Abstract
Inositol phosphates are a large and diverse family of signalling molecules. While genetic studies have discovered important functions for them, the biochemistry behind these roles is often not fully characterized. A key obstacle in inositol phosphate research in mammalian cells has been the lack of straightforward techniques for their purification and analysis. Here we describe the ability of titanium dioxide (TiO2) beads to bind inositol phosphates. This discovery allowed the development of a new purification protocol that, coupled with gel analysis, permitted easy identification and quantification of InsP6 (phytate), its pyrophosphate derivatives InsP7 and InsP8, and the nucleotides ATP and GTP from cell or tissue extracts. Using this approach, InsP6, InsP7 and InsP8 were visualized in Dictyostelium extracts and a variety of mammalian cell lines and tissues, and the effects of metabolic perturbation on these were explored. TiO2 bead purification also enabled us to quantify InsP6 in human plasma and urine, which led to two distinct but related observations. Firstly, there is an active InsP6 phosphatase in human plasma, and secondly, InsP6 is undetectable in either fluid. These observations seriously question reports that InsP6 is present in human biofluids and the advisability of using InsP6 as a dietary supplement.
Keywords: phytic acid, IP6, IP7, IP8, blood
2. Background
Inositol is present in all eukaryotes, most archaea and some bacteria [1], but only nucleated cells have taken advantage of the metabolic stability of this sugar to evolve the complex array of phosphorylated signalling molecules known as inositol phosphates (if water soluble) or inositides (if lipid-bound) [2]. Attention has been drawn to the soluble inositol phosphates by the elucidation of the signalling pathway that connects receptor activation, via phospholipase C, to the release of the second messenger Ins(1,4,5)P3 [3]. A variety of inositol phosphates are also generated using Ins(1,4,5)P3 as precursor through a series of kinases and phosphatases [2]. Notably, the sequential action of the kinases IPMK (inositol polyphosphate multikinase, also known as Ipk2) [4] and IP5-2kinase (also known as IPPK or Ipk1) [5] convert Ins(1,4,5)P3 to InsP6 (inositol hexakisphosphate; phytic acid) [6,7]. InsP6 was originally discovered as a phosphate storage molecule in plant seeds but is now known to be present in all eukaryotic cells. It is the most abundant intracellular inositol phosphate species, with concentrations ranging between 10 and 50 µM in mammalian cells [8,9]. The social amoeba Dictyostelium discoideum has the highest known (non-plant seed) concentration, where InsP6 levels can reach 0.5 mM [8,10,11]. Biophysical studies have indicated that at cytoplasmic pH and salt concentrations, InsP6 exists in a neutral pentamagnesium form with a solubility limit of 49 μM [12], so it is likely that in D. discoideum some of the InsP6 is compartmentalized in vesicles, or that this organism has unusual cytoplasmic conditions permitting higher InsP6 solubility.
In mammals, were extracellular InsP6 to exist, it should only do so complexed to proteins, since it would be expected to precipitate in the prevailing salt and pH conditions. The suggested presence of InsP6 in human biofluids is currently relevant as ‘natural products’ companies are selling InsP6 as a supposedly beneficial dietary supplement. The claim is that dietary InsP6 is absorbed by the intestinal mucosa and transported in plasma, both of which assumptions have proved highly divisive. Specific InsP6 transporters are yet to be identified in mammals, and a molecule as polar as InsP6 cannot diffuse through the plasma membrane; thus its intestinal absorption is unlikely. By a variety of assays, the InsP6 concentration in human plasma has been calculated as 0.2–0.5 µM [13,14], but a more direct and highly specific mass assay was unable to confirm these values [8], showing that InsP6 could be present in human plasma at only sub-nanomolar concentrations, if at all.
Many signalling roles have been attributed to InsP6, notably a role in the control of nuclear–cytoplasm mRNA export [15]. The InsP6-derived inositol pyrophosphates have their own signalling roles and have been implicated in the pathophysiology of important human diseases such as diabetes, obesity, cancer, blood coagulation and viral infection (for reviews, see [16–18]). In these studies, the cellular biochemistry of InsP6, InsP7 and InsP8 is often incompletely characterized, however. This is due to the technical difficulty of accurate measurement, which requires radioactive metabolic labelling and HPLC analysis [19]. These cumbersome techniques have held back InsP6 and inositol pyrophosphate research, which consequently lags far behind sister fields such as InsP3/Ca2+ and the inositol lipids [2]. The development of new methods to facilitate the analysis of InsP6 and inositol pyrophosphates is therefore imperative. A few years ago, a polyacrylamide gel electrophoresis (PAGE)-based method was developed for this purpose. This technique can easily resolve highly phosphorylated inositol species that are then visualized and quantified by staining with toluidine blue or DAPI [20,21]. This method has been well received as it does not require radioactive tracers and is now in common use for in vitro studies.
Because of the abundance of InsP6, InsP7 and InsP8 in D. discoideum, the PAGE technique can be used to study inositol pyrophosphate metabolism in this amoeba [10]. For mammalian extracts, however, the lower concentrations of inositol phosphates and thus larger extraction volumes have precluded direct analysis by PAGE. Here we describe a new inositol phosphate extraction method that overcomes these limitations, allowing the direct analysis of unlabelled InsP6 and inositol pyrophosphates extracted from mammalian cells and tissues. Since this newly developed technology allows the extraction of inositol phosphates from large sample volumes, we also used it to test for the presence of InsP6 in human biofluids in an attempt to resolve the controversy [8,22,23] surrounding this issue.
3. Material and methods
3.1. Cell culture and treatment
Mammalian, plant and fly cells used were gifts from several different laboratories and were grown in standard conditions for each cell type. HCT116 cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS, in 5% CO2. Vegetative state Dictyostelium discoideum cells were grown at 22°C in a shaking HL5 medium supplemented with 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Invitrogen). For sodium fluoride treatment, 90% confluent HeLa, MCF7 and HCT116 cells (2× 14 cm dishes) were treated with 10 mM sodium fluoride (Sigma) for 1 h before harvesting by trypsinization. For oligomycin treatment, the cells were pre-treated with glucose-free DMEM for 30 min, before addition of 5 μM oligomycin (Sigma) for 3 h. Cells were harvested by trypsinization.
