieces of the Puzzle, ed. Brack, A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 119-146.
- 8.Cronin, J. R. (1976) Origins Life 7, 337-342. [DOI] [PubMed] [Google Scholar]
- 9.Kuan, Y. J., Charnley, S. B., Huang, H.-C., Tseng, W.-L. & Kisiel, Z. (2003) Astrophys. J. 593, 848-867. [Google Scholar]
- 10.Kerridge, J. F. (1999) Space Sci. Rev. 90, 275-288. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz, A. W. & Chang, S. (2002) in Life's Origin, ed. Schopf, J. W. (Univ. of California Press, Berkeley), pp. 46-77.
- 12.Engel, M. H. & Macko, S. A. (2001) Precambr. Res. 106, 35-45. [Google Scholar]
- 13.Ehrenfreund, P., Glavin, D. P., Botta, O., Cooper, G. & Bada, J. L. (2001) Proc. Natl. Acad. Sci. USA 98, 2138-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oró, J. (1961) Nature 190, 389-390. [Google Scholar]
- 15.Jessberger, E. K. (1999) Space Sci. Rev. 90, 91-97. [Google Scholar]
- 16.Chyba, C. F. & Sagan, C. (1992) Nature 355, 125-132. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenfreund, P. (1997) Science 283, 1123-1124. [DOI] [PubMed] [Google Scholar]
- 18.Van der Velden, W. & Schwartz, A. W. (1977) Geochim. Cosmochim. Acta 41, 961-968. [Google Scholar]
- 19.Stoks, P. G. & Schwartz, A. W. (1979) Nature 282, 709-710. [Google Scholar]
- 20.Cooper, G., Kimmich, N., Belisle, W., Sarinana, J., Brabham, K. & Garrel, L. (2001) Nature 414, 879-883. [DOI] [PubMed] [Google Scholar]
- 21.Gesteland, R., Cech, T. R. & Atkins, J. F. (1999) The RNA World (Cold Spring Harbor Lab. Press, Plainview, NY).
- 22.Beier, M., Reck, F., Wagner, T., Krishnamurthy, R. & Eschenmoser, A. (1999) Science 283, 699-703. [DOI] [PubMed] [Google Scholar]
- 23.Joyce, G. F. (2002) Nature 418, 214-221. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen, P. E. (1993) Origins Life Evol. Biosphere 23, 323-327. [DOI] [PubMed] [Google Scholar]
- 25.Egholm, M., Burchardt, O., Nielsen, P. E. & Berg, R. H. (1992) J. Am. Chem. Soc. 114, 1895-1897. [Google Scholar]
- 26.Egholm, M., Nielsen, P. E., Burchardt, O. & Berg, R. H. (1992) J. Am. Chem. Soc. 114, 9677-9678. [Google Scholar]
- 27.Egholm, M., Burchardt, O., Christensen, L., Behrens, C., Freier, S. M., Driver, D. A., Berg, R. H., Kim, S. K., Norden, B. & Nielsen, P. E. (1993) Nature 365, 566-568. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt, J. G., Christensen, L., Nielsen, P. E. & Orgel, L. E. (1997) Nucleic Acids Res. 25, 4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt, J. G., Nielsen, P. E. & Orgel, L. E. (1997) Nucleic Acids Res. 25, 4797-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koppitz, M., Nielsen, P. E. & Orgel, L. E. (1998) J. Am. Chem. Soc. 120, 4563-4569. [DOI] [PubMed] [Google Scholar]
- 31.Böhler, C., Nielsen, P. E. & Orgel, L. E. (1995) Nature 376, 578-581. [DOI] [PubMed] [Google Scholar]
- 32.Wittung, P., Nielsen, P. E., Burchardt, O., Egholm, M. & Nordén, B. (1994) Nature 368, 561-563. [DOI] [PubMed] [Google Scholar]
- 33.Kozlov, I. A., Orgel, L. E. & Nielsen, P. E. (2000) Angew. Chem. Int. Ed. 39, 4292-4295. [DOI] [PubMed] [Google Scholar]
- 34.Kozlov, I. A., Orgel, L. E. & Nielsen, P. E. (2000) Angew. Chem. 112, 4462-4464. [Google Scholar]
- 35.Nelson, K. E., Levy, M. & Miller, S. L. (2000) Proc. Natl. Acad. Sci. USA 97, 3868-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel, M. H., Macko, S. A., Qian, Y. & Silfer, J. A. (1995) Adv. Space Res. 15, 99-106. [DOI] [PubMed] [Google Scholar]
- 37.Brack, A. & Orgel, L. E. (1975) Nature 256, 383-387. [DOI] [PubMed] [Google Scholar]
- 38.Brack, A. (1993) Pure Appl. Chem. 65, 1143-1151. [Google Scholar]
- 39.Brack, A. (1987) Origins Life Evol. Biosphere 17, 367-379. [DOI] [PubMed] [Google Scholar]
- 40.Pillinger, C. T. (1984) Geochim. Cosmochim. Acta 48, 2739-2766. [Google Scholar]
- 41.Thiemann, W. H.-P. & Meierhenrich, U. (2001) Origins Life Evol. Biosphere 31, 199-210. [DOI] [PubMed] [Google Scholar]