3.2. Titanium dioxide bead extraction
All steps in the extraction until elution were performed at 4°C to avoid acid degradation of inositol pyrophosphates. First, the titanium dioxide (TiO2) beads (Titansphere TiO 5 µm; GL Sciences) were weighed and prepared by washing once in water then once in 1 M perchloric acid (PA). Generally 4–5 mg of beads was used for each sample. After centrifuging at 3500g for 1 min, the beads were resuspended in PA.
Cells were harvested as appropriate and washed in PBS. A small aliquot was removed for later protein quantification, enabling normalization. The cells were pelleted and extracted using 800 µl PA (pH 1). After resuspension in the acid, samples were kept on ice with vortexing for 10 min, then centrifuged at 18 000g for 5 min, at 4°C. The supernatants were removed into new eppendorfs and TiO2 beads added (4 mg in 50 μl PA). Samples were vortexed briefly then rotated at 4°C for 15 min; the inositol phosphates and other molecules were adsorbed onto the beads at this point. Beads were pelleted by centrifuging at 3500g for 1 min, and then washed twice in PA with supernatants discarded. To elute, 200 µl 10% ammonium hydroxide (pH 10) was added to the beads. Samples were vortexed briefly before rotation for 5 min. After centrifuging, the supernatants (containing the inositol phosphates) were transferred into new eppendorfs. The elution procedure was repeated on the beads to ensure full recovery, and the second supernatants added to the first. The samples were then vacuum evaporated to 50 µl for PAGE or other further analysis. Alternatively, samples were evaporated until at pH 7 then stored at 4°C or −20°C.
The protocol used for TiO2 extraction from Dictyostelium PA extracts, diluted InsP6 standards and radioactive 3H-Ins(1,4,5)P3 (PerkinElmer) or 3H-InsP6 (Amersham) was the same as above, except that the standards were directly added to 1 ml PA. For the radioactive experiments, 5 ml of Ultima Gold (PerkinElmer) scintillation cocktail was added to the TiO2 eluate and the samples were counted in a β-counter.
Mouse liver and brain were collected from newborn (P1) pups and rapidly frozen. PA (2 ml) was added to approximately 0.5 g of tissues, equivalent to one liver or two brains. The organs were rapidly homogenized in an electric blender and incubated in ice for 10 min. The samples were centrifuged at more than 15 000g for 15 min and the supernatant used for TiO2 bead extraction.
3.3. Plasma, serum and urine extraction
Bovine and horse serum and plasma were bought from Life Technologies and Sigma Aldrich. Human serum and plasma were bought from TCS Biosciences and Sigma Aldrich. Alternatively, human plasma was prepared from anonymous donors. Two 20 ml samples of blood from each volunteer were collected into tubes pre-filled with 1.6 mg of EDTA per ml of blood (5.5 mM) and immediately cooled on ice for 10 min. One of the samples was then spiked with 1 nmol of InsP6 prior to removal of cells and platelets by centrifugation at 1500g for 10 min at 4°C. This yielded plasma for analysis. To extract InsP6, half volume of 2 M PA was added to 10–20 ml of serum or plasma and the sample rotated at 4°C for 30 min. The denatured proteins were removed by centrifugation at 15 000g at 4°C for 30 min. The supernatant was subjected to the extraction procedure as described above, using 5 mg of TiO2 beads.
Human urine was obtained from anonymous donors. After centrifugation at 2000g at 4°C for 5 min to remove any epithelial cells, which were discarded, the samples were split in half; one half was spiked with 2 nmol InsP6. Concentrated PA was added to a final concentration of 1 M and rotated at 4°C for 30 min. The denatured proteins were removed by centrifugation at 15 000g at 4°C for 30 min. The supernatant was subjected to the extraction procedure as described above.
3.4. Enzymatic treatment
Cell extracts were treated with apyrase (New England Biolabs) following the manufacturer's instructions.
3.5. Polyacrylamide gel electrophoresis of inositol phosphates
PAGE was performed as previously described [20]. Briefly, 35% polyacrylamide/TBE gels were used to resolve the TiO2-extracted samples. Samples were mixed with either orange G or bromophenol blue loading buffers. The gels were pre-run for 30 min at 300 V and run overnight at 4°C at 600 V and 6 mA, until the orange G had run through two-thirds of the gel. Gels were stained as previously described [20] with either DAPI or toluidine blue. Gels stained with toluidine blue were scanned using a desktop computer scanner for image analysis. ImageJ was used for densitometry (n = 3 per experiment), and amounts of inositol pyrophosphates are expressed as a ratio of their band density over InsP6. Nucleotides and polyP standards were bought from Sigma Aldrich, while InsP6, InsP5, InsP4 and InsP3 were bought from Sichem.
4. Results
4.1. Titanium dioxide binds to inositol phosphates
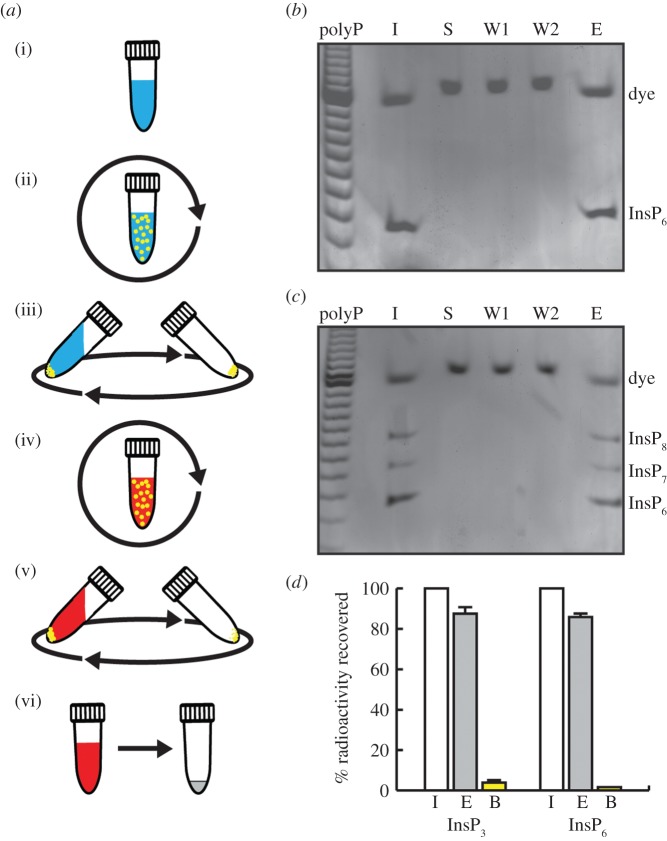
The ability of titanium dioxide (TiO2) to bind with very high affinity to phosphate groups is used in phosphopeptide enrichment protocols, an essential step in modern phosphoproteomic studies [24]. We used this TiO2 property [25] to develop a simple enrichment method (schematized in figure 1a) to extract inositol phosphates from acidic solutions, normally 1 M PA [19]. Initially, a specific amount of InsP6 diluted in PA was incubated with TiO2 beads for 30 min. After two washes with PA, InsP6 was eluted from the beads by a pH change induced by 10% ammonium hydroxide. After removing the ammonium hydroxide and reducing the volume using a centrifugal evaporator, the samples were resolved by PAGE and visualized with toluidine blue staining, demonstrating an almost complete recovery of the input InsP6 (figure 1b). We next tested this procedure on a D. discoideum extract, and recovered quantifiable levels of InsP6 and its pyrophosphate derivatives InsP7 and InsP8 (figure 1c). To precisely quantify the inositol phosphate recovery, radioactive 3H-Ins(1,4,5)P3 and 3H-InsP6 tracers were each mixed with 2 nmol of Ins(1,4,5)P3 and InsP6 and subjected to TiO2 enrichment. These experiments demonstrated that 87 ± 4.6 and 84 ± 3.5% (average ± s.d., n = 4) for Ins(1,4,5)P3 and InsP6, respectively, of input radioactivity was recovered after TiO2 elution, while about 2–4% remained attached to the TiO2 beads (figure 1d). The TiO2 beads are in fact completely efficient at binding and releasing inositol phosphates; the small loss is intrinsic to the manual handling involved.
Figure 1.
TiO2 purifies inositol phosphates. (a) Flowchart describing the five-step TiO2 bead extraction procedure. (i) Acidic solution (blue) containing inositol phosphates is incubated with (ii) TiO2 beads (yellow) for 10 min, before (iii) spinning and washing the beads twice with 1 M PA. Elution occurs by incubation (iv) at basic pH (red) with subsequent spinning and recovering the supernatant (v). This is evaporated (vi) to concentrate and neutralize (grey) the extract. (b) InsP6 diluted in 1 M PA was purified using TiO2 and subjected to PAGE with toluidine blue staining. I, input; S, supernatant; W1 and W2, washes; E, eluted. While all the eluted InsP6 was loaded on the gel only 1/10 (approx. 100 µl) of the S, W1 and W2 fractions were loaded. The acid in these fractions results in slightly compressed and slower migration of the orange G dye. (c) As (b), but using a PA extraction from vegetative state D. discoideum cells as input. These toluidine blue-stained gels are representative of experiments performed at least three times. (d) To calculate the exact percentage of recovery, radioactive 3H-Ins(1,4,5)P3 and 3H-InsP6 were subjected to TiO2 purification. The radioactivity recovered (E) and radioactivity remaining on TiO2 beads (B) were normalized to the respective radioactive input (I). The graph showing the average ± s.d. (n = 4) is representative of two independent experiments with matching results.
4.2. Titanium dioxide purifies inositol phosphates and nucleotides from mammalian cell extracts
The lower concentration of inositol phosphates in mammalian cells has previously rendered extracts from these cells impracticable for PAGE analysis. Either the volume is too large to load onto the gel, or volume reduction by evaporation results in salt concentrations that cause aberrant gel migration. The ability to concentrate inositol phosphates using TiO2 beads overcomes these limitations.
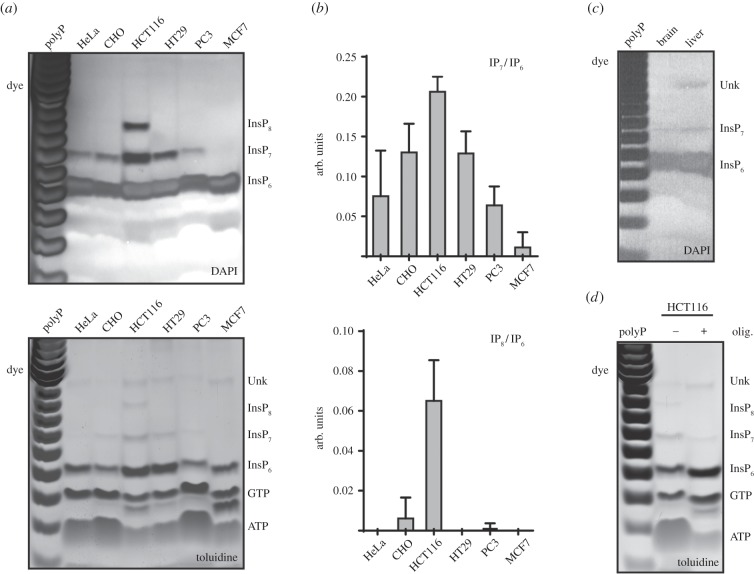
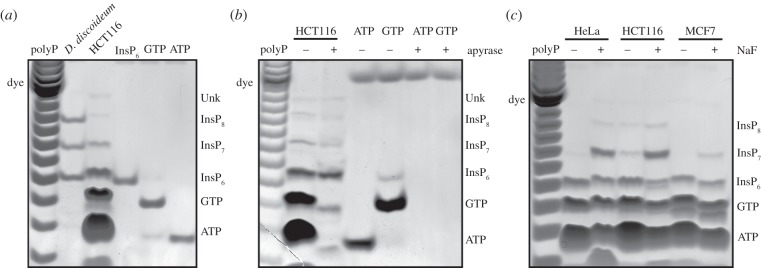
We tested TiO2 enrichment on extracts from HCT116 cells (human colon cancer cell line). PAGE analysis (figure 2a) of the phosphate-rich molecules extracted revealed the presence of three inositol phosphate bands that co-migrate with D. discoideum-extracted InsP6, InsP7 and InsP8 [10]. Interestingly, unlike D. discoideum extracts, mammalian cell extracts revealed extra bands that co-migrate with the nucleotide standards ATP and GTP. We confirmed the identity of the bands presumed to be ATP and GTP by treating the TiO2-purified samples with apyrase, an enzyme that specifically hydrolyses nucleotides (figure 2b). We also detected a faint, slower migrating band of unknown nature (labelled Unk), which is particularly abundant in liver extract (figure 3c). This band does not represent an InsP9 species since it is not fully degraded after phytase treatment unlike the InsP6, InsP7 and InsP8 bands (data not shown). The partial action of phytase on this unknown band suggests a complex molecule containing an inositol phosphate group. Inositol pyrophosphates in mammalian cells are known to be dramatically regulated by sodium fluoride (NaF) [26]. To confirm the identity of the observed InsP7 and InsP8 bands, we incubated three human cell lines with NaF: firstly, MCF7 cells, which usually have undetectable levels of inositol pyrophosphates; secondly, HeLa cells, which have detectable levels of InsP7; and thirdly, HCT116 cells, which have detectable levels of InsP7 and InsP8. PAGE analysis after TiO2 extraction revealed that NaF treatment increases inositol pyrophosphate levels, and decreased the level of their precursor InsP6, in all three cell lines (figure 2c).
Figure 2.
TiO2 beads purify nucleotides and inositol phosphates from mammalian cells. (a) PA extracts from vegetative D. discoideum (4 × 106 cells) and the human HCT116 cell line (80 × 106 cells) were subjected to TiO2 enrichment. After resolving the extract with PAGE, phosphate-rich molecules were visualized by toluidine blue staining for comparison with InsP6, ATP and GTP nucleotide standards. (b) TiO2-purified HCT116 extract and nucleotide standards were subjected to apyrase treatment, before resolution by PAGE and staining with toluidine blue. (c) Two 14 cm dishes of 80% confluent HeLa, HCT116 and MCF7 cells were treated with 10 mM sodium fluoride (NaF) for 1 h, before purification of inositol phosphates with TiO2 beads and resolution by PAGE with toluidine blue staining. The gels presented are representative of experiments performed at least three times.
Figure 3.
Visualizing InsP6, InsP7 and InsP8 from mammalian cells and organs. (a) Cells from six different human lines were collected and washed in PBS. A small aliquot was taken for determination of protein concentration, while the rest was PA extracted and subjected to TiO2 bead enrichment. Extracts relative to equivalent amounts of protein for each cell line (approx. 35 mg) were loaded on two parallel gels subsequently stained by DAPI (top) and toluidine blue (bottom). (b) Densitometry of toluidine blue-stained gel from three independent experiments was used to calculate ratios of InsP7 and InsP8 over their precursor InsP6. (c) Mouse brain and liver (approximately 0.5 g) were homogenized and extracted with PA. After TiO2 purification the inositol phosphates were resolved by PAGE and stained with DAPI. (d) Two 14 cm dishes of 80% confluent HCT116 cells were pre-treated in glucose-free medium for 30 min before addition of 5 µM oligomycin for 3 h. The TiO2 extracts were then resolved by PAGE and stained with toluidine blue. The gels presented are representative of experiments performed three times.
4.3. Screening of mammalian cell lines and tissues for the presence of inositol pyrophosphates
Next, we decided to screen 27 mammalian, one plant and one Drosophila cell line for the presence of inositol pyrophosphates, to identify the best cell line(s) for studying the different aspects of inositol pyrophosphate metabolism. TiO2 purification was performed from 90% confluent cells grown in two 14 cm adherent culture dishes or shaking culture, as appropriate. The use of DAPI to better visualize inositol pyrophosphates [20] revealed the presence of InsP7 in almost all cells analysed (electronic supplementary material, figure S1), while InsP8 is easily detectable in mouse ES cells, Drosophila S2 and the human HCT116 cell line, where inositol pyrophosphates seem to be particularly abundant. An exact comparative analysis cannot be achieved since cell density, size and shape differ greatly between cell lines. Furthermore, DAPI staining is not linear, unlike toluidine blue, the staining intensity of which depends only on the number of phosphates [10]. Therefore, we chose several human cell lines to investigate their relative inositol pyrophosphate levels more thoroughly, normalizing the different extracts by protein mass. The normalized analysis of six mammalian cell lines by DAPI confirmed the screening result (figure 3a). Parallel toluidine blue staining confirmed a variable amount of InsP7 and especially InsP8 between the cell lines (figure 3a,b).
Coupling the TiO2 method with PAGE analysis also allows extraction and investigation of inositol pyrophosphates from previously intractable sources, including animal organs such as mouse brain or liver, where InsP6 and InsP7 can be easily detected (figure 3c).
4.4. Effect of altered energetic metabolism on inositol pyrophosphates levels
The ability to enrich and analyse InsP7 and InsP8 from mammalian extracts has the potential to revolutionize this field of research. As inositol pyrophosphates have been linked to cellular and organismal metabolism [27–29], we took advantage of the TiO2 method to observe their changes after metabolic perturbation. Inositol pyrophosphates were TiO2-extracted from glucose-starved HCT116 cells treated with the oxidative phosphorylation inhibitor oligomycin for 3 h. PAGE analysis showed the disappearance of InsP8 and a substantial reduction in InsP7, with a concomitant increase in InsP6 (figure 3d).
4.5. Absence of InsP6 in human blood revealed by titanium dioxide extraction
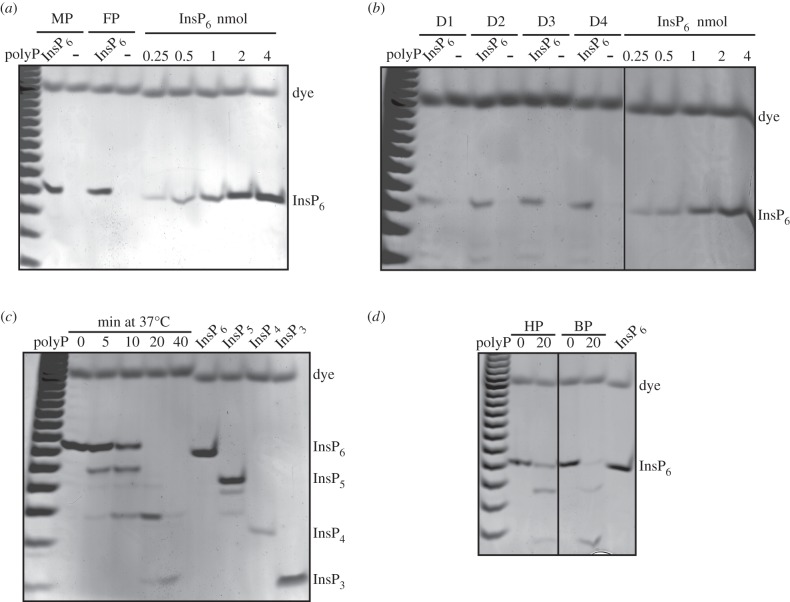
Since large volumes of acidified fluid can be subject to TiO2 bead extraction, this gave us the opportunity to assay InsP6 in biofluids. Initially, we used commercially available serum from bovine, equine and human sources. We extracted 20 ml of serum with TiO2 beads and analysed the extracts by PAGE. While we were able to detect an almost complete InsP6 recovery in the spiked samples, we did not recover any InsP6 in non-spiked serum (electronic supplementary material, figure S2A,B). We next analysed human plasma from a commercial source. Similar to serum, TiO2 extraction and PAGE analysis showed that InsP6 could not be recovered from non-spiked samples of human plasma (figure 4a). The lower limit of InsP6 standard detection on PAGE is about 0.25 nmol (figures 4a,b and 5), therefore TiO2-extracting 20 ml of plasma with a recovery of approximately 85% (figure 1e) indicates that the lower limit of plasma InsP6 we are able to extract and detect is approximately 15 nM. Consequently, we conclude that substantially less than 15 nM InsP6 is present in human plasma, in agreement with the enzymatic radio-assay previously reported [8].
Figure 4.
Absence of InsP6 and presence of inositol phosphate phosphatase in human plasma. All the extracts were resolved by PAGE and visualized with toluidine blue staining. (a) EDTA was added to 20 ml of commercial human plasma from male (MP) and female (FP); InsP6 was added to the spiked aliquot (InsP6; 2 nmol). The samples were then acidified and subjected to TiO2 enrichment. (b) Plasma from healthy anonymous donors (D1 to D4) was prepared as described in Material and methods, with spiking (InsP6; 1 nmol), and subjected to TiO2 extraction. (c) 4 nmol of InsP6 was added to 1 ml of human plasma and incubated at 37°C for the indicated time before acidification and extraction of inositol phosphates with the TiO2 procedure. Standards: InsP6 (4 nmol); InsP5 (6 nmol of Ins(1,3,4,5,6)P5); InsP4 (5 nmol of Ins(1,4,5,6)P4); InsP3 (20 nmol of Ins(1,4,5)P3). (d) 4 nmol of InsP6 was added to 1 ml of a different source of human plasma (HP) and bovine plasma (BP) before incubation at 37°C for the indicated time, followed by acidification of the samples and TiO2 extraction. The gels presented are representative of experiments performed two to four times.
Figure 5.
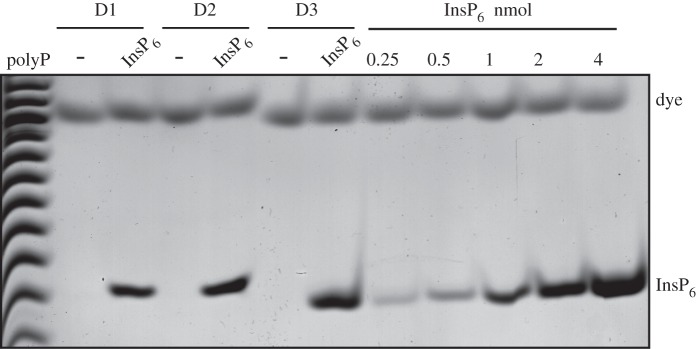
Absence of InsP6 in human urine. Freshly collected urine from healthy anonymous donors (D1–D3) was centrifuged to remove any epithelial cells. The samples were divided into two aliquots (25 ml each for D1 and D2, 10 ml for D3), EDTA was added and InsP6 (2 nmol) was supplemented into the spiked (InsP6) aliquots. The samples were then PA extracted and subjected to TiO2 enrichment. The extracted inositol phosphates were resolved by PAGE and visualized with toluidine blue staining. The gel presented is representative of three experimental repeats.
Conversely, using less direct assays, others have suggested that the InsP6 concentration in human plasma is in the 0.2–0.5 µM range [13,14]. They suggested that the previous failure [8] to detect InsP6 in plasma was due to losses during plasma preparation [23]. To investigate this possibility, we here prepared plasma from anonymous donors following the extraction protocol described in [13,14] (which uses EDTA as an anti-coagulant [23]). While we were able to detect a good recovery of InsP6 from plasma when whole blood was spiked with 1 nmol (0.05 µM) of InsP6, we were again unable to detect any InsP6 in non-spiked human plasma (figure 4b). Interestingly, in the spiked samples, other faster migrating bands of weak intensity, probably lower forms of InsPx, were detected besides InsP6, and we only recovered spiked InsP6 with a high efficiency if the blood was cooled on ice before spiking. These observations suggested the presence of a phosphatase activity in plasma. To test this directly, we incubated InsP6-spiked human plasma at 37°C. Just 5 min of incubation resulted in substantial conversion of InsP6 to InsP5 (figure 4c); 20 min led to the complete removal of InsP6, while after 40 min all the exogenous InsP6 was converted to InsP3 and even lower forms of inositol phosphates. It is likely that a recently reported secreted mammalian phosphatase, MINPP1 (multiple inositol polyphosphate phosphatase) [30], is responsible for the observed activity. To further confirm the presence of phosphatase activity in plasma, a different source of human plasma together with bovine plasma was tested for the presence of this enzymatic activity (figure 4d). InsP6 phosphatase activity was detectable in both cases, indicating that this activity may be a common characteristic of mammals.
4.6. Absence of InsP6 in human urine revealed by titanium dioxide extraction
Another human biofluid in which InsP6 has been contentiously reported is urine, with some literature indicating that it reaches 1–3 µM concentration [31]. Therefore, we processed 10–25 ml of urine from anonymous donors using TiO2 and visualized the extraction on PAGE. Similar to the serum and plasma experiments, we were unable to detect any InsP6 in non-spiked urine (figure 5). Thus, less than 12 nM InsP6 is present in human urine, a maximal value in accordance with the earlier report using a specific enzymatic assay [8].
5. Discussion
TiO2 beads and pre-packed columns are useful tools for the enrichment of phosphopeptides and have contributed hugely to the development and application of phosphoproteomic studies [24]. We have taken advantage of this TiO2 phosphate binding property [25] to develop a new inositol phosphate purification protocol. Crucially, it allows for the purification of inositol phosphates from large volumes of acidified extracts and so makes feasible the extraction/enrichment of the low concentration inositol phosphates in mammalian extracts.
The direct analysis of mammalian InsP6 and its pyrophosphate derivatives InsP7 and InsP8 by coupling TiO2 enrichment with PAGE analysis negates the requirement for HPLC and 3H-inositol labelling. This new procedure therefore simplifies inositol pyrophosphate analysis in particular and also solves the often forgotten but ever-present problem of determining the labelling time necessary for metabolic equilibrium. Among the new research possibilities opened up by TiO2 purification is the analysis of inositol phosphates from animal tissues. Animal welfare and monetary considerations made this previously troublesome, as it would require treating the live animal with 3H-inositol tracer; analysis of human tissues and fluids was completely unattainable. The method described permits the extraction and analysis of inositol phosphates from animal organs including but not limited to mouse brain or liver, where InsP6 and InsP7 can be detected, or chicken egg white, where InsP5 and InsP6 are particularly abundant (Saiardi laboratory, unpublished data).
The ability of InsP3 to bind to TiO2 indicates that lower phosphorylated inositol species can also be purified from biological samples. As these stain poorly by toluidine blue they cannot be quantified by gel electrophoresis, but mass assays for other inositol phosphates exist that, coupled with TiO2 purification, can be used in quantifying lower phosphorylated inositol species from biological specimens. Beyond the soluble inositol phosphates, it will be interesting to take advantage of this newly discovered ability of TiO2 to bind and purify phosphate groups attached to an inositol ring to develop new inositol lipid purification methods, since the current approaches are essentially adaptations of Folch's extraction method, developed more than 60 years ago [32].
The parallel analysis of D. discoideum and mammalian cell extracts revealed quite different patterns of extracted molecules. While InsP6, InsP7 and InsP8 are extracted from both amoeba and mammalian cells, the nucleotides ATP and GTP are only visible in the mammalian extract. A quantitative comparison between the two experimental models is inappropriate since the number of cells extracted and analysed is different (in figure 2, 4 × 106 cells for D. discoideum were compared to 80 × 106 human HCT116 cells). However, the relative proportion between inositol pyrophosphate and nucleotides is unquestionably different in the two experimental models analysed. Since inositol pyrophosphates are able to regulate energetic metabolism and specifically are inversely connected with ATP level [27], we might speculate that the high levels of inositol pyrophosphates present in D. discoideum are lowering nucleotide levels. This hypothesis is currently under investigation.
We have also taken advantage of the high efficiency of TiO2 extraction to independently re-address the question of whether human body fluids such as plasma and urine contain any InsP6 (the relevance of this question is discussed below). In contrast with some other groups (e.g. [13,14]), but in agreement with an earlier study that used a specific and sensitive enzyme-based InsP6 assay [8], we find that there is no InsP6 present, with a detection limit more than an order of magnitude below the levels claimed to be there by others. We have carefully controlled for InsP6 recovery using InsP6-spiked controls (including adding exogenous InsP6 to whole fresh blood), and it is important to stress that our discovery of a highly active phosphatase in human (and bovine) plasma, probably secreted MINPP1 [30], which hydrolyses any spiked InsP6 within minutes at 37°C, in itself rules out the possibility of any InsP6 being present in plasma in vivo. Eiseman et al. [33] reported that in rats the half-life of intravenously injected radiolabelled InsP6 is 8 min, suggesting that the presence of an active InsP6 phosphatase in plasma is not confined to humans and cattle. Given the potent ion-chelating power of InsP6, it actually makes evolutionary sense to immediately remove such a compound (which might be released by cell lysis or damage) from extracellular fluids. We should add that the available evidence suggests that ingested InsP6, if absorbed through the gut, enters the blood plasma exclusively as inositol [33], with possibly also some small amounts of inositol monophosphate [34]; the dephosphorylation of InsP6 before absorption is apparently caused by gut flora [35].
These observations have relevance for the reported effects of dietary InsP6 on, for example, kidney stone formation [36,37] and other calcifications [38], or on cancer growth [39]. Understanding these effects does not need to invoke InsP6 in extracellular fluids, as they can readily be explained either by InsP6 acting as a chelator of cations (e.g. Ca2+, Fe3+) in the gut and thus altering uptake [8], or because InsP6 is a major source of our dietary inositol [8]. In the latter context, in studies on cancer where inositol has been compared directly with InsP6, the two have similar efficacies (e.g. [40] and see [39] for other references).
This discussion then points to a key question: if all the effects of dietary InsP6 (other than as a source of inositol) are mediated by modulating cation absorption from the gut, could taking InsP6 supplements ever be harmful? In humans on a poor diet the answer is clearly ‘yes’ [41], and there is a significant effort in the plant breeding world to produce low InsP6 varieties of maize and rice to reduce these deleterious effects [42]. Shamshuddin [43] has argued that the ion-chelating effect of InsP6 in the gut is not harmful in well-fed individuals, but this has only been examined for a few divalent cations (e.g. Zn2+ and Cu2+ [44,45]), while trivalent metals, whose affinity for InsP6 is very much higher than divalents [46] and which are essential dietary components (e.g. Cr3+ [47]), have not been studied in this context. Overall, this leads us to the conclusion that chronically altering cation absorption from the gut by artificially loading the diet with a non-specific chelator [39] in the hope that it might impact indirectly on cancer or other pathologies seems highly inadvisable.
Supplementary Material
Acknowledgements
We thank the members of the Saiardi laboratory for discussion, suggestions and helpful comments.
Ethics statement
All volunteers gave informed consent prior to donating blood. These experiments were carried out in accordance with the University of Cambridge Research Ethics Committee guidance ‘University of Cambridge Policy on the Ethics of Research Involving Human Participants and Personal Data’.
Author contributions
M.S.C.W., S.J.B., R.F.I. and A.S. designed the research. M.S.C.W., S.J.B., F.P. and A.S. performed experiments. M.S.C.W., S.J.B., R.F.I. and A.S. wrote the manuscript.
Funding statement
The work is supported by the Medical Research Council (MRC) core support to the MRC/UCL Laboratory for Molecular Cell Biology University Unit (MC_U122680443). S.J.B. was supported by the British Pharmacological Society and the Wellcome Trust.
Conflict of interests
The authors declare no competing financial interests.
References
- 1.Michell RH. 2008. Inositol derivatives: evolution and functions. Nat. Rev. Mol. Cell Biol. 9, 151–161 (doi:10.1038/nrm2334) [DOI] [PubMed] [Google Scholar]
- 2.Irvine RF, Schell MJ. 2001. Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2, 327–338 (doi:10.1038/35073015) [DOI] [PubMed] [Google Scholar]
- 3.Irvine RF. 2003. 20 years of Ins(1,4,5)P3, and 40 years before. Nat. Rev. Mol. Cell Biol. 4, 586–590. [DOI] [PubMed] [Google Scholar]
- 4.Saiardi A, Nagata E, Luo HR, Sawa A, Luo X, Snowman AM, Snyder SH. 2001. Mammalian inositol polyphosphate multikinase synthesizes inositol 1,4,5-trisphosphate and an inositol pyrophosphate. Proc. Natl Acad. Sci. USA 98, 2306–2311 (doi:10.1073/pnas.041614598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbsky JW, Wilson MP, Kisseleva MV, Majerus PW, Wente SR. 2002. The synthesis of inositol hexakisphosphate. Characterization of human inositol 1,3,4,5,6-pentakisphosphate 2-kinase. J. Biol. Chem. 277, 31 857–31 862 (doi:10.1074/jbc.M205682200) [DOI] [PubMed] [Google Scholar]
- 6.Raboy V. 2003. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 64, 1033–1043 (doi:10.1016/S0031-9422(03)00446-1) [DOI] [PubMed] [Google Scholar]
- 7.Shears SB. 2001. Assessing the omnipotence of inositol hexakisphosphate. Cell Signal 13, 151–158 (doi:10.1016/S0898-6568(01)00129-2) [DOI] [PubMed] [Google Scholar]
- 8.Letcher AJ, Schell MJ, Irvine RF. 2008. Do mammals make all their own inositol hexakisphosphate? Biochem. J. 416, 263–270 (doi:10.1042/BJ20081417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver KG, Putney JW, Jr, Obie JF, Shears SB. 1992. The interconversion of inositol 1,3,4,5,6-pentakisphosphate and inositol tetrakisphosphates in AR4-2J cells. J. Biol. Chem. 267, 21 528–21 534. [PubMed] [Google Scholar]
- 10.Pisani F, Livermore T, Rose G, Chubb JR, Gaspari M, Saiardi A. 2014. Analysis of Dictyostelium discoideum inositol pyrophosphate metabolism by gel electrophoresis. PLoS ONE 9, e85533 (doi:10.1371/journal.pone.0085533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephens LR, Irvine RF. 1990. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature 346, 580–583 (doi:10.1038/346580a0) [DOI] [PubMed] [Google Scholar]
- 12.Veiga N, Torres J, Dominguez S, Mederos A, Irvine RF, Diaz A, Kremer C. 2006. The behaviour of myo-inositol hexakisphosphate in the presence of magnesium(II) and calcium(II): protein-free soluble InsP6 is limited to 49 µM under cytosolic/nuclear conditions. J. Inorg. Biochem. 100, 1800–1810 (doi:10.1016/j.jinorgbio.2006.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grases F, Simonet BM, Prieto RM, March JG. 2001. Variation of InsP(4),InsP(5) and InsP(6) levels in tissues and biological fluids depending on dietary phytate. J. Nutr. Biochem. 12, 595–601 (doi:10.1016/S0955-2863(01)00178-4) [DOI] [PubMed] [Google Scholar]
- 14.Tur F, Tur E, Lentheric I, Mendoza P, Encabo M, Isern B, Grases F, Maraschiello C, Perello J. 2013. Validation of an LC-MS bioanalytical method for quantification of phytate levels in rat, dog and human plasma. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 928, 146–154 (doi:10.1016/j.jchromb.2013.03.023) [DOI] [PubMed] [Google Scholar]
- 15.Folkmann AW, Noble KN, Cole CN, Wente SR. 2011. Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export. Nucleus 2, 540–548 (doi:10.4161/nucl.2.6.17881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty A, Kim S, Snyder SH. 2011. Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 4, re1 (doi:10.1126/scisignal.2001958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shears SB. 2009. Diphosphoinositol polyphosphates: metabolic messengers? Mol. Pharmacol. 76, 236–252 (doi:10.1124/mol.109.055897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson MS, Livermore TM, Saiardi A. 2013. Inositol pyrophosphates: between signalling and metabolism. Biochem. J. 452, 369–379 (doi:10.1042/BJ20130118) [DOI] [PubMed] [Google Scholar]
- 19.Azevedo C, Saiardi A. 2006. Extraction and analysis of soluble inositol polyphosphates from yeast. Nat. Protoc. 1, 2416–2422 (doi:10.1038/nprot.2006.337) [DOI] [PubMed] [Google Scholar]
- 20.Losito O, Szijgyarto Z, Resnick AC, Saiardi A. 2009. Inositol pyrophosphates and their unique metabolic complexity: analysis by gel electrophoresis. PLoS ONE 4, e5580 (doi:10.1371/journal.pone.0005580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loss O, Azevedo C, Szijgyarto Z, Bosch D, Saiardi A. 2011. Preparation of quality inositol pyrophosphates. J. Vis. Exp. 55, e3027 (doi:10.3791/3027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvine RF. 2014. Absence of detectable inositol hexakisphosphate (phytate) in plasma. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 960, 253–254 (doi:10.1016/j.jchromb.2013.12.015) [DOI] [PubMed] [Google Scholar]
- 23.Perello J, Grases F. 2014. Phytate levels in biological fluids of mammals. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 960, 255–257 (doi:10.1016/j.jchromb.2013.12.016) [DOI] [PubMed] [Google Scholar]
- 24.Pinkse MW, Uitto PM, Hilhorst MJ, Ooms B, Heck AJ. 2004. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal. Chem. 76, 3935–3943 (doi:10.1021/ac0498617) [DOI] [PubMed] [Google Scholar]
- 25.Matsuda H, Nakamura H, Nakajima T. 1990. New ceramic Titania: selective adsorbent for organic phosphates. Anal. Sci. 6, 911–912 (doi:10.2116/analsci.6.911) [Google Scholar]
- 26.Menniti FS, Miller RN, Putney JW, Jr, Shears SB. 1993. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J. Biol. Chem. 268, 3850–3856. [PubMed] [Google Scholar]
- 27.Szijgyarto Z, Garedew A, Azevedo C, Saiardi A. 2011. Influence of inositol pyrophosphates on cellular energy dynamics. Science 334, 802–805 (doi:10.1126/science.1211908) [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty A, et al. 2010. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 (doi:10.1016/j.cell.2010.11.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wundenberg T, Mayr GW. 2012. Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol. Chem. 393, 979–998 (doi:10.1515/hsz-2012-0133) [DOI] [PubMed] [Google Scholar]
- 30.Windhorst S, Lin H, Blechner C, Fanick W, Brandt L, Brehm MA, Mayr GW. 2013. Tumour cells can employ extracellular Ins(1,2,3,4,5,6)P(6) and multiple inositol-polyphosphate phosphatase 1 (MINPP1) dephosphorylation to improve their proliferation. Biochem. J. 450, 115–125 (doi:10.1042/BJ20121524) [DOI] [PubMed] [Google Scholar]
- 31.Grases F, Isern B, Perello J, Sanchis P, Prieto RM, Costa-Bauza A. 2006. Absorption of myo-inositol hexakisphosphate (InsP6) through the skin in humans. Pharmazie 61, 652. [PubMed] [Google Scholar]
- 32.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- 33.Eiseman J, Lan J, Guo J, Joseph E, Vucenik I. 2011. Pharmacokinetics and tissue distribution of inositol hexaphosphate in C.B17 SCID mice bearing human breast cancer xenografts. Metabolism: Clin. Exp. 60, 1465–1474 (doi:10.1016/j.metabol.2011.02.015) [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto K, Vucenik I, Shamsuddin AM. 1993. [3H]phytic acid (inositol hexaphosphate) is absorbed and distributed to various tissues in rats. J. Nutr. 123, 713–720. [DOI] [PubMed] [Google Scholar]
- 35.Wise A, Gilburt DJ. 1982. Phytate hydrolysis by germfree and conventional rats. Appl. Environ. Microbiol. 43, 753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grases F, Garcia-Gonzalez R, Torres JJ, Llobera A. 1998. Effects of phytic acid on renal stone formation in rats. Scand. J. Urol. Nephrol. 32, 261–265 (doi:10.1080/003655998750015412) [DOI] [PubMed] [Google Scholar]
- 37.Curhan GC, Willett WC, Knight EL, Stampfer MJ. 2004. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 164, 885–891 (doi:10.1001/archinte.164.8.885) [DOI] [PubMed] [Google Scholar]
- 38.Grases F, Perello J, Prieto RM, Simonet BM, Torres JJ. 2004. Dietary myo-inositol hexaphosphate prevents dystrophic calcifications in soft tissues: a pilot study in Wistar rats. Life Sci. 75, 11–19 (doi:10.1016/j.lfs.2003.11.030) [DOI] [PubMed] [Google Scholar]
- 39.Vucenik I, Shamsuddin AM. 2006. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 55, 109–125 (doi:10.1207/s15327914nc5502_1) [DOI] [PubMed] [Google Scholar]
- 40.Vucenik I, Yang GY, Shamsuddin AM. 1995. Inositol hexaphosphate and inositol inhibit DMBA-induced rat mammary cancer. Carcinogenesis 16, 1055–1058 (doi:10.1093/carcin/16.5.1055) [DOI] [PubMed] [Google Scholar]
- 41.Raboy V. 2008. Demonizing phytate. Nat. Biotechnol. 26, 497–498 (doi:10.1038/nbt0508-497) [DOI] [PubMed] [Google Scholar]
- 42.Raboy V. 2007. The ABCs of low-phytate crops. Nat. Biotechnol. 25, 874–875 (doi:10.1038/nbt0807-874) [DOI] [PubMed] [Google Scholar]
- 43.Shamsuddin AM. 2008. Demonizing phytate. Nat. Biotechnol. 26, 496–497 (doi:10.1038/nbt0508-496b) [DOI] [PubMed] [Google Scholar]
- 44.Grases F, Simonet BM, Perello J, Costa-Bauza A, Prieto RM. 2004. Effect of phytate on element bioavailability in the second generation of rats. J. Trace Elements Med. Biol. 17, 229–234 (doi:10.1016/S0946-672X(04)80023-3) [DOI] [PubMed] [Google Scholar]
- 45.Harland B. 2006. Rat plasma copper and zinc concentrations were not negatively affected by high phytate content of ‘Black Seed’. FASEB J. 20, A196. [Google Scholar]
- 46.Torres J, Dominguez S, Cerda MF, Obal G, Mederos A, Irvine RF, Diaz A, Kremer C. 2005. Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J. Inorg. Biochem. 99, 828–840 (doi:10.1016/j.jinorgbio.2004.12.011) [DOI] [PubMed] [Google Scholar]
- 47.Fessler TA. 2013. Trace elements in parenteral nutrition: a practical guide for dosage and monitoring for adult patients. Nutr. Clin. Practice 28, 722–729 (doi:10.1177/0884533613506596) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